16 July 2020: Database Analysis
Estimation of Hub Genes and Infiltrating Immune Cells in Non-Smoking Females with Lung Adenocarcinoma by Integrated Bioinformatic Analysis
Jie Li1ABCDEF, Ben Wang2BCDF, Xin Li3BF, Yuxi Zhu1AE*DOI: 10.12659/MSM.922680
Med Sci Monit 2020; 26:e922680
Abstract
BACKGROUND: In recent years, the morbidity and mortality rates of lung adenocarcinoma in non-smoking females have been increasing dramatically. Although much research has been done with some progress, the molecular mechanism remains unclear. In this study we aimed to estimate hub genes and infiltrating immune cells in non-smoking females with lung adenocarcinoma.
MATERIAL AND METHODS: Firstly, we obtained differentially expressed genes (DEGs) by GEO2R analysis based on 3 independent mRNA microarray datasets of GSE10072, GSE31547, and GSE32863. The DAVID database was utilized for functional enrichment analysis of DEGs. Moreover, we identified hub genes with prognostic value by STRING, Cytoscape, and Kaplan Meier plotter. Subsequently, these genes were further analyzed by Gene Expression Profiling Interactive Analysis, Oncomine, Tumor Immune Estimation Resource, and Human Protein Atlas. Finally, the immune infiltration analysis was performed by CIBERSORT and The Cancer Genome Atlas with R packages.
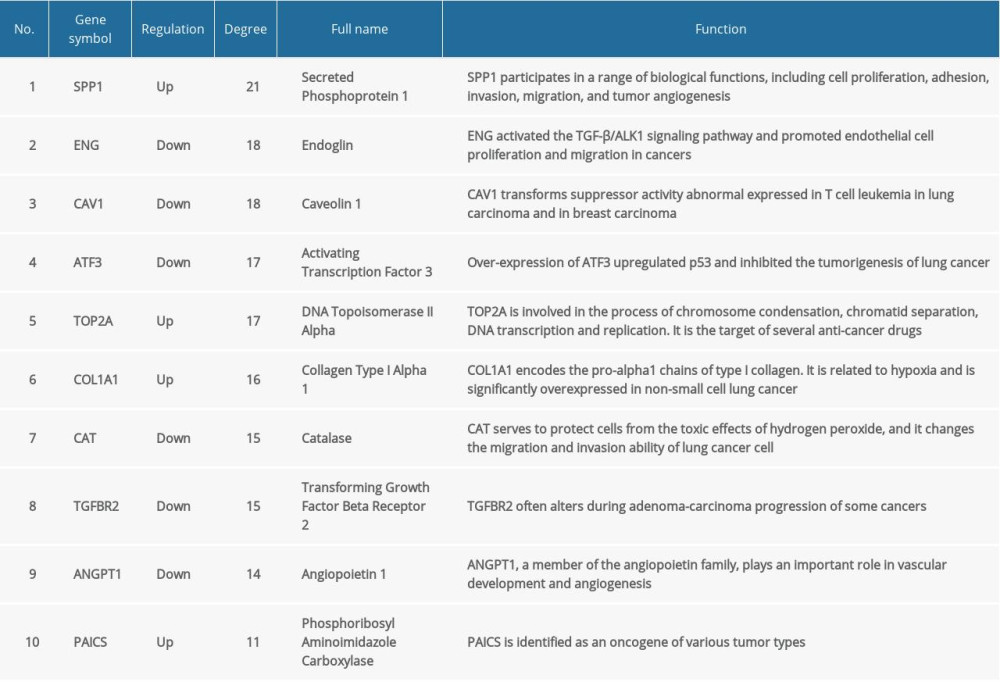
RESULTS: We found 315 DEGs enriching in the extracellular matrix organization, cell adhesion, integrin binding, angiogenesis, and hypoxic response. And among these DEGs, we identified 10 hub genes (SPP1, ENG, ATF3, TOP2A, COL1A1, PAICS, CAV1, CAT, TGFBR2, and ANGPT1) of significant prognostic value. Simultaneously, we illustrated the distribution and differential expressions of 22 immune cell subtypes. and dendritic cells resting and macrophages M1 were identified with prognostic significance.
CONCLUSIONS: The results indicated that 10 hub genes and 2 immune cell subtypes might be promising biomarkers for lung adenocarcinoma in non-smoking females. This finding needs to be further evaluated.
Keywords: Tobacco, Smokeless, Tumor Markers, Biological, Adenocarcinoma of Lung, Computational Biology, Databases, Genetic, Gene Expression Profiling, gene ontology, Gene Regulatory Networks, Lymphocytes, Tumor-Infiltrating, Microarray Analysis, Protein Interaction Maps
Background
Lung cancer has become the chief cause of malignancy deaths worldwide, and adenocarcinoma is the most common histologic type of lung cancer [1]. Previously, smoking was thought to be the major cause of lung adenocarcinoma (LUAD). However, the morbidity of LUAD has increased in never-smokers, especially in females [2]. Studies have shown that non-smoking lung cancer should be considered as a separate subtype [3]. Epidemiological, pathological, and molecular evidence suggested that estrogen appears to participate in the carcinogenic effect of lung cancer besides smoking [4,5]. A study in South Korea found morbidity differences in gender and histological subtypes in smoking-related lung cancer. Compared with males, females were more likely to develop non-smoking related LUAD, thus, gender was also an independent prognostic factor [6,7]. One study reported that females benefited significantly more from immunotherapy for lung cancer than males [8]. Additionally, some studies have indicated that anti-estrogen could reduce non-small cell lung cancer (NSCLC) cell proliferation [9]. Therefore, more attention should be paid to the treatment and prognostic evaluation of LUAD in non-smoking females [10].
Although its pathogenesis remains unclear, the application of bioinformatics analysis in precision medicine might contribute to finding the key biomarkers in the big data era [11]. Data mining in cancers has played a vital part in cancer diagnosis and management [12]. Consequently, we explored the promising molecular mechanism of LUAD in non-smoking females by bioinformatics. We identified the differentially expressed genes (DEGs) between LUAD and normal samples of non-smoking females by data mining. Simultaneously, CIBERSORT and The Cancer Genome Atlas (TCGA) were utilized for immune infiltration analysis. Finally, we found 10 hub genes and 2 immune cell subtypes as promising biomarkers for LUAD in non-smoking females, which provided useful information for further exploration.
Material and Methods
MICROARRAY DATA:
We downloaded qualified datasets from the Gene Expression Omnibus (GEO) database (
IDENTIFICATION OF DEGS:
GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r), a web application using BioConductor R packages [13], could compare DEGs from 2 or more datasets in the GEO series. It was universally applied in various bioinformatics analyses [14–16], and it provided the native R script for researchers to replicate their analyses. We utilized GEO2R to screen DEGs between LUAD tissue samples and normal tissue samples of non-smoking females. |log FC| >1 and P<0.01 was set as the cutoff criterion. Moreover, we replicated this analysis by the native R script to ensure the reliability of the present study.
FUNCTIONAL ENRICHMENT ANALYSES OF DEGS:
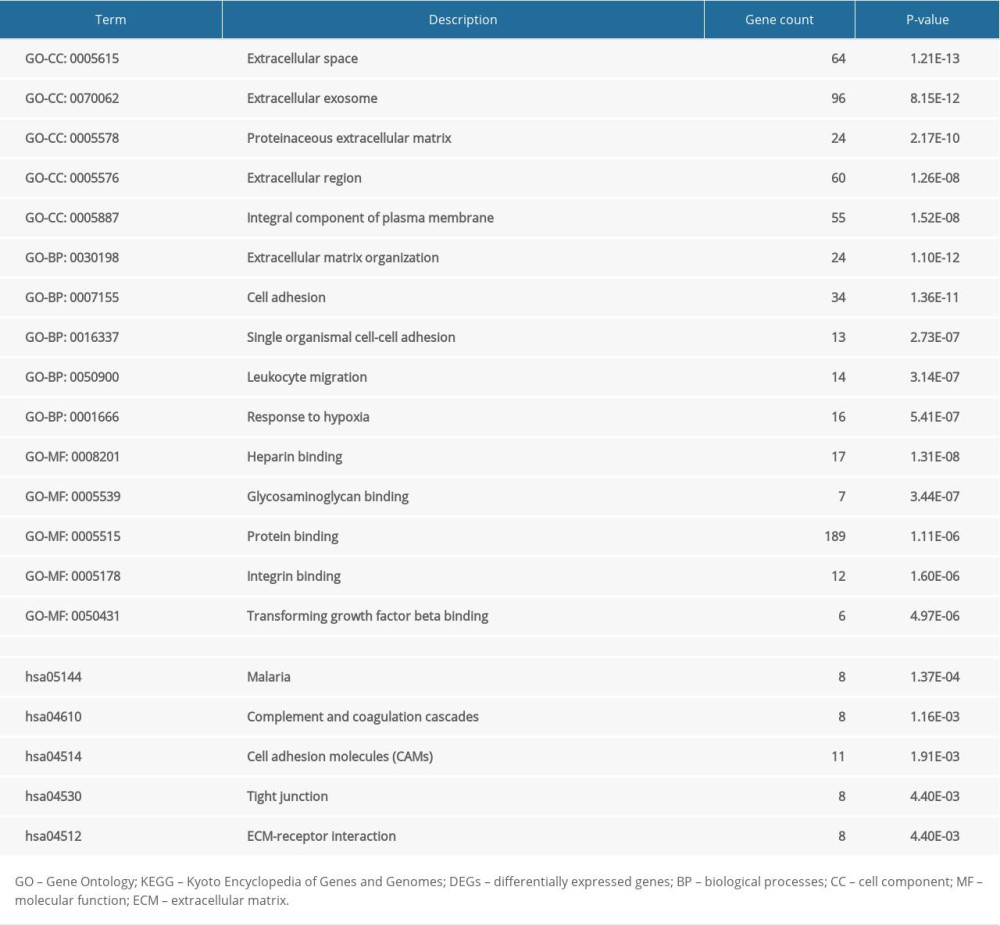
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of DEGs were performed by the DAVID database (http://david.ncifcrf.gov) (version 6.8) [17]. P<0.05 was set as statistically significant.
PPI NETWORK AND MODULE ANALYSIS:
STRING (http://string-db.org) (version 10.0) provides the prediction of quality-controlled protein-protein association networks. We performed the STRING database to construct a protein-protein interaction (PPI) network for DEGs, and combined score >0.4 was set as statistically significant. Cytoscape (version 3.6.1) [18] was performed to visualize molecular interaction networks. The plugin Molecular Complexity Detection (MCODE) (version 1.5.1) in Cytoscape was used to identify the most important module from the PPI network. And the condition was set as follows: Degree cutoff=2, k-core=5, max. Depth=100, and node score cutoff=0.2. Subsequently, functional enrichment analysis was performed for genes in this module by the online bioinformatics database Metascape (http://metascape.org/) [19].
HUB GENES SELECTION AND ANALYSIS:
The plugin cytoHubba of Cytoscape was performed to calculate the degree of genes in the PPI network. DEGs with degrees >10 were selected as hub genes. The Kaplan Meier plotter (http://kmplot.com/analysis/) [20] is an online platform to estimate the prognostic value of thousands of genes in several cancer types based on the data from GEO, Genomic Expression Archive, and TCGA database. And we performed overall survival (OS) analysis of hub genes in LUAD with non-smoking females by Kaplan Meier plotter. Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn) [21] provides the differential analysis based on the Genotype-Tissue Expression and the TCGA database. Moreover, we visualized the differential expression of the most significant hub genes in LUAD by GEPIA. Finally, further analyses were performed on SPP1, the hub gene with the highest degree found by cytoHubba. Tumor Immune Estimation Resource (TIMER; https://cistrome.shinyapps.io/timer/) [22] was used to assess the expression profile of SPP1 in various human tumors based on TCGA database. And a meta-analysis of expression of SPP1 in LUAD compared with normal tissues in different datasets was estimated based on the Oncomine database (http://www.oncomine.com) [23]. SPP1 protein expression analysis in LUAD tissues and normal tissues was performed by the Human Protein Atlas (https://www.proteinatlas.org) [24].
DISTRIBUTION AND PROGNOSTIC ANALYSIS OF INFILTRATING IMMUNE CELLS IN NON-SMOKING FEMALE LUAD:
Firstly, we downloaded the Transcriptome Profiling data and Clinical data of female LUAD from TCGA database. Among them, 34 normal female lung tissue samples and 47 non-smoking female LUAD tissue samples were included in this study (as for only 5 normal non-smoking female lung tissue samples were available in TCGA, we included all of 34 normal female lung tissue samples as the control group). And the raw data was converted to which could be matched with CIBERSORT [25] by Practical Extraction and Report Language (Perl). Moreover, we randomized the converted data by limma packages (version 3.8). After deleting samples with P>0.05, 32 normal samples and 42 tumor samples were left. Then we predicted the distribution of 22 infiltrating immune cells in these samples by CIBERSORT. Finally, vioplot packages and survival packages were performed to illustrate the distribution and prognostic analysis of 22 infiltrating immune cells of non-smoking female LUAD.
Results
IDENTIFICATION OF DEGS:
The detailed sample information of the included datasets was presented in Supplementary Table 1. We identified 315 overlapped DEGs among 3 datasets (Figure 1), consisting of 254 downregulated DEGs and 61 upregulated DEGs (Supplementary Table 2). Notably, the regulation of these 315 DEGs was consistent in all these 3 datasets.
FUNCTIONAL ENRICHMENT ANALYSES OF DEGS:
The whole results of GO and KEGG enrichment analyses for 315 DEGs were presented in Supplementary Table 3, and the top 5 GO and KEGG terms were visualized in Table 1.
PPI NETWORK AND MODULE ANALYSIS:
The PPI network of 315 DEGs was constructed (Figure 2), including 315 nodes and 708 edges. And the most significant module was illustrated in Figure 3A. Subsequently, our results suggested that DEGs in this module were mostly enriched in ERK1 and ERK2 cascade, vasculature development, cellular response to hormone stimulus and adrenomedullin receptor signaling pathway (Figure 3B).
HUB GENE SCREENING AND ANALYSIS:
In total, we identified 36 DEGs as hub genes with degrees >10 (Supplementary Table 4). Subsequently, 10 hub genes (Table 2) were screened out with prognostic value (Figure 4). Our results indicated that over-expression of SPP1, ENG, ATF3, TOP2A, COL1A1, and PAICS was related to worse OS for non-smoking females with LUAD (P<0.05). On the other hand, under-expression of CAV1, CAT, TGFBR2, and ANGPT1 was associated with a poorer OS for non-smoking females with LUAD (P<0.05). As illustrated in Figure 5, compared with normal tissues, the expressing of SPP1, TOP2A, COL1A1, and PAICS increased in LUAD tissues, while ENG, ATF3, CAV1, CAT, TGFBR2, and ANGPT1 decreased based on GEPIA. These results were coordinated with the results of differential expression analysis based on the GEO database, which validated the reliability of GEO analysis indirectly. Among these 10 most significant hub genes, SPP1 accounts for the highest degree of 21, suggesting the potential significance. The result of TIMER indicated that SPP1 was overexpressed in some cancers compared with normal tissues, including LUAD, breast invasive carcinoma (BRCA), colon adenocarcinoma (COAD), liver hepatocellular carcinoma (LIHC), stomach adenocarcinoma (STAD), uterine corpus endometrial carcinoma (UCEC), etc., (Figure 6A). A meta-analysis based on Oncomine datasets revealed that SPP1 was over-expressed in LUAD compared with normal tissues (Figure 6B). As shown in Figure 6C, SPP1 protein was higher expressed in patients with LUAD compared with normal tissue.
DISTRIBUTION AND PROGNOSTIC ANALYSIS OF INFILTRATING IMMUNE CELLS IN NON-SMOKING FEMALE LUAD:
The detailed clinical information of included samples was shown in Supplementary Table 5. The distribution of 22 kinds of infiltrating immune cells of non-smoking female LUAD indicated that T cells CD4 memory resting, macrophages M2 and macrophages M0 accounted for the largest proportion (Figure 7A). The results revealed that a series of cells are differentially expressed between tumor tissues and normal tissues with P-value <0.05. Some cells have higher expression in tumor tissue than that in normal tissue, including plasma cells, T cells regulatory, macrophages M1, and dendritic cells resting. In contrast, some cells have lower expression in tumor tissue than that in normal tissue, consisting of T cells CD4 memory resting, natural killer (NK) cells resting, monocytes, macrophages M0, mast cells resting, and neutrophils. In addition, among these 22 infiltrating immune cells, only dendritic cells resting and macrophages M1 were found to be statistically significant (P<0.05) for prognostic value. As shown in Figure 7B and 7C, lower expression of dendritic cells resting indicated a poor prognosis, while lower expression of macrophages M1 suggested a better prognosis.
Discussion
In the present study, we found 10 prognostic hub genes and 2 kinds of significant infiltrating immune cells of LUAD in non-smoking females, which were verified with multiple databases. The biological functions and signaling pathways enriched in DEGs might participate in the tumorigenesis and development of LUAD in non-smoking females. Notably, this work was repeated 3 times by 3 individual researchers to ensure the reliability of the results.
Among these 10 most significant hub genes with prognostic value,
In order to further explore the pathogenesis of LUAD in non-smoking females, we screened the most important module (Figure 3A) from the PPI network. The DEGs in this module were mostly enriched in vasculature development, transforming growth factor (TGF)-beta signal pathway, and cellular response to hormone stimulus (Figure 3B), which could be involved in oncogenesis and progress of LUAD in non-smoking females. Among them,
Our results indicated that over-expression of
Furthermore, the immune microenvironment of LUAD in non-smoking females might also contribute to the tumorigenesis. A model of ovarian cancer indicated that increased immune infiltrates contributed to tumor progression, including dendritic cells and macrophages [60], which supported our findings (Figure 7A). However, Figure 7B illustrated that lower expression level of dendritic cells resting resulted in poor prognosis. As for relapsed colorectal cancer patients, fewer tumor-infiltrating dendritic cells were detected [61]. On the other hand, the increased tumor-infiltrating dendritic cells were observed in a mouse model of ovarian cancer as tumor progressed [62]. This suggested that during the process of tumorigenesis and development, the amount, subtypes, and functions of dendritic cells were changing [63], and gender and smoking might be included as impact factors to understand its complex functions. Accumulated evidence revealed the essential value of TAMs, M1 phenotype of TAMs, was identified as a tumor-suppressing factor in LUAD [64]. Based on TCGA and CIBERSORT, our results suggested that lower level of macrophages M1 was associated with better prognosis, which is contrary to previous reports, suggesting that female and smoking might be independent factors. It has been reported that the ratio of macrophages M1 increased from 26% to 84% with smoking severity [65]. Regrettably, the impact of gender and smoking on macrophages M1 in LUAD has not been assessed in previous studies, and further research is urgently demanded.
Conclusions
Importantly, in this study, the mechanisms of LUAD in non-smoking females were explored by bioinformatics methods, and promising biomarkers and possible signaling pathways were identified and validated based on multiple databases were combined to confirm these results. Ten hub genes and 2 immune cell subtypes were found with prognostic significance, including
Figures
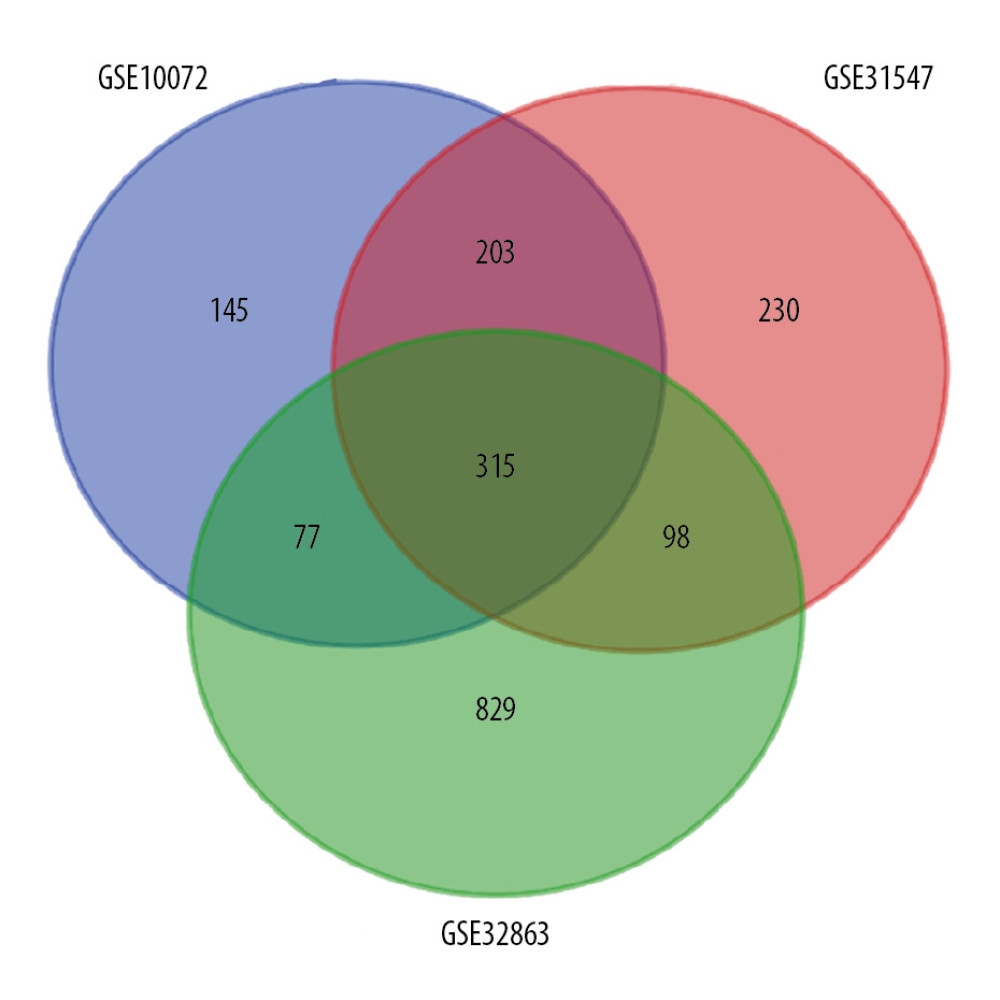 Figure 1. Venn diagram for overlapping DEGs in 3 microarray datasets. |log FC| >1 and P<0.01 was set as the cutoff criterion. There were 315 overlapped DEGs among 3 datasets (GSE10072, GSE31547, GSE32863) identified. DEGs – differentially expressed genes.
Figure 1. Venn diagram for overlapping DEGs in 3 microarray datasets. |log FC| >1 and P<0.01 was set as the cutoff criterion. There were 315 overlapped DEGs among 3 datasets (GSE10072, GSE31547, GSE32863) identified. DEGs – differentially expressed genes. 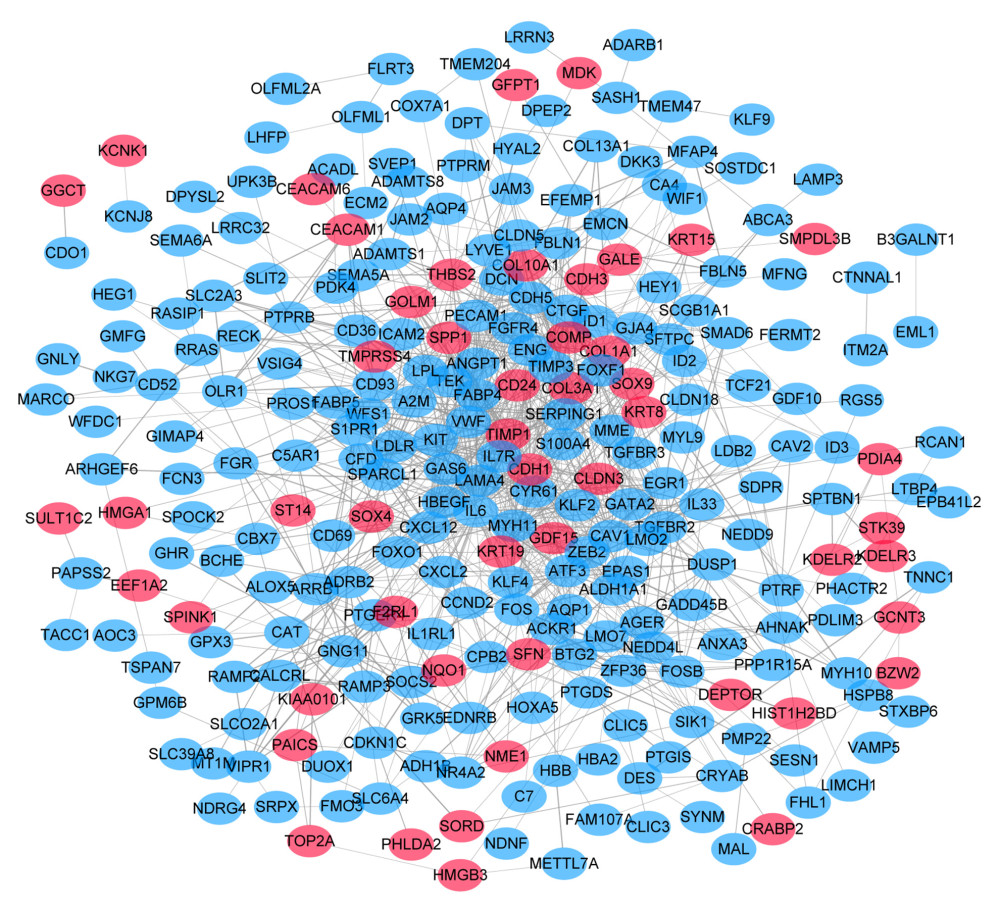 Figure 2. PPI network of 315 DEGs construction using STRING and Cytoscape. This network includes 315 nodes and 708 edges. Nodes stand for the DEGs and edges stand for the association of DEGs. Red nodes represent upregulated DEGs, while blue nodes represent downregulated DEGs. PPI – protein–protein interactions; DEGs – differentially expressed genes.
Figure 2. PPI network of 315 DEGs construction using STRING and Cytoscape. This network includes 315 nodes and 708 edges. Nodes stand for the DEGs and edges stand for the association of DEGs. Red nodes represent upregulated DEGs, while blue nodes represent downregulated DEGs. PPI – protein–protein interactions; DEGs – differentially expressed genes. 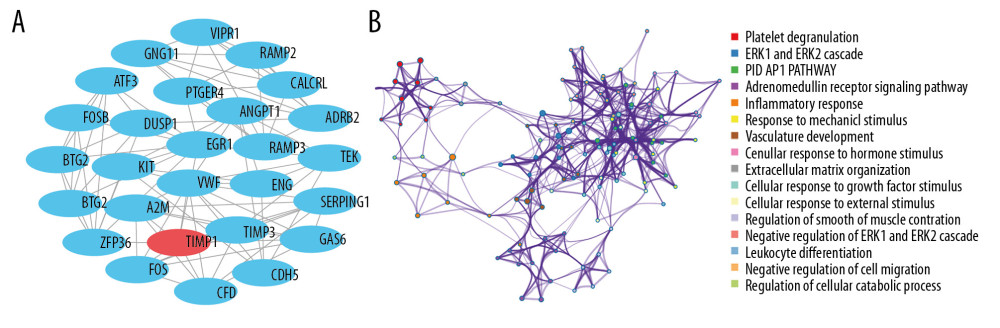 Figure 3. Analysis of the most significant module. (A) Identification of the most significant module from PPI network using MCODE plugin of Cytoscape. Red nodes stand for upregulated DEGs, while blue nodes represent downregulated DEGs. (B) Functional enrichment analysis of the most significant module performed by Metascape. PPI – protein–protein interactions; MCODE – Molecular Complexity Detection; DEGs – differentially expressed genes.
Figure 3. Analysis of the most significant module. (A) Identification of the most significant module from PPI network using MCODE plugin of Cytoscape. Red nodes stand for upregulated DEGs, while blue nodes represent downregulated DEGs. (B) Functional enrichment analysis of the most significant module performed by Metascape. PPI – protein–protein interactions; MCODE – Molecular Complexity Detection; DEGs – differentially expressed genes. 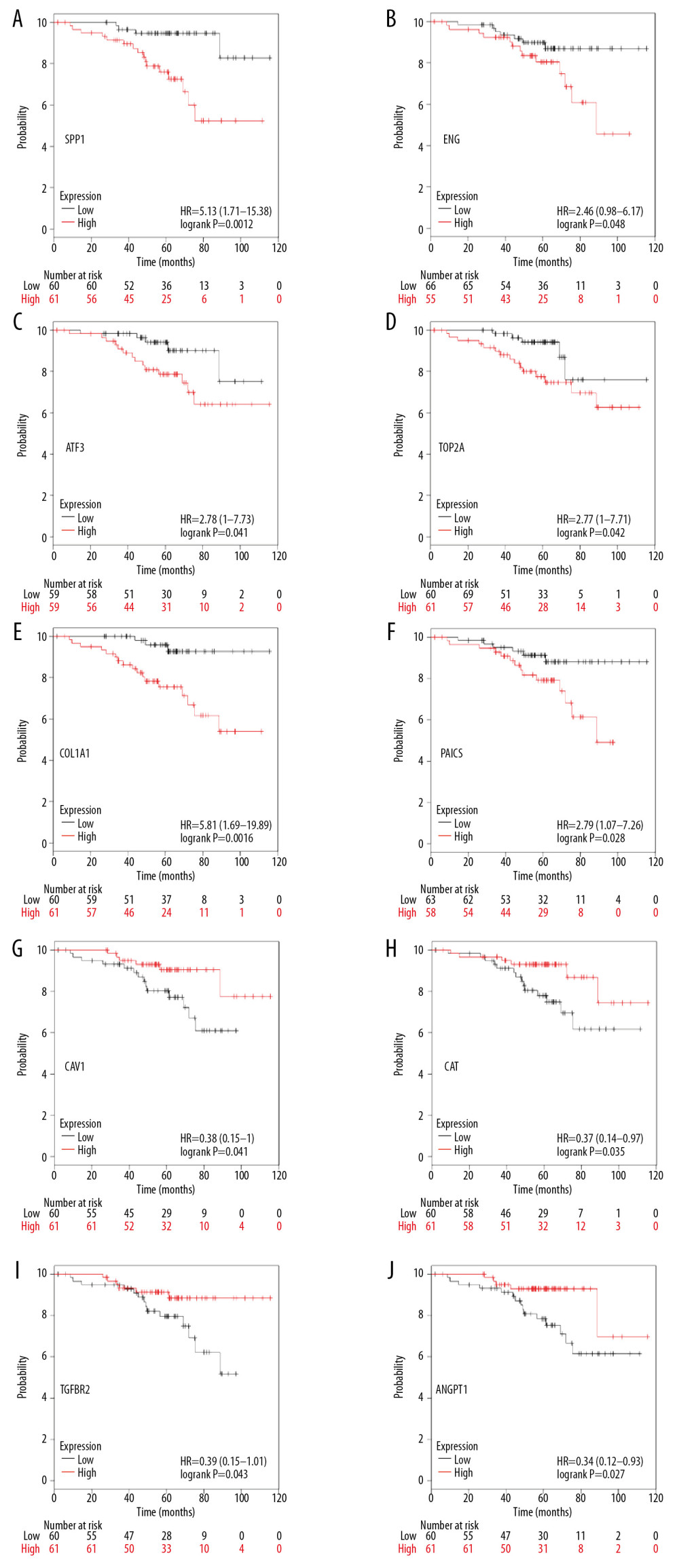 Figure 4. Overall survival analysis of 10 most significant hub genes in non-smoking females with LUAD based on Kaplan Meier plotter platform using the data of GEO, GEA, and TCGA databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEO – Gene Expression Omnibus; GEA – Genomic Expression Archive; TCGA – The Cancer Genome Atlas.
Figure 4. Overall survival analysis of 10 most significant hub genes in non-smoking females with LUAD based on Kaplan Meier plotter platform using the data of GEO, GEA, and TCGA databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEO – Gene Expression Omnibus; GEA – Genomic Expression Archive; TCGA – The Cancer Genome Atlas. 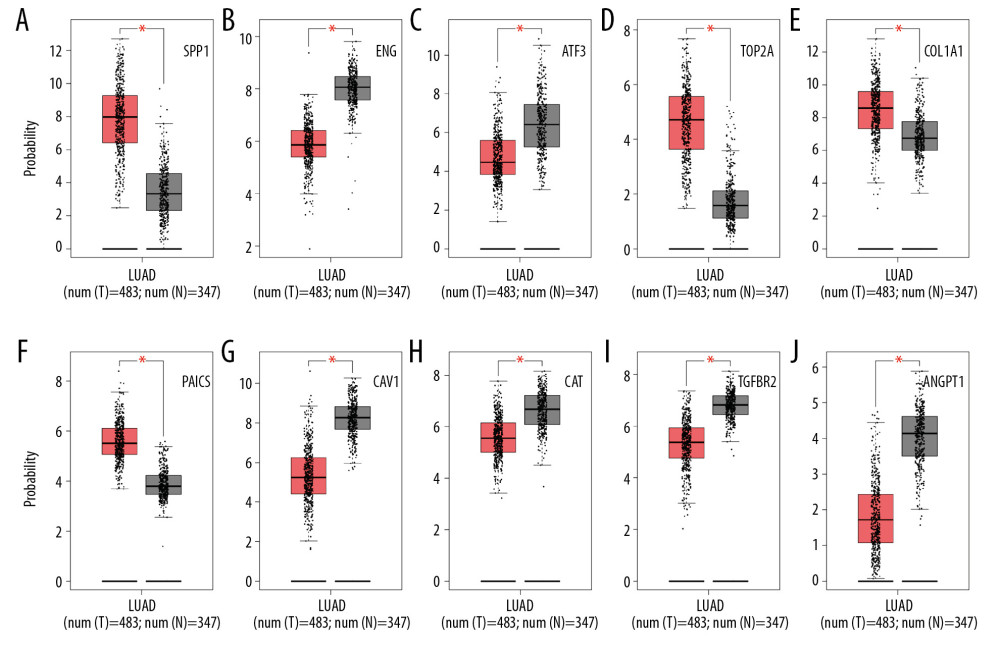 Figure 5. The differential expression analysis of 10 most significant hub genes in LUAD based on GEPIA using the data of TCGA and GTEx databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEPIA – Gene Expression Profiling Interactive Analysis; TCGA – The Cancer Genome Atlas; GTEx – Genotype-Tissue Expression.
Figure 5. The differential expression analysis of 10 most significant hub genes in LUAD based on GEPIA using the data of TCGA and GTEx databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEPIA – Gene Expression Profiling Interactive Analysis; TCGA – The Cancer Genome Atlas; GTEx – Genotype-Tissue Expression. 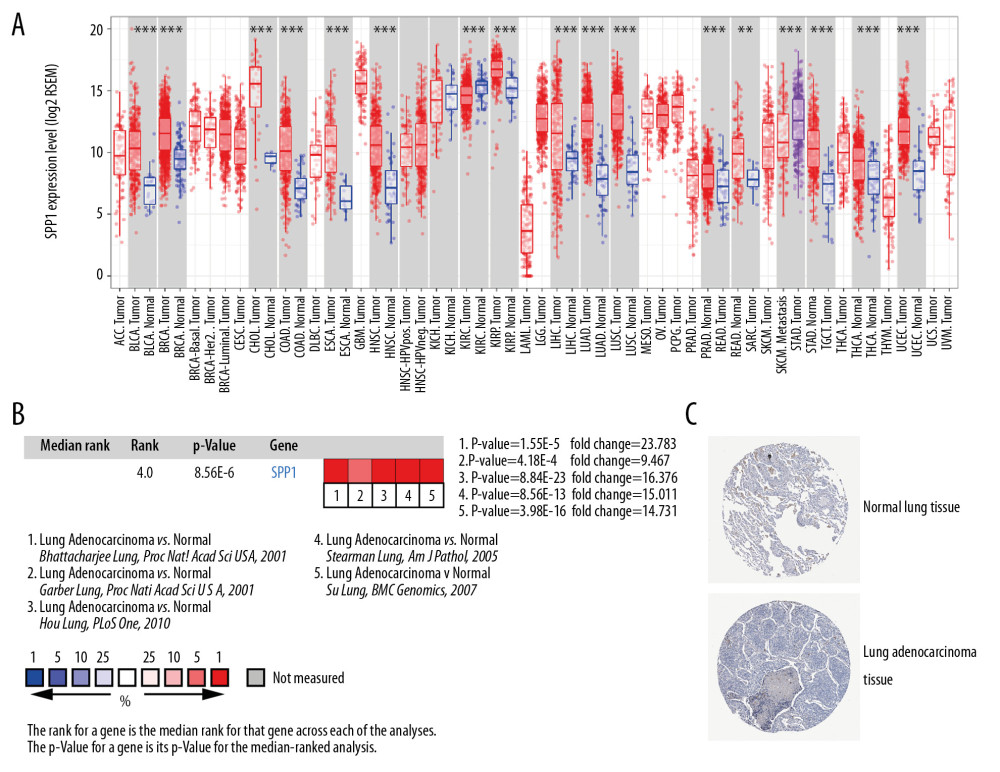 Figure 6. The upregulation of SPP1 was validated in different databases. (A) The expression profiling of SPP1 in various tumor types performed by TIMER according to the TCGA database. The red columns stand for tumor samples and the blue columns stand for normal samples. *** Represents that the P<0.001, and ** represents that the P<0.01. (B) A meta-analysis of SPP1 across 5 analyses in the Oncomine database showed that SPP1 was upregulated in LUAD. (C) Immunohistochemistry results of SPP1 protein expression in LUAD tissue and normal tissue from HPA database. TIMER – Tumor Immune Estimation Resource; TCGA – The Cancer Genome Atlas; HPA – Human Protein Atlas; LUAD – lung adenocarcinoma.
Figure 6. The upregulation of SPP1 was validated in different databases. (A) The expression profiling of SPP1 in various tumor types performed by TIMER according to the TCGA database. The red columns stand for tumor samples and the blue columns stand for normal samples. *** Represents that the P<0.001, and ** represents that the P<0.01. (B) A meta-analysis of SPP1 across 5 analyses in the Oncomine database showed that SPP1 was upregulated in LUAD. (C) Immunohistochemistry results of SPP1 protein expression in LUAD tissue and normal tissue from HPA database. TIMER – Tumor Immune Estimation Resource; TCGA – The Cancer Genome Atlas; HPA – Human Protein Atlas; LUAD – lung adenocarcinoma. 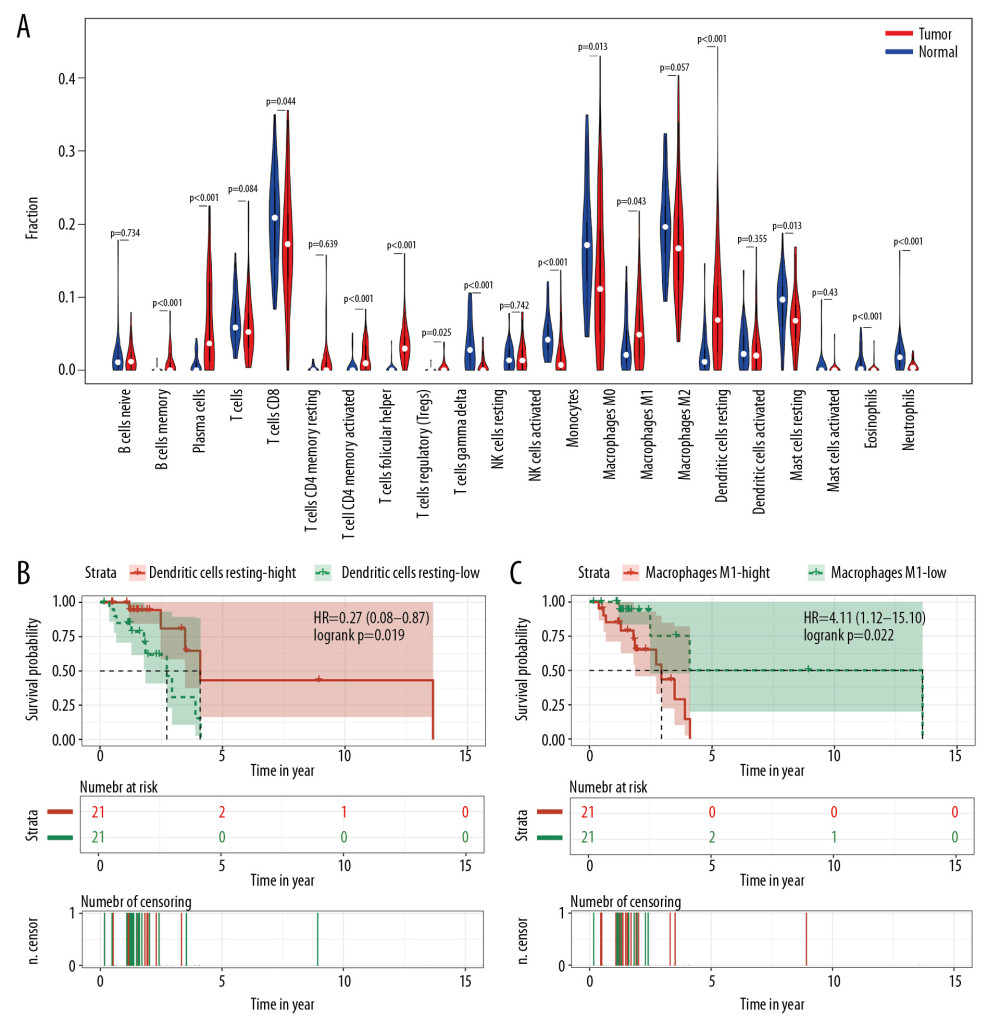 Figure 7. Distribution and prognostic analysis of infiltrating immune cells in non-smoking females with LUAD based on TCGA database and CIBERSORT. (A) Distribution landscape of infiltrating immune cell in non-smoking females with LUAD. The overall survival analysis of dendritic cells resting (B) and macrophages M1 (C). LUAD – lung adenocarcinoma; TCGA – The Cancer Genome Atlas.
Figure 7. Distribution and prognostic analysis of infiltrating immune cells in non-smoking females with LUAD based on TCGA database and CIBERSORT. (A) Distribution landscape of infiltrating immune cell in non-smoking females with LUAD. The overall survival analysis of dendritic cells resting (B) and macrophages M1 (C). LUAD – lung adenocarcinoma; TCGA – The Cancer Genome Atlas. References
1. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69; 7-34
2. Cancer Genome Atlas Research Network, Comprehensive molecular profiling of lung adenocarcinoma: Nature, 2014; 511; 543-50
3. Donner I, Katainen R, Sipila LJ, Germline mutations in young non-smoking women with lung adenocarcinoma: Lung Cancer, 2018; 122; 76-82
4. Cheng TD, Darke AK, Redman MW, Smoking, sex, and non-small cell lung cancer: Steroid hormone receptors in tumor tissue (S0424): J Natl Cancer Inst, 2018; 110; 734-42
5. Hsu LH, Chu NM, Kao SH, Estrogen, estrogen receptor and lung cancer: Int J Mol Sci, 2017; 18(8) pii: E1713
6. O’Keeffe LM, Taylor G, Huxley RR, Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis: BMJ Open, 2018; 8; e021611
7. Kinoshita FL, Ito Y, Morishima T, Sex differences in lung cancer survival: Long-term trends using population-based cancer registry data in Osaka, Japan: Jpn J Clin Oncol, 2017; 47; 863-69
8. Conforti F, Pala L, Bagnardi V, Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis: Lancet Oncol, 2018; 19; 737-46
9. Skov BG, Fischer BM, Pappot H, Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival: Lung Cancer, 2008; 59; 88-94
10. Sun S, Schiller JH, Gazdar AF, Lung cancer in never smokers – a different disease: Nat Rev Cancer, 2007; 7; 778-90
11. Nakagawa H, Fujita M, Whole genome sequencing analysis for cancer genomics and precision medicine: Cancer Sci, 2018; 109; 513-22
12. Wu D, Wang X, Application of clinical bioinformatics in lung cancer-specific biomarkers: Cancer Metastasis Rev, 2015; 34; 209-16
13. Barrett T, Wilhite SE, Ledoux P, NCBI GEO: Archive for functional genomics data sets – update: Nucleic Acids Res, 2013; 41; D991-95
14. Feng H, Gu ZY, Li Q, Identification of significant genes with poor prognosis in ovarian cancer via bioinformatical analysis: J Ovarian Res, 2019; 12; 35
15. Sun C, Yuan Q, Wu D, Identification of core genes and outcome in gastric cancer using bioinformatics analysis: Oncotarget, 2017; 8; 70271-80
16. Zhang L, Peng R, Sun Y, Identification of key genes in non-small cell lung cancer by bioinformatics analysis: Peer J, 2019; 7; e8215
17. Jiao X, Sherman BT, Huang da W, DAVID-WS: A stateful web service to facilitate gene/protein list analysis: Bioinformatics, 2012; 28; 1805-6
18. Doncheva NT, Morris JH, Gorodkin J, Jensen LJ, Cytoscape stringApp: Network analysis and visualization of proteomics data: J Proteome Res, 2019; 18; 623-32
19. Tripathi S, Pohl MO, Zhou Y, Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding: Cell Host Microbe, 2015; 18; 723-35
20. Gyorffy B, Surowiak P, Budczies J, Lanczky A, Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer: PLoS One, 2013; 8; e82241
21. Tang Z, Li C, Kang B, GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses: Nucleic Acids Res, 2017; 45; W98-102
22. Li T, Fan J, Wang B, TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells: Cancer Res, 2017; 77; e108-10
23. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles: Neoplasia, 2007; 9; 166-80
24. Uhlen M, Fagerberg L, Hallstrom BM, Proteomics. Tissue-based map of the human proteome: Science, 2015; 347; 1260419
25. Newman AM, Liu CL, Green MR, Robust enumeration of cell subsets from tissue expression profiles: Nat Methods, 2015; 12; 453-57
26. Choe EK, Yi JW, Chai YJ, Park KJ, Upregulation of the adipokine genes ADIPOR1 and SPP1 is related to poor survival outcomes in colorectal cancer: J Surg Oncol, 2018; 117; 1833-40
27. Chen X, Xiong D, Ye L, SPP1 inhibition improves the cisplatin chemo-sensitivity of cervical cancer cell lines: Cancer Chemother Pharmacol, 2019; 83; 603-13
28. Insua-Rodríguez J, Pein M, Hongu T, Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis: EMBO Mol Med, 2018; 10 pii: e9003
29. Hu Z, Lin D, Yuan J, Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer: Clin Cancer Res, 2005; 11; 4646-52
30. Blasberg JD, Pass HI, Goparaju CM, Reduction of elevated plasma osteopontin levels with resection of non-small-cell lung cancer: J Clin Oncol, 2010; 28; 936-41
31. Hao C, Cui Y, Owen S, Human osteopontin: Potential clinical applications in cancer (review): Int J Mol Med, 2017; 39; 1327-37
32. Ogata T, Ueyama T, Nomura T, Osteopontin is a myosphere-derived secretory molecule that promotes angiogenic progenitor cell proliferation through the phosphoinositide 3-kinase/Akt pathway: Biochem Biophys Res Commun, 2007; 359; 341-47
33. Pang X, Xie R, Zhang Z, Identification of SPP1 as an extracellular matrix signature for metastatic castration-resistant prostate cancer: Front Oncol, 2019; 9; 924
34. Das R, Philip S, Mahabeleshwar GH, Osteopontin: it’s role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression: IUBMB Life, 2005; 57; 441-47
35. Babarović E, Valković T, Budisavljević I, The expression of osteopontin and vascular endothelial growth factor in correlation with angiogenesis in monoclonal gammopathy of undetermined significance and multiple myeloma: Pathol Res Pract, 2016; 212; 509-16
36. Wang X, Zhang F, Yang X, Secreted phosphoprotein 1 (SPP1) contributes to second-generation EGFR tyrosine kinase inhibitor resistance in non-small cell lung cancer: Oncol Res, 2019; 27; 871-77
37. Zhang Y, Du W, Chen Z, Xiang C, Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma: Exp Cell Res, 2017; 359; 449-57
38. Chang WL, Lin MY, Kuo HY: Future Oncol, 2017; 13; 1415-25
39. Banerjee A, Rose R, Johnson GA, The influence of estrogen on hepatobiliary osteopontin (SPP1) expression in a female rodent model of alcoholic steatohepatitis: Toxicol Pathol, 2009; 37; 492-501
40. Maneechotesuwan K, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ, Simvastatin up-regulates adenosine deaminase and suppresses osteopontin expression in COPD patients through an IL-13-dependent mechanism: Respir Res, 2016; 17; 104
41. Bishop E, Theophilus EH, Fearon IM: BMC Cardiovasc Disord, 2012; 12; 75
42. Fisher TE, Molskness TA, Villeda A, Vascular endothelial growth factor and angiopoietin production by primate follicles during culture is a function of growth rate, gonadotrophin exposure and oxygen milieu: Hum Reprod, 2013; 28; 3263-70
43. Yao S, Dong SS, Ding JM, Sex-specific SNP-SNP interaction analyses within topologically associated domains reveals ANGPT1 as a novel tumor suppressor gene for lung cancer: Genes Chromosomes Cancer, 2019 [Epub ahead of print]
44. Michael IP, Orebrand M, Lima M, Angiopoietin-1 deficiency increases tumor metastasis in mice: BMC Cancer, 2017; 17; 539
45. Lebrin F, Goumans MJ, Jonker L, Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction: EMBO J, 2004; 23; 4018-28
46. Dallas NA, Samuel S, Xia L, Endoglin (CD105): A marker of tumor vasculature and potential target for therapy: Clin Cancer Res, 2008; 14; 1931-37
47. Du A, Jiang Y, Fan C, NDRG1 Downregulates ATF3 and inhibits cisplatin-induced cytotoxicity in lung cancer A549 cells: Int J Med Sci, 2018; 15; 1502-7
48. Li X, Zhou X, Li Y: Thorac Cancer, 2017; 8; 181-91
49. Wu Q, Zhang B, Sun Y, Identification of novel biomarkers and candidate small molecule drugs in non-small-cell lung cancer by integrated microarray analysis: Onco Targets Ther, 2019; 12; 3545-63
50. Zhang Z, Wang Y, Zhang J, COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway: Mol Med Rep, 2018; 17; 5037-42
51. Gallenne T, Ross KN, Visser NL, Systematic functional perturbations uncover a prognostic genetic network driving human breast cancer: Oncotarget, 2017; 8; 20572-87
52. Zhang R, Xu J, Zhao J, Bai JH, Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A: J Cell Biochem, 2018; 119; 7256-63
53. Tsai JY, Lee MJ, Dah-Tsyr Chang M, Huang H, The effect of catalase on migration and invasion of lung cancer cells by regulating the activities of cathepsin S, L, and K: Exp Cell Res, 2014; 323; 28-40
54. Yan Y, Xu Z, Qian L, Identification of CAV1 and DCN as potential predictive biomarkers for lung adenocarcinoma: Am J Physiol Lung Cell Mol Physiol, 2019; 316; L630-43
55. Malkoski SP, Haeger SM, Cleaver TG, Loss of transforming growth factor beta type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma: Clin Cancer Res, 2012; 18; 2173-83
56. Singh DP, Kaur G, Bagam P, Membrane microdomains regulate NLRP10- and NLRP12-dependent signalling in A549 cells challenged with cigarette smoke extract: Arch Toxicol, 2018; 92; 1767-83
57. Koomägi R, Stammler G, Manegold C, Expression of resistance-related proteins in tumoral and peritumoral tissues of patients with lung cancer: Cancer Lett, 1996; 110; 129-36
58. Szymanowska-Narloch A, Jassem E, Skrzypski M, Molecular profiles of non-small cell lung cancers in cigarette smoking and never-smoking patients: Adv Med Sci, 2013; 58; 196-206
59. Alzoubi KH, Halboup AM, Alomari MA, Khabour OF, The neuroprotective effect of vitamin E on waterpipe tobacco smoking-induced memory impairment: The antioxidative role: Life Sci, 2019; 222; 46-52
60. Scarlett UK, Rutkowski MR, Rauwerdink AM, Ovarian cancer progression is controlled by phenotypic changes in dendritic cells: J Exp Med, 2012; 209; 495-506
61. Krempski J, Karyampudi L, Behrens MD, Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer: J Immunol, 2011; 186; 6905-13
62. Kocián P, Šedivcová M, Drgáč J, Tumor-infiltrating lymphocytes and dendritic cells in human colorectal cancer: Their relationship to KRAS mutational status and disease recurrence: Hum Immunol, 2011; 72; 1022-28
63. Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL, Tumor-infiltrating dendritic cells in cancer pathogenesis: J Immunol, 2015; 194; 2985-91
64. Rakaee M, Busund LR, Jamaly S, Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry: Neoplasia, 2019; 21; 282-93
65. Bazzan E, Turato G, Tine M, Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity: Respir Res, 2017; 18; 40
Figures
 Figure 1. Venn diagram for overlapping DEGs in 3 microarray datasets. |log FC| >1 and P<0.01 was set as the cutoff criterion. There were 315 overlapped DEGs among 3 datasets (GSE10072, GSE31547, GSE32863) identified. DEGs – differentially expressed genes.
Figure 1. Venn diagram for overlapping DEGs in 3 microarray datasets. |log FC| >1 and P<0.01 was set as the cutoff criterion. There were 315 overlapped DEGs among 3 datasets (GSE10072, GSE31547, GSE32863) identified. DEGs – differentially expressed genes. Figure 2. PPI network of 315 DEGs construction using STRING and Cytoscape. This network includes 315 nodes and 708 edges. Nodes stand for the DEGs and edges stand for the association of DEGs. Red nodes represent upregulated DEGs, while blue nodes represent downregulated DEGs. PPI – protein–protein interactions; DEGs – differentially expressed genes.
Figure 2. PPI network of 315 DEGs construction using STRING and Cytoscape. This network includes 315 nodes and 708 edges. Nodes stand for the DEGs and edges stand for the association of DEGs. Red nodes represent upregulated DEGs, while blue nodes represent downregulated DEGs. PPI – protein–protein interactions; DEGs – differentially expressed genes. Figure 3. Analysis of the most significant module. (A) Identification of the most significant module from PPI network using MCODE plugin of Cytoscape. Red nodes stand for upregulated DEGs, while blue nodes represent downregulated DEGs. (B) Functional enrichment analysis of the most significant module performed by Metascape. PPI – protein–protein interactions; MCODE – Molecular Complexity Detection; DEGs – differentially expressed genes.
Figure 3. Analysis of the most significant module. (A) Identification of the most significant module from PPI network using MCODE plugin of Cytoscape. Red nodes stand for upregulated DEGs, while blue nodes represent downregulated DEGs. (B) Functional enrichment analysis of the most significant module performed by Metascape. PPI – protein–protein interactions; MCODE – Molecular Complexity Detection; DEGs – differentially expressed genes. Figure 4. Overall survival analysis of 10 most significant hub genes in non-smoking females with LUAD based on Kaplan Meier plotter platform using the data of GEO, GEA, and TCGA databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEO – Gene Expression Omnibus; GEA – Genomic Expression Archive; TCGA – The Cancer Genome Atlas.
Figure 4. Overall survival analysis of 10 most significant hub genes in non-smoking females with LUAD based on Kaplan Meier plotter platform using the data of GEO, GEA, and TCGA databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEO – Gene Expression Omnibus; GEA – Genomic Expression Archive; TCGA – The Cancer Genome Atlas. Figure 5. The differential expression analysis of 10 most significant hub genes in LUAD based on GEPIA using the data of TCGA and GTEx databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEPIA – Gene Expression Profiling Interactive Analysis; TCGA – The Cancer Genome Atlas; GTEx – Genotype-Tissue Expression.
Figure 5. The differential expression analysis of 10 most significant hub genes in LUAD based on GEPIA using the data of TCGA and GTEx databases, including SPP1 (A), ENG (B), ATF3 (C), TOP2A (D), COL1A1 (E), PAICS (F), CAV1 (G), CAT (H), TGFBR2 (I), ANGPT1 (J). LUAD – lung adenocarcinoma; GEPIA – Gene Expression Profiling Interactive Analysis; TCGA – The Cancer Genome Atlas; GTEx – Genotype-Tissue Expression. Figure 6. The upregulation of SPP1 was validated in different databases. (A) The expression profiling of SPP1 in various tumor types performed by TIMER according to the TCGA database. The red columns stand for tumor samples and the blue columns stand for normal samples. *** Represents that the P<0.001, and ** represents that the P<0.01. (B) A meta-analysis of SPP1 across 5 analyses in the Oncomine database showed that SPP1 was upregulated in LUAD. (C) Immunohistochemistry results of SPP1 protein expression in LUAD tissue and normal tissue from HPA database. TIMER – Tumor Immune Estimation Resource; TCGA – The Cancer Genome Atlas; HPA – Human Protein Atlas; LUAD – lung adenocarcinoma.
Figure 6. The upregulation of SPP1 was validated in different databases. (A) The expression profiling of SPP1 in various tumor types performed by TIMER according to the TCGA database. The red columns stand for tumor samples and the blue columns stand for normal samples. *** Represents that the P<0.001, and ** represents that the P<0.01. (B) A meta-analysis of SPP1 across 5 analyses in the Oncomine database showed that SPP1 was upregulated in LUAD. (C) Immunohistochemistry results of SPP1 protein expression in LUAD tissue and normal tissue from HPA database. TIMER – Tumor Immune Estimation Resource; TCGA – The Cancer Genome Atlas; HPA – Human Protein Atlas; LUAD – lung adenocarcinoma. Figure 7. Distribution and prognostic analysis of infiltrating immune cells in non-smoking females with LUAD based on TCGA database and CIBERSORT. (A) Distribution landscape of infiltrating immune cell in non-smoking females with LUAD. The overall survival analysis of dendritic cells resting (B) and macrophages M1 (C). LUAD – lung adenocarcinoma; TCGA – The Cancer Genome Atlas.
Figure 7. Distribution and prognostic analysis of infiltrating immune cells in non-smoking females with LUAD based on TCGA database and CIBERSORT. (A) Distribution landscape of infiltrating immune cell in non-smoking females with LUAD. The overall survival analysis of dendritic cells resting (B) and macrophages M1 (C). LUAD – lung adenocarcinoma; TCGA – The Cancer Genome Atlas. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952