02 July 2020: Clinical Research
Tumor Location and Survival Outcomes in Lung Adenosquamous Carcinoma: A Propensity Score Matched Analysis
Xinlin Shi1ABCEF, Xiangrong Shao1B, Yawen Zhang1BC, Feng Wu1AE*, Yujian Tao1ABDOI: 10.12659/MSM.922138
Med Sci Monit 2020; 26:e922138
Abstract
BACKGROUND: There is little information in the literature available on lung adenosquamous carcinoma (LASC). The association between tumor location and survival outcomes in LASC is poorly understood. Our study was designed to probe the effect of tumor location on survival outcomes of LASC.
MATERIAL AND METHODS: Patients with LASC between 2004 and 2015 were identified using the Surveillance, Epidemiology and End Results (SEER) databases. The patients were divided into 2 groups, a main bronchus group and a peripheral group, according to their primary sites. The Propensity Score Matching (PSM) method was used to reduce possible bias between groups. The primary endpoints were overall survival (OS) and cancer-specific survival (CSS).
RESULTS: A total of 3176 patients, afflicted with LASC between 2004 and 2015, were extracted from the SEER databases. Of these, 212 patients were found to be eligible for analysis after a propensity 1: 1 nearest neighbor matched analysis. After PSM, multivariate Cox regression analysis showed that primary site, American Joint Committee on Cancer (AJCC) stage, T stage and surgery were independent predictors of LASC in both OS and CSS. Kaplan-Meier survival analysis showed that patients with LASC located in a peripheral site had better survival outcomes than those with LASC located in the main bronchus. In subgroup analysis, the advantages of tumor located in a peripheral site were more pronounced in female patients and AJCC stage I patients.
CONCLUSIONS: Tumor location may have an impact on the survival outcomes of patients with LASC. Patients with LASC located in a peripheral site had better survival outcomes than patients with LASC located in the main bronchus, particularly in female patients and AJCC stage I patients.
Keywords: Carcinoma, Adenosquamous, Location Directories and Signs, SEER Program, Adenocarcinoma of Lung, Lung, Multivariate Analysis, Neoplasm Grading, propensity score
Background
Lung cancer is one of the most common devastating tumors with a high incidence and mortality rate worldwide [1]. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are the major histopathologic types of lung cancer. NSCLC usually includes adenocarcinoma (AC), squamous cell carcinoma (SCC), and adenosquamous carcinoma (ASC). According to the 2015 WHO pathological classification, lung adenosquamous carcinoma (LASC) has been categorized as a carcinoma; it contains components of both AC and SCC with each component constituting at least 10% of the tumor [2]. The proportion of AC or SCC is also an essential factor that affects the prognosis of LASC; the predominant histological subtype of AC may be an independent prognostic factor for LASC [3]. LASC is widely known as an extremely rare subtype of lung cancer, only accounting for approximately 0.4–4% of all lung carcinoma [4,5].
Because of the low incidence of LASC and a subsequent lack of large-scale clinical outcome data, there is little information currently available on LASC. However, previous studies have shown that LASC is relatively aggressive and has worse survival outcomes compared with AC and SCC [6,7]. Although LASC has the same clinical manifestations as other subtypes of NSCLC, it was usually diagnosed at an advanced stage due to its high frequency of lymph node metastasis, vascular invasion, and parietal pleural involvement [8,9]. Age, sex, histologic grade, and tumor stage are considered to be potential prognostic factors, but the effects of tumor location on survival outcomes of LASC are still poorly understood. Thus, it is desirable to conduct large-scale multicenter clinical studies to evaluate the effect of tumor location on survival outcomes of patients with LASC. The SEER database is an open database that consists of 18 population-based cancer registries and has numerous case series representing 30% of the US population [10]. We analyzed the data extracted from the SEER databases through regular and Propensity Score Matching (PSM) methods.
Material and Methods
ETHICS STATEMENT:
We conducted this study by acquiring clinical data from the SEER databases; the username of the SEER database is 12158-Nov2018. SEER*Stat 8.3.5 software was applied for extraction. Due to the openness of the SEER databases, our research was exempted from the need for approval by the Ethics Committee of the Affiliated Hospital of Yangzhou University.
DATA EXTRACTION:
Data of all the patients with LASC between 2004 and 2015 were extracted from the SEER databases. Inclusion criteria for our study were listed as follows: 1) the histology/behavior codes (ICD-0-3 8560/3) and restriction on site recode ICD-0-3/WHO 2008 (International Classification of Diseases for Oncology, Third Edition) to “Lung and Bronchus” were applied to confirm LASC; 2) patients with year of diagnosis were between 2004 and 2015; 3) patients with active follow-ups; 4) patients with survival time more than 0 days. Exclusion criteria were as follows: 1) patients with the history of other tumors; 2) patients without accurate diagnosis based on pathological confirmation; 3) patients with unknown clinical characteristics information including age, race, sex, tumor primary site, American Joint Committee on Cancer (AJCC) stage group, cause of death, surgery, radiation and chemotherapy; and 4) patients with incomplete survival data.
Covariates were listed as follows: age, sex, primary site, grade, TNM stage (6th edition), cancer-specific death, surgery, radiation, chemotherapy, survival month and vital status. The primary endpoints were overall survival (OS) and cancer-special survival (CSS).
A total of 3176 patients with LASC were recruited in this study and were divided into 2 groups, according to tumor location. In our study, the main bronchus was defined as a tumor located in primary bronchus, including left or right primary bronchus; the peripheral site was defined as a tumor located in a position other than primary bronchus including upper lobe, middle lobe, lower lobe, and overlapping lesion of the lung. The group of main bronchus contains 107 patients, and the peripheral group contains 3069 patients.
PROPENSITY SCORE MATCHING (PSM):
The Propensity Score Matching (PSM) method was used to reduce potential bias between the main bronchus group and the peripheral group. In our study, we conducted a propensity 1: 1 nearest neighbor matched analysis to minimize potential bias between groups. The propensity score was calculated using the logistic regression model. Chi-squared tests were used to examine the covariate balance before and after PSM.
STATISTICAL ANALYSIS:
In our study, OS and CSS were calculated using Kaplan-Meier survival analysis. A chi-squared test was conducted to test the statistical difference of age, sex, grade, AJCC stage, AJCC T stage, AJCC N stage, AJCC M stage, surgery, radiation and chemotherapy between the groups.
Univariate and multivariate Cox proportional hazards regression models were used to evaluate the risk of mortality and conduct subgroup analyses. All statistical analyses were conducted with SPSS 22.0 (Chicago, IL, USA). GraphPad-Prism 8.0 software was used to generate survival curves. Microsoft Excel 2016 software was used to generate a forest plot.
Results
BASELINE CHARACTERISTICS OF PATIENTS WITH LASC BEFORE AND AFTER PSM:
A total of 3176 patients were selected for this study before PSM, 212 were deemed eligible for analysis after a propensity 1: 1 nearest neighbor matched analysis. According to primary tumor sites, the 212 patients were divided into 2 groups, with each group containing 106 patients. Baseline characteristics of patients before PSM and after PSM are shown in Table 1. Such characteristics included: age, sex, race, AJCC stage, T stage, N stage, M stage, surgery, and radiation and chemotherapy. Before PSM, covariates-such as age, grade, AJCC stage, T stage, N stage, M stage, surgery, radiation, and chemotherapy had a significant difference between groups(all P-value <0.05). After PSM, there were no major differences in the multiple variables that were compared between the main bronchus group and the peripheral group (all P-value >0.05).
SURVIVAL OUTCOMES OF PATIENTS WITH LASC BEFORE AND AFTER PSM:
Before PSM, the 5-year OS and CSS rates were 25.44% and 32.10%, respectively. The OS and CSS curves are shown in Figure 1. The peripheral group had better survival outcomes compared with the main bronchus group both in OS and CSS (Figure 2). The 5-year OS and CSS rates in the peripheral group were 26.09% and 32.89%, and in main bronchus group was 7.06% and 8.79%. After PSM, curves of OS and CSS were shown in Figure 3. Similar results between groups were also obtained for the survival curve (Figure 4). The 5-year OS and CSS rates in the peripheral group were 10.77% and 18.02%, and in the main bronchus group were 7.13% and 8.89%.
UNIVARIATE AND MULTIVARIATE ANALYSIS AFTER 1: 1 PSM ANALYSIS:
After PSM, factors including age, sex, race, primary site, grade, AJCC stage, T stage, N stage, M stage, surgery, radiation, and chemotherapy were selected as factors with which to conduct univariate survival analysis. Upon univariate analyses, the presence of factors-including primary sites, AJCC stage, T stage, M stage, surgery and chemotherapy were the predictive prognosis of patient survival outcomes (all P-value >0.05). These factors were associated with OS and CSS (Tables 2, 3). Tumors located in the main bronchus, advanced cancer stage, T4 stage, M1 stage, without surgery, and without chemotherapy were associated with worse survival outcomes both in OS and CSS. Cox regression analysis was performed to identify independent predictors of LASC. Significant univariate factors were additionally analyzed in regression models. Multivariate Cox regression analysis showed that the primary site, AJCC stage, T stage and surgery were independent predictors of LASC both in OS and CSS (P-value <0.05).
SUBGROUP ANALYSIS FOR OS AND CSS AFTER 1: 1 PSM ANALYSIS:
Subgroup analysis showed that a tumor located in a peripheral location was associated with better survival outcomes (Figures 5, 6). OS was statistically significant in the subgroups stratified by female (hazard ratio [HR]: 1.510, 95% confidence interval [CI]: 1.001–2.277, P=0.039); other race (HR: 0.173, 95% CI: 0.008–3.515, P=0.008); unknown stage (HR: 1.561, 95% CI: 1.008–2.418, P=0.030); AJCC stage I (HR: 5.022, 95% CI: 0.626–40.310, P=0.025); T4 stage (HR: 1.475, 95% CI: 0.978–2.225, P=0.047); undergoing radiation (HR: 1.606, 95% CI: 0.992–2.2559, P=0.035). CSS was statistically significant in the subgroups stratified by female (HR: 1.599, 95% CI: 1.017–2.390, P=0.033); white race (HR: 1.437, 95% CI: 1.028–2.010, P=0.025); other race (HR: 0.173, 95% CI: 0.008–3.515, P=0.008); AJCC stage I (HR: 7.435, 95% CI: 0.853–64.810, P=0.025); without chemotherapy (HR: 1.571, 95% CI: 1.044–3.142, P=0.034). All subgroup analysis of OS and CSS showed survival rates in favor of tumors located in peripheral sites, especially for female and AJCC stage I patients.
Discussion
LASC is a rare subgroup of lung cancer, accounting for only 0.4–4% of all lung carcinomas [7,11]. Little information is available on the clinical characteristics of LASC due to its low incidence [12]. Additionally, LASC is more challenging to diagnose than AC and SCC since it is a mixture of the 2. A low under sampling puncture can lead to an incorrect diagnosis of squamous cell carcinoma or adenocarcinoma. Instead, the key to a successful diagnosis lies in pathological diagnosis by cytology or tissue, and resection is a relatively safe and reliable diagnostic method. The accuracy of non-resection approaches for the diagnosis of LASC is relatively low, and many LASC patients may miss the best time for interventions because of the non-resected biopsies. Uramoto et al. described a case of pulmonary adenosquamous carcinoma, which was confirmed via histological examination of the resection specimen that showed only adenocarcinoma in the biopsy and only squamous cell carcinoma in the bronchial wash and brushing specimens [13]. Therefore, the correct tumor classification of diagnosis is necessary.
Some studies have been shown that LASC was more commonly seen in male smokers who were over the age of 65 years old [6,14]. In our study, the number of enrolled patients with age over 60 years was 2498 (78.65%); this was a similar result in a review conducted by Li and Lu [15]. Besides, the male-to-female ratio was 1.19: 1 (1729 versus 1447) in our research, which was lower than the previously reported in the literature. However, a report comes from SEER databases showed a similar result: the male-to-female ratio was 1.24: 1 (2349 versus 1896) [16]. Considering that both studies included more than 3000 patients, we have reason to believe that the data of this research are worthy of belief. The primary condition of patients, their pathological grade, and additional therapy received are favorable prognostic factors for LASC, which are parallel to our study. According to multivariate analysis, primary site, AJCC stage, T stage and surgery were all independent predictors of LASC. Besides, the proportion of AC or SCC is also an essential factor that affects the prognosis of LASC. LASC, with balanced adenomatous and squamous components, was associated with better survival outcomes than those with one predominant component [9,17]. However, in contrast to these results, some authors also observed that SCC-predominant histology represents a better prognosis of ASC [18]. Therefore, further research on this topic is warranted.
In our study, the 5-year OS rate was 25.44%, which is similar to previously reported ranged from 6.2% to 25.4% [19]. Compared with AC or SCC, LASC usually Nakagawa et al. found that LASC also had a significantly poorer prognosis than AC or SCC (the 5-survival rate was 6.2% versus 41.5%) [20]. Besides, LASC tended to be diagnosed at a more advanced stage, with a high rate of lymph node metastasis, more frequent vascular invasion, and parietal pleural involvement than AC [21]. Similarly, high cell grading, advanced stage, and intratumoral perineural invasion were more prevalent in ASC than the single histology tumor [22]. Surgery is the main treatment of LASC, but the rate of survival at post-surgery is unsatisfactory. In comparison, Maeda et al. had shown that the 5-year survival rate was 23.3% for ASC patients, 58% for AC patients, and 40.8% for SCC patients [6]. The site of lesion is essential for the decision of surgery, and there may be a few mortalities related or consequences of surgery. In our study, the proportion of operation in main bronchus group and peripheral group was 12.14% versus 38.64%, according to subgroup analysis results, surgery is not the major factor for the less OS and CSS between 2 groups(
To our knowledge, this is the first paper to describe tumor location relating to the survival outcomes of LASC. In our study, Kaplan-Meier survival analysis and subgroup analysis showed that patients with LASC located in peripheral sites had better survival outcomes than those located in the main bronchus. The 5-year survival rates of OS and CSS in the peripheral group were higher than in the main bronchus group. Patients of LASC, particularly female patients, other race, AJCC stage I, and those with peripherally located tumors, have a significantly better prognosis. The possible reasons are as follows. First, Watanabe et al. found that larger tumor size, subjective symptoms, and a more advanced disease stage (stage III or stage IV) were more prevalent among main bronchus located LASC than among peripherally located LASC [14]. Second, the frequency of obstructive pneumonia was higher in patients with main bronchus-located tumors than in those with peripherally located tumors, which suggests a poor prognosis [27]. The incidence of LASC varies significantly in different locations, and tumors are most often deemed to be peripheral by radiology [27]. LASC might originate from a peripherally located cancer stem cell [8]. Third, in a previous report, it was shown that main bronchus located LASC had a larger SCC component than peripherally located LASC [27], and the proportion of AC or SCC may have an impact on survival outcomes of LASC. Last but not least, higher SUVmax was also a significant prognostic factor for the recurrence of LASC [7]. Maximum standardized uptake value (SUVmax) is higher in main bronchus-located tumors than in peripherally located tumors. Shimoji et al. found that the SUVmax of main bronchus tumors ranged from 2.0 to 24.5, with a median SUV max of 9.3. In comparison, they found that the median SUVmax of the peripheral tumors was 8.1, which was significantly lower than that of the central tumors [28]. This is in keeping with the results of the previous studies in NSCLC [29].
Not surprisingly, in patients with lung adenocarcinoma, tumor location can be used as a useful predictor for lymph node metastasis and survival outcomes [30,31]. Adenocarcinoma of the lung located in a central location had a high risk of lymph node metastasis and poor prognosis. The situation is different for lung squamous cell carcinoma. Serval studies have shown that tumor location was not significantly associated with OS in lung squamous cell carcinoma [32–34]. However, peripheral squamous cell carcinoma of the lung has advantages compared with central lung squamous cell carcinoma, such as an earlier stage, good differentiation, less pleural invasion, less frequent lymph node metastasis, and lymphovascular invasion.
Several limitations of our study deserve mention. First, there was a lack of blood index data, such as tumor markers like carcinoembryonic antigen, cytokeratin-19-fragment; besides, genetic mutation detection (including EGFR, KRAS, ROS1, and BRAF mutation) is very popular among NSCLC patients, which can provide guidance for clinicians to make a reasonable treatment plan. Second, information regarding patients’ comorbidities and smoking history is not included because they are not available in SEER databases. Third, this was a retrospective study, as opposed to a randomized experiment. Fourth, PSM might increase the bias due to matching as it does not account for dormant and unobserved confounding variables.
Conclusions
Tumor location may have an impact on the survival outcomes of LASC. Patients with LASC located in a peripheral site had better survival outcomes than those located in main bronchus, particularly in female and AJCC stage I patients.
Figures
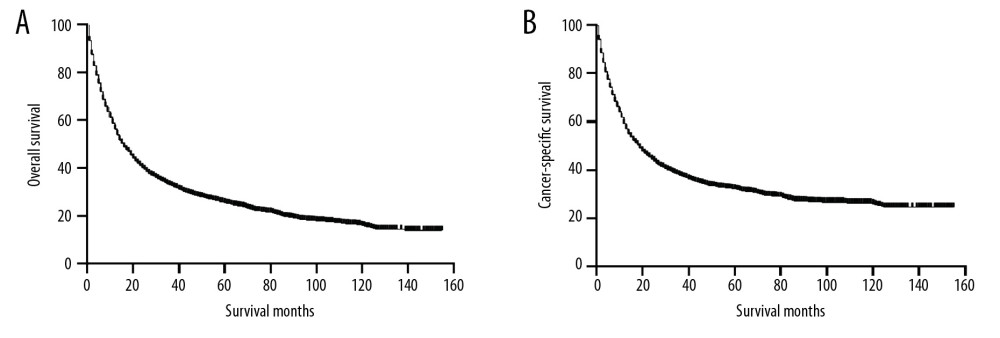 Figure 1. Kaplan-Meier curves of OS (A) and CSS (B) before PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 1. Kaplan-Meier curves of OS (A) and CSS (B) before PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. 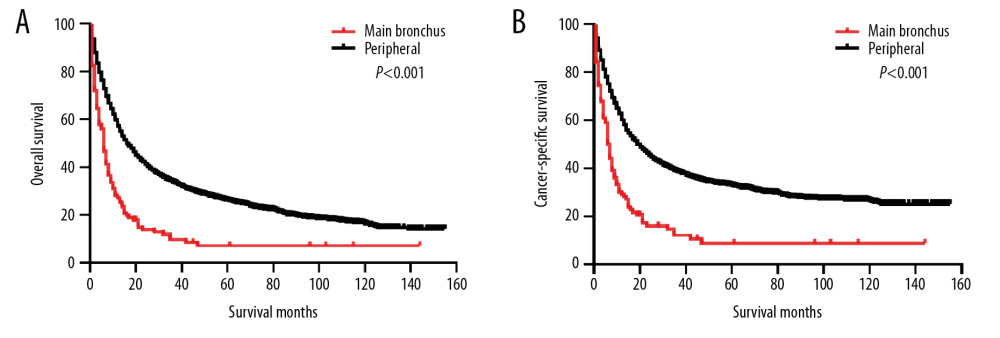 Figure 2. Kaplan-Meier curves of OS and CSS before PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, before 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 2. Kaplan-Meier curves of OS and CSS before PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, before 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. 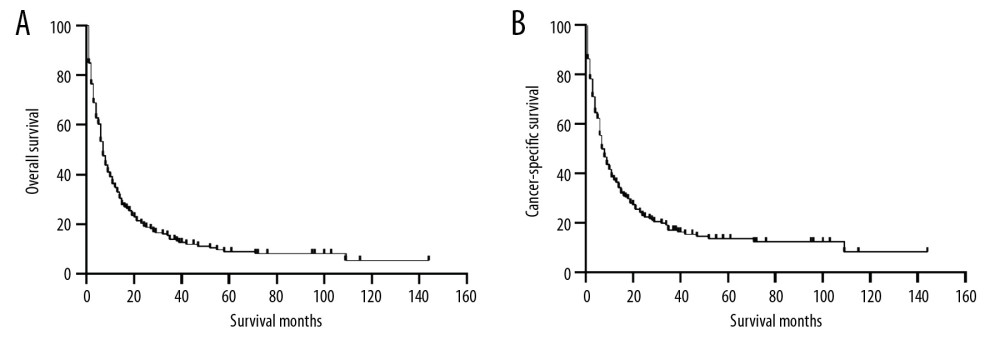 Figure 3. Kaplan-Meier curves of OS (A) and CSS (B) after PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 3. Kaplan-Meier curves of OS (A) and CSS (B) after PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. 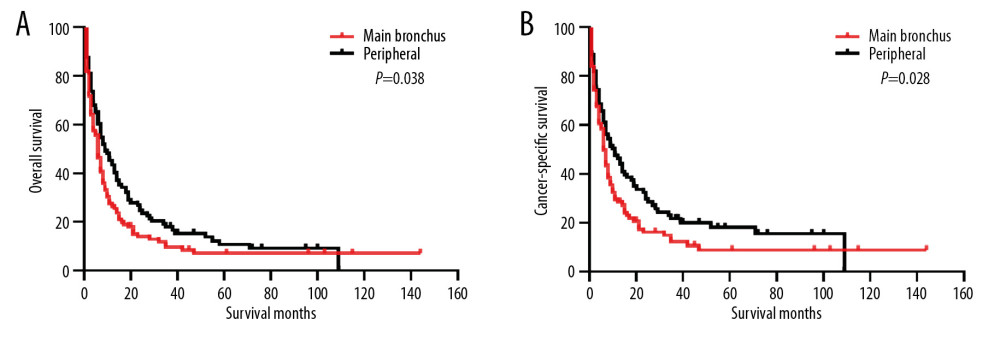 Figure 4. Kaplan-Meier curves of OS and CSS after PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, after 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 4. Kaplan-Meier curves of OS and CSS after PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, after 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. 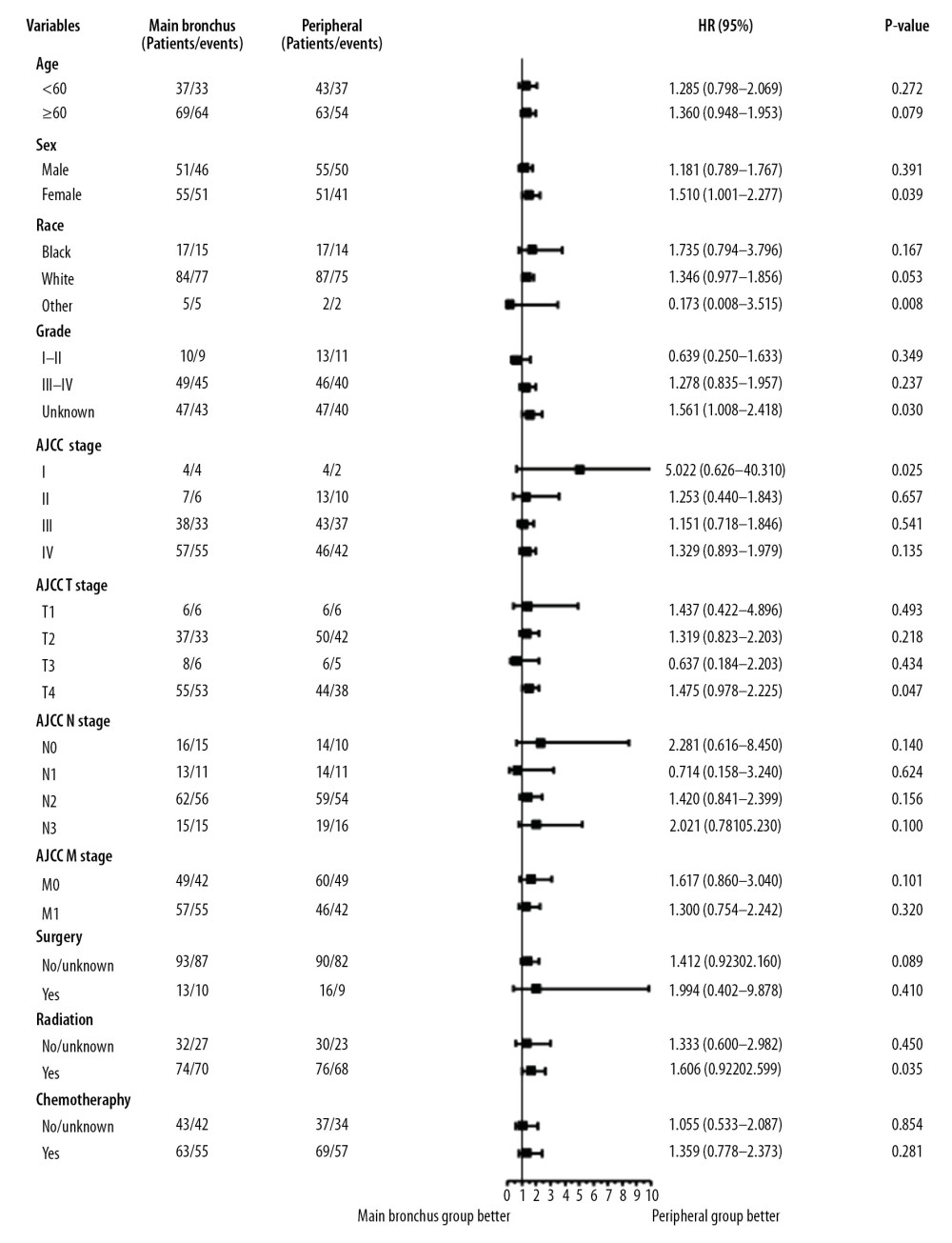 Figure 5. Subgroup analysis between main bronchus and peripheral groups for OS after PSM. OS – overall survival; PSM – Propensity Score Matching.
Figure 5. Subgroup analysis between main bronchus and peripheral groups for OS after PSM. OS – overall survival; PSM – Propensity Score Matching. 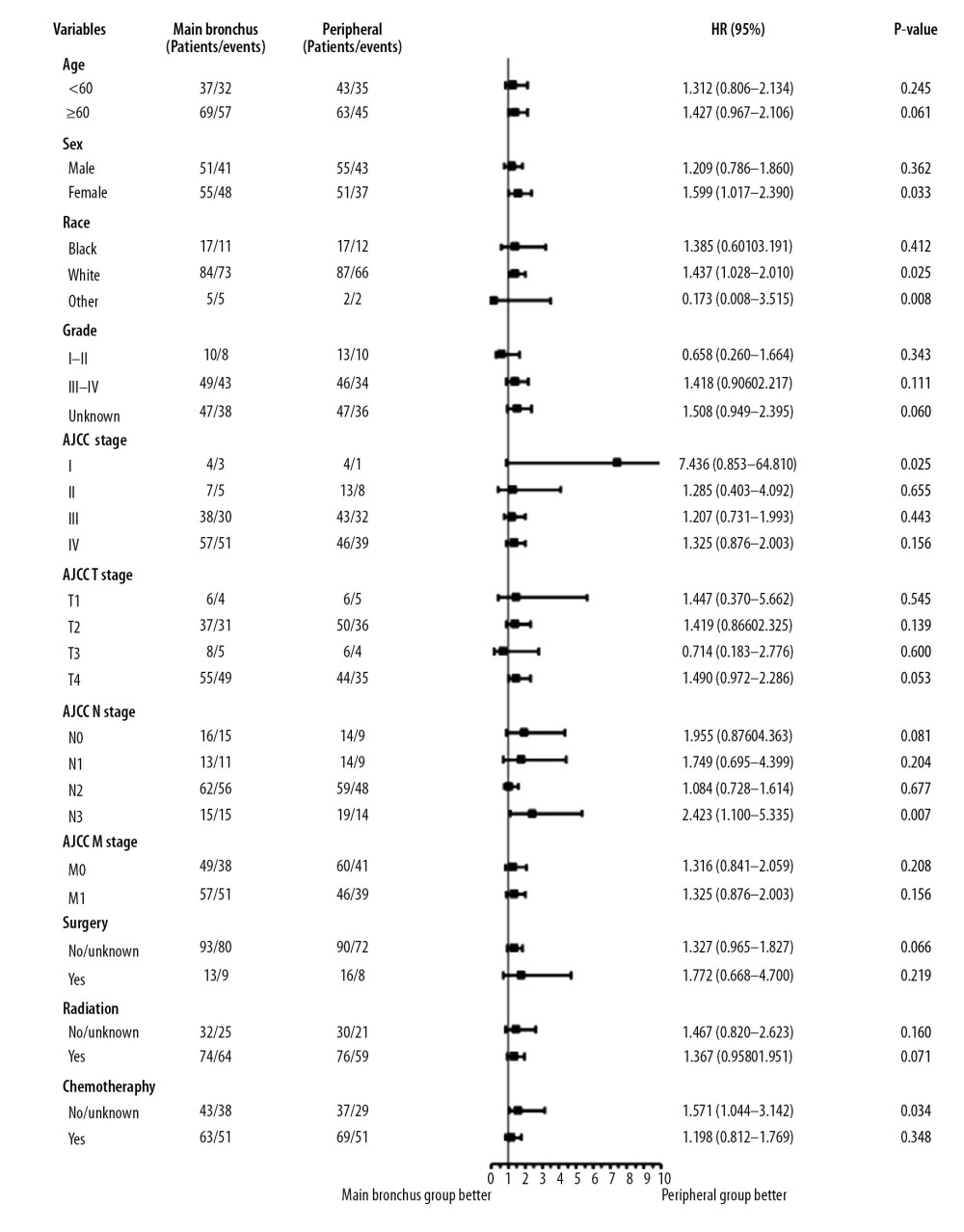 Figure 6. Subgroup analysis between main bronchus and peripheral groups for CSS after PSM. CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 6. Subgroup analysis between main bronchus and peripheral groups for CSS after PSM. CSS – cancer-specific survival; PSM – Propensity Score Matching. References
1. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
2. Travis WD, Brambilla E, Burke AP, Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart: J Thorac Oncol, 2015; 10(9); 1240-42
3. Zhu L, Jiang L, Yang J, Clinical characteristics and prognosis of patients with lung adenosquamous carcinoma after surgical resection: results from two institutes: J Thorac Dis, 2018; 10(4); 2397-402
4. Teng XDWorld Health Organization classification of tumours, pathology and genetics of tumours of the lung: Zhonghua Bing Li Xue Za Zhi, 2005; 34(8); 544-46 [in Chinese]
5. Rao N, Adenosquamous carcinoma: Semin Diagn Pathol, 2014; 31(4); 271-77
6. Maeda H, Matsumura A, Kawabata T, Adenosquamous carcinoma of the lung: Surgical results as compared with squamous cell and adenocarcinoma cases: Eur J Cardiothorac Surg, 2012; 41(2); 357-61
7. Filosso PL, Ruffini E, Asioli S, Adenosquamous lung carcinomas: A histologic subtype with poor prognosis: Lung Cancer, 2011; 74(1); 25-29
8. Lee Y, Chung JH, Kim SE, Adenosquamous carcinoma of the lung: CT, FDG PET, and clinicopathologic findings: Clin Nucl Med, 2014; 39(2); 107-12
9. Mordant P, Grand B, Cazes A, Adenosquamous carcinoma of the lung: Surgical management, pathologic characteristics, and prognostic implications: Ann Thorac Surg, 2013; 95(4); 1189-95
10. Duggan MA, Anderson WF, Altekruse S, The Surveillance, Epidemiology, and End Results (SEER) program and pathology: Toward strengthening the critical relationship: Am J Surg Pathol, 2016; 40(12); e94-102
11. Takamori S, Noguchi M, Morinaga S, Clinicopathologic characteristics of adenosquamous carcinoma of the lung: Cancer, 1991; 67(3); 649-54
12. Naunheim KS, Taylor JR, Skosey C, Adenosquamous lung carcinoma: Clinical characteristics, treatment, and prognosis: Ann Thorac Surg, 1987; 44(5); 462-66
13. Uramoto H, Yamada S, Hanagiri T, Clinicopathological characteristics of resected adenosquamous cell carcinoma of the lung: Risk of coexistent double cancer: J Cardiothorac Surg, 2010; 5; 92
14. Watanabe Y, Tsuta K, Kusumoto M, Clinicopathologic features and computed tomographic findings of 52 surgically resected adenosquamous carcinomas of the lung: Ann Thorac Surg, 2014; 97(1); 245-51
15. Li C, Lu H, Adenosquamous carcinoma of the lung: Onco Targets Ther, 2018; 11; 4829-35
16. Wang J, Lian B, Ye L, Clinicopathological characteristics and survival outcomes in adenosquamous carcinoma of the lung: A population-based study from the SEER database: Oncotarget, 2018; 9(8); 8133-46
17. Zhao H, Yang H, Yao F, Improved survival associated with a balanced structure between adenomatous and squamous components in patients with adenosquamous carcinoma of the lung: Eur J Surg Oncol, 2016; 42(11); 1699-706
18. Guo Y, Jia L, Shao GG, Clinicopathological characteristics and prognosis of patients with adenosquamous lung carcinoma: J Huazhong Univ Sci Technolog Med Sci, 2015; 35(3); 350-55
19. Pan F, Cui S, Wang W, Survival analysis for lung adenosquamous carcinoma patients with brain metastasis: J Cancer, 2018; 9(20); 3707-12
20. Nakagawa K, Yasumitu T, Fukuhara K, Poor prognosis after lung resection for patients with adenosquamous carcinoma of the lung: Ann Thorac Surg, 2003; 75(6); 1740-44
21. Cakir E, Demirag E, Aydin M, Unsal E, Clinicopathologic features and prognostic significance of lung tumours with mixed histologic patterns: Acta Chir Belg, 2009; 109(4); 489-93
22. Ruffini E, Rena O, Oliaro A, Lung tumors with mixed histologic pattern. Clinico-pathologic characteristics and prognostic significance: Eur J Cardiothorac Surg, 2002; 22(5); 701-7
23. Zhang C, Yang H, Lang B, Surgical significance and efficacy of epidermal growth factor receptor tyrosine kinase inhibitors in patients with primary lung adenosquamous carcinoma: Cancer Manag Res, 2018; 10; 2401-7
24. Fan L, Yang H, Yao F, Clinical outcomes of epidermal growth factor receptor tyrosine kinase inhibitors in recurrent adenosquamous carcinoma of the lung after resection: Onco Targets Ther, 2017; 10; 239-45
25. Morodomi Y, Okamoto T, Takenoyama M, Clinical significance of detecting somatic gene mutations in surgically resected adenosquamous cell carcinoma of the lung in japanese patients: Ann Surg Oncol, 2015; 22(8); 2593-98
26. Hu M, Zhang B, Xu J, Clinical outcomes of different generations of EGFR Tyrosine kinase inhibitors in advanced lung adenosquamous carcinoma: Mol Diagn Ther, 2019; 23(6); 773-79
27. Ishida T, Kaneko S, Yokoyama H, Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features: Am J Clin Pathol, 1992; 97(5); 678-85
28. Shimoji M, Nakajima T, Yamatani C, A clinicopathological and immunohistological re-evaluation of adenosquamous carcinoma of the lung: Pathol Int, 2011; 61(12); 717-22
29. Al-Sarraf N, Gately K, Lucey J, Clinical implication and prognostic significance of standardised uptake value of primary non-small cell lung cancer on positron emission tomography: Analysis of 176 cases: Eur J Cardiothorac Surg, 2008; 34(4); 892-97
30. Sun W, Yang X, Liu Y, Primary tumor location is a useful predictor for lymph node metastasis and prognosis in lung adenocarcinoma: Clin Lung Cancer, 2017; 18(1); e49-55
31. Ketchedjian A, Daly BD, Fernando HC, Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma: J Thorac Cardiovasc Surg, 2006; 132(3); 544-48
32. Lin MW, Huang YL, Yang CY, The differences in clinicopathologic and prognostic characteristics between surgically resected peripheral and central lung squamous cell carcinoma: Ann Surg Oncol, 2019; 26(1); 217-29
33. Funai K, Yokose T, Ishii G, Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung: Am J Surg Pathol, 2003; 27(7); 978-84
34. Hayashi T, Sano H, Egashira R, Difference of morphology and immunophenotype between central and peripheral squamous cell carcinomas of the lung: Biomed Res Int, 2013; 2013 157838
Figures
 Figure 1. Kaplan-Meier curves of OS (A) and CSS (B) before PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 1. Kaplan-Meier curves of OS (A) and CSS (B) before PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. Figure 2. Kaplan-Meier curves of OS and CSS before PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, before 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 2. Kaplan-Meier curves of OS and CSS before PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, before 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. Figure 3. Kaplan-Meier curves of OS (A) and CSS (B) after PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 3. Kaplan-Meier curves of OS (A) and CSS (B) after PSM. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. Figure 4. Kaplan-Meier curves of OS and CSS after PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, after 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 4. Kaplan-Meier curves of OS and CSS after PSM. OS (A) and CSS (B) between main bronchus and peripheral groups, after 1: 1 PSM analysis. OS – overall survival; CSS – cancer-specific survival; PSM – Propensity Score Matching. Figure 5. Subgroup analysis between main bronchus and peripheral groups for OS after PSM. OS – overall survival; PSM – Propensity Score Matching.
Figure 5. Subgroup analysis between main bronchus and peripheral groups for OS after PSM. OS – overall survival; PSM – Propensity Score Matching. Figure 6. Subgroup analysis between main bronchus and peripheral groups for CSS after PSM. CSS – cancer-specific survival; PSM – Propensity Score Matching.
Figure 6. Subgroup analysis between main bronchus and peripheral groups for CSS after PSM. CSS – cancer-specific survival; PSM – Propensity Score Matching. Tables
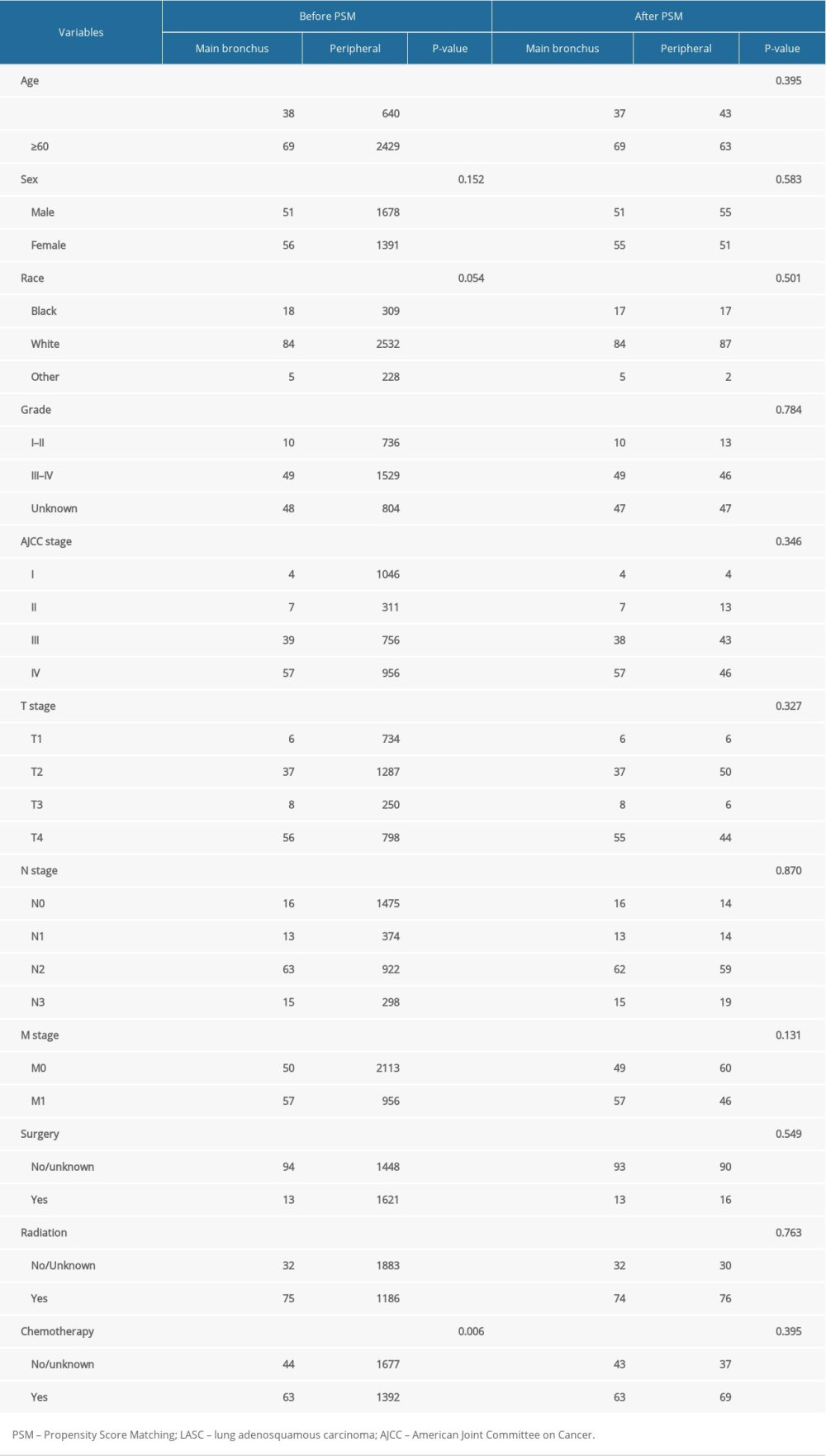 Table 1. Baseline characteristics for patients with LASC before and after PSM.
Table 1. Baseline characteristics for patients with LASC before and after PSM.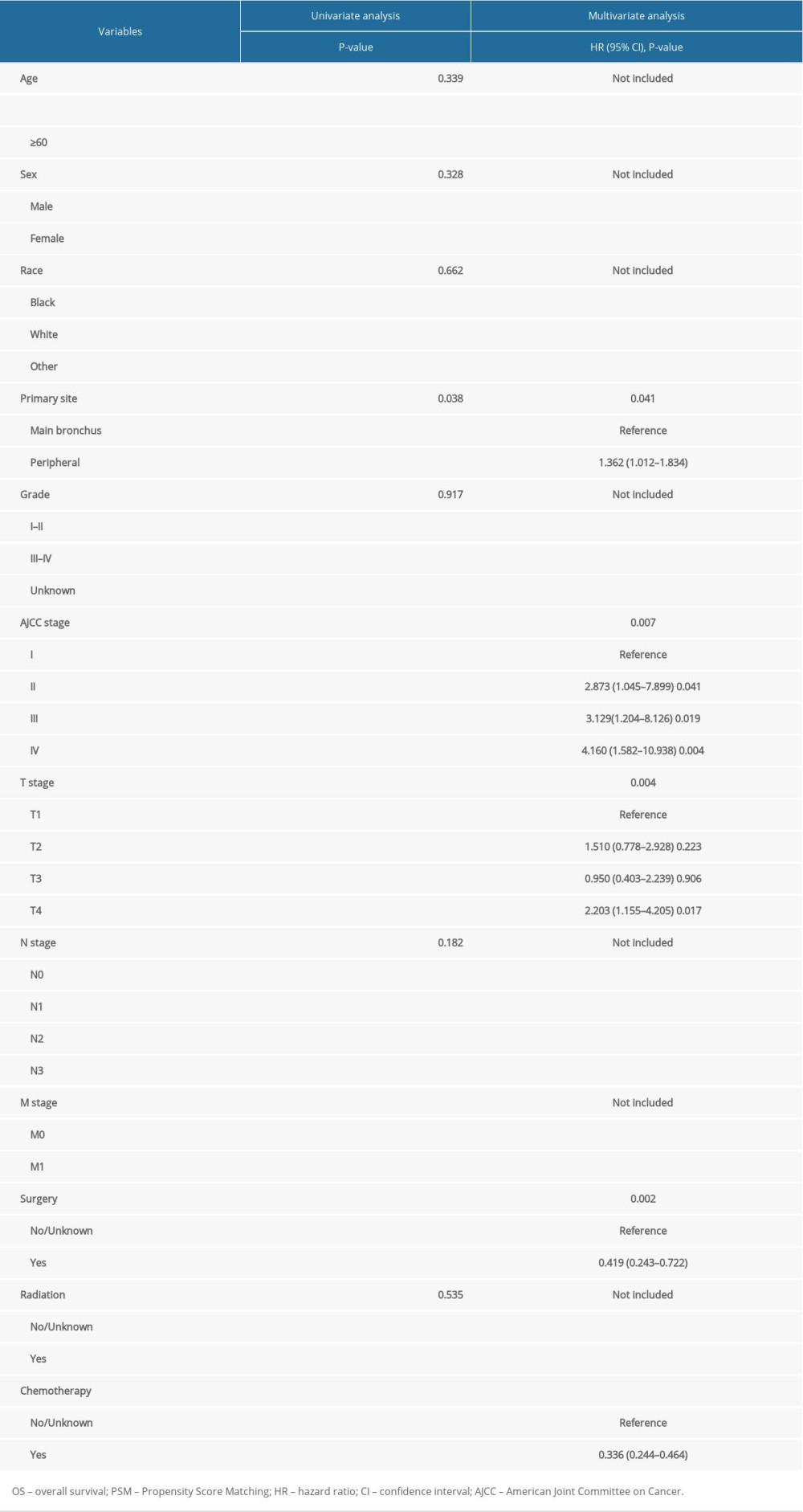 Table 2. Univariate and multivariate analyses of OS after PSM.
Table 2. Univariate and multivariate analyses of OS after PSM.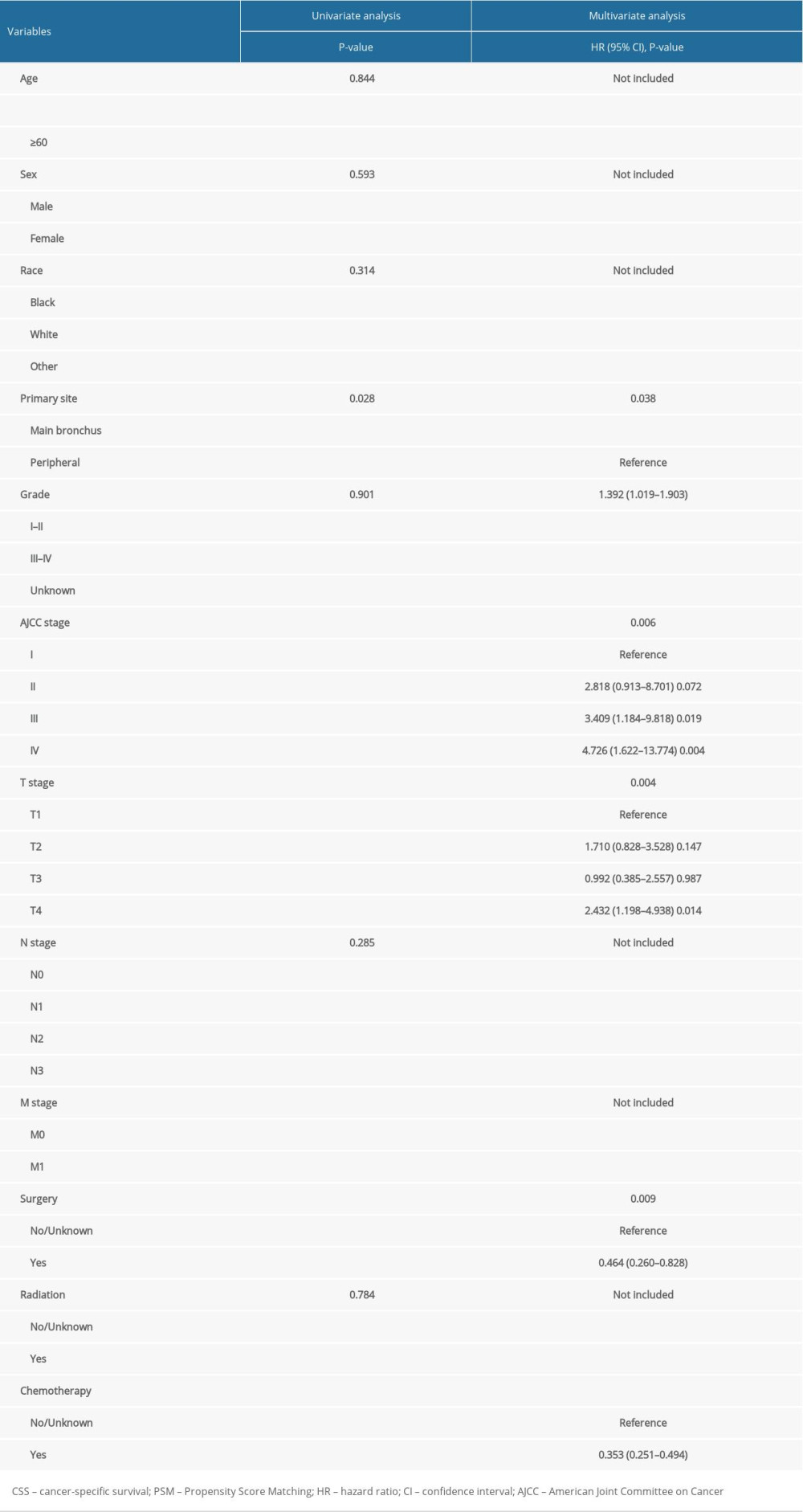 Table 3. Univariate and multivariate analyses of CSS after PSM.
Table 3. Univariate and multivariate analyses of CSS after PSM. Table 1. Baseline characteristics for patients with LASC before and after PSM.
Table 1. Baseline characteristics for patients with LASC before and after PSM. Table 2. Univariate and multivariate analyses of OS after PSM.
Table 2. Univariate and multivariate analyses of OS after PSM. Table 3. Univariate and multivariate analyses of CSS after PSM.
Table 3. Univariate and multivariate analyses of CSS after PSM. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








