19 April 2021: Lab/In Vitro Research
Loss of Speckle-Type POZ Protein Promotes Prostate Cancer Cell Migration and Invasion Through Upregulation of MCP-1
Junlin Shi12ABCDEFG, Ji Cao3AG, Xiaomei Lu12B, Langlin Fan12B, Hongwei Guo12G, Jiejun Fu12ABCDEFG*DOI: 10.12659/MSM.929199
Med Sci Monit 2021; 27:e929199
Abstract
BACKGROUND: The goal of this study is to verify that the loss of speckle-type POZ protein (SPOP) promotes the migration and invasion of prostate cancer cells, and that this process is brought about by an increase in MCP-1.
MATERIAL AND METHODS: SPOP knockout C4-2 cells (C4-2 SPOP-/-) were verified by western blotting. Transwell and wound-healing assays were applied to verify different migration and invasion abilities between the C4-2 SPOP-/- and control cells. We used an antibody array to find different soluble chemokine factors in the C4-2 SPOP-/- cells. ELISA and qRT-PCR were applied for confirmation. To test MCP-1 function in conditioned medium, a transwell assay was applied with or without anti-MCP-1 antibody.
RESULTS: The western blot showed that SPOP was knocked out in sgSPOP-1 and sgSPOP-2 (different clones of C4-2 SPOP-/-). The transwell and wound-healing assays indicated that, compared with control cells, sgSPOP-1 and sgSPOP-2 had stronger migration and invasion abilities. The antibody array found that the expression of MCP-1 was upregulated in sgSPOP-1 and sgSPOP-2 conditioned medium. This result was verified by ELISA and qRT-PCR. In the prostate cancer cells, migration and invasion activity was greatly increased in C4-2 SPOP-/- conditioned medium, while this activity was decreased after anti-MCP-1 antibody neutralization.
CONCLUSIONS: Our findings suggest that the loss of SPOP in C4-2 cells promotes increased cell migration and invasion abilities. This may be realized by upregulating the expression of MCP-1. The inhibition of MCP-1 expression may be an effective treatment for SPOP-mutant prostate cancer.
Keywords: Genes, Tumor Suppressor, Prostatic Neoplasms, Receptors, CCR2, Antibodies, Blocking, Chemokine CCL2, Mutation, Nuclear Proteins, Repressor Proteins, Tumor Cells, Cultured, Wound Healing
Background
Prostate cancer (PCa) has the highest incidence of any male malignant tumor in the United States [1]. Metastasis is among the main features of malignant tumors, leading to a high mortality rate from cancer; therefore, the key factors that initiate this process must be determined.
Speckle-type POZ (pox virus and zinc finger protein) protein (SPOP) is an adaptor protein of E3 ubiquitin ligase that was first identified in the serum of a scleroderma patient by Yasuo Nagai [2]. It is a member of the MATH-BTB protein family; it contains 2 domains: the C-terminal BTB domain and the N-terminal MATH domain. The BTB domain is responsible for binding Cul 3 to form a functional E3 ubiquitin ligase complex, while the function of the MATH domain involves substrate recognition and interaction [3,4]. SPOP substrate research has shown that the BTB protein plays a role in regulating a variety of cellular processes, including hormone-dependent signaling, cell cycle regulation and apoptosis, cell differentiation, and epigenetic control [5-8].
Many studies have shown that SPOP is the most common gene found to be mutated in PCa (10–15%), and that it is concentrated in the MATH-encoding region [9,10]. This suggests that its interaction with substrate proteins may be impaired by mutations. Therefore, some substrates of CRL3SPOP, such as the androgen receptor (AR), are the subjects of specific background research in the field of PCa biology [11,12]. Other substrates of CRL3SPOP include steroid receptor coactivator 3 (SRC-3) [6], the DEK oncogene [13], and ETS-related gene (ERG) [14–17]. Until now, there has been only a small amount of research on the relationship between metastasis and SPOP. Chen et al reported that SPOP suppressed invasion by osteosarcoma via the PI3K/AKT/NF-kappa B signaling pathway [18]. Xu et al said that the restoration of SPOP could inhibit miRNA-543-induced gastric cancer cell migration and invasion [19]. Duan et al also wrote a preview showing that ERG rearrangements or SPOP mutations lead to elevated ERG levels, thereby promoting cell invasion in PCa [15]. This preview referenced the results of Gan and An, who found that SPOP mutations in PCa inhibit the degradation of ERG, leading to enhanced levels of ERG and the promotion of cell invasion [16,17].
Despite the relevant studies mentioned above, the specific mechanisms through which SPOP mutations promote tumor metastasis are unclear. In recent years, more attention has been paid to the effects of the tumor microenvironment on tumor metastasis [20,21]. In the current study, we have focused on the microenvironment to explain cell metastasis in SPOP-mutant PCa. With a view toward further revealing the molecular mechanism underlying SPOP-mutant PCa, we first obtained SPOP knockout PCa cells and verified the change in their metastasis ability. Furthermore, we found that MCP-1 was highly expressed in SPOP knockout cell-conditioned medium (CM) and that it may be the key molecule that promotes SPOP-mutant PCa metastasis.
Material and Methods
CELL CULTURE:
The SPOP knockout human PCa cells, C4-2SPOP−/− sgSPOP-1 and sgSPOP-2, and the control cells were gifts of Dr. Wenyi Wei at Harvard Medical School. The cells were cultured in Royal Park Memorial Institute (RPMI 1640) medium (GIBCO, California, USA), containing 10% Fetal Bovine Serum (FBS) and streptomycin, at 37°C in a humid, 5% CO2 atmosphere. The cells were routinely tested for mycoplasma contamination.
PREPARATION OF CELL-CONDITIONED MEDIUM:
CM from sgSPOP-1, sgSPOP-2, and control cells was harvested for soluble factors, which were measured and subsequently verified by enzyme-linked immunosorbent assay (ELISA) [22].
WESTERN BLOTTING:
C4-2SPOP−/− sgSPOP-1 and sgSPOP-2, as well as control cells, were lysed in a radio immunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail. After centrifugation, the supernatant was collected, the concentration was measured, and denaturation was allowed to occur. Then, the whole-cell lysates (50 μg) were resolved through SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and then the proteins were transferred from the gel to a polyvinylidene difluoride (PVDF) membrane and incubated in a blocking buffer that consisted of 5% nonfat milk with tris buffered saline with Tween (TBST) buffer, for 1 h at room temperature. After washing with TBST buffer, the membranes were incubated with a primary antibody to SPOP (Proteintech, Wuhan, China) and tubulin (Cell Signaling Technology, Boston, USA) overnight at 4°C. After another TBST washing, followed by another 1 h of incubation with a secondary HRP antibody, at room temperature, immunoreactive proteins were detected using an enhanced chemiluminescence SuperSignal substrate (Thermo Fisher Scientific, Massachusetts, USA).
TRANSWELL INVASION ASSAYS AND TRANSWELL MIGRATION ASSAYS:
Non-FBS medium (500 μL) was put into 8.0 μm 24-well plate chamber inserts (Corning 3422 for migration and BD Biosciences for invasion, Massachusetts and New Jersey, USA, respectively). After removal from the refrigerator, the Matrigel-medium mix was allowed to solidify through incubation at 37°C for 2 h. Then, the plates were removed from the incubator, and 5×104 cells in serum-free medium were seeded in the top chamber. The bottom of each insert was filled with 750 μl of medium supplemented with 10% FBS. For the specific factor blocking assay, RPMI 1640 medium and CM with 2 μg/ml monoclonal mouse anti-human MCP-1 (eBioscience, California, USA) were placed in the lower chambers. For each sample, the procedure was repeated 3 times. After 24 h of cell incubation, the cells were washed with phosphate buffer saline (PBS) and fixed with 4% paraformaldehyde for 18 min. Cotton swabs were used to clear up the cells on the top of the insert, while 0.5% crystal violet blue was used to stain the cells that adhered to the bottom. After 15 min of standing at room temperature while protected from light, they were washed with H2O. The images were obtained using a microscope and the cells were counted in 15 different view fields using ImageJ software. An invasive index was used to calculate the ratio of the cells that had invaded through the Matrigel-coated membrane to the cells that migrated through the uncoated membrane [22].
WOUND-HEALING ASSAY:
C4-2SPOP−/− sg-SPOP-1 and sg-SPOP-2 cells and control cells were plated in six-well plates at a high concentration of 5×105 cells/well. After 24 h of incubation, the cells were treated with 20 μg/ml mitomycin for 3 h to inhibit proliferation. Scratches were made in the plate, using a 200 μl pipette tip. Images were collected at 0, 10, and 18 h after scratching. The experiment was repeated 3 times. Wound closure areas were quantified using Photoshop and ImageJ software.
ANTIBODY ARRAY:
Human Chemokine Array C1 from RayBiotech (Atlanta, GA, USA) contains 38 repeated antibodies spotted on a single membrane. Experiments were implemented according to the manufacturer’s protocol, and the results were analyzed by both Lane 1D software from Sage Creation (Beijing, China) and software from RayBiotech.
ELISA:
To measure the expression/secretion levels of soluble factors, the conditioned media were harvested from C4-2SPOP−/− sgSPOP-1 and sgSPOP-2 and control cells. An MCP-1 ELISA kit from NeoBioscience (Shenzhen, China) was used to measure the levels of MCP-1 in the conditioned medium. This was performed according to the manufacturer’s protocol.
QUANTITATIVE PCR (QRT-PCR):
TRIzol (Invitrogen, California, USA) was used to extract total RNA from C4-2SPOP−/− sgSPOP-1 and sgSPOP-2 cells, and control cells, according to the manufacturer’s instructions. The total RNA concentration was detected by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). A High-Capacity cDNA Reverse Transcription Kit (Invitrogen) was used to convert RNA into cDNA, according to the manufacturer’s protocol.
The following primers were used:
Additionally, qRT-PCR was performed using the ABI 7500 system (Applied Biosystems, California, USA). The experiment was conducted independently in 3 biological replicates, and GAPDH was used as the housekeeping control gene.
STATISTICAL ANALYSIS:
StatView software (Abacus Concepts, Berkeley, CA) was used to perform statistical analysis. The significance of the in vitro cell migration, cell invasion, ELISA, and qPCR between the C4-2SPOP−/− (sgSPOP-1, sgSPOP-2) and control groups was determined by a
Results
SPOP KNOCKOUT PROMOTES C4-2 CELL MIGRATION AND INVASION:
Western blotting was used to confirm that sgSPOP-1 and sgSPOP-2 (Figure 1A) cells did not express SPOP, but control cells did express SPOP (Figure 1B). The results showed that SPOP was knocked out in sgSPOP-1 and sgSPOP-2.
To further investigate the metastasis-related function of SPOP, transwell assays were implemented between sgSPOP-1 and sgSPOP-2 (without SPOP expression) and control cells (with SPOP expression). The results revealed that sgSPOP-1 and sgSPOP-2 cells showed stronger migration and invasion abilities compared with control cells (Figure 1C). Subsequently, a scratch wound-healing assay was performed, and it showed that sgSPOP-1 and sgSPOP-2 cells had higher migration abilities than the control cells. This was consistent with the results of the transwell assay (Figure 1D).
:
C4-2 SPOP−/− cells showed stronger migration and invasion abilities than C4-2 control cells. To characterize the changes in soluble factors secreted by C4-2 SPOP−/−, CM was collected from sgSPOP-1 and sgSPOP-2 and control cell cultures, and a Human Chemokine Antibody Array (RayBiotech) was applied to detect the expression of chemokines in these media. After normalization, the results demonstrated that the expression of MCP-1 in sgSPOP-1 and sgSPOP-2 was upregulated compared with that in the control cells (Figure 2A).
To verify the antibody array data, ELISA was applied to characterize the secretion levels of MCP-1 in the collected CM. In accordance with the results of the arrays, the expression/secretion levels of MCP-1 in the C4-2 SPOP−/− cells were significantly higher than those in the C4-2 control cells (Figure 2B). Furthermore, this result was also confirmed by qRT-PCR, in which the expression of MCP-1 was higher in the C4-2 SPOP−/− cells (Figure 2C).
Since MCP-1 in the CM enhanced the migration and invasion of PCa cells, and since MCP-1 was expressed at higher levels in the CM of C4-2 SPOP−/− cells, we focused on the association between MCP-1 and C4-2 SPOP−/− cell migration and invasion. To test whether MCP-1 in the CM of C4-2 SPOP−/− cells affects their migration and invasion, we conducted a transwell assay. Many migrating and invading cells were recognized in the CM-treated group, whereas fewer migrating and invading cells were found in the control medium (Figure 3). The CM with the anti-MCP-1 antibody reduced the number of migrating and invading cells (P<0.01 for sgSPOP-1 migration and P<0.05 for sgSPOP-2 migration; P<0.05 for both sgSPOP-1 and sgSPOP-2 invasion).
Discussion
Among men worldwide, PCa is the type of malignant tumor with the highest incidence. Recurrence and metastasis are the biggest challenges for clinical treatment [23]. In PCa, SPOP is the most common mutated gene, and there are reports showing that SPOP is closely related to tumor cell metastasis [16,17,19,24]. We hypothesized that the loss of SPOP may promote PCa metastasis by changing the tumor microenvironment. Certain protein networks regulate the occurrence of metastasis. Blocking SPOP mutation or the mutation of related key molecules may benefit patients who are at risk of metastasis.
In the current study, we first obtained SPOP knockout PCa cells through a lentiCRISPR/Cas 9 system (C4-2SPOP−/− and control cells). Then, western blot was used to verify SPOP expression. After determining that the loss of SPOP creates stability, we found that C4-2SPOP−/− showed stronger migration and invasion abilities compared with control cells, through transwell and wound-healing assays. This result is consistent with the previous results of other scholars [15].
Next, we wanted to know how SPOP suppresses tumor metastasis. Our results suggested that the loss of SPOP changed the microenvironment that promotes tumor cell migration and invasion. By using antibody arrays, we identified that the MCP-1 level dramatically increased in the C4-2SPOP−/− cells’ CM compared with the control cells’ CM. ELISA and qRT-PCR verified this finding. These results show that the loss of SPOP may promote PCa cell migration and invasion through upregulation of MCP-1 in the tumor microenvironment. To confirm this finding, we used an anti-MCP-1 antibody to neutralize MCP-1 in the CM of C4-2SPOP−/−. A transwell assay was again used to compare the migration and invasion abilities of C4-2SPOP−/− cells in the following media: RPMI 1640, CM, and CM+anti-MCP-1. C4-2SPOP−/− cells showed decreased migration and invasion abilities in CM+anti-MCP-1, which suggested that MCP-1 may be a key molecule enabling metastasis in SPOP-mutant PCa.
MCP-1, also known as CCL2, belongs to the C-C chemokine subfamily. It has been shown to be important for recruitment of monocytes, activation of the process of acute inflammation, and development of cancer. Extensive research has indicated that MCP-1 could induce the occurrence of EMT and promote cell proliferation [25–27], angiogenesis [28–31], and metastasis [29,32,33]. Higher levels of MCP-1 after neoadjuvant chemotherapy may lead to recurrence and metastasis [34]. Additionally, in the tumor microenvironment, higher MCP-1 expression may enhance the likelihood of cancer cell invasion by activating both autocrine and paracrine pathways [35]. These findings are consistent with our finding that MCP-1 secreted by SPOP-mutant PCa cells may promote cell migration and invasion.
Much research has shown that SPOP is a frequent mutation factor in PCa. Additionally, its structure, cellular function, functional consequences of mutation, and possible mechanisms in PCa have been explored in many reports. An et al and Gan et al reported that cancer-associated SPOP mutations were not sufficient to promote ERG ubiquitylation. Furthermore, they found that ERG stabilization is important for enhancing the migration and invasion activities of SPOP-mutant cells [16,17]. Our research focused on changes in the tumor microenvironment and found that SPOP could regulate the secretion of MCP-1, thereby further regulating cell migration and invasion in PCa. Although SPOP may affect the metastasis of PCa through its substrate, according to the result of the transwell assay with or without anti-MCP-1 antibody, the occurrence of PCa cell migration and invasion is at least partially achieved through the involvement of MCP-1.
Studies have shown that SPOP can mediate the degradation of BRD4 [36,37]. On the one hand, in SPOP-deficient PCa cells, enhanced BRD4 transcription co-activation and increased ERG expression jointly promoted cell migration. On the other hand, BRD4 plays an important role in the production of CCL2. It upregulates the expression of MCP-1 via the activation of the NF-κB signaling pathway. In other words, SPOP regulates MCP-1 in an indirect way. Many phase I and phase II clinical trials have shown the efficacy of the monoclonal antibody carlumab targeting MCP-1 in solid tumors [36–38]. Prostate cancer patients may also benefit from this approach.
Conclusions
The loss of SPOP can promote PCa cell migration and invasion. In this study, we explored the possible mechanisms underlying this promotion using antibody arrays and verified array results. However, to better understand the molecular mechanisms behind the association of SPOP and MCP-1 with metastasis, further studies are needed. Additionally, in vivo studies are necessary. MCP-1 and related pathway inhibitors can be used to block the migration and invasion of PCa, thereby preventing tumor recurrence and metastasis.
Figures
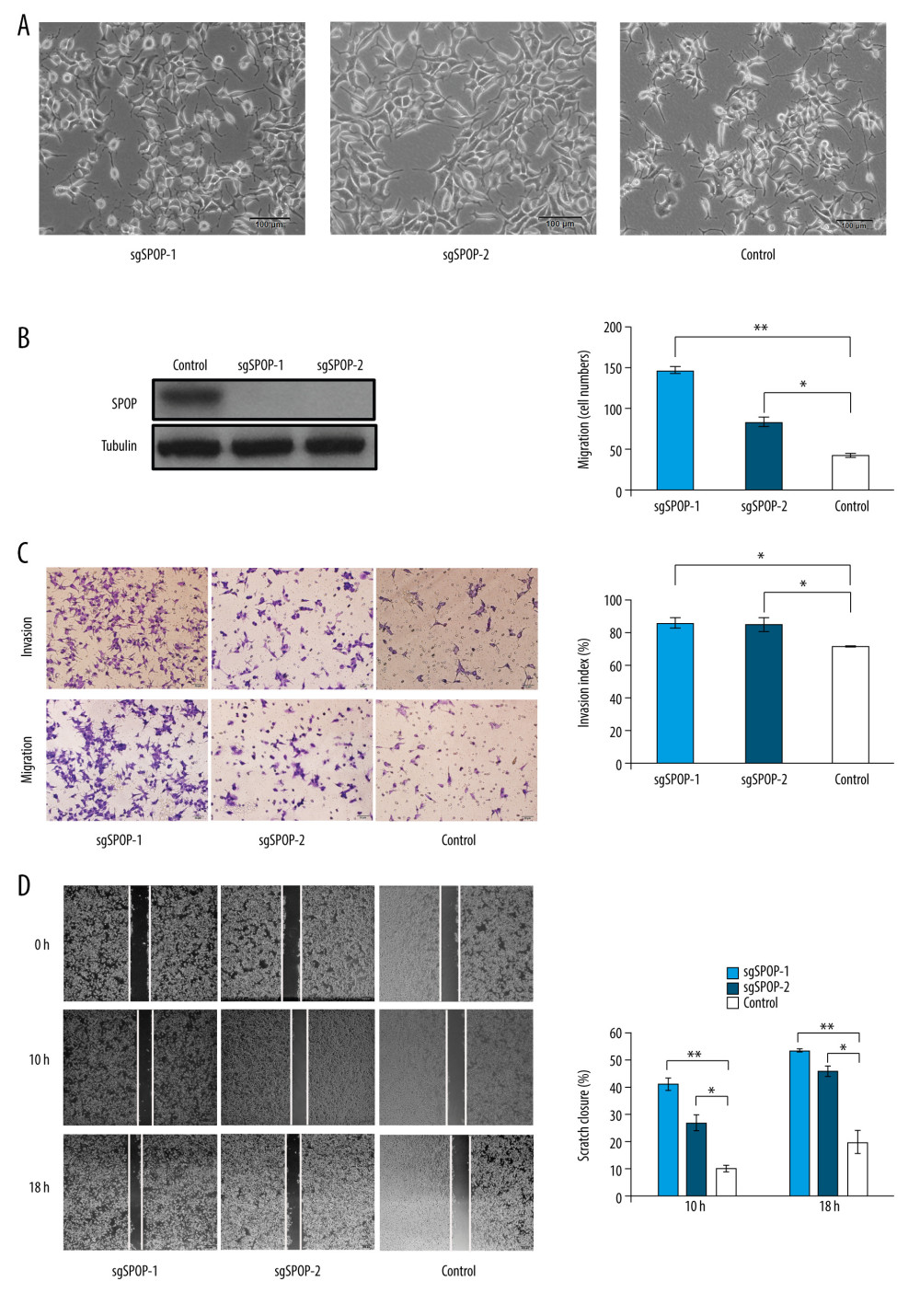 Figure 1. SPOP knockout promotes C4-2 cell migration and invasion. (A) Photomicrograph of sgSPOP-1 and sgSPOP-2 compared with parent cell line C4-2 (control). (B) Western blotting with anti-SPOP antibody confirms the presence of SPOP in control C4-2 cells and absence in both sgSPOP-1 and sgSPOP-2 cells. (C) A transwell assay was used to explore cell migration and invasion ability in sgSPOP-1, sgSPOP-2, and control cells. The results are presented as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. (D) The migration ability of sgSPOP-1 cells, sgSPOP-2 cells, and control cells was investigated using a scratch wound-healing assay. The results are shown as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01.
Figure 1. SPOP knockout promotes C4-2 cell migration and invasion. (A) Photomicrograph of sgSPOP-1 and sgSPOP-2 compared with parent cell line C4-2 (control). (B) Western blotting with anti-SPOP antibody confirms the presence of SPOP in control C4-2 cells and absence in both sgSPOP-1 and sgSPOP-2 cells. (C) A transwell assay was used to explore cell migration and invasion ability in sgSPOP-1, sgSPOP-2, and control cells. The results are presented as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. (D) The migration ability of sgSPOP-1 cells, sgSPOP-2 cells, and control cells was investigated using a scratch wound-healing assay. The results are shown as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. 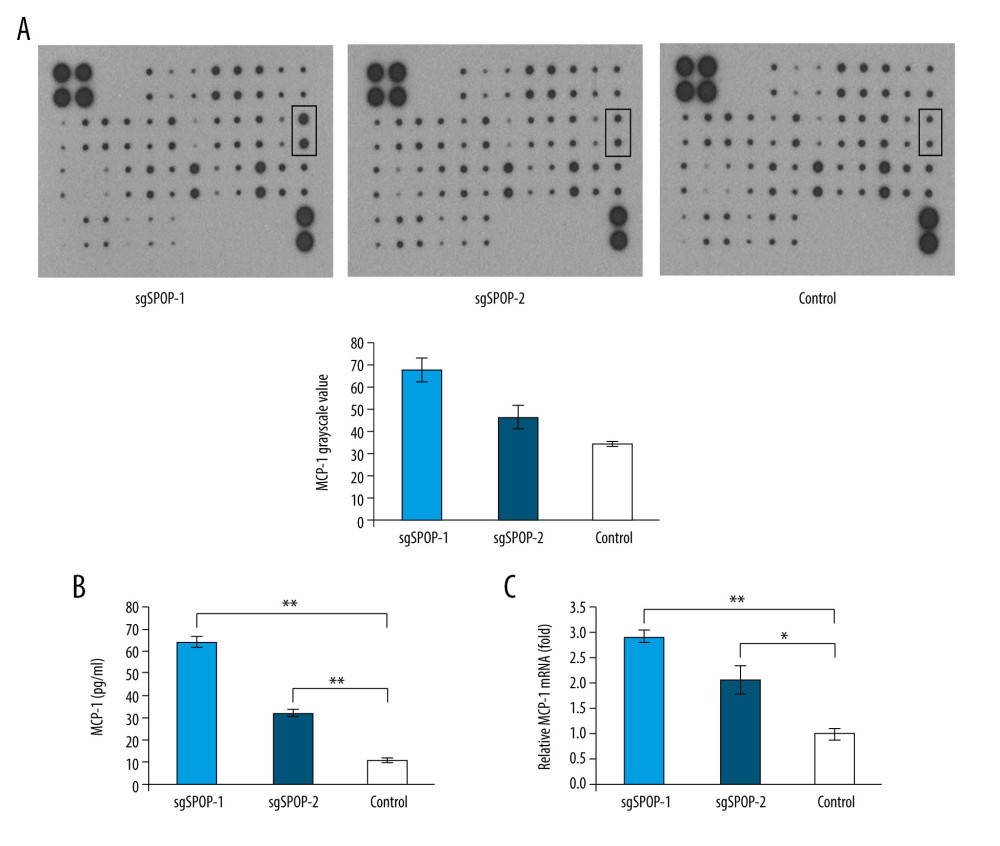 Figure 2. The difference in soluble factors in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected by chemokine antibody arrays and verified by ELISA and qRT-PCR. (A) The difference in expressed chemokines in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected through chemokine antibody array. (B) ELISA was used to verify the MCP-1 expression level in the conditioned medium. The results are presented as the mean±SD of 3 independent experiments; ** P<0.01. (C) qRT-PCR measurement of the MCP-1 mRNA expression levels. The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01.
Figure 2. The difference in soluble factors in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected by chemokine antibody arrays and verified by ELISA and qRT-PCR. (A) The difference in expressed chemokines in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected through chemokine antibody array. (B) ELISA was used to verify the MCP-1 expression level in the conditioned medium. The results are presented as the mean±SD of 3 independent experiments; ** P<0.01. (C) qRT-PCR measurement of the MCP-1 mRNA expression levels. The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. 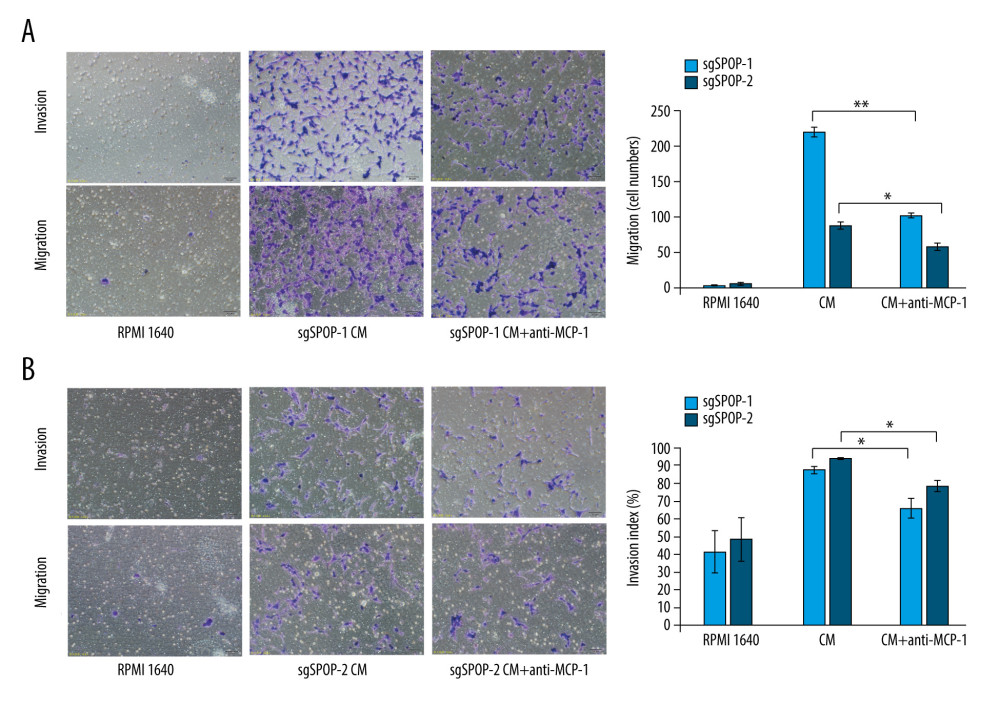 Figure 3. The migration and invasion of sgSPOP-1 and sgSPOP-2 cells in response to their CM. (A) A total of 5×104 sgSPOP-1 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-1 CM; Lane 3, sgSPOP-1 CM with anti-MCP-1 (2 μg/ml). (B) A total of 5×104 sgSPOP-2 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-2 CM; Lane 3, sgSPOP-2 CM with anti-MCP-1 (2 μg/ml). The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. CM – conditioned medium.
Figure 3. The migration and invasion of sgSPOP-1 and sgSPOP-2 cells in response to their CM. (A) A total of 5×104 sgSPOP-1 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-1 CM; Lane 3, sgSPOP-1 CM with anti-MCP-1 (2 μg/ml). (B) A total of 5×104 sgSPOP-2 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-2 CM; Lane 3, sgSPOP-2 CM with anti-MCP-1 (2 μg/ml). The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. CM – conditioned medium. References
1. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69; 7-34
2. Nagai Y, Kojima T, Muro Y, Identification of a novel nuclear speckle-type protein, SPOP: FEBS Lett, 1997; 418; 23-26
3. Mani RS, The emerging role of speckle-type POZ protein (SPOP) in cancer development: Drug Discov Today, 2014; 19(9); 1498-502
4. Zhuang M, Calabrese MF, Liu J, Structures of SPOP-substrate complexes: Insights into molecular architectures of BTB-Cul3 ubiquitin ligases: Mol Cell, 2009; 36; 39-50
5. Kim B, Nam HJ, Pyo KE, Breast cancer metastasis suppressor 1 (BRMS1) is destabilized by the Cul3-SPOP E3 ubiquitin ligase complex: Biochem Biophys Res Commun, 2011; 415; 720-26
6. Li C, Ao J, Fu J, Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1: Oncogene, 2011; 30; 4350-64
7. An J, Wang C, Deng Y, Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants: Cell Rep, 2014; 6; 657-69
8. La M, Kim K, Park J, Daxx-mediated transcriptional repression of MMP1 gene is reversed by SPOP: Biochem Biophys Res Commun, 2004; 320; 760-65
9. Barbieri CE, Baca SC, Lawrence MS, Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer: Nat Genet, 2012; 44(6); 685-89
10. Cancer Genome Atlas Research Network, The molecular taxonomy of primary prostate cancer: Cell, 2015; 163(4); 1011-25
11. Yan Y, An J, Yang Y, Dual inhibition of AKT-mTOR and AR signaling by targeting HDAC3 in PTEN- or SPOP-mutated prostate cancer: EMBO Mol Med, 2018; 10(4); e8478
12. Blattner M, Liu D, Robinson BD, SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling: Cancer Cell, 2017; 31; 436-51
13. Lin D, Dong X, Wang K, Identification of DEK as a potential therapeutic target for neuroendocrine prostate cancer: Oncotarget, 2015; 6; 1806-20
14. Shoag J, Liu D, Blattner M, SPOP mutation drives prostate neoplasia without stabilizing oncogenic transcription factor ERG: Journal Clin Invest, 2018; 128; 381-86
15. Duan S, Pagano M, SPOP mutations or ERG rearrangements result in enhanced levels of ERG to promote cell invasion in prostate cancer: Mol Cell, 2015; 59; 883-84
16. An J, Ren S, Murphy SJ, Truncated ERG oncoproteins from TMPRSS2-ERG Fusions are resistant to SPOP-mediated proteasome degradation: Mol Cell, 2015; 59; 904-16
17. Gan W, Dai X, Lunardi A, SPOP promotes ubiquitination and degradation of the ERG oncoprotein to suppress prostate cancer progression: Mol Cell, 2015; 59; 917-30
18. Chen L, Pei H, Lu SJ, SPOP suppresses osteosarcoma invasion via PI3K/AKT/NF-κB signaling pathway: Eur Rev Med Pharmacol Sci, 2018; 22(3); 609-15
19. Xu J, Wang F, Wang X, miRNA-543 promotes cell migration and invasion by targeting SPOP in gastric cancer: Onco Targets Ther, 2018; 11; 5075-82
20. Ren B, Cui M, Yang G, Tumor microenvironment participates in metastasis of pancreatic cancer: Mol Cancer, 2018; 17(1); 108
21. Xie HY, Shao ZM, Li DQ, Tumor microenvironment: Driving forces and potential therapeutic targets for breast cancer metastasis: Chin J Cancer, 2017; 36(1); 36
22. Shi JL, Wang LH, Zou CL, Tumor microenvironment promotes prostate cancer cell dissemination via the Akt/mTOR pathway: Oncotarget, 2018; 9; 9206-18
23. Yamamoto KN, Nakamura A, Haeno H, The evolution of tumor metastasis during clonal expansion with alterations in metastasis driver genes: Sci Rep, 2015; 5; 15886
24. Zhang J, Bu X, Wang H, Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance: Nature, 2018; 553; 91-95
25. Lee WJ, Jo SY, Lee MH, The Effect of MCP-1/CCR2 on the proliferation and senescence of epidermal constituent cells in solar lentigo: Int J Mol Sci, 2016; 17; 948
26. Kuper C, Beck FX, Neuhofer W, Autocrine MCP-1/CCR2 signaling stimulates proliferation and migration of renal carcinoma cells: Oncol Lett, 2016; 12; 2201-9
27. Braganhol E, Kukulski F, Levesque SA, Nucleotide receptors control IL-8/CXCL8 and MCP-1/CCL2 secretions as well as proliferation in human glioma cells: Biochim Biophys Acta, 2015; 1852; 120-30
28. Passaro C, Borriello F, Vastolo V, The oncolytic virus dl922–947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma: Oncotarget, 2016; 7; 1500-15
29. Liu X, Jing X, Cheng X, FGFR3 promotes angiogenesis-dependent metastasis of hepatocellular carcinoma via facilitating MCP-1-mediated vascular formation: Med Oncol, 2016; 33; 46
30. Adini I, Adini A, Bazinet L, Melanocyte pigmentation inversely correlates with MCP-1 production and angiogenesis-inducing potential: FASEB J, 2015; 29; 662-70
31. Wang S, Xu M, Li F, Ethanol promotes mammary tumor growth and angiogenesis: The involvement of chemoattractant factor MCP-1: Breast Cancer Res Treat, 2012; 133; 1037-48
32. Dutta P, Sarkissyan M, Paico K, MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis: Breast Cancer Res Treat, 2018; 170; 477-86
33. Min A, Zhu C, Wang J, Focal adhesion kinase knockdown in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis via downregulating MCP-1/CCL2 expression: J Biochem Mol Toxicol, 2015; 29; 70-76
34. Vyas D, Laput G, Vyas AK, Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis: Onco Targets Ther, 2014; 7; 1015-23
35. Santander AM, Lopez-Ocejo O, Casas O, Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: The role of obesity and inflammation in breast adipose tissue: Cancers, 2015; 7; 143-78
36. Mu JF, Sun PF, Ma ZM, BRD4 promotes tumor progression and NF-κB/CCL2-dependent tumor-associated macrophage recruitment in GIST: Cell Death Dis, 2019; 935; 1-11
37. Dai XP, Gan WJ, Li XN, Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4: Nat Med, 2017; 23; 1063-71
38. Gao J, Wei B, Liu M, Endothelial p300 promotes portal hypertension and hepatic fibrosis through CCL2-mediated angiocrine signaling, 2020 [Online ahead of print]
Figures
 Figure 1. SPOP knockout promotes C4-2 cell migration and invasion. (A) Photomicrograph of sgSPOP-1 and sgSPOP-2 compared with parent cell line C4-2 (control). (B) Western blotting with anti-SPOP antibody confirms the presence of SPOP in control C4-2 cells and absence in both sgSPOP-1 and sgSPOP-2 cells. (C) A transwell assay was used to explore cell migration and invasion ability in sgSPOP-1, sgSPOP-2, and control cells. The results are presented as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. (D) The migration ability of sgSPOP-1 cells, sgSPOP-2 cells, and control cells was investigated using a scratch wound-healing assay. The results are shown as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01.
Figure 1. SPOP knockout promotes C4-2 cell migration and invasion. (A) Photomicrograph of sgSPOP-1 and sgSPOP-2 compared with parent cell line C4-2 (control). (B) Western blotting with anti-SPOP antibody confirms the presence of SPOP in control C4-2 cells and absence in both sgSPOP-1 and sgSPOP-2 cells. (C) A transwell assay was used to explore cell migration and invasion ability in sgSPOP-1, sgSPOP-2, and control cells. The results are presented as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. (D) The migration ability of sgSPOP-1 cells, sgSPOP-2 cells, and control cells was investigated using a scratch wound-healing assay. The results are shown as the mean±SD of 3 independent replicates; * P<0.05, ** P<0.01. Figure 2. The difference in soluble factors in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected by chemokine antibody arrays and verified by ELISA and qRT-PCR. (A) The difference in expressed chemokines in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected through chemokine antibody array. (B) ELISA was used to verify the MCP-1 expression level in the conditioned medium. The results are presented as the mean±SD of 3 independent experiments; ** P<0.01. (C) qRT-PCR measurement of the MCP-1 mRNA expression levels. The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01.
Figure 2. The difference in soluble factors in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected by chemokine antibody arrays and verified by ELISA and qRT-PCR. (A) The difference in expressed chemokines in the conditioned media of sgSPOP-1 cells, sgSPOP-2 cells, and control cells, as detected through chemokine antibody array. (B) ELISA was used to verify the MCP-1 expression level in the conditioned medium. The results are presented as the mean±SD of 3 independent experiments; ** P<0.01. (C) qRT-PCR measurement of the MCP-1 mRNA expression levels. The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. Figure 3. The migration and invasion of sgSPOP-1 and sgSPOP-2 cells in response to their CM. (A) A total of 5×104 sgSPOP-1 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-1 CM; Lane 3, sgSPOP-1 CM with anti-MCP-1 (2 μg/ml). (B) A total of 5×104 sgSPOP-2 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-2 CM; Lane 3, sgSPOP-2 CM with anti-MCP-1 (2 μg/ml). The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. CM – conditioned medium.
Figure 3. The migration and invasion of sgSPOP-1 and sgSPOP-2 cells in response to their CM. (A) A total of 5×104 sgSPOP-1 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-1 CM; Lane 3, sgSPOP-1 CM with anti-MCP-1 (2 μg/ml). (B) A total of 5×104 sgSPOP-2 cells were seeded to test for migration and invasion ability. Lane 1, RMPI 1640; Lane 2, sgSPOP-2 CM; Lane 3, sgSPOP-2 CM with anti-MCP-1 (2 μg/ml). The results are presented as the mean±SD of 3 independent experiments; * P<0.05, ** P<0.01. CM – conditioned medium. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








