09 September 2020: Clinical Research
Clinical Characteristics and Treatment Outcomes in Human Immunodeficiency Virus (HIV)-Infected Patients with Liver Abscess: A Retrospective Study of 53 Patients
Wei Zhang1AE, Hongwei Yu1BC, Na Luo2BC, Zhongjie Hu1AE*DOI: 10.12659/MSM.923761
Med Sci Monit 2020; 26:e923761
Abstract
BACKGROUND: Although episodes of liver abscess (LA) have been reported in patients infected with the human immunodeficiency virus (HIV), specific symptoms in these patients remain unclear.
MATERIAL AND METHODS: The clinical characteristics, laboratory findings, treatments, and final clinical outcomes of LA in 53 HIV-infected patients were analyzed.
RESULTS: The most common clinical manifestations were fever (92.5%), chills (41.5%), and abdominal pain (37.7%). The mean CD4⁺ T cell count in these HIV-infected patients at admission was 328.09±236.192 cells/µL. LA and blood cultures were positive in six (17.6%) and two (5.4%) patients, respectively. Thirteen strains of pathogens, including Staphylococcus, Corynebacterium, and Candida, were detected in LA cultures. Forty-four (95.7%) of 46 patients were successfully treated with antibiotics plus image-guided percutaneous aspiration, drainage, or surgery, whereas four (57.1%) of the remaining seven patients were successfully treated with antibiotics alone. Septic shock [odds ratio (OR)=8.970; 95% confidence interval (CI)=0.840–92.110; p=0.014] and ascites (OR=7.057; 95% CI=0.683–72.957; p=0.016) were found to be independent risk factors for poor prognosis. The clinical characteristics of LA in HIV-infected patients were nonspecific, with bacteria being the primary pathogens.
CONCLUSIONS: Antibiotics plus image-guided percutaneous drainage can effectively improve treatment outcomes in HIV-infected patients with LA.
Keywords: HIV, liver abscess, Anti-Bacterial Agents, Drainage, HIV Infections, Surgery, Computer-Assisted, young adult
Background
Liver abscess (LA) is classified as a pus-filled suppurated mass in the liver parenchyma, probably caused by infection and multiplication of microorganisms [1]. The incidence of this rare condition varies across regions. For example, the incidence of LA was found to be 1.1–1.3 per 100,000 individuals in western countries [2–4] and 17.6 per 100,000 individuals in Asian countries [5]. Moreover, the incidence of LA in the United States has increased from 3.59 to 4.0 per 100,000 individuals [6]. The mortality rate of LA is relatively high, between 10% and 40% [7]. In recent years, a generally immunocompromised state has been regarded as the key risk factor for LA, although other factors, including diabetes mellitus (DM), cirrhosis, sex, and age have been associated with the onset of LA [8].
Acquired immunodeficiency syndrome (AIDS), characterized by an immunodeficient and immunocompromised state, is an infectious disease caused by human immunodeficiency virus (HIV). HIV-infected patients are susceptible to a number of infections [9, 10], with LA being one of the most common and severe infections reported. In Taiwan, the incidence of amoebic LA in HIV-infected patients has increased markedly [11]. However, the specific characteristics of LA in Chinese patients with underlying HIV infection remain largely unknown.
We have had 8 years of experience in the diagnosis and management of LA in HIV-infected patients. This retrospective study was therefore performed to identify the clinical manifestations; evaluate the imaging, pathological, and microbiological characteristics; and determine the preferred treatment plan for these patients. This study also sought to identify factors that could predict the persistence of LA in HIV-infected patients.
Material and Methods
PATIENTS:
This retrospective study included HIV-infected patients with LA who were admitted to Beijing Youan Hospital, Capital Medical University, China, between January 2010 and December 2018, inclusive. The study procedure complied with the principles of the Declaration of Helsinki and was approved by Beijing Youan Hospital, which waived the requirement for written informed consent owing to the retrospective nature of the study. Only the first episode of LA experienced by each patient during the study period was included in the statistical analyses.
POPULATION:
The hospital records of Beijing Youan Hospital were subjected to a computerized search using ICD-9 codes to identify HIV-infected patients diagnosed with LA during the study period. Patient characteristics were recorded at admission, with readmissions considered single admissions.
DATA COLLECTION AND DEFINITIONS:
Data collected from patients’ electronic medical records included general information, medical history, clinical symptoms, findings at physical examination, laboratory data, imaging results, treatments, complications, and outcomes. LA was diagnosed based on clinical characteristics, imaging results, microbiological findings, and/or pathological findings. Patients aged <18 years, those with hydatid disease of the liver or secondary pyogenic LA, and those with incomplete data were excluded.
The included patients were categorized into two groups, those exhibiting improved clinical signs and symptoms as well as reduced or absorbed LA (improvement group) and those without significant improvements in clinical signs and symptoms and those who were discharged or died during hospitalization (non-improvement group).
STATISTICAL ANALYSIS:
All data were analyzed using SPSS 20.0. Parametric variables were analyzed using Student’s
Results
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS:
The demographic characteristics of the 53 HIV-infected patients diagnosed with LA between January 2010 and December 2018 are presented in Table 1. These 53 patients included 52 (98.1%) men and one (1.9%) woman, of mean±SD age 36.96±10.86 years (range, 20–71 years). Forty-eight (90.6%) patients were successfully treated, whereas five (9.4%) showed no improvements in clinical signs and symptoms and were discharged or died during hospitalization.
The most common HIV transmission route was homosexual contact (
Of the 53 HIV-infected patients, 23 (43.4%) developed LA 2 months to 7 years after being diagnosed with AIDS. Of these 23 patients, 15 (65.2%) received antiretroviral therapy (ART). The other 30 (56.6%) patients were unaware of HIV infection until developing LA (ART-naïve patients), with 23 (76.7%) of these 30 patients receiving ART during the subsequent course of their disease.
Among the 53 study patients, 23 (43.4%) had syphilis and one (1.9%) had diabetes mellitus. Two (3.8%) patients were coinfected with hepatitis B virus (HBV) and one (1.9%) with hepatitis C virus (HCV). No patient was infected with both HBV and HCV. Three (5.7%) patients reported a history of alcohol abuse, and 24 (45.3%) patients had gallbladder diseases.
SYMPTOMS AND SIGNS:
The most frequent symptom experienced by these patients was fever (
Clinically, 23 (43.4%) patients had splenomegaly, 18 (34.0%) had right upper quadrant pain, seven (13.2%) had hepatomegaly, and four (7.6%) were positive for Murphy’s sign at admission (Table 2).
LABORATORY AND IMAGING FINDINGS:
As shown in Table 3, white blood cell counts were elevated (>9.5×109/L) in 26 (49.1%) patients and anemia in 46 (86.8%). Abnormal results included elevated alanine aminotransferase (32.7%), aspartate aminotransferase (53.9%), total bilirubin (23.1%), direct bilirubin (32.79%), and alkaline phosphatase (53.3%) activities. The mean CD4+ T cell count at admission in all 53 HIV-infected patients was 328.09±236.19 cells/μL, with 17 (32.1%) patients having CD4+ T cell counts <200 cells/μL.
Forty (75.5%) patients had a single LA, whereas 13 (24.5%) had multiple LAs. LA was present in the right lobe in 33 (62.2%) patients, the left lobe in nine (17.0%), the caudate lobe in two (3.8%), and both lobes in nine (17.0%). LA diameter ranged from 2.10 to 16.30 cm, being <5 cm in six (11.3%) patients, 5<≤10 cm in 31 (58.5%), and >10 cm in 16 (30.2%).
MICROBIOLOGICAL CHARACTERISTICS:
LA culture results were available for 34 (64.2%) of the 53 study patients. Of these 34 patients, six (17.6%) had positive bacterial cultures, with the most commonly identified species being
TREATMENT AND OUTCOMES:
Patients were started on empirical antibiotic treatment at the onset of infection, with antibiotics subsequently changed based on the results of susceptibility tests. Seven (13.2%) patients received medical treatment alone without interventional treatment or surgery (Table 4), with four (57.1%) successfully treated with antibiotics alone. Of the three patients who did not respond to antibiotic treatment, two were discharged from the hospital for financial reasons and the third died of lymphadenoma during hospitalization.
Of the 53 patients, 44 (83.0%) underwent ultrasound or computed tomography (CT)-guided percutaneous drainage, with eight (18.2%) of these 44 patients undergoing drainage 2–3 times. Three patients (6.8%) experienced major complications following interventional drainage. One developed biliary fistulae, which were resolved with medical treatment, whereas two presented with LA rupture, which were resolved using interventional hemostatic therapy. Of the two patients with ruptured abscesses, one died of respiratory failure during hospitalization and the other was discharged owing to worsening of the condition.
Two (3.8%) of these 53 patients underwent successful surgical treatment. One patient required surgery because of LA rupture. The other patient, who was coinfected with HBV, underwent surgery to determine whether the condition was malignant. Five (9.4%) patients developed septic shock. The mean length of hospitalization was 27.9±17.8 days (range, 3–82 days).
The improvement and non-improvement groups were compared to analyze the risk factors associated with final treatment outcome. Multivariable analysis showed that ascites [odds ratio (OR)=8.970; 95% confidence interval (CI)=0.840–92.110;
Discussion
In the 1990s, the etiology of LA mainly included biliary tract infection, portal vein seeding, direct extension, hepatic arterial seeding, penetrating trauma, and cryptogenic causes [12]. More recently, however, LA has been associated with malignancy or its treatments, including radiofrequency ablation and tumor embolization, and immunosuppression [13–15]. Blood transfusion through the hepatic artery [16] and skin abscesses [17] have also been associated with the development of LA in HIV-infected patients. Approximately 50% of the HIV-infected patients in the present study were found to have gallbladder diseases. These patients often exhibit a combination of infections, such as pneumonia, oral
A study of 23 HIV-infected patients with LA in Thailand reported that
Typical symptoms of LA include the triad of fever, right upper quadrant pain, and hepatomegaly [4]. Other symptoms include nausea, vomiting, weight loss, and diarrhea. Jaundice is less common in these patients [20]. In the current study, most patients had fever; but <50% each had right upper quadrant pain and hepatomegaly. Some patients reported chronic febrile illness symptoms, such as long-term diarrhea, loss of appetite, and weight loss. LA in our HIV-infected patients did not always present with typical clinical symptoms. Moreover, many of these patients present with a combination of several opportunistic infections and malignant tumors, the symptoms of which might overlap with those of LA, resulting in a considerable diagnostic delay. Therefore, additional imaging examinations are required for diagnosis of LA in HIV-infected patients.
Ultrasound and CT are highly sensitive for the diagnosis of LA, with contrast-enhanced CT having a sensitivity of approximately 100% [13,20]. However, this condition must be distinguished from amoebic LA, cysts, tumors, and tumor-associated LA. Apart from imaging, LA can be diagnosed using LA smear, special staining, indirect hemagglutination assays for antiamoebic antibodies, and tumor markers. Similar to previous studies, we found that the most common LA location was the right liver lobe (62.2%, 33/53) [14,21], with most of our patients presenting with a single LA (75.5%, 40/53).
At the beginning of the 20th century, the mortality rate from LA was as high as 75%–80% owing to the lack of effective treatment methods [12]. Although antibiotics are presently the primary treatment for LA, no recommendations have been proposed regarding antibiotic treatment of HIV-infected patients with LA. Rather, the principles for treating non-HIV-infected patients are generally applied. Empirical antibiotic treatment is designed to treat gram-negative bacilli, gram-positive cocci, and anaerobic bacteria. Treatment with a third-generation cephalosporin plus metronidazole or piperacillin/tazobactam has shown curative effects in patients with LA [22, 23]. In addition to treatment with a third-generation cephalosporin and piperacillin/tazobactam, patients in the current study were administered metronidazole to treat anaerobic and/or amoebic infections. If antibiotic treatment is ineffective in HIV-infected patients, the possibility of opportunistic or atypical pathogenic infections should be considered. Percutaneous drainage should be considered another first-line treatment, as it reduces the mortality rate from LA to 10–30% [24]. Cultures of drained LA specimens could aid in determining the most appropriate antibiotic treatment. However, percutaneous drainage was reported unsuccessful in 15–36% of treatment patients, a failure likely due to the presence of multilumen LAs and drainage fluid adhesion [23,25,26]. Generally, percutaneous drainage is performed in patients with hemorrhage and biliary fistulae [27]. In this study, three (6.8%) of the 44 patients who developed LAs and experienced major complications related to interventional drainage showed effective improvement after treatment. Treatment selection is dependent on LA size and consistency [8], with few reports recommending surgical drainage in patients with an LA diameter >5 cm. In the current study, 39 (88.6%) of the 44 patients with large LAs (>5 cm) were treated with percutaneous drainage plus antibiotics, with a success rate of 38–39%, indicating that percutaneous drainage was effective in treating large LAs.
Factors such as male sex, cirrhosis, LA rupture, sepsis, multidrug resistant infection, anemia, and air cavity formation have been associated with increased mortality in patients with LA. Septic shock or ascites associated with severe infections has been associated with a high mortality rate. High-efficiency ART can induce immune reconstitution and impede the clinical progress of HIV/AIDS in HIV-infected patients, effectively altering prognosis, even in patients with severe complications. The poor prognosis of patients in the current study was not associated with highly active ART. Studies are needed to evaluate the effect of highly active ART in HIV-infected patients with LA.
This study had several limitations. First, it was a retrospective single-center study, indicating limitations in sample number and generalization. Moreover, owing to the limitations of the methods of detection, relevant data regarding the involved pathogens were likely insufficient.
Conclusions
The clinical characteristics and laboratory findings of HIV-infected patients with LA are nonspecific, suggesting that the possibility of LA be considered in HIV-infected patients with fever, abdominal pain, and other non-specific manifestations. LA specimens should be tested for pathogens, including fungi and amoebae in addition to bacteria. Recommended treatment methods for these patients include antibiotic treatment combined with image-guided percutaneous suction and drainage, which may be more effective than simple antibiotic treatment, thereby improving prognosis.
Tables
Table 1. Demographic characteristics of 53 HIV-infected patients with liver abscess.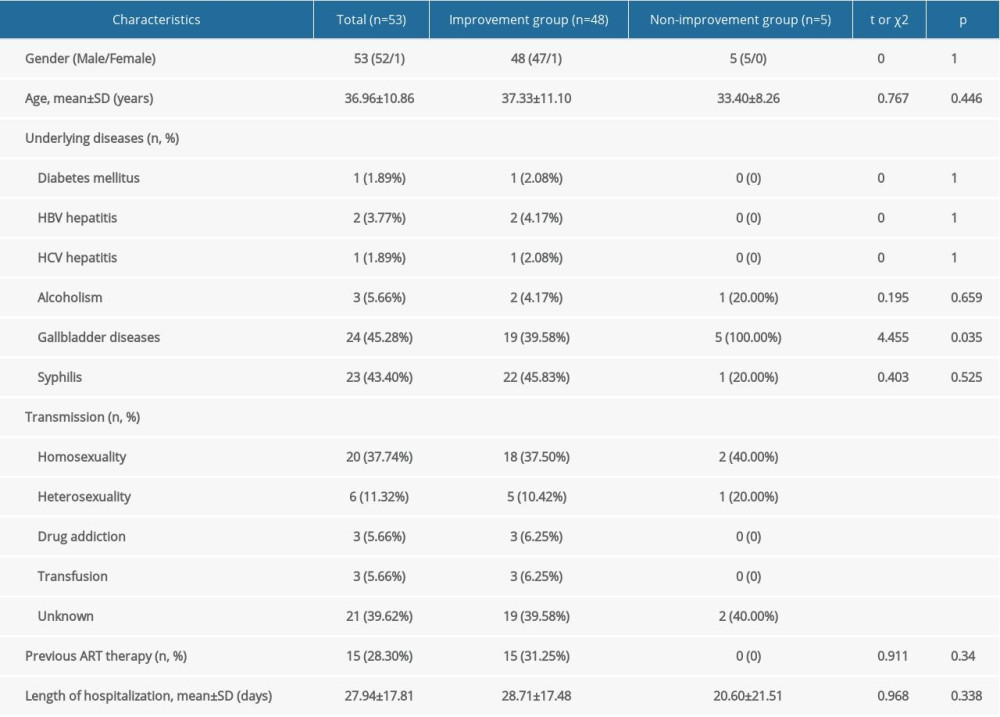 Table 2. Clinical manifestations in 53 HIV-infected patients with LA.
Table 2. Clinical manifestations in 53 HIV-infected patients with LA.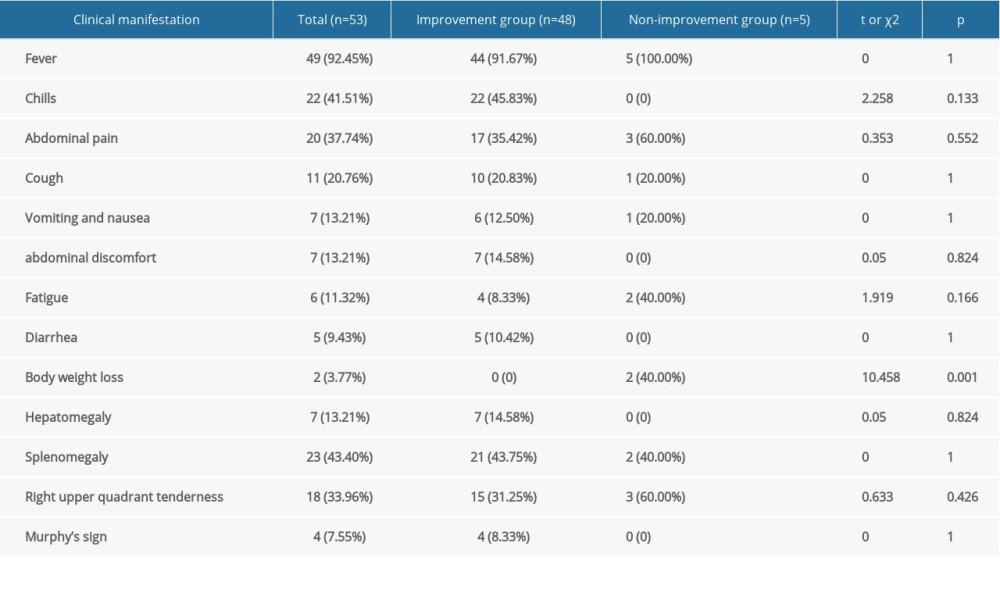 Table 3. Laboratory results of 53 HIV-infected patients with liver abscess.
Table 3. Laboratory results of 53 HIV-infected patients with liver abscess.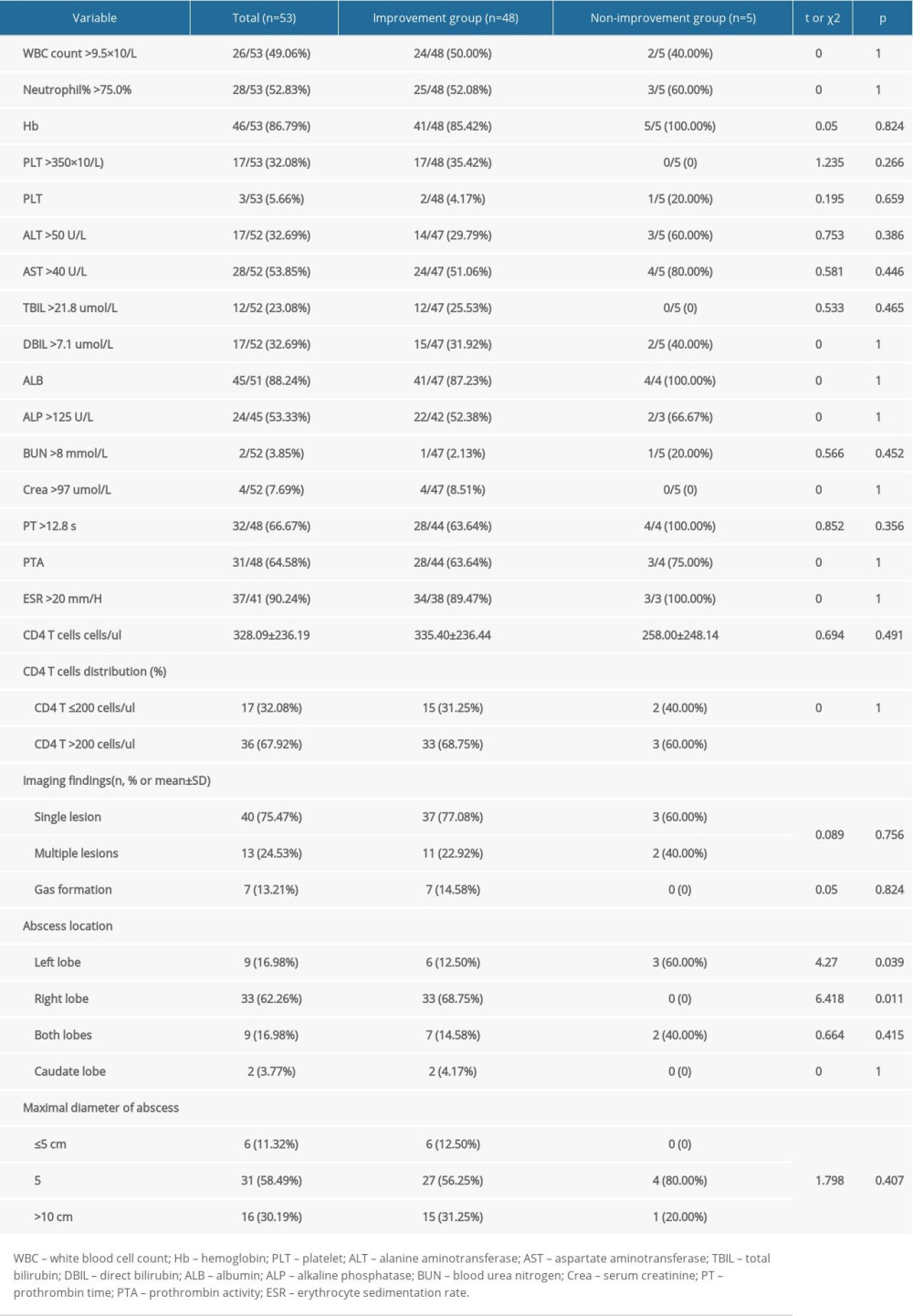 Table 4. Treatments and complications in 53 HIV-infected patients with LA.
Table 4. Treatments and complications in 53 HIV-infected patients with LA.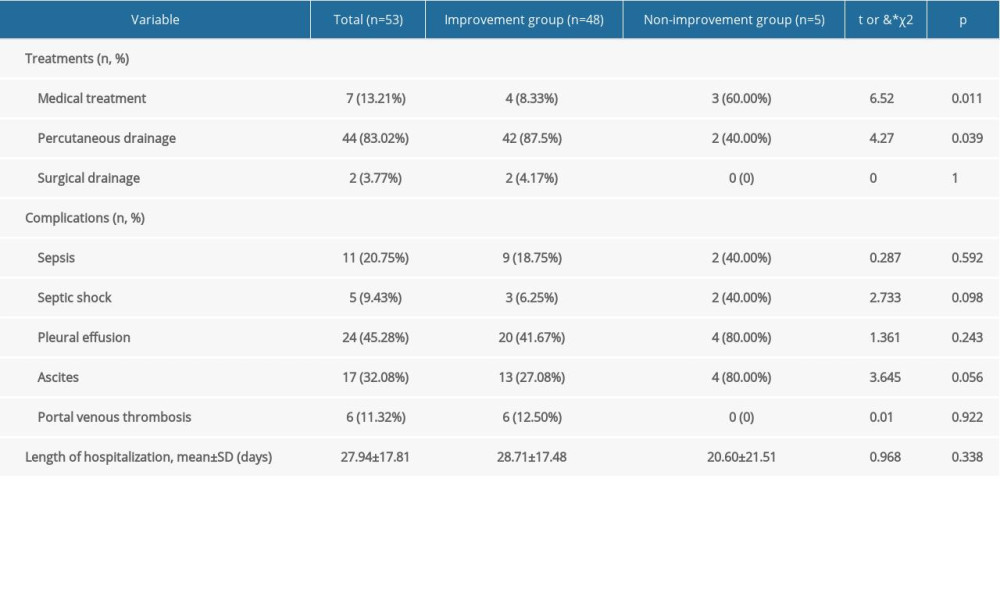
References
1. Chiche L, Dargère S, Le Pennec V, Pyogenic-liver abscess: Diagnosis and management: Gastroenterol Clin Biol, 2008; 32; 1077-91
2. Hansen P, Schønheyder H, Pyogenic hepatic abscess. A 10-year population-based retrospective study: APMIS, 1998; 106; 396-402
3. Mølle I, Thulstrup AM, Vilstrup H, Sørensen HT, Increased risk and case fatality rate of pyogenic liver abscess in patients with liver cirrhosis: A nationwide study in Denmark: Gut, 2001; 48; 260-63
4. Kaplan GG, Gregson DB, Laupland KB, Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess: Clin Gastroenterol Hepatol, 2004; 2; 1032-38
5. Tsai FC, Huang YT, Chang LY, Wang JT, Pyogenic liver abscess as endemic disease, Taiwan: Emerg Infect Dis, 2008; 14; 1592-600
6. Meddings L, Myers RP, Hubbard J, A population-based study of pyogenic liver abscesses in the United States: Incidence, mortality, and temporal trends: Am J Gastroenterol, 2010; 105; 117-24
7. Law ST, Li KK, Is hepatic neoplasm-related pyogenic liver abscess a distinct clinical entity?: World J Gastroenterol, 2012; 18; 1110-16
8. Mavilia MG, Molina M, Wu GY, The evolving nature of hepatic abscess: A review: J Clin Transl Hepatol, 2016; 4; 158-68
9. Ehrahim RA, Farid EMA, Yousif A, Jamsheer AE, Microbiological infections in HIV positive Bahraini patients with low CD4+ T-lymphocyte count: J Commun Dis, 2002; 34; 160-70
10. Prabhu S, Harwell JI, Kumarasamy N, Advanced HIV: Diagnosis, treatment, and prevention: Lancet HIV, 2019; 6; e540-51
11. Hsu MS, Hsieh SM, Chen MY, Association between amebic liver abscess and human immunodeficiency virus infection in Taiwanese subjects: BMC Infect Dis, 2008; 8; 48
12. Huang CJ, Pitt HA, Lipsett PA, Pyogenic hepatic abscess: Changing trends over 42 years: Ann Surg, 1996; 223; 600-9
13. Mezhir JJ, Fong Y, Jacks LM, Current management of pyogenic liver abscess: Surgery is now second-line treatment: J Am Coll Surg, 2010; 210; 975-83
14. Kurland JE, Brann OS, Pyogenic and amebic liver abscesses: Curr Gastroenterol Rep, 2004; 6; 273-79
15. Pearce NW, Knight R, Irving H, Non operative management of pyogenic liver abscess: HPB )Oxford), 2003; 5; 91-95
16. Johannsen EC, Sifri CD, Madoff LC, Pyogenic liver abscesses: Infect Dis Clin North Am, 2000; 14; 547-63
17. Sayana S, Ricaurte JC, Khanlou H, An unusual pathogen for a liver abscess in a human immunodeficiency virus-infected individual: Am J Med Sci, 2010; 339; 290-91
18. Wiwanitkit V, Causative agents of liver abscess in HIV-seropositive patients: A 10-year case series in Thai hospitalized patients: Trop Doct, 2005; 35; 115-17
19. Price JC, Thio CL, Liver disease in the HIV-infected individual: Clin Gastroenterol Hepatol, 2010; 8; 1002-12
20. Rahimian J, Wilson T, Oram V, Holzman RS, Pyogenic liver abscess: Recent trends in etiology and mortality: Clin Infect Dis, 2004; 39; 1654-59
21. Vidal JE, da Silva PR, Schiavon Nogueira R: Rev Inst Med Trop São Paulo, 2003; 45; 115-57
22. Lübbert C, Wiegand J, Karlas T, Therapy of liver abscesses: Viszeralmedizin, 2014; 30; 334-41
23. Tan YM, Chung AYF, Chow PKH, An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm: Ann Surg, 2005; 241; 485-90
24. Kuo SH, Lee YT, Li CR, Mortality in Emergency Department Sepsis score as a prognostic indicator in patients with pyogenic liver abscess: Am J Emerg Med, 2013; 31; 916-21
25. Alkofer B, Dufay C, Parienti JJ, Are pyogenic liver abscesses still a surgical concern? A Western experience: HPB Surg, 2012; 2012 316013
26. Hope WW, Vrochides DV, Newcomb WL, Optimal treatment of hepatic abscess: Am Surg, 2008; 74; 178-82
27. Pang TCY, Fung T, Samra J, Pyogenic liver abscess: an audit of 10 years’ experience: World J Gastroenterol, 2011; 17; 1622-30
Tables
 Table 1. Demographic characteristics of 53 HIV-infected patients with liver abscess.
Table 1. Demographic characteristics of 53 HIV-infected patients with liver abscess. Table 2. Clinical manifestations in 53 HIV-infected patients with LA.
Table 2. Clinical manifestations in 53 HIV-infected patients with LA. Table 3. Laboratory results of 53 HIV-infected patients with liver abscess.
Table 3. Laboratory results of 53 HIV-infected patients with liver abscess. Table 4. Treatments and complications in 53 HIV-infected patients with LA.
Table 4. Treatments and complications in 53 HIV-infected patients with LA. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








