22 February 2021: Clinical Research
Morbidity and All-Cause Mortality Following Radical Prostatectomy Compared with Observation for Localized Prostate Cancer in Chinese Men: A Non-Randomized Retrospective Study
Xi Zhang1ABF, Xiang Li1ABF, Qiwei Yu1BDF, Jun Ma1CDF, Xuemin Zeng1BCF, Li Xue2DEF*DOI: 10.12659/MSM.928596
Med Sci Monit 2021; 27:e928596
Abstract
BACKGROUND: The Chinese 2018 guidelines and the current 2014 Chinese Urological Association guidelines for prostate cancer recommend radical prostatectomy for Chinese men with localized prostate cancer as the first choice, but it has treatment-related adverse effects. This study aimed to study morbidity and all-cause mortality following radical prostatectomy compared with observation for localized prostate cancer in Chinese men from a single center.
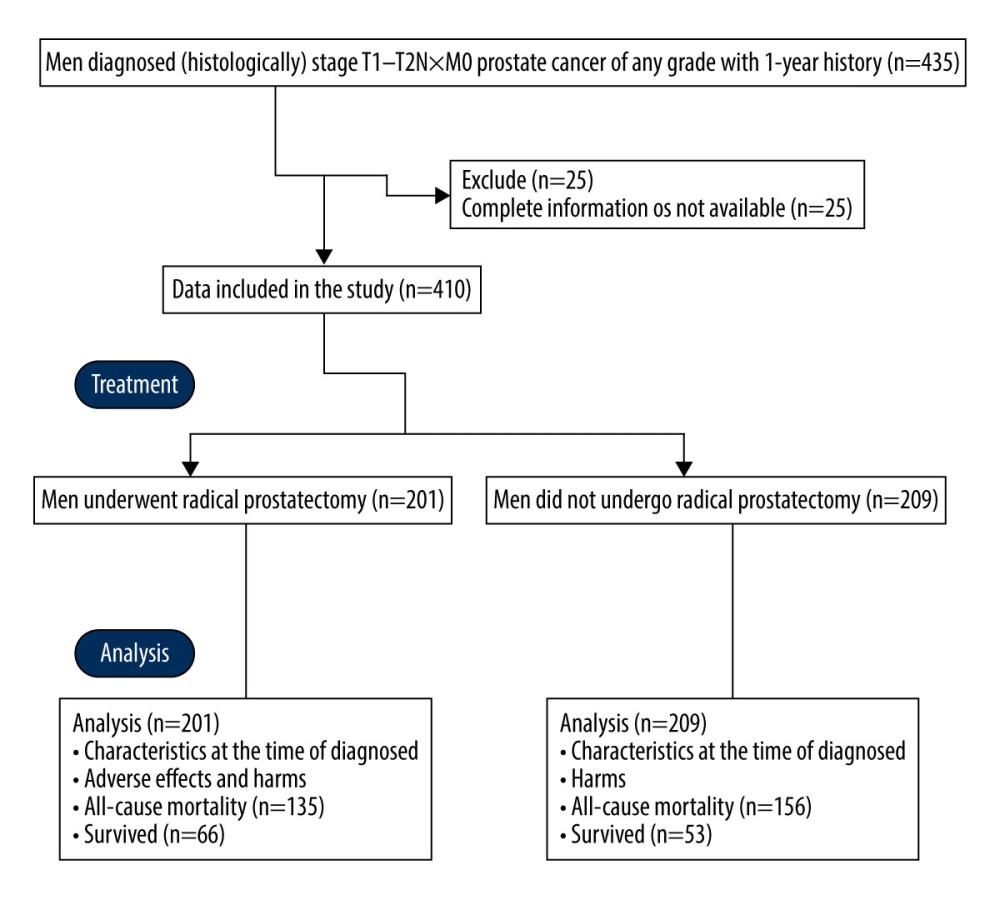
MATERIAL AND METHODS: Men diagnosed (histologically) as stage T1–T2N×M0 prostate cancer of any grade with 1-year history were included in the analysis. A total of 201 men underwent radical prostatectomy (RP cohort) and 209 men did not undergo radical prostatectomy (OS cohort).
RESULTS: During follow-up (17–24 years), 135 (67%) men died in the RP cohort and 156 (75%) men died in the OS cohort (P=0.103). All-cause mortality was lower for men with prostate-specific antigen level >10 ng/mL (P<0.0001), Gleason score ≥7 (P=0.004), and high D’Amico tumor risk scores (P=0.007) if they underwent radical prostatectomy. Age ≥65 years (P=0.041), Gleason score ≥7 (P=0.049), and tumor stage ≥2c (P=0.045) were associated with all-cause mortality.
CONCLUSIONS: The findings from this study showed that radical prostatectomy has no significant beneficial effects when compared with observation for Chinese men with localized prostate cancer, unless they had a prostate-specific antigen level >10 ng/mL, Gleason score ≥7, and high D’Amico tumor risk scores.
Keywords: Erectile Dysfunction, Mortality, Neoplasm Grading, Prostate-Specific Antigen, Prostatectomy, Prostatic Neoplasms, Asians, Morbidity, Prostate
Background
Prostate cancer is a common tumor in Chinese men [1] and 30% of newly diagnosed Chinese men with prostate cancer have a clinically localized stage of cancer [2]. At present, limited information is available on prostate cancer in Chinese men concerning race-based pathological differences [3]. The randomized trials on North American men [4] and English men [5] with localized prostate cancer showed that radical prostatectomy does not reduce mortality, but has treatment-related adverse effects, as compared with active monitoring. However, randomized trials involving Scandinavian men [6] and North American men (PIVOT: Prostate Cancer Intervention versus Observation Trial) [7,8] reported that men with localized prostate cancer benefit from radical prostatectomy. Guidelines like the European Association of Urology-European Society for Radiotherapy & Oncology-International Society of Geriatric Oncology (EAU-ESTRO-SIOG) guidelines part I [9] and the American Urological Association and Society of Urologic Oncology (AUA/ASTRO/SUO) guideline part II [10] for the management of localized prostate cancer have recommended a risk-stratified clinical framework, but the Chinese Urological Association (CUA) has no clear guidelines [2] of risk stratification for localized prostate cancer, and the current 2014 CUA guidelines for prostate cancer [11] are based on the European and American guidelines and are not suitable for the actual situation of Chinese men with localized prostate cancer [12]. However, the Chinese guidelines 2018 [2] and the current 2014 CUA guidelines for prostate cancer [11] recommend radical prostatectomy for localized prostate cancer as the first choice for low-, intermediate-, and high-risk prostate cancer in Chinese men. The 2018 Chinese guidelines [2] recommended watchful waiting for patients with life expectancy ≤10 years and active surveillance for patients with life expectancy >10 years, but it is difficult to estimate life expectancy of patients. The EAU-ESTRO-SIOG guidelines part I [9] recommend watchful waiting for all stages of localized prostate cancer and radical prostatectomy for intermediate- and high-risk prostate cancer. AUA/ASTRO/SUO guideline part II [10] recommends radical prostatectomy for patients with life expectancy >10 years and age <65 years, but the same question remains of how to calculate life expectancy of patients. The AUA/ASTRO/SUO guideline part II also recommends watchful waiting for patients with all pathological and radiological diagnoses at regular time intervals during follow-up, but does not give limits for life expectancy and age for localized prostate cancer patients if they are put on watchful waiting. All of these guidelines were developed in White patients, not for Chinese, especially Han Chinese, patients with localized prostate cancer.
The objective of the present non-randomized retrospective study was to study morbidity and all-cause mortality following radical prostatectomy compared with observation for localized prostate cancer in Chinese men from a single center. We also assessed the risk factors for all-cause mortality among the enrolled men.
Material and Methods
ETHICS APPROVAL AND CONSENT TO PARTICIPATE:
The designed protocol (2018YBXM-SF-13-5, dated 19 August 2020) was approved by the Review Boards of the Kunshan Hospital of Traditional Chinese Medicine, Kunshan, China, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and the Chinese Urological Association. The study adhered to the laws of China and the 2008 Helsinki Declaration. The enrolled men provided signed informed consent regarding pathology and surgery (if performed) during hospitalization. Data of men were retrospectively collected from the institutes after obtaining written consent from authorities.
STUDY POPULATION:
Men (age >40 years) with histologically confirmed stage T1-T2N×M0 prostate cancer of any grade and with a 1-year history were included in the analyses. Data of men who had incomplete information available in the institutes’ records were excluded from analyses.
Data regarding baseline tumor characteristics, age, all-cause mortality, and health problems during follow-up of the included patients were collected from the medical records of the institutes.
COMORBIDITIES:
Comorbidities were assessed using the Charlson score, which ranges from 0 to 4, with 0 indicating fully active. The higher score, the poorer the functional status [13].
TUMOR RISK:
Tumor risk was assessed using the D’Amico tumor risk scores. The D’Amico risk groups were stratified into low-, intermediate-, or high-risk of biochemical recurrence after surgery according to the clinical TNM stage (tumor, nodule, and metastasis stage), the biopsy Gleason score, and the preoperative prostate-specific antigen level [14,15]. Low-risk was defined as tumor stage 2a or less, prostate-specific antigen level less than 10 ng/mL, and Gleason score 6 (3+3) or less. Intermediate-risk was defined as tumor stage 2b, prostate-specific antigen level 10–20 ng/mL, and Gleason score 7 (4+3 or 3+4). High-risk was defined as tumor stage 2c or more, prostate-specific antigen level more than 20 ng/mL, and Gleason score 8 or more [16].
PROSTATE CANCER RISK ASSESSMENT (CAPRA) SCORES:
CAPRA was assessed according to the guidelines of the University of California, San Francisco, Cancer of the Prostate Risk Assessment score in the range of 0–10 using Gleason score, positive biopsies, age, and the clinical TNM stage [17]. For men with Gleason score ≤6 (3+3), the assigned CAPRA points for Gleason score were zero and for men with Gleason score 8–10, the assigned CAPRA points for Gleason score were 3. It was classified as low-risk, intermediate-risk, and high-risk [7,8].
ADVERSE EFFECTS AND HEALTH PROBLEMS:
Radical proctectomy (all surgeries were performed by urologist(s) with a minimum of 3 years of experiences of the institutes)-related complications within 1 month after surgery and health problems within 2 years after diagnosis (self-reported) for all enrolled men were collected and analyzed.
STATISTICAL ANALYSIS:
The study assumed that a minimum of 35±5% men would survive the follow-up period (17–24 years). The sample size was calculated on the basis of 80% power and 5% two-sided type-I error (α=0.05 and β=0.2) at a 95% confidence level. The minimum number of men required in each cohort (the sample size) was 199. SPSS V25.0 IBM Corporation, Armonk, NY, USA was used for statistical analysis. The Fisher exact test was performed to assess differences between constant data, and an unpaired
Results
STUDY POPULATION:
From 15 November 1995 to 12 February 2003, a total of 435 men (age >40 years) were diagnosed (histologically) as having stage T1-T2N×M0 prostate cancer of any grade with a 1-year history at the Department of Urology of the Kunshan Hospital of Traditional Chinese Medicine, Kunshan, China and the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China. Among them, complete information of 25 men was not available in the patients’ records of the institutes; therefore, these data were excluded from the study. Among the 410 included men, 201 men underwent radical prostatectomy (RP cohort) and 209 men did not undergo radical prostatectomy (OS cohort). The men decided on their own either to undergo radical prostatectomy or not to receive any surgery. Data regarding baseline tumor characteristics, age, comorbidities, health status, all-cause mortality during follow-up period, tumor risk, and CAPRA scores were collected from medical records of the institutes and were analyzed (Figure 1).
CHARACTERISTICS AT THE TIME OF DIAGNOSIS:
There were no significant differences in the characteristics of men at the time of diagnosis between the 2 cohorts (P>0.05 for all, Table 1).
ALL-CAUSE MORTALITY:
As of 1 August 2020, a total of 291 (71%) men had died. The minimum follow-up time was 17 years and the maximum follow-up time was 24 years. A total of 135 (67%) men had died in the RP cohort and 156 (75%) men had died in the OS cohort. The number of deaths was not significantly different (P=0.103) between the 2 cohorts. Mean survival was 15 years in the RP cohort and 14 years in the OS cohort. There was a 4% reduction in all-cause mortality and an increase in survival 1 year after radical prostatectomy at 24 years of follow-up. All-cause mortality was lower in men with prostate-specific antigen level >10 ng/mL (P<0.0001), Gleason score ≥7 (p=0.004), and high D’Amico tumor risk scores (P=0.007) if they underwent radical prostatectomy, irrespective of the other characteristics at the time of diagnosis (Table 2).
RISK FACTORS FOR ALL-CAUSE MORTALITY:
Univariate analysis showed that age ≥65 years (P=0.012), prostate-specific antigen level >10 ng/mL (P=0.023), Charlson score ≥1 (P=0.045), Gleason score ≥7 (P=0.041), tumor stage ≥2c (P=0.035), and high CAPRA scores (P=0.042) were associated with all-cause mortality. Multivariate analysis showed that age ≥65 years (P=0.041), Gleason score ≥7 (P=0.049), and tumor stage ≥2c (P=0.045) were associated with all-cause mortality (Table 3).
ADVERSE EFFECTS OF RADICAL PROSTATECTOMY:
Within 1 month after radical prostatectomy, wound infections had occurred in 9 (4%) men, urinary tract infection occurred in 7 (2%) men, bleeding requiring transfusion was reported in 2 (1%) men, bowel dysfunction was reported in 2 (1%) men, and urinary catheterization was required in 20 (10%) men. The details of the adverse effects that occurred in men in the RP cohort within 1 month after radical prostatectomy are reported in Table 4. After 2 years, rates of urinary incontinence (P=0.001), erectile dysfunction (P<0.0001), and bowel dysfunctions (P=0.034) were significantly higher among men of the RP cohort than those of the OS cohort. The detailed self-reported health problems among men 2 years after diagnosis are reported in Table 5. However, there were no significant differences in anxiety and depression (developed from the hospital anxiety and depression scale) between men in the 2 cohorts during the follow-up period.
Discussion
We found that during a 24-year follow-up, radical prostatectomy had no significant beneficial effects when compared with observation. The results of the current study agree with the those of randomized trials on North American men [4,7,8], Scandinavian men [6], and English men [5] with localized prostate cancer. The PIVOT trial [7,8] had type II error and reported that 74% of patients with low-grade localized prostate cancer without prostatectomy survived the follow-up period [18]. In general, radical prostatectomy has no beneficial effects on Chinese men with localized prostate cancer compared to observation.
We found that all-cause mortality was lower in men with prostate-specific antigen level >10 ng/mL, Gleason score ≥7, and high D’Amico tumor risk scores if they underwent radical prostatectomy, irrespective of the other characteristics of men at the time of diagnosis. Our findings for all-cause mortality agreed with the those of randomized trials on North American men [4,7,8] and English men [5] with localized prostate cancer. Radical prostatectomy was beneficial in Chinese men who had prostate-specific antigen levels >10 ng/mL, Gleason score ≥7, and high D’Amico tumor risk scores.
Age ≥65 years, Gleason score ≥7, and tumor stage ≥2c were independently associated with all-cause mortality. The results of risk factor analysis for all-cause mortality of the current study agreed with those of randomized trials in North American men [4,7,8], Scandinavian men [6], and English men [5] with localized prostate cancer. We found no significant difference between watchful waiting and radical prostatectomy in Chinese men with localized prostate cancer with age ≥65 years, Gleason score ≥7, and/or tumor stage ≥2c.
The general surgical issues, urinary incontinence, and erectile dysfunction were assessed among men who underwent radical proctectomy. The results concerning adverse effects of radical proctectomy and health problems during the follow-up period of the current study agreed with those of randomized trials on North American men [4,7,8], Scandinavian men [6], and English men [5], as well as with results from a non-randomized prospective study of English men [19], and a retrospective study of Chinese men [20] with localized prostate cancer. Proctectomy has been reported to be associated with clinically worse outcomes [21], while radical prostatectomy is associated with more adverse effects and health problems than observation in Chinese men.
The present study has certain limitations, such as the small sample size, the use of a single center, and a focus on disease progression and tumor progression, which requires surgery, and distant metastases were not considered. The expertise of surgeons has effects on surgery-related outcomes [7], but we did not assess the effect of urologists’ experiences during the follow-up. In addition, retrospective analysis leads to selection bias. The mortality included in the study was all-cause mortality; therefore, patients who died due to only prostate cancer were not considered separately. The D’Amico tumor risk scores and the CAPRA scores parameters used in the study were based on studies of White patients with localized prostate cancer. These parameters need to be validated for Chinese populations before being used for research in China.
Conclusions
The findings from this study showed that radical prostatectomy has no significant beneficial effects when compared with observation for Chinese men with localized prostate cancer, unless they have a prostate-specific antigen level >10 ng/mL, Gleason score ≥7, and high D’Amico tumor risk scores. Age ≥65 years, Gleason score ≥7, and tumor stage ≥2c were independently associated with all-cause mortality. Also, radical prostatectomy has adverse effects related to surgery and significant health problems related to the urinogenital system during follow-up. The 2018 Chinese guidelines and the current 2014 Chinese Urological Association guidelines for prostate cancer need to be updated to develop clear recommendations for management of localized prostate cancer in Chinese men.
Tables
Table 1. Characteristics of men at the time of diagnosis.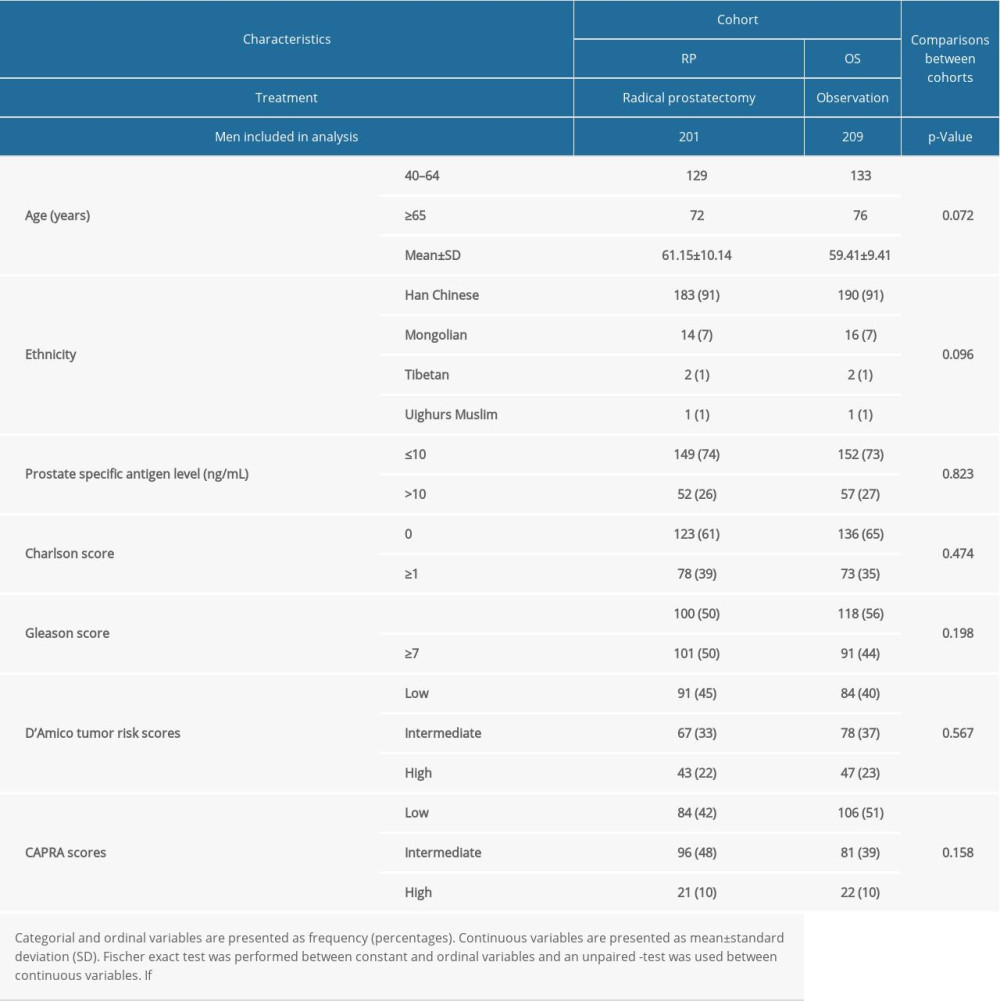 Table 2. All-cause mortality during follow-up time according to characteristics of men.
Table 2. All-cause mortality during follow-up time according to characteristics of men.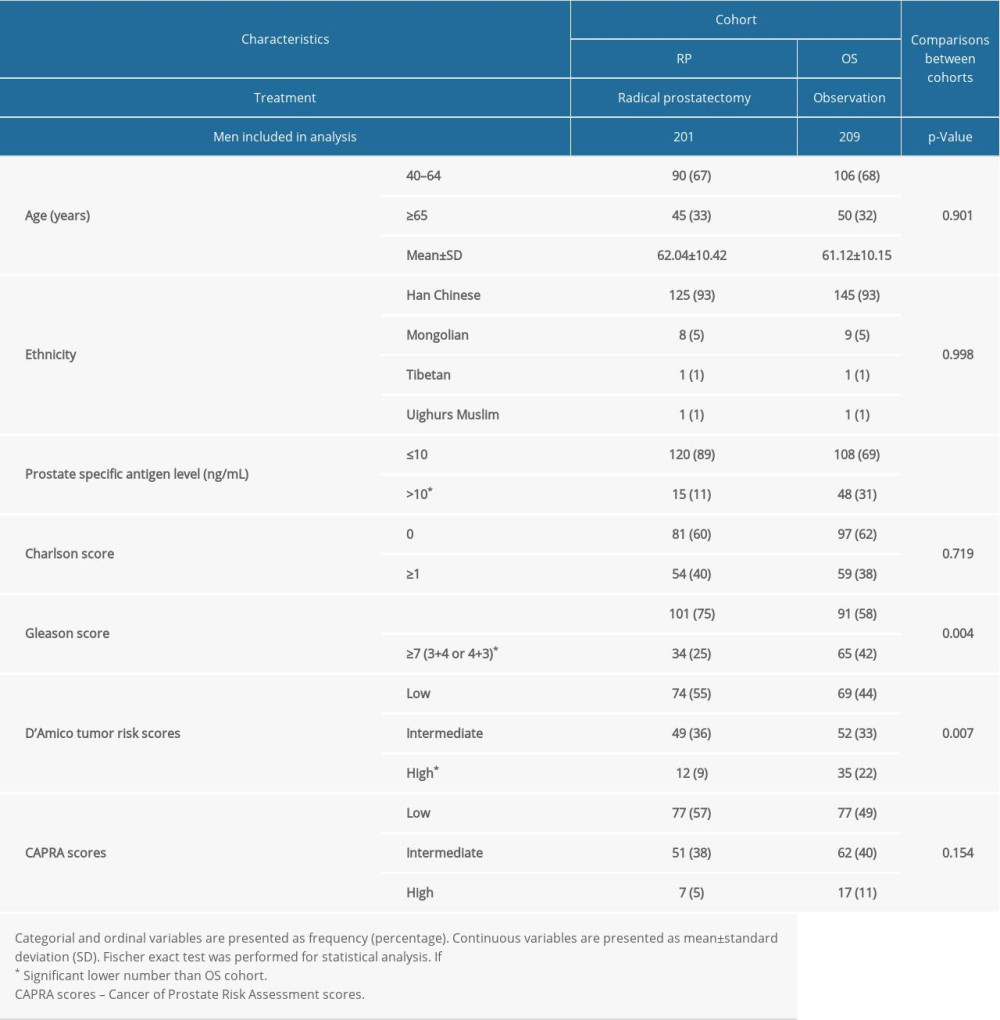 Table 3. Risk factor for all-cause mortality.
Table 3. Risk factor for all-cause mortality.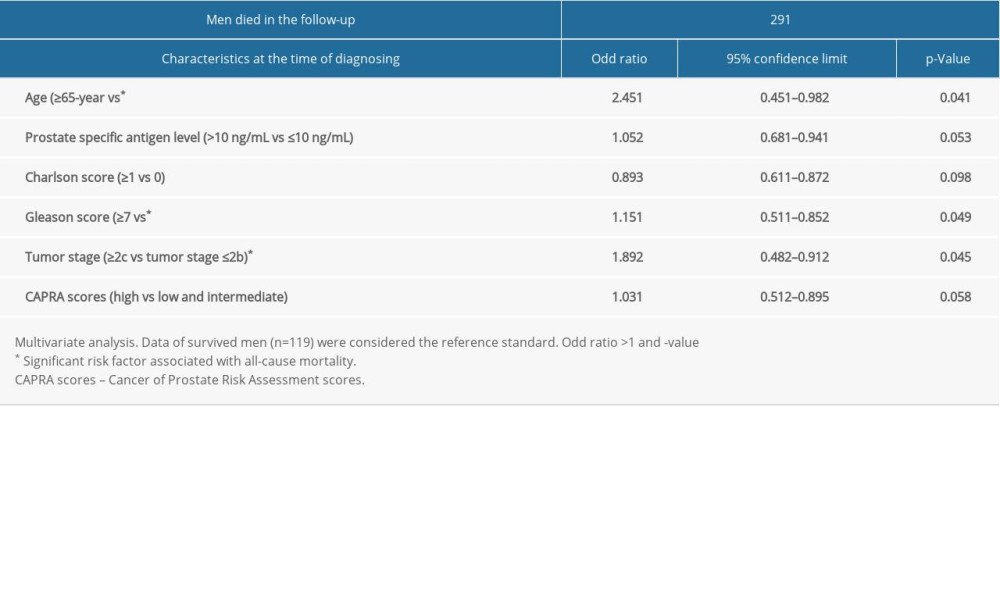 Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy.
Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy.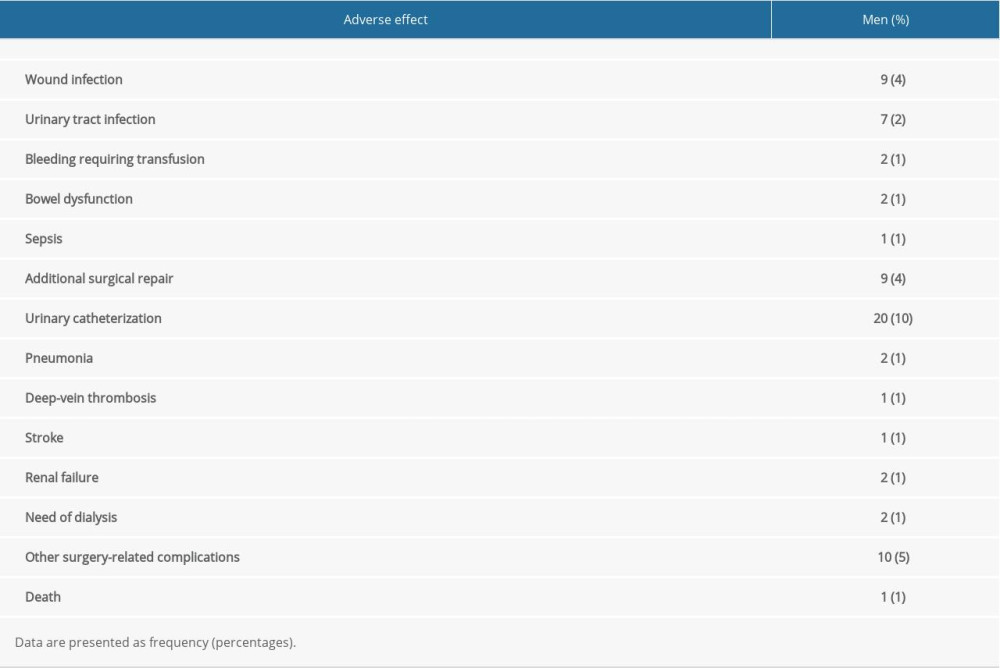 Table 5. Self-reported health problems in men 2 years after diagnosis.
Table 5. Self-reported health problems in men 2 years after diagnosis.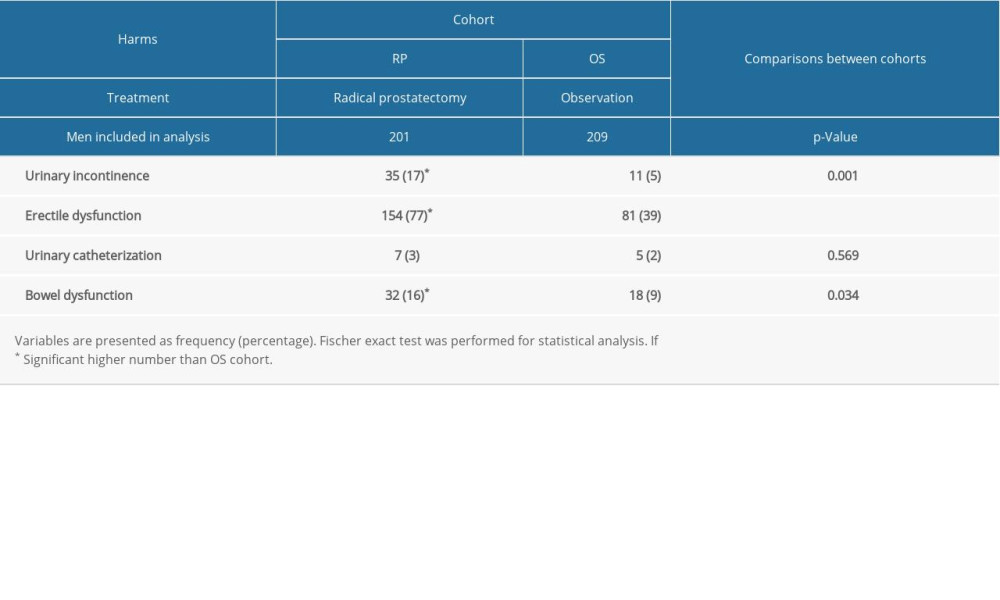
References
1. Xu P, Wang YHPrevention and treatment of erectile dysfunction after prostatectomy: An update: Zhonghua Nan Ke Xue, 2017; 23(7); 656-62 [in Chinese]
2. National Health Commission of the People’s Republic of China, Chinese guidelines for diagnosis and treatment of prostate cancer 2018 (English version): Chin J Cancer Res, 2019; 31; 67-83
3. Zhu Y, Yang XQ, Han CT, Pathological features of localized prostate cancer in China: A contemporary analysis of radical prostatectomy specimens: PLoS One, 2015; 10; e0121076
4. Wilt TJ, Jones KM, Barry MJ, Follow-up of prostatectomy versus observation for early prostate cancer: N Engl J Med, 2017; 377; 132-42
5. Hamdy FC, Donovan JL, Lane JA, 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer: N Engl J Med, 2016; 375; 1415-24
6. Bill-Axelson A, Holmberg L, Garmo H, Radical prostatectomy or watchful waiting in prostate cancer – 29-year follow-up: N Engl J Med, 2018; 379; 2319-29
7. Wilt TJ, Vo TN, Langsetmo L, Radical prostatectomy or observation for clinically localized prostate cancer: Extended follow-up of the prostate cancer intervention versus observation trial (PIVOT): Eur Urol, 2020; 77; 713-24
8. Wilt TJ, Brawer MK, Jones KM, Radical prostatectomy versus observation for localized prostate cancer: N Engl J Med, 2012; 367; 203-13
9. Mottet N, Bellmunt J, Bolla M, EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent: Eur Urol, 2017; 71; 618-29
10. Sanda MG, Cadeddu JA, Kirkby E, Clinically localized prostate cancer. AUA/ASTRO/SUO guideline. Part II: Recommended approaches and details of specific care options: J Urol, 2018; 199; 990-97
11. Wang J, Wang QRevision and significance of the guidelines on prostate cancer in 2014: J Mod Urol, 2015; 12; 844-47 [in Chinese]
12. Wei Y, Liu L, Li X, Current treatment for low-risk prostate cancer in China: A national network survey: J Cancer, 2019; 10; 1496-502
13. Sivaraman A, Ordaz Jurado G, Cathelineau X, Older patients with low Charlson score and high-risk prostate cancer benefit from radical prostatectomy: World J Urol, 2016; 34; 1367-72
14. D’Amico AV, Whittington R, Malkowicz SB, Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer: JAMA, 1998; 280; 969-74
15. D’Amico AV, Whittington R, Malkowicz SB, Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era: J Urol, 2001; 166; 2185-88
16. Meynard C, Huertas A, Dariane C, Tumor burden and location as prognostic factors in patients treated by iodine seed implant brachytherapy for localized prostate cancers: Radiat Oncol, 2019; 15; 1
17. Cooperberg MR, Pasta DJ, Elkin EP, The University of California, San Francisco Cancer of the Prostate Risk Assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy: J Urol, 2005; 173; 1938-42
18. Sharma V, Karnes RJ, Prostatectomy versus observation for early prostate cancer: N Engl J Med, 2017; 377; 1302
19. Donovan JL, Hamdy FC, Lane JA, Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer: N Engl J Med, 2016; 375; 1425-37
20. Huang W, Zhang Y, Shen BH, Outcomes of health-related quality of life after open, laparoscopic, or robot-assisted radical prostatectomy in China: Cancer Manag Res, 2019; 11; 899-907
21. Hoffman KE, Penson DF, Zhao Z, Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer: JAMA, 2020; 323; 149-63
Tables
 Table 1. Characteristics of men at the time of diagnosis.
Table 1. Characteristics of men at the time of diagnosis. Table 2. All-cause mortality during follow-up time according to characteristics of men.
Table 2. All-cause mortality during follow-up time according to characteristics of men. Table 3. Risk factor for all-cause mortality.
Table 3. Risk factor for all-cause mortality. Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy.
Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy. Table 5. Self-reported health problems in men 2 years after diagnosis.
Table 5. Self-reported health problems in men 2 years after diagnosis. Table 1. Characteristics of men at the time of diagnosis.
Table 1. Characteristics of men at the time of diagnosis. Table 2. All-cause mortality during follow-up time according to characteristics of men.
Table 2. All-cause mortality during follow-up time according to characteristics of men. Table 3. Risk factor for all-cause mortality.
Table 3. Risk factor for all-cause mortality. Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy.
Table 4. Adverse effects reported in men of the RP cohort within 1 month after radical prostatectomy. Table 5. Self-reported health problems in men 2 years after diagnosis.
Table 5. Self-reported health problems in men 2 years after diagnosis. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952