24 October 2020: Clinical Research
Risk Factors Affecting Clinical Outcome in Patients with Carbapenem-Resistant : A Retrospective Study
Gefei He1DEFG, Juanjuan Huang1DEF, Shiqiong Huang1B, Ji Sun1C, Yulv Zhou2B, Hong Tan3B, Hui Shen4C, Zhuan Li1AG*, Jiyang Liu1AGDOI: 10.12659/MSM.925693
Med Sci Monit 2020; 26:e925693
Abstract
BACKGROUND: The increased prevalence of carbapenem-resistant K. pneumoniae (CRKP) poses a great threat worldwide. Early identification of CRKP in patients is paramount. Moreover, fully understanding the risk factors affecting clinical outcome and actively providing targeted treatment can improve the cure rate of patients with CRKP. Therefore, our study aimed to describe the clinical characteristics and identify the risk factors affecting clinical outcomes in patients with CRKP.
MATERIAL AND METHODS: From January 2016 to September 2017, CRKP strains and clinical data from 97 hospitalized patients were collected. We first performed an antibiotic susceptibility test on CRKP strains using the Kirby-Bauer disc agar diffusion method. Logistic regression analysis was then performed to analyze risk factors.
RESULTS: According to clinical outcome, among the 97 CRKP patients, 67 were in the effective group and 30 patients were in the noneffective group. Risk factors found to correlate with poor clinical outcome in patients with CRKP included ICU admission, arteriovenous catheterization, indwelling gastric tube, indwelling urethral catheter, tracheal intubation, mechanical ventilation, hypoproteinemia, and exposure to carbapenems. Multivariate analysis showed that hypoproteinemia (OR: 2.83, p=0.042), presence of an indwelling gastric tube (OR: 4.54, p=0.005), and exposure to carbapenems (OR: 2.77, p=0.045) negatively affected clinical outcome in patients with CRKP.
CONCLUSIONS: Adverse risk factors correlated with poor clinical outcomes in patients with CRKP were determined. This could be of help in identifying high-risk patients with whom clinicians should take extra precautions and adjust therapeutic strategy to supplement conventional basic treatment with additional measures.
Keywords: Fatal Outcome, Klebsiella pneumoniae, Risk Factors, Aged, 80 and over, Carbapenems, Drug Resistance, Bacterial, Klebsiella Infections
Background
The rapid spread of CRKP has remained an urgent global threat, despite the efforts of researchers to control its spread [7,8]. Unfortunately, CRKP is becoming prevalent in China, bringing new challenges to clinical anti-infective treatment [9,10]. Of note, several studies have concluded that CRKP could increase the mortality rate from
Material and Methods
ETHICS APPROVAL:
This study was approved by the Institutional Ethics Committee of the First Hospital of Changsha and was conducted in accordance with the Declaration of Helsinki. All enrolled participants provided written informed consent.
STUDY DESIGN AND PATIENTS:
This study was conducted at the First Hospital of Changsha, a tertiary-care teaching hospital with 1700 beds. Data on
DATA COLLECTION AND DEFINITIONS:
All data were collected by reviewing and recording medical histories, including: the clinical department(s) where CRKP strains were isolated; the patient’s age, sex, and length of hospital stay; comorbidities such as liver insufficiency, cardiac insufficiency, renal insufficiency, diabetes mellitus, hypertension, malignancy, and hypoproteinemia (plasma albumin <30 g/l); hospitalization (ICU, hospital history, and APCHE II score), mechanical ventilation, and invasive procedures (e.g., arteriovenous catheterization, indwelling gastric tube, indwelling urethral catheter, tracheal intubation, peripherally inserted central venous catheters, or indwelling jejunal tube); and history of antimicrobials taken in the past 90 days, including the use of carbapenems, tigecycline, and compound sulfamethoxazole.
Based on other studies [18,19], our clinical outcomes included the clinical manifestations and auxiliary examination results of patients, such as body temperature, laboratory test results for blood, liver and kidney function, coagulation function, and infection-related biomarkers (procalcitonin and C-reactive protein), as well as microbial culture results, drug susceptibility tests, and imaging results. For any patient with 2 or more positive test results, only the clinical data related to the first positive result were collected. Each patient was assigned to the effective or noneffective group after drug administration for 28 days or death.
The inclusion criteria for the effective group were as follows. 1) Full cure: all clinical signs and symptoms of the patients with CRKP had disappeared. 2) Improvement: clinical signs and symptoms of the patients with CRKP had partially disappeared, or patients were transferred from the intensive care unit (ICU) to the general ward to continue treatment, or the laboratory test results had improved. The inclusion criteria for the noneffective group were either a worsening of clinical signs and symptoms or death.
ANTIBIOTIC SUSCEPTIBILITY TEST:
The susceptibility of CRKP strains to 18 antibiotics (ampicillin, ampicillin/sulbactam, piperacillin/sulbactam, cefazolin, ceftazidime pentahydrate, ceftriaxone sodium, cefepime, cefotetan, aztreonam, imipenem, ertapenem, amikacin, gentamicin, tobramycin, levofloxacin, ciprofloxacin, furadantin, and compound sulfamethoxazole) was determined by the Kirby-Bauer disc agar diffusion method. Tigecycline drug sensitivity was determined according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards. The rest of the antibacterial results were determined according to the standards recommended by the Clinical and Laboratory Standards Institute (CLSI) in 2017.
The extent of drug resistance was defined as follows. Multidrug resistance (MDR): resistant to 3 or more antibacterial drugs in the antibacterial spectrum. Extensive drug resistance (XDR): resistant to almost all antibacterials except 1–2 antibacterials. Pan-drug resistance (PDR): resistant to all types of antibacterial drugs [20].
STATISTICAL ANALYSIS:
Numbers and percentages were used to represent categorical variables. Continuous variables are expressed as mean±standard deviation (SD) (normally distributed). Chi-square test and
Results
CHARACTERISTICS OF PATIENTS:
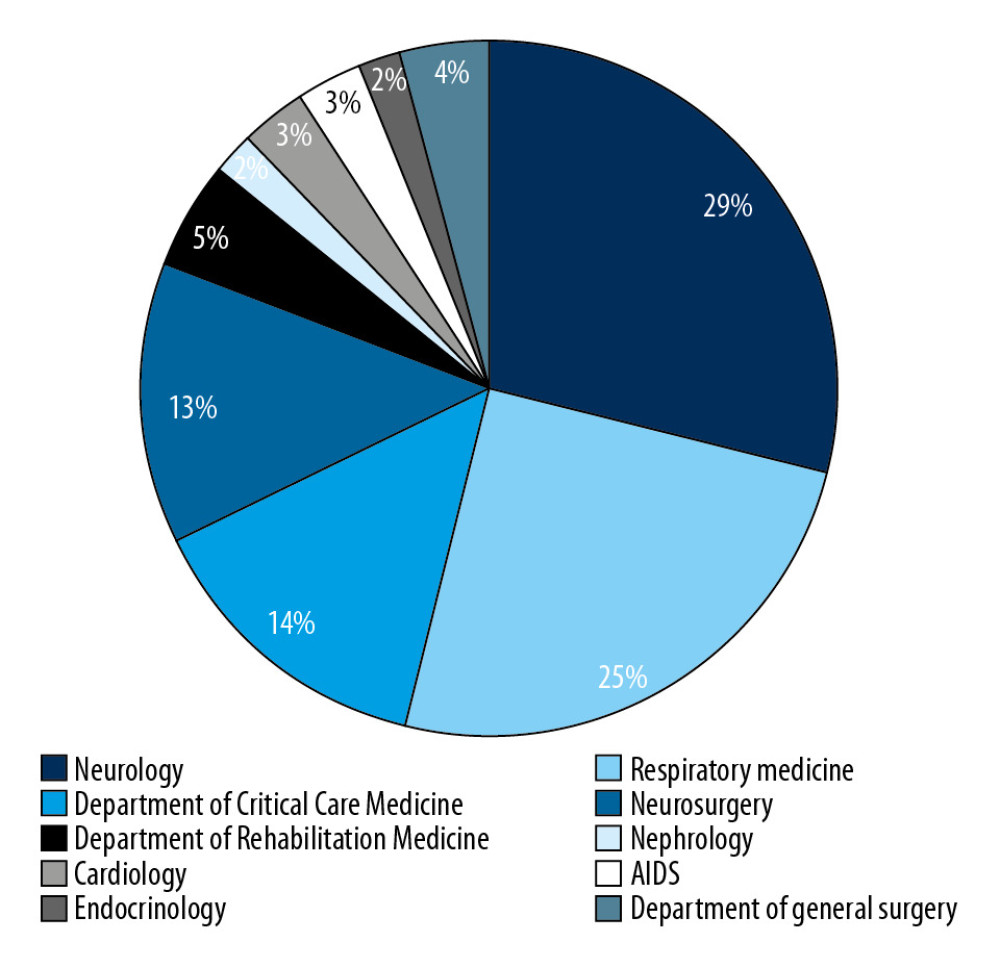
We identified a total of 97 unique cases of patients with CRKP during the study period. Isolates were obtained from different clinical samples such as: sputum (n=62, 63.92%), bronchoalveolar lavage (n=15, 15.46%), urine (n=11, 11.34%), blood (n=2, 2.06%), bile (n=1, 1.03%), pus (n=1, 1.03%), fluid (n=2, 2.06%), ascites (n=2, 2.06%), and throat swab (n=1, 1.03%). As shown in Figure 1, most of the study patients were from the Departments of Neurology, Respiratory Medicine, Critical Care Medicine, and Neurosurgery. Furthermore, we distributed patients with CRKP into effective and noneffective groups based on the clinical outcome of the patient, with 67 cases (69%) identified as effective and 30 cases (31%) identified as noneffective.
The basic clinical features of each dataset are listed in Table 1. The mean age of the patients was 71.2±16.37 and 71.57±18.26 years in the effective and noneffective groups, respectively, and male patients accounted for 57% of the total study population. Compared with the effective group, a significantly larger percentage of patients in the noneffective group were admitted to the ICU (73.33% vs. 45.78%, P=0.009) and received mechanical ventilation (80% vs. 53.73%, P=0.014), as well as higher APCHE2 scores (14.73±5.90 vs. 11.48±5.31 5, P=0.008). A larger fraction of patients in the noneffective group received invasive procedures compared with the effective group, including arteriovenous catheterization (70% vs. 43.28%, P=0.015), indwelling gastric tube (50% vs. 23.88%, P=0.011), and indwelling urethral catheter (86.67% vs. 62.28%, P=0.017). Moreover, we observed that patients in the noneffective group were more likely to be prescribed carbapenems than those in the effective group (70% vs. 41.79%, P=0.01).
ANTIBIOTIC SUSCEPTIBILITY TEST:
The drug resistance rates for aminoglycosides, β-lactams, carbapenems, quinolones, and sulfa drugs among the 97 CRKP isolates are summarized in Table 2. The drug sensitivity results demonstrated that compound sulfamethoxazole had the lowest resistance rate (21.65%), followed by amikacin (48.45%), gentamicin (59.79%), tobramycin (63.92%), levofloxacin (94.85%), and ciprofloxacin (94.85%). The remaining antibiotics had resistance rates of 100%. The percentages of MDR, XDR, and PDR were 94%, 3%, and 3%, respectively (Table 3). The most frequently isolated MDR strains, from the Neurology Department, are shown in Table 3.
RISK FACTOR ANALYSIS:
To identify risk factors for poor clinical outcomes in patients with CRKP, we conducted a retrospective study. Clinical variables are listed in Table 4. Firstly, we performed a univariate analysis of these clinical variables. In this univariate analysis, the following factors were negatively correlated with good clinical outcomes: ICU admission (OR: 3.39, p=0.011), exposure to arteriovenous catheterization (OR: 3.06, p=0.017), indwelling gastric tube (OR: 3.19, p=0.013), indwelling urethral catheter (OR: 3.87, p=0.023), tracheal intubation (OR: 2.90, p=0.019), mechanical ventilation (OR: 3.44, p=0.017), hypoproteinemia (OR: 2.47, p=0.049), and exposure to carbapenems (OR: 3.25, p=0.012).
The multivariate analyses of the effective and noneffective groups were carried out with the adjustment of the logistic regression model for 8 variables, wherein the risk factors dramatically differed from the univariate analyses. As shown in Table 5, hypoproteinemia (OR: 2.83, p=0.042), presence of an indwelling gastric tube (OR: 4.54, p=0.005), and exposure to carbapenems (OR: 2.77, p=0.045) were found to be independent risk factors for poor clinical outcomes in patients with CRKP.
Discussion
Currently, carbapenem-resistant
In the present study, we found that clinical outcome was associated with various factors, including ICU stay, exposure to arteriovenous catheterization, indwelling gastric tube, indwelling urethral catheter, tracheal intubation, mechanical ventilation, hypoproteinemia, and exposure to carbapenems. Among these, presence of an indwelling gastric tube, hypoproteinemia and exposure to carbapenems were independent risk factors for poor clinical outcome in patients with CRKP in multivariate analyses. These findings indicated that clinicians should attach great importance to appropriate antibiotic use and aseptic invasive procedures.
In our study, we found that CRKP had a low resistance rate to compound sulfamethoxazole (21.65%), which was similar to the findings in a previous report [26].
The multivariate analyses showed that exposure to carbapenems, hypoproteinemia, and an indwelling gastric tube were independent risk factors for poor clinical outcome in patients with CRKP. Emerging evidence has indicated that carbapenem administration is an independent risk factor for CRKP infection, as well as for
Conclusions
We found that the presence of an indwelling gastric tube, hypoproteinemia, and exposure to carbapenems were risk factors for poor clinical outcome in patients with CRKP. These findings may provide a theoretical foundation for the adjustment of clinical therapeutic strategies. Clinicians should pay close attention to the condition of CRKP-infected patients and ensure rational use of carbapenems. In addition, strengthened monitoring of the hospital environment could improve the prognoses of patients with CRKP.
Tables
Table 1. Characteristics of 97 patients with CRKP in the effective and noneffective groups.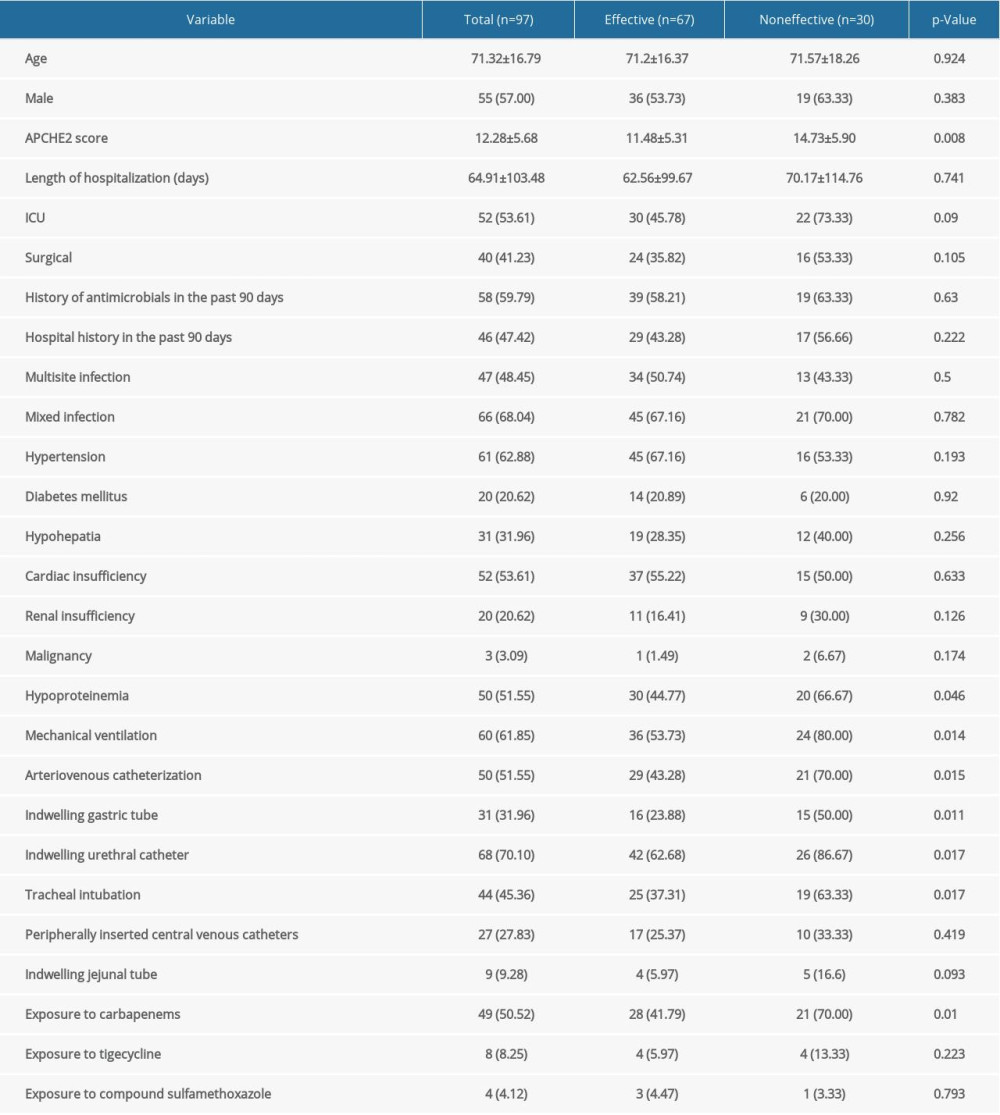 Table 2. Drug sensitivity of CRKP strains.
Table 2. Drug sensitivity of CRKP strains.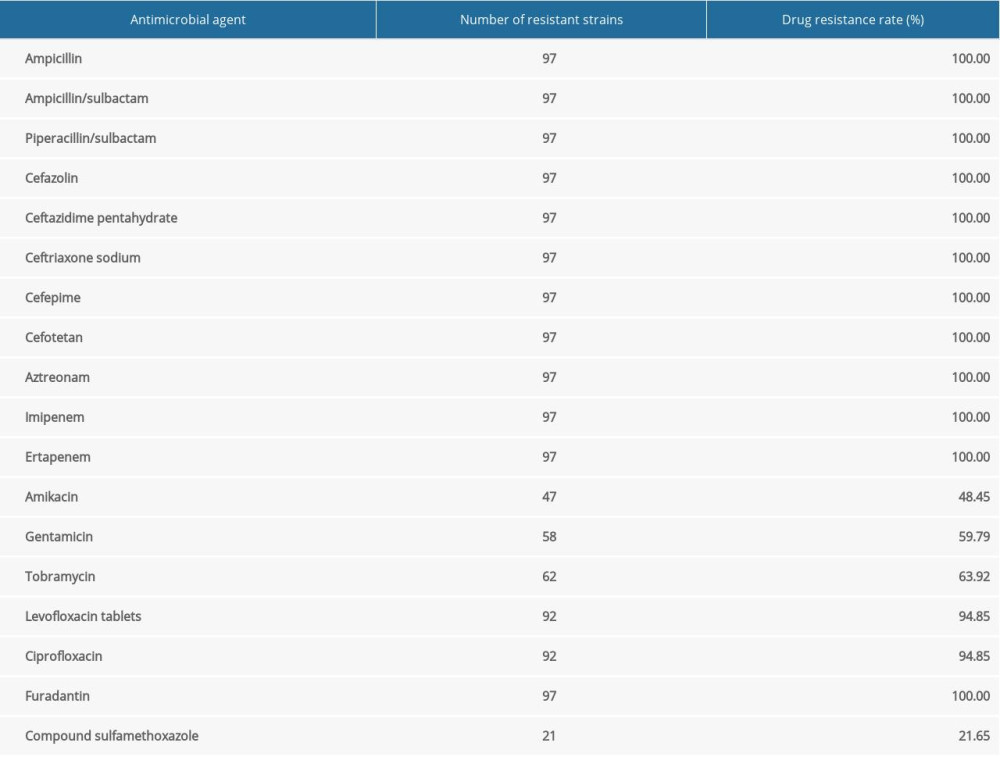 Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated.
Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated.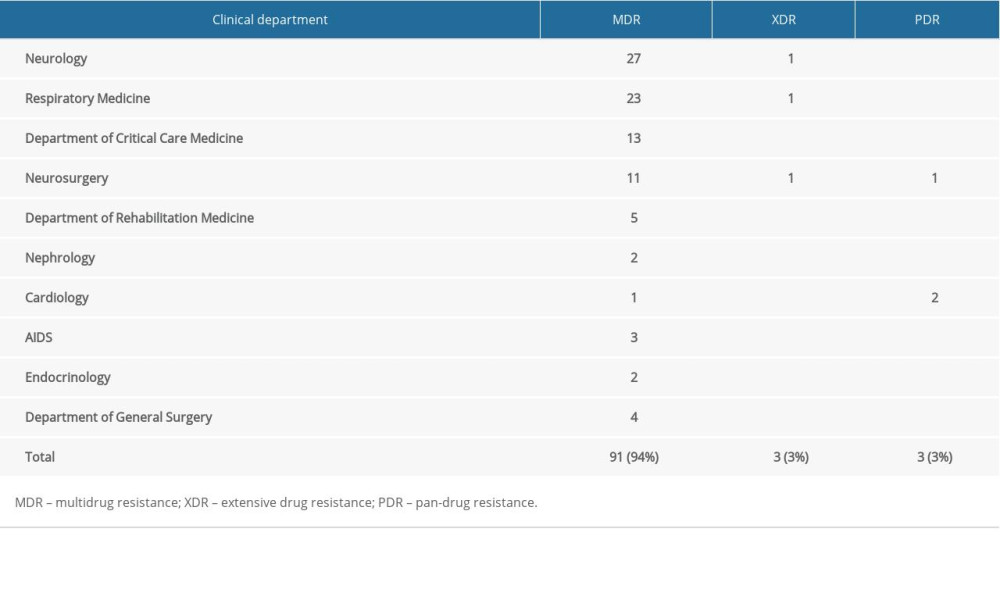 Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP.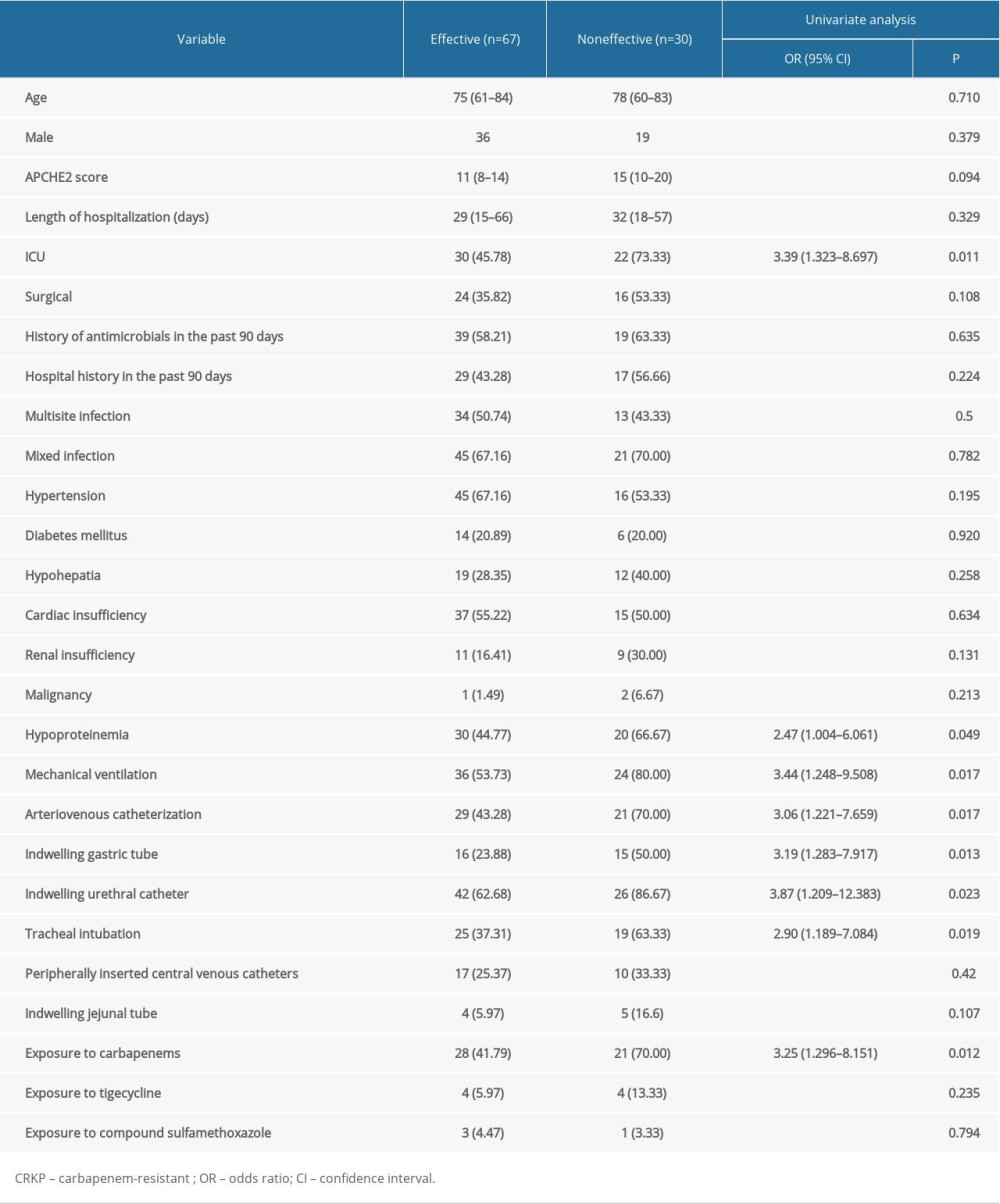 Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP.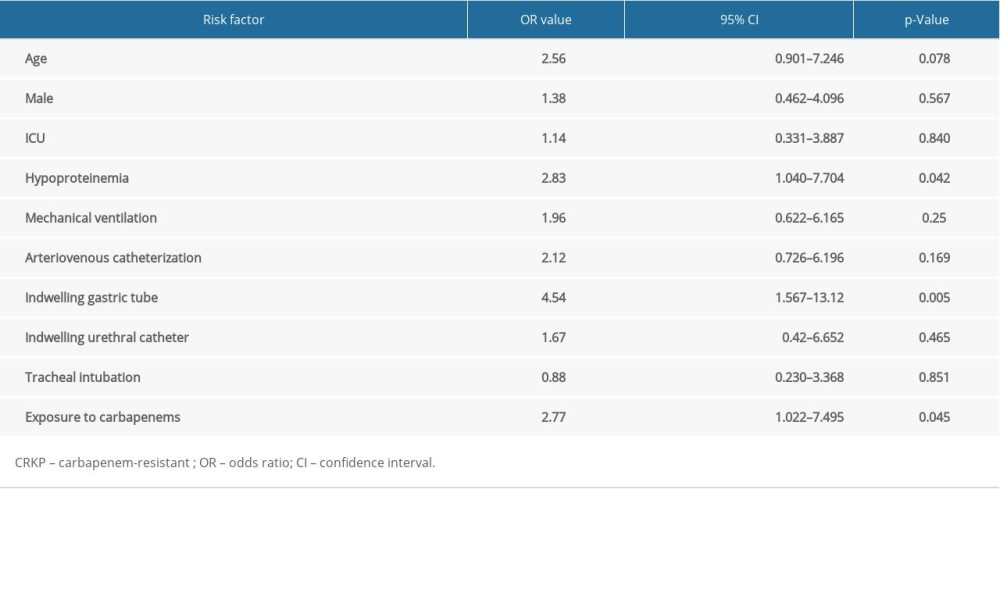
References
1. Daikos GL, Markogiannakis A, Souli M, Tzouvelekis LS: Expert Rev Anti Infect Ther, 2012; 10(12); 1393-404
2. Broberg CA, Palacios M, Miller VL: F1000Prime Rep, 2014; 6; 64
3. Neuhauser MM, Weinstein RA, Rydman R, Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use: JAMA, 2003; 289(7); 885-88
4. Huang S, Zhu P, Sun B, Modulation of YrdC promotes hepatocellular carcinoma progression via MEK/ERK signaling pathway: Biomed Pharmacother, 2019; 114; 108859
5. Huang SQ, Sun B, Xiong ZP, The dysregulation of tRNAs and tRNA derivatives in cancer: J Exp Clin Cancer Res, 2018; 37(1); 101
6. Hu FP, Guo Y, Zhu DM, Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014: Clin Microbiol Infect, 2016; 22(Suppl 1); S9-14
7. Girmenia C, Serrao A, Canichella M: Mediterr J Hematol Infect Dis, 2016; 8(1); e2016032
8. Tumbarello M, Trecarichi EM, De Rosa FG: J Antimicrob Chemother, 2015; 70(7); 2133-43
9. Xu A, Zheng B, Xu YC, National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013: Clin Microbiol Infect, 2016; 22(Suppl 1); S1-8
10. Zhou J, Li G, Ma X: Am J Infect Control, 2015; 43(10); 1122-24
11. Bratu S, Landman D, Haag R: Arch Intern Med, 2005; 165(12); 1430-35
12. Gasink LB, Edelstein PH, Lautenbach E: Infect Control Hosp Epidemiol, 2009; 30(12); 1180-85
13. Raneros AB, Minguela A, Rodriguez RM, Correction: Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition: Oncotarget, 2018; 9(67); 32881
14. Kofteridis DP, Valachis A, Dimopoulou D: J Infect Chemother, 2014; 20(5); 293-97
15. Liu B, Yi H, Fang J: Infect Drug Resist, 2019; 12; 1089-98
16. Jiao Y, Qin Y, Liu J: Pathog Glob Health, 2015; 109(2); 68-74
17. Zhang Y, Guo LY, Song WQ: BMC Infect Dis, 2018; 18(1); 248
18. Simon L, Gauvin F, Amre DK, Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis: Clin Infect Dis, 2004; 39(2); 206-17
19. Christ-Crain M, Opal SM, Clinical review: The role of biomarkers in the diagnosis and management of community-acquired pneumonia: Crit Care, 2010; 14(1); 203
20. Magiorakos AP, Srinivasan A, Carey RB, Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance: Clin Microbiol Infect, 2012; 18(3); 268-81
21. Munoz-Price LS, Poirel L: Lancet Infect Dis, 2013; 13(9); 785-96
22. Tzouvelekis LS, Markogiannakis A, Psichogiou M: Clin Microbiol Rev, 2012; 25(4); 682-707
23. Canton R, Akova M, Carmeli Y, Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe: Clin Microbiol Infect, 2012; 18(5); 413-31
24. Nordmann P, Poirel L, The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide: Clin Microbiol Infect, 2014; 20(9); 821-30
25. Zheng B, Dai Y, Liu Y: Front Microbiol, 2017; 8; 1061
26. Lombardi F, Gaia P, Valaperta R: Biomed Res Int, 2015; 2015 871947
27. Su J, Li D, Guo Q: Microb Drug Resist, 2019; 25(2); 152-56
28. Tian L, Tan R, Chen Y: Antimicrob Resist Infect Control, 2016; 5; 48
29. Teo J, Cai Y, Tang S, Risk factors, molecular epidemiology and outcomes of ertapenem-resistant, carbapenem-susceptible Enterobacteriaceae: A case-case-control study: PLoS One, 2012; 7(3); e34254
30. Brizendine KD, Richter SS, Cober ED, van Duin D: Antimicrob Agents Chemother, 2015; 59(1); 553-57
31. Hoxha A, Kärki T, Giambi CStudy Working Group: J Hosp Infect, 2016; 92(1); 61-66
32. Lee CR, Lee JH, Park KS: Front Microbiol, 2016; 7; 895
33. Zheng X, Wang JF, Xu WL: Antimicrob Resist Infect Control, 2017; 6; 102
34. Gagliotti C, Giordani S, Ciccarese V: Am J Infect Control, 2014; 42(9); 1006-8
35. Zavascki AP, Cruz RP, Goldani LZ, Risk factors for imipenem-resistant Pseudomonas aeruginosa: A comparative analysis of two case-control studies in hospitalized patients: J Hosp Infect, 2005; 59(2); 96-101
36. Lee SO, Kim NJ, Choi SH: Antimicrob Agents Chemother, 2004; 48(1); 224-28
37. Markley JD, Bernard S, Bearman G, Stevens MP, De-escalating antibiotic use in the inpatient setting: Strategies, controversies, and challenges: Curr Infect Dis Rep, 2017; 19(4); 17
38. Xu L, Sun X, Ma X: Ann Clin Microbiol Antimicrob, 2017; 16(1); 18
39. Joles JA, Rabelink TJ, Braam B, Koomans HA, Plasma volume regulation: defences against edema formation (with special emphasis on hypoproteinemia): Am J Nephrol, 1993; 13(5); 399-412
Tables
 Table 1. Characteristics of 97 patients with CRKP in the effective and noneffective groups.
Table 1. Characteristics of 97 patients with CRKP in the effective and noneffective groups. Table 2. Drug sensitivity of CRKP strains.
Table 2. Drug sensitivity of CRKP strains. Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated.
Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated. Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP. Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP. Table 1. Characteristics of 97 patients with CRKP in the effective and noneffective groups.
Table 1. Characteristics of 97 patients with CRKP in the effective and noneffective groups. Table 2. Drug sensitivity of CRKP strains.
Table 2. Drug sensitivity of CRKP strains. Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated.
Table 3. Departments from which CRKP strains with MDR, XDR, and PDR were isolated. Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 4. Univariate analysis of risk factors for poor clinical outcome in patients with CRKP. Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP.
Table 5. Multivariate analysis of risk factors for poor clinical outcome in patients with CRKP. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952