25 August 2020: Lab/In Vitro Research
Network Pharmacology to Identify the Pharmacological Mechanisms of a Traditional Chinese Medicine Derived from in Patients with Rheumatoid Arthritis
Tao Jiang12ACDEF, Bo Kong1ACDF, Wei Yan1BF, Changgui Wu1BF, Min Jiang2AEG*, Xing Xu2AEG, Xiaobing Xi12AEGDOI: 10.12659/MSM.922639
Med Sci Monit 2020; 26:e922639
Abstract
BACKGROUND: This study used a network pharmacology approach to identify the pharmacological mechanisms of a traditional Chinese medicine derived from Trachelospermum jasminoides (Lindl.) Lem. in patients with rheumatoid arthritis (RA).
MATERIAL AND METHODS: Known compounds of T. jasminoides were obtained from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database, the Shanghai Institute of Organic Chemistry of Chinese Academy of Science, Chemistry (CASC) database, and a literature search. Putative targets of identified compounds were predicted by SwissTargetPrediction. RA-related targets were achieved from the Therapeutic Target database, Drugbank database, Pharmacogenomics Knowledgebase, and Online Mendelian Inheritance in Man database. The protein-protein interaction (PPI) network was built by STRING. CluGO was utilized for Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis.
RESULTS: A total of 354 potential targets were predicted for the 17 bioactive compounds in T. jasminoides; 69 of these targets overlapped with RA-related targets. A PPI network was composed and 2 clusters of 59 and 42 nodes each were excavated. GO and KEGG enrichment analysis of the overlapping targets and the 2 clusters was mainly grouped into immunity, inflammation, estrogen, anxiety, and depression processes.
CONCLUSIONS: Our study illustrated that T. jasminoides alleviates RA through the interleukin-17 signaling pathway, the tumor necrosis factor signaling pathway, and other immune and inflammatory-related processes. It also may exert effects in regulating cell differentiation and potentially has anti-anxiety, anti-depression, and estrogen-like effects.
Keywords: Arthritis, Rheumatoid, Pharmacologic Actions, Protein Interaction Maps, Apocynaceae, Databases, Genetic, Drugs, Chinese Herbal, gene ontology
Background
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by erosive arthritis [1]. The principal clinical manifestations of RA are joint swelling and tenderness, followed by cartilage destruction and joint space narrowing. In the late stage, severe bone destruction and absorption lead to joint rigidity, deformity, and dysfunction. In addition, RA can also harm a wide variety of body organs, including the heart, skin, eyes, and blood vessels [2]. RA affects about 0.24% of the world’s population and has a higher morbidity among women and the elderly [3]. The prevalence is expected to increase substantially in the coming decades due to increases in aging populations throughout the world.
The primary treatment goal for RA is to attain remission as soon as possible, yet many patients do not adequately respond to treatment or achieve this goal and must rely on drug maintenance [4]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently used to relieve inflammation and improve joint function, but they have various side effects and are therefore not suitable for long-term use [5]. Disease-modifying antirheumatic drugs (DMARDs) help to slow joint destruction progression. However, approximately 30% to 50% of patients do not respond well to traditional DMARDs, and all biological DMARDs affect the immune system and increase the risk of infection [6]. Furthermore, DMARDs are costly, with annual prices ranging from $10 000 to $36 000 [7]. Therefore, novel drugs are needed to meet the clinical demands.
Studies of complementary therapies such as traditional Chinese medicine (TCM) against RA have yielded promising results [8]. The TCM wind-dampness dispelling herbs have been commonly been used in managing RA in East Asia for a long time. Luo Shi Teng, which is derived from the dry vines of
In our current study, a network pharmacology method was employed to uncover the potential mechanisms for
Material and Methods
:
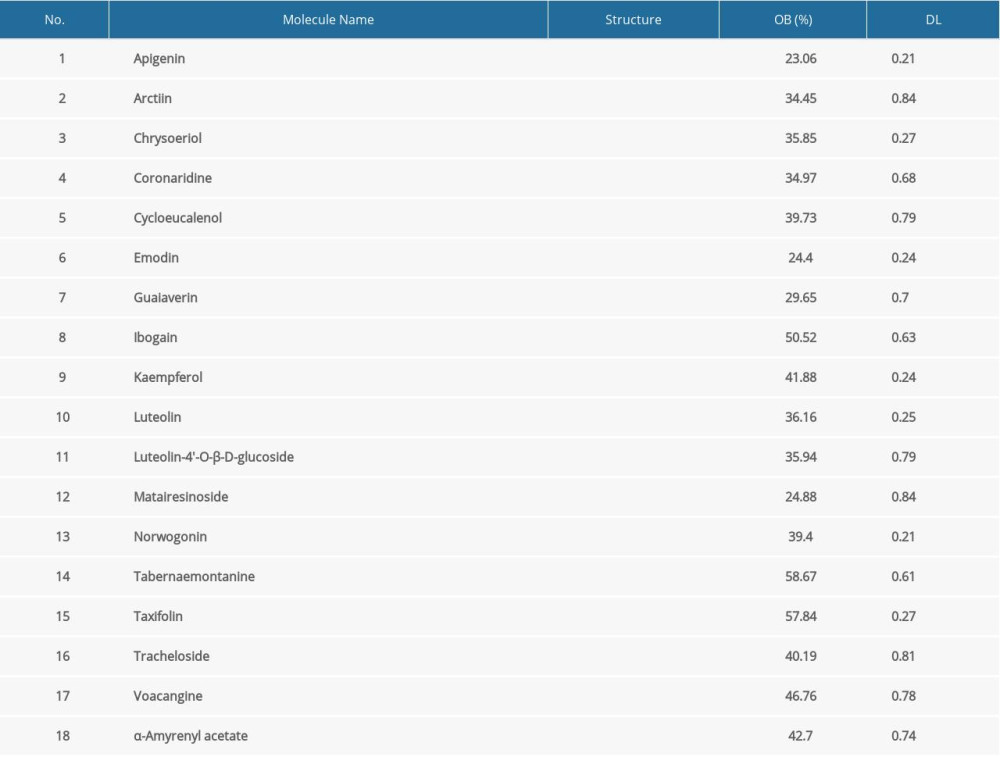
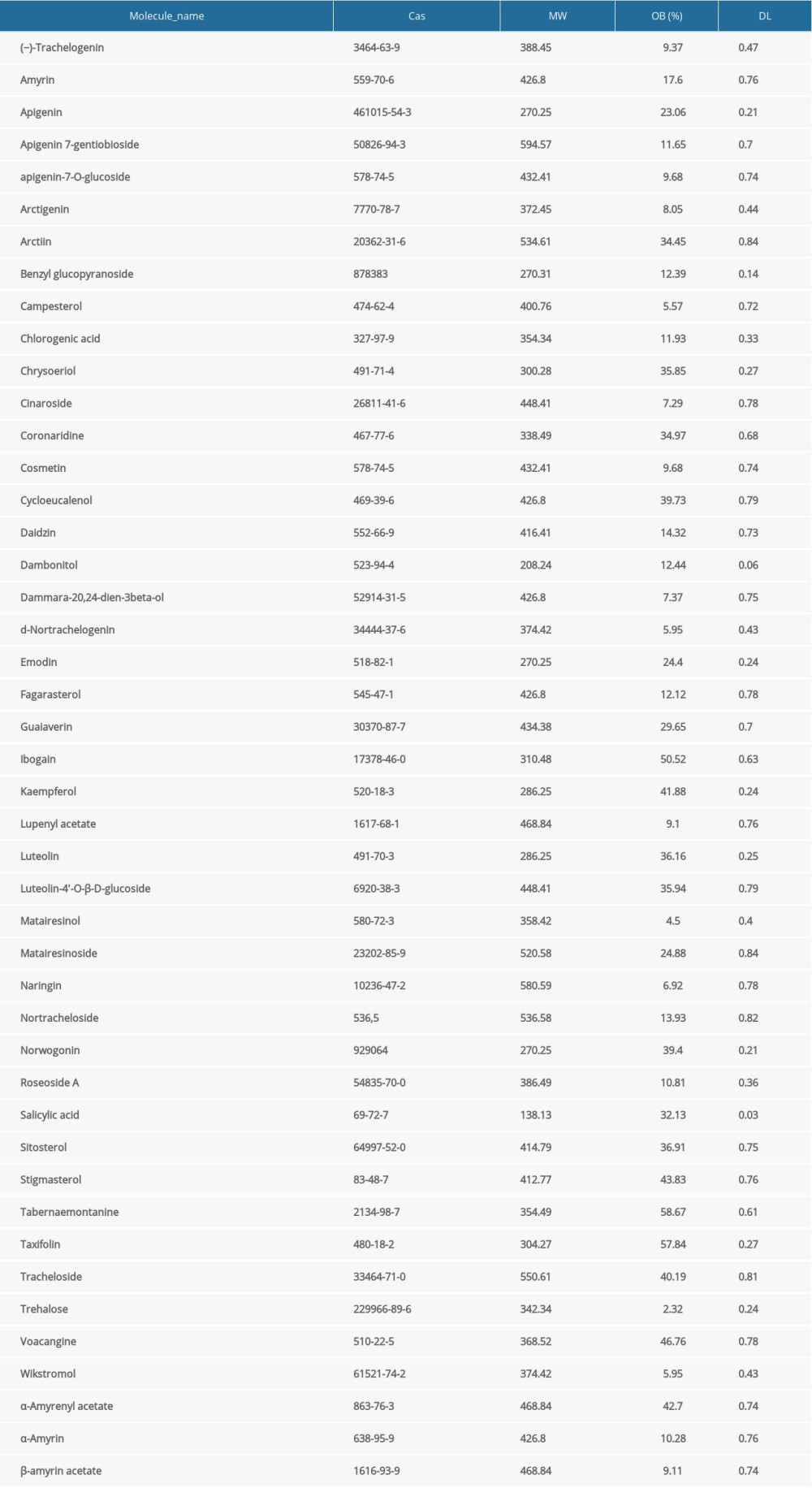
Compounds derived from T. jasminoides were identified from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (http://lsp.nwu.edu.cn/tcmsp.php, updated on May 31, 2014) [13] and the Shanghai Institute of Organic Chemistry of Chinese Academy of Science, Chemistry (CASC) database (http://202.127.145.134/scdb/, updated on February 11, 2018). To supplement this information, published literature was screened for known compounds of T. jasminoides. Oral bioavailability (OB) and drug-likeness (DL) of the compounds were obtained from the TCMSP. OB is an essential pharmacokinetic property in drug screening that indicates the ability of a given compound to be delivered to systemic circulation via oral administration [14]. DL reflects a complex balance of molecular and structural characteristics that determines whether a particular molecule is similar to a known drug [15]. In this research, compounds with OB ≥20% and DL ≥0.18 were classified as bioactive compounds for further analysis [16].
PUTATIVE TARGETS RELATED TO THE IDENTIFIED COMPOUNDS:
SwissTargetPrediction Server (http://www.swisstargetprediction.ch/, updated on April 5, 2018) was used to predict the putative targets of the bioactive compounds based on the molecular similarity [17]. Structure information of the identified compounds was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/, updated on October 26, 2017). Gene information including the gene name and the gene ID was confirmed by Uniprot database (https://www.uniprot.org). The species was restricted to Homo sapiens.
MINING KNOWN RA-ASSOCIATED TARGETS:
Data on known RA-related targets were assembled from the following databases, using “rheumatoid arthritis” as the key term: (i) Therapeutic Target Database (TTD, http://db.idrblab.net/ttd/, updated on November 11, 2019) [18]; (ii) Drugbank database (https://www.drugbank.ca, updated on December 20, 2017) [19]; (iii) Pharmacogenomics Knowledgebase (PharmGKB) database (https://www.pharmgkb.org/, updated on December 28, 2017) [20]; and (iv) Online Mendelian Inheritance in Man (OMIM) database (http://omim.org/, updated on January 3, 2018) [21].
NETWORK CONSTRUCTION AND TOPOLOGY ANALYSIS:
A compound-target (C-T) network was constructed and visualized with candidate compounds and their corresponding targets using Cytoscape 7.0. The nodes in the generated networks represented compounds and their targets, and the edges represented the correspondence between them. The STRING provides both predicted and experimentally proved protein-protein interaction (PPI) information [22]. STRING v11.0 (https://string-db.org/) was employed to build a PPI network with the compound targets and RA-related target data. The degree centrality (DC) is a key network topology parameter that refers to the number of connections of one specific node with others. Nodes with higher DC are considered to be more central [23]. Therefore, we selected nodes with a DC that was equal or greater than twice the median to form a core PPI network. Finally, the most significant submodules were excavated by Molecular Complex Detection (MCODE) plug-in.
ENRICHMENT ANALYSIS:
A Cytoscape plugin “ClueGO” was utilized to perform Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis under the criterion of
Results
:
A total of 46 compounds from T. jasminoides were identified from TCMSP, CASC, and the literature search (Supplementary Table 1). Among them, 18 bioactive compounds met the screening criteria of OB > 20% and DL >0.18 (Table 1). Notably, plant sterols including stigmasterol, sitosterol, and campesterol were excluded because the side effect of steroids have been widely reported. A total of 1118 putative human protein targets were predicted based on the structures of the candidate compounds. After duplicates were deleted, 354 potential targets were retained. The interactions between bioactive compounds and their putative targets are shown in Figure 1.
SCREENING FOR RA-RELATED TARGETS:
RA-related targets were collected from 4 available sources: 70 targets from the TTD, 153 targets from the Drugbank, 104 targets from the PharmGKB, and 20 targets from the OMIM were obtained. Among these, 69 overlapped with compound targets (Figure 2). These overlapping targets mainly interact with chrysoeriol, kaempferol, apigenin, luteolin, and norwogonin, and they were considered the main active compounds against RA.
ENRICHMENT ANALYSIS FOR THE OVERLAPPING TARGETS:
The canonical pathways related to RA pathology were extracted from KEGG enrichment. The pathways included 2 inflammation-related pathways, tumor necrosis factor (TNF) signaling pathway (KEGG: 04668, P=1.12E-03) and nuclear factor kappa-B (NF-κB) signaling pathway (KEGG: 04064, P=3.96E-03); 5 immune-related pathways, interleukin (IL)-17 signaling pathway (KEGG: 04657, P=3.54E-05), Th17 cell differentiation (KEGG: 04659, P=9.66E-04), Th1 and Th2 cell differentiation (KEGG: 04658, P=3.43E-03), T-cell receptor signaling pathway (KEGG: 04660, P=2.54E-02), and B-cell receptor signaling pathway (KEGG: 04662, P=4.42E-02); and 2 hormone-related pathways, steroid hormone biosynthesis (KEGG: 00140, P=1.02E-06) and ovarian steroidogenesis (KEGG: 04913, P=1.08E-04). As shown in Figure 3A, 3B, most pathways can be classified into the IL-17 signaling pathway and the steroid hormone biosynthesis function groups.
In addition, biological processes based on GO enrichment were clustered into 13 function groups (Figure 3C, 3D). The literature screening indicated that the following processes were related to RA pathology: hormone metabolic process (GO: 0042445, P=1.29E-11), reactive oxygen species metabolic process (GO: 0072593, P=3.08E-09), regulation of reactive oxygen species metabolic process (GO: 2000377, P=3.08E-09), regulation of macrophage chemotaxis (GO: 0010758, P=3.08E-03), neurotransmitter metabolic process (GO: 0042133, P=1.69E-05), and regulation of synaptic transmission, GABAergic (GO: 0032228, P=4.18E-03).
PPI NETWORK CONSTRUCTION AND TOPOLOGY ANALYSIS:
A PPI network provides information on interactions between compounds and diseases, and it helps to mine the underlying mechanisms of compounds against diseases. After compound targets and RA-related targets were input into STRING, a PPI network was generated with 585 nodes and 11 826 edges (Figure 4A). Subsequently, nodes with DC that were equal to or greater than twice the median (DC ≥60) of all nodes were identified as core targets based on the topology analysis. The core PPI network is composed of 124 nodes and 3246 edges (Figure 4B). The MCODE plugin mined 2 dense clusters from the core PPI network, and these clusters included 59 and 42 nodes, respectively (Figure 4C, 4D).
ENRICHMENT ANALYSIS OF THE 2 DENSE CLUSTERS:
KEGG enrichment of 2 clusters included 6 inflammatory pathways, including TNF signaling pathway (KEGG: 04668, P=5.04E-05), NF-κB signaling pathway (KEGG: 04064, P=1.90E-04), phosphatidylinositol 3′-kinase (PI3K)-Akt signaling pathway (KEGG: 04151, P=9.85E-12), vascular endothelial growth factor (VEGF) signaling pathway (KEGG: 04370, P=5.49E-08), HIF-1 signaling pathway (KEGG: 04115, P=3.11E-10), and Fc epsilon RI signaling pathway (KEGG: 04664, P=3.02E-02); 4 immunology pathways, including IL-17 signaling pathway (KEGG: 04657, P=1.77E-04), T-cell receptor signaling pathway (KEGG: 04660, P=3.43E-06), B-cell receptor signaling pathway (KEGG: 04662, P=5.14E-06), and chemokine signaling pathway (KEGG: 04062, P=1.29E-03); and 2 estrogen-related pathways, including estrogen signaling pathway (KEGG: 04915, P=9.33E-09) and gonadotropin-releasing hormone (GnRH) signaling pathway (KEGG: 04912, P=3.35E-04). The top 20 KEGG signaling pathways are shown in Figure 5A, 5B.
Immune system processes (Figure 5C, 5D) based on GO enrichment included regulation of adaptive immune response (GO: 0002821, P=1.43E-06), regulation of immunoglobulin production (GO: 0002637, P=4.49E-05), immunoglobulin secretion (GO: 0048305, P=8.24E-05), monocyte differentiation (GO: 0030224, P=1.10E-02), and macrophage activation (GO: 0042116, P=1.91E-02), among others.
Discussion
In the present research, we screened 18 bioactive compounds of
A complex network of cytokines and chemokines regulates the inflammatory environment of the synovial cavity and is accompanied by infiltration of leukocytes; therefore, various therapeutic targets are present. TNF and IL-6 receptor inhibitors have been widely recognized as acceptable anti-RA agents [29]. Other cytokines in the context of RA pathogenesis, such as IL-17, are now targets for second-generation agents [30]. However, due to molecular and cellular heterogeneity between patients, many patients do not respond to a single blocker, and even among patients who initially respond, few people experience complete relief and many lose their response over time [31]. Therefore, new strategies have been adapted to achieve higher response rates, and combination therapy has been considered an option. Fischer et al. [32] found that simultaneously blocking TNFα and IL-17 in human mesenchymal stem cells inhibited cytokine, chemokine, and matrix enzyme reactions and blocked tissue destruction and arthritis more strongly compared with a single blockade. In the present study, we analyzed the interactions between bioactive compounds and RA-related targets and found that the
IL-17 is a potent proinflammatory cytokine secreted by T helper cell 17, neutrophils, mast cells, and eosinophils [30]. It is highly expressed in synovial fluid and serum in RA patients and is positively correlated with disease activity and severity [31]. IL-17 was found to significantly inhibit the development of collagen-induced arthritis in IL-17A-deficient mice, as well as reduce synovial hyperplasia, cell infiltration, and bone erosion [33]. In addition, IL-17 also disrupts the balance of nuclear Factor-κ B ligand (RANKL)-osteoprotegerin by stimulating the expression of the receptor activator of RANKL and promoting osteoclast differentiation and bone erosion [34]. Given the important role of IL-17 in RA pathogenesis, IL-17-targeted RA treatment strategies are attracting increasing attention. The European Medicines Agency recommended approval of secukinumab, a new IL-17A inhibitor developed by Novartis in 2015. Phase III clinical trials showed that secukinumab diminished disease activity and improved symptoms in patients with active RA who do not respond to TNF inhibitors [35].
TNF, in particular TNF-α, is a critical cytokine in RA pathophysiology that modulates immune response in several ways. TNF-α is a autocrine stimulating factor that induces the expansion of other inflammatory cytokines, including IL-1, IL-6, and IL-8 [36]. It also induces the activation of leukocytes and endothelial cells, regulates angiogenesis, and stimulates nociceptors. More importantly, Bertolini et al. [37] showed that TNF has the potential to drive osteoclast formation and inhibit osteoblast differentiation and thus disrupt the balance between bone destruction and formation, which may explain the joint damage in RA. TNF inhibitors, including infliximab, adalimumab, etanercept, and others, have been widely used in clinical RA therapy. Although anti-TNF therapy has significantly improved the prognosis of RA, only two-thirds of RA patients respond to anti-TNF therapy [38].
With the deepening understanding of RA pathophysiology, new potential therapeutic targets on immunity and inflammation are constantly being proposed [39]. In the present research, we further excavated the HIF-1 signaling pathway, NF-κB signaling pathway, PI3K-Akt signaling pathway, Fc epsilon RI signaling pathway, chemokine signaling pathway, VEGF signaling pathway, and the reactive oxygen species metabolic process as possible approaches for
More importantly, our research suggests that
Antidepressant and anti-anxiety efforts have received increasing attention in the treatment of RA [49,50]. Depression and anxiety are caused by perceived pain in RA patients. A prospective multicenter study indicated that depression and anxiety may reduce the likelihood of joint relief during RA treatment [51]. GO analysis of overlapping targets between compound targets and RA-related targets suggested that
The phytoestrogen effects of flavonoids have been extensively studied [55,56]. Various flavonoids from
This study has some limitations. First, due to the screening criteria, this study included only compounds that were considered to be biologically active. Secondly, although many potential pathways have been screened through network pharmacology, further experimental verification is still required, which should be our next step in research.
Conclusions
Herbal medicines are an important part of complementary and alternative medicine. In the present study, we excavated the main bioactive compounds and uncovered the potential mechanisms for
Figures
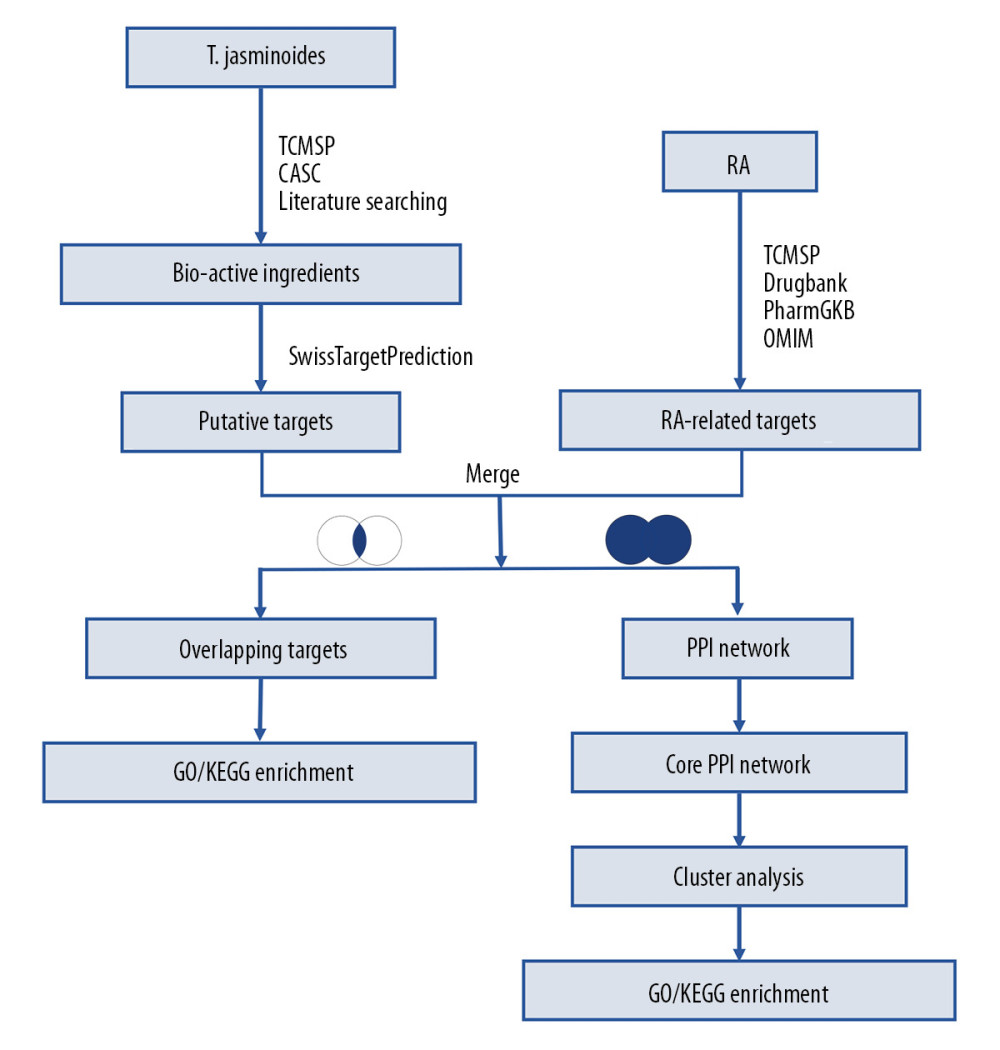 Figure 1. The technical framework of this study.
Figure 1. The technical framework of this study. 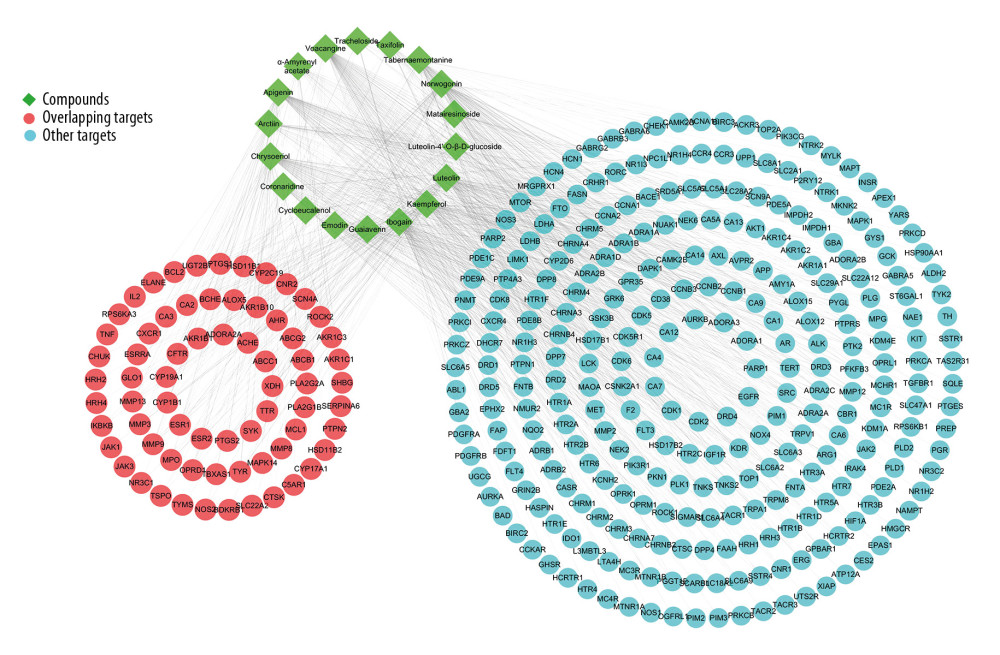 Figure 2. The Compound-Target network for the bioactive compounds and the putative human protein targets. The green nodes represent the bioactive compounds, the red nodes represent the overlapping targets between compound targets and rheumatoid arthritis (RA)-related targets, and the blue nodes represent other putative compound targets.
Figure 2. The Compound-Target network for the bioactive compounds and the putative human protein targets. The green nodes represent the bioactive compounds, the red nodes represent the overlapping targets between compound targets and rheumatoid arthritis (RA)-related targets, and the blue nodes represent other putative compound targets. 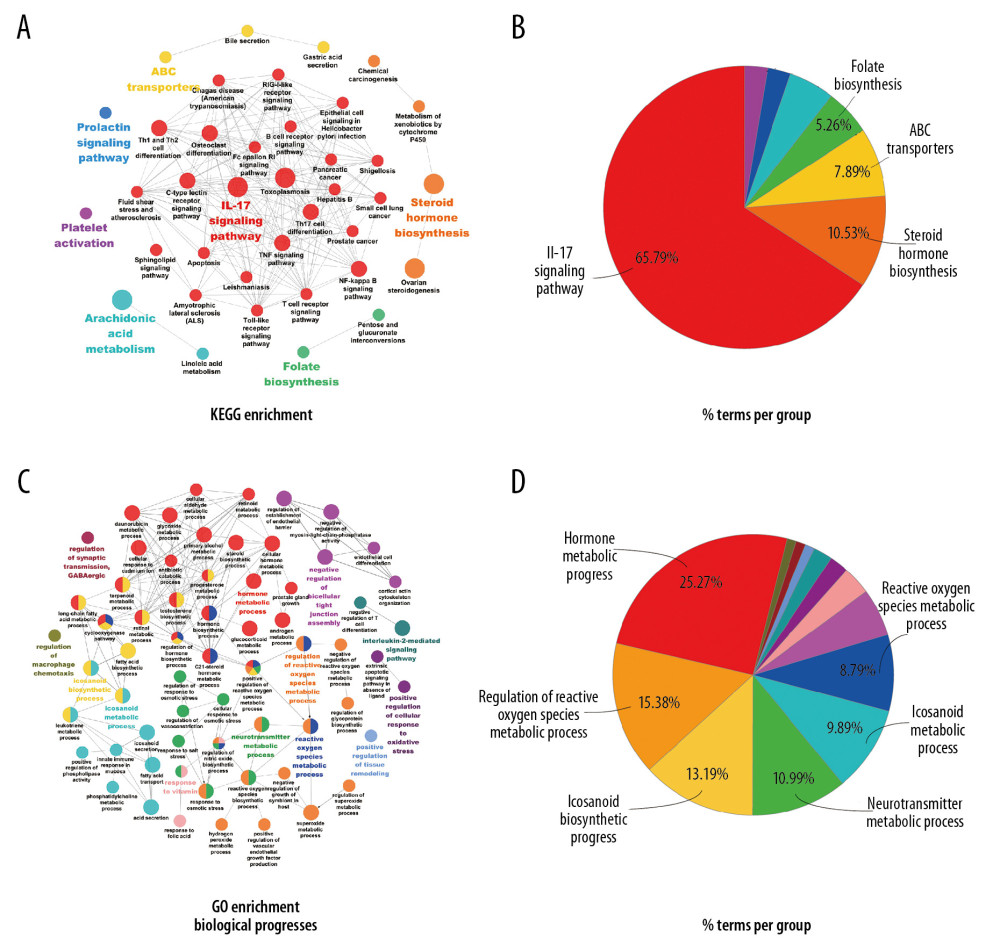 Figure 3. Enrichment analysis of the overlapping targets. (A) Overlapping targets enriched in the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways. (B) The pie chart of function groups based on KEGG enrichment. (C) Overlapping targets enriched in the biological processes based on Gene Ontology (GO) enrichment. (D) The pie chart of function groups based on biological processes.
Figure 3. Enrichment analysis of the overlapping targets. (A) Overlapping targets enriched in the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways. (B) The pie chart of function groups based on KEGG enrichment. (C) Overlapping targets enriched in the biological processes based on Gene Ontology (GO) enrichment. (D) The pie chart of function groups based on biological processes. 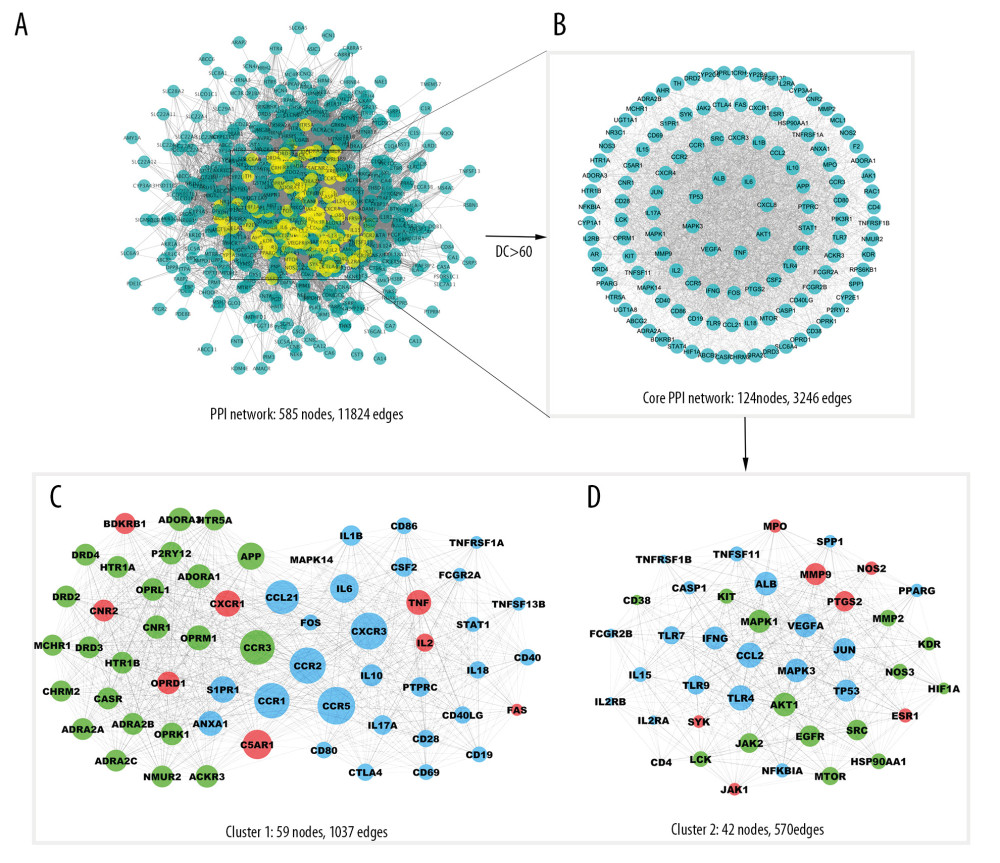 Figure 4. Protein-protein interaction (PPI) network construction and topology analysis. (A) The PPI network of compound targets and rheumatoid arthritis (RA)-related targets. (B) The Core PPI network excavated from A. (C, D) Clusters of the core PPI network excavated from B. The green, blue, red nodes in C and D represent compound targets, RA-related targets, and the overlapping targets respectively. The size of the nodes in C and D is illustrated in descending order of degree centrality (DC) with a bigger size means the node being more “central.”
Figure 4. Protein-protein interaction (PPI) network construction and topology analysis. (A) The PPI network of compound targets and rheumatoid arthritis (RA)-related targets. (B) The Core PPI network excavated from A. (C, D) Clusters of the core PPI network excavated from B. The green, blue, red nodes in C and D represent compound targets, RA-related targets, and the overlapping targets respectively. The size of the nodes in C and D is illustrated in descending order of degree centrality (DC) with a bigger size means the node being more “central.” 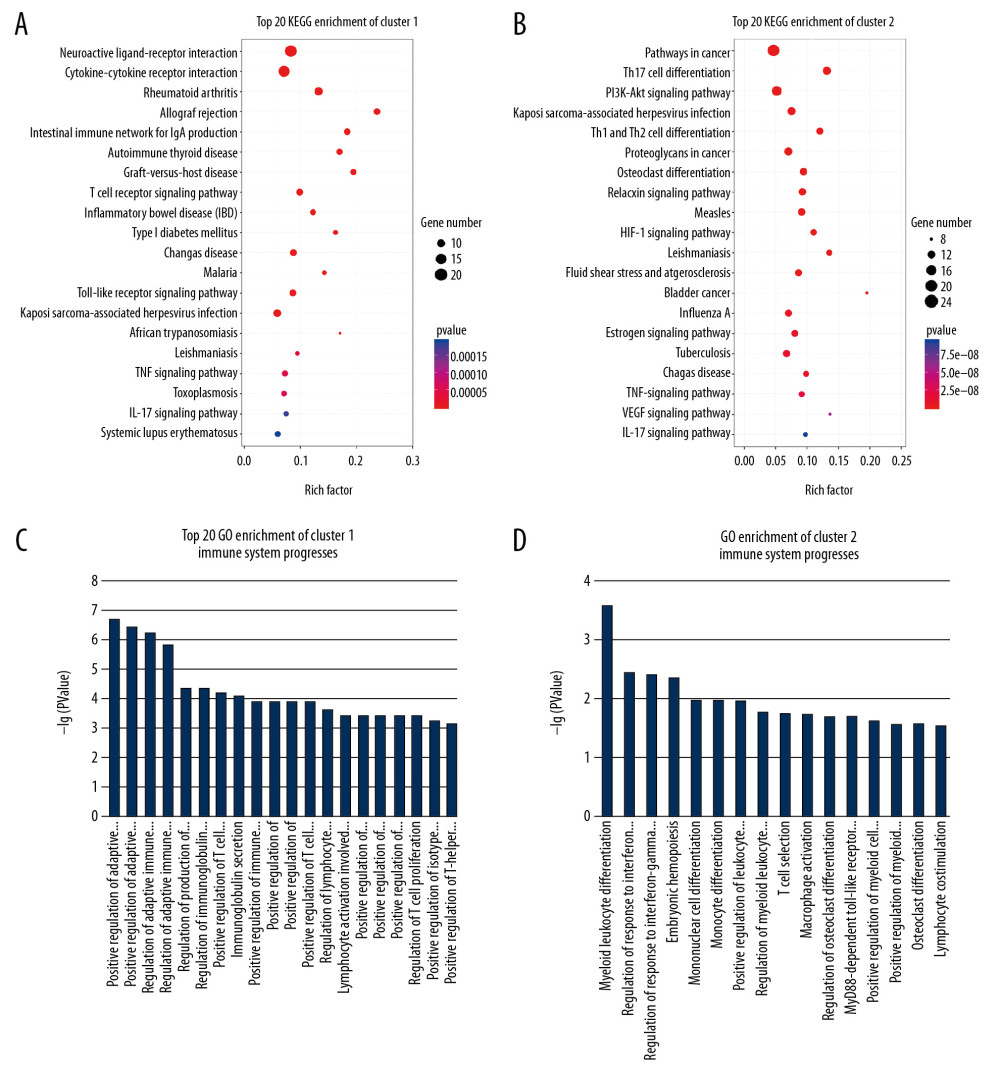 Figure 5. Enrichment analysis of the clusters. The bubble charts of Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment results for cluster 1 (A) and cluster 2 (B). The bar charts of the immune system progresses based on Gene Ontology (GO) enrichment for cluster 1 (C) and cluster 2 (D).
Figure 5. Enrichment analysis of the clusters. The bubble charts of Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment results for cluster 1 (A) and cluster 2 (B). The bar charts of the immune system progresses based on Gene Ontology (GO) enrichment for cluster 1 (C) and cluster 2 (D). 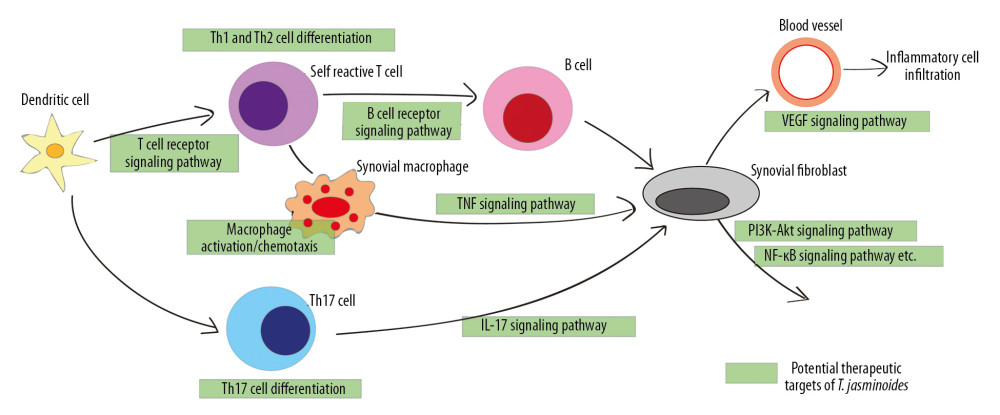 Figure 6. A proposed model illustrates the potential mechanisms of the T. jasminoides against rheumatoid arthritis. The green squares represent the potential therapeutic targets of T. jasminoides.
Figure 6. A proposed model illustrates the potential mechanisms of the T. jasminoides against rheumatoid arthritis. The green squares represent the potential therapeutic targets of T. jasminoides. References
1. Smolen JS, Aletaha D, McInnes IB, Rheumatoid arthritis: Lancet, 2016; 388(10055); 2023-38
2. Prete M, Racanelli V, Digiglio L, Extra-articular manifestations of rheumatoid arthritis: an update: Autoimmun Rev, 2011; 11(2); 123-31
3. Cross M, Smith E, Hoy D, The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study: Ann Rheum Dis, 2014; 73(7); 1316-22
4. Burmester GR, Pope JE, Novel treatment strategies in rheumatoid arthritis: Lancet, 2017; 389(10086); 2338-48
5. Crofford LJ, Use of NSAIDs in treating patients with arthritis: Arthritis Res Ther, 2013; 15(Suppl 3); S2
6. Mackintosh JA, Stainer A, De Sadeleer LJ, Rheumatoid arthritis: Pulmonary manifestations of systemic diseases (ERS Monograph), 2019; 44-67, Sheffield, UK, European Respiratory Society
7. Wasserman AM, Diagnosis and management of rheumatoid arthritis: Am Fam Physician, 2011; 84(11); 1245-52
8. Xing Q, Fu L, Yu Z, Zhou X, Efficacy and safety of integrated traditional Chinese medicine and Western medicine on the treatment of rheumatoid arthritis: A meta-analysis: Evid Based Complement Alternat Med, 2020; 2020 4348709
9. Zhao Z, He X, Zhao Y: Molecules, 2017; 22(9); 1406
10. Sheu MJ, Chou PY, Cheng HC: J Ethnopharmacol, 2009; 126(2); 332-38
11. Hopkins AL, Network pharmacology: the next paradigm in drug discovery: Nat Chem Biol, 2008; 4(11); 682-90
12. Yuan H, Ma Q, Cui H, How can synergism of traditional medicines benefit from network pharmacology?: Molecules, 2017; 22(7); 1135
13. Ru J, Li P, Wang J, TCMSP: A database of systems pharmacology for drug discovery from herbal medicines: J Cheminform, 2014; 6; 13
14. Tian S, Li Y, Wang J, ADME evaluation in drug discovery. 9. Prediction of oral bioavailability in humans based on molecular properties and structural fingerprints: Mol Pharm, 2011; 8(3); 841-51
15. Tian S, Wang J, Li Y, The application of in silico drug-likeness predictions in pharmaceutical research: Adv Drug Deliv Rev, 2015; 86; 2-10
16. Song W, Ni S, Fu Y, Wang Y, Uncovering the mechanism of Maxing Ganshi Decoction on asthma from a systematic perspective: A network pharmacology study: Sci Rep, 2018; 8(1); 17362
17. Daina A, Michielin O, Zoete V, SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules: Nucleic Acids Res, 2019; 47(W1); W357-64
18. Li YH, Yu CY, Li XX, Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics: Nucleic Acids Res, 2018; 46(D1); D1121-27
19. Wishart DS, Feunang YD, Guo AC, DrugBank 5.0: A major update to the DrugBank database for 2018: Nucleic Acids Res, 2018; 46(D1); D1074-82
20. Whirl-Carrillo M, McDonagh EM, Hebert JM, Pharmacogenomics knowledge for personalized medicine: Clin Pharmacol Ther, 2012; 92(4); 414-17
21. Hamosh A, Scott AF, Amberger J, Online mendelian inheritance in man (OMIM): Hum Mutat, 2000; 15(1); 57-61
22. Szklarczyk D, Gable AL, Lyon D, STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets: Nucleic Acids Res, 2019; 47(D1); D607-13
23. Bu D, Su Z, Zou J, Study of the mechanism underlying therapeutic effect of Compound Longmaining on myocardial infarction using a network pharmacology-based approach: Biomed Pharmacother, 2019; 118; 109234
24. Zhuang Z, Ye G, Huang B, Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB: Med Sci Monit, 2017; 23; 3925-31
25. Yoon HY, Lee EG, Lee H, Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs: Int J Mol Med, 2013; 32(4); 971-77
26. Aziz N, Kim MY, Cho JY: J Ethnopharmacol, 2018; 225; 342-58
27. Li X, Han Y, Zhou Q, Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis: J Cell Mol Med, 2016; 20(1); 170-80
28. Li F, Lang F, Zhang H, Apigenin alleviates endotoxin-induced myocardial toxicity by modulating inflammation, oxidative stress, and autophagy: Oxid Med Cell Longev, 2017; 2017 2302896
29. Nakagawa J, Koyama Y, Kawakami A, A novel scoring system based on common laboratory tests predicts the efficacy of TNF-inhibitor and IL-6 targeted therapy in patients with rheumatoid arthritis: A retrospective, multicenter observational study: Arthritis Res Ther, 2017; 19(1); 185
30. Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T, The role of IL-17 and related cytokines in inflammatory autoimmune diseases: Mediators Inflamm, 2017; 2017 3908061
31. Kellner H, Targeting interleukin-17 in patients with active rheumatoid arthritis: Rationale and clinical potential: Ther Adv Musculoskelet Dis, 2013; 5(3); 141-52
32. Fischer JAA, Hueber AJ, Wilson S, Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: Development and characterization of a novel bispecific antibody: Arthritis Rheumatol, 2015; 67(1); 51-62
33. Nakae S, Nambu A, Sudo K, Iwakura Y, Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice: J Immunol, 2003; 171(11); 6173-77
34. Ganesan R, Rasool M, Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis: Mol Immunol, 2017; 91; 134-44
35. Genovese MC, Durez P, Richards HB, Efficacy and safety of secukinumab in patients with rheumatoid arthritis: A phase II, dose-finding, double-blind, randomised, placebo controlled study: Ann Rheum Dis, 2013; 72(6); 863-69
36. Brennan FM, Jackson A, Chantry D, Inhibitory effect of TNFα antibodies on synovial cell interleukin-1 production in rheumatoid arthritis: Lancet, 1989; 334(8657); 244-47
37. Bertolini DR, Nedwin GE, Bringman TS: Nature, 1986; 319(6053); 516-18
38. Cuchacovich M, Bueno D, Carvajal R, Clinical parameters and biomarkers for anti-TNF treatment prognosis in rheumatoid arthritis patients: Clin Rheumatol, 2014; 33(12); 1707-14
39. Cheung TT, McInnes IB, Future therapeutic targets in rheumatoid arthritis?: Semin Immunopathol, 2017; 39(4); 487-500
40. Park SY, Lee SW, Kim HY, HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation: Eur J Immunol, 2015; 45(4); 1216-27
41. MacDonald IJ, Liu SC, Su CM, Implications of angiogenesis involvement in arthritis: Int J Mol Sci, 2018; 19(7); 2012
42. Brouwer E, Gouw ASH, Posthumus MD, Hypoxia inducible factor-1-alpha (HIF-1α) is related to both angiogenesis and inflammation in rheumatoid arthritis: Clin Exp Rheumatol, 2009; 27(6); 945-51
43. Wakahara S, Fujii Y, Nakao T, Gene expression profiles for FcɛRI, cytokines and chemokines upon FcɛRI activation in human cultured mast cells derived from peripheral blood: Cytokine, 2001; 16(4); 143-52
44. Smallwood MJ, Nissim A, Knight AR, Oxidative stress in autoimmune rheumatic diseases: Free Radic Biol Med, 2018; 125; 3-14
45. Monney L, Sabatos CA, Gaglia JL, Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease: Nature, 2002; 415(6871); 536-41
46. Quayle AJ, Chomarat P, Miossec P, Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0-like) cytokine patterns: Scand J Immunol, 1993; 38(1); 75-82
47. van Hamburg JP, Tas SW, Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis: J Autoimmun, 2018; 87; 69-81
48. Boissier MC, Assier E, Falgarone G, Bessis N, Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm: Joint Bone Spine, 2008; 75(4); 373-75
49. Boyden SD, Hossain IN, Wohlfahrt A, Lee YC, Non-inflammatory causes of pain in patients with rheumatoid arthritis: Curr Rheumatol Rep, 2016; 18(6); 30
50. Matcham F, Norton S, Scott DL, Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: Secondary analysis of a randomized controlled trial: Rheumatol (Oxford), 2015; 55(2); 268-78
51. Michelsen B, Kristianslund EK, Sexton J, Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study: Ann Rheum Dis, 2017; 76(11); 1906-10
52. Möhler H, The GABA system in anxiety and depression and its therapeutic potential: Neuropharmacology, 2012; 62(1); 42-53
53. Chilmonczyk Z, Bojarski AJ, Pilc A, Sylte I, Functional selectivity and antidepressant activity of serotonin 1A receptor ligands: Int J Mol Sci, 2015; 16(8); 18474-506
54. Zangrossi H, Graeff FG, Serotonin in anxiety and panic: Contributions of the elevated T-maze: Neurosci Biobehav Rev, 2014; 46(P3); 397-406
55. Chen MN, Lin CC, Liu CF, Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review: Climacteric, 2015; 18(2); 260-69
56. Rietjens IMCM, Louisse J, Beekmann K, The potential health effects of dietary phytoestrogens: Br J Pharmacol, 2017; 174(11); 1263-80
57. Long X, Fan M, Bigsby RM, Nephew KP, Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-α-dependent and estrogen receptor-α-independent mechanisms: Mol Cancer Ther, 2008; 7(7); 2096-108
58. Shim JH, Stavre Z, Gravallese EM, Bone loss in rheumatoid arthritis: Basic mechanisms and clinical implications: Calcif Tissue Int, 2018; 102(5); 533-46
59. Fu SW, Zeng GF, Zong SH, Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats: Nutr Res, 2014; 34(6); 467-77
Figures
 Figure 1. The technical framework of this study.
Figure 1. The technical framework of this study. Figure 2. The Compound-Target network for the bioactive compounds and the putative human protein targets. The green nodes represent the bioactive compounds, the red nodes represent the overlapping targets between compound targets and rheumatoid arthritis (RA)-related targets, and the blue nodes represent other putative compound targets.
Figure 2. The Compound-Target network for the bioactive compounds and the putative human protein targets. The green nodes represent the bioactive compounds, the red nodes represent the overlapping targets between compound targets and rheumatoid arthritis (RA)-related targets, and the blue nodes represent other putative compound targets. Figure 3. Enrichment analysis of the overlapping targets. (A) Overlapping targets enriched in the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways. (B) The pie chart of function groups based on KEGG enrichment. (C) Overlapping targets enriched in the biological processes based on Gene Ontology (GO) enrichment. (D) The pie chart of function groups based on biological processes.
Figure 3. Enrichment analysis of the overlapping targets. (A) Overlapping targets enriched in the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways. (B) The pie chart of function groups based on KEGG enrichment. (C) Overlapping targets enriched in the biological processes based on Gene Ontology (GO) enrichment. (D) The pie chart of function groups based on biological processes. Figure 4. Protein-protein interaction (PPI) network construction and topology analysis. (A) The PPI network of compound targets and rheumatoid arthritis (RA)-related targets. (B) The Core PPI network excavated from A. (C, D) Clusters of the core PPI network excavated from B. The green, blue, red nodes in C and D represent compound targets, RA-related targets, and the overlapping targets respectively. The size of the nodes in C and D is illustrated in descending order of degree centrality (DC) with a bigger size means the node being more “central.”
Figure 4. Protein-protein interaction (PPI) network construction and topology analysis. (A) The PPI network of compound targets and rheumatoid arthritis (RA)-related targets. (B) The Core PPI network excavated from A. (C, D) Clusters of the core PPI network excavated from B. The green, blue, red nodes in C and D represent compound targets, RA-related targets, and the overlapping targets respectively. The size of the nodes in C and D is illustrated in descending order of degree centrality (DC) with a bigger size means the node being more “central.” Figure 5. Enrichment analysis of the clusters. The bubble charts of Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment results for cluster 1 (A) and cluster 2 (B). The bar charts of the immune system progresses based on Gene Ontology (GO) enrichment for cluster 1 (C) and cluster 2 (D).
Figure 5. Enrichment analysis of the clusters. The bubble charts of Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment results for cluster 1 (A) and cluster 2 (B). The bar charts of the immune system progresses based on Gene Ontology (GO) enrichment for cluster 1 (C) and cluster 2 (D). Figure 6. A proposed model illustrates the potential mechanisms of the T. jasminoides against rheumatoid arthritis. The green squares represent the potential therapeutic targets of T. jasminoides.
Figure 6. A proposed model illustrates the potential mechanisms of the T. jasminoides against rheumatoid arthritis. The green squares represent the potential therapeutic targets of T. jasminoides. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952