16 December 2021: Animal Study
Forkhead Box Protein M1 Promotes Nasopharyngeal Carcinoma Cell Tumorigenesis Possibly via the Wnt/β-Catenin Signaling Pathway
Chao Yu1ABEFG*, Hongyan Chen2AEF, Yanli Zhao1B, Yuedong Zhang1BCDDOI: 10.12659/MSM.931970
Med Sci Monit 2021; 27:e931970
Abstract
BACKGROUND: Forkhead box protein M1 (FoxM1) is an important transcription factor involved in the development and progression of various malignancies. However, its role in nasopharyngeal carcinoma (NPC) remains largely unknown. This study aimed to assess the effect of FoxM1 on NPC cell tumorigenesis as well as the underlying mechanism.
MATERIAL AND METHODS: NPC cell lines CNE-1 and CNE-2 were treated with vehicle and FoxM1 inhibitor thiostrepton or transfected with small interfering RNA. CCK-8 assay, flow cytometric assay, and Hoechst 33258 staining were performed to assess the viability, apoptosis and nuclear morphological impairment, and cell cycle, respectively. The expression of apoptosis-related caspase-3 and caspase-9 was detected by western blot analysis The tumor growth in the mouse xenograft model of NPC treated with thiostrepton or control was assessed. The expression of Wnt/β-catenin signaling proteins p27, FoxM1, S phase kinase-associated protein 2 (SKP2), and Cyclin D1 were determined both in cells and xenograft tissues by western blot analysis.
RESULTS: Inhibition of FoxM1 by thiostrepton significantly suppressed NPC cell viability, induced apoptosis, increased cell cycle arrest, impaired nuclear morphology, and reduced NPC cell-derived tumor xenograft growth. Mechanistically, inhibition or knockdown of FoxM1 inactivated the Wnt/β-catenin signaling pathway, as demonstrated by altered expression of Wnt/β-catenin signaling-related genes, including p27, SKP2, and cyclin D1, in both NPC cells and xenograft tissues.
CONCLUSIONS: We identified FoxM1 as a novel regulator of NPC cell tumorigenesis in vitro and in vivo. Targeting FoxM1 could be a promising therapeutic strategy against NPC.
Keywords: Cell Proliferation, Forkhead Box Protein M1, nasopharyngeal carcinoma, Animals, Blotting, Western, carcinogenesis, Cell Line, Tumor, Humans, Male, Mice, Nude, Nasopharyngeal Neoplasms, Neoplasm Transplantation
Background
Nasopharyngeal carcinoma (NPC) arises from the epithelium lining the nasopharynx, and although NPC is rare globally, the incidence of NPC is quite high, at approximately 1/5000 in southern China and Southeast Asia [1]. The pathogenesis of NPC is not fully understood. In general, viral infections, genetic mutations, and environmental factors are thought to contribute to the initiation, development, and progression of NPC [2–5]. NPC is a potentially curable disease. Standard treatment options for NPC include endoscopic surgery [6,7], and combined use of radiotherapy and chemotherapy [1]. However, these therapies have limitations such as adverse effects and modest risks of regional recurrence and distant metastasis [8]. Therefore, the identification of potential novel therapeutic targets for NPC treatment is urgently needed [9].
Forkhead box protein M1 (FoxM1) is a key member of the Forkhead family of transcription factors and is constitutively expressed in regenerating and proliferating mammalian cells [10,11]. FoxM1 has been reported to play a central role in cell proliferation and cell cycle progression [12–15]. Additionally, FoxM1 expression has been found to be dysregulated in a variety of cancer types. FoxM1 overexpression is responsible for cancer progression [16–19] and poor prognosis [17,19–21], suggesting that FOXM1 may act as a prognostic marker in patients with cancer [20–22].
Previous studies have found that FoxM1 is upregulated in NPC and overexpression of FoxM1 is associated with lymph node metastasis and advanced tumor stage in NPC, and FoxM1 expression may be an adverse prognostic marker in NPC patients [23,24]. However, the function of FoxM1 has not yet been determined in NPC. In this study, we examined the function of FoxM1 as well as the underlying mechanism in the regulation of NPC cell behavior by performing loss-of-function assays in NPC cells and an NPC mouse xenograft model. Our study establishes a functional association between FoxM1 and NPC development and provides valuable clues for the identification of potential therapeutic targets and the development of novel therapeutic strategies against NPC.
Material and Methods
CELL CULTURE AND REAGENTS:
The CNE-1 and CNE-2 human Epstein-Barr virus (EBV)-negative NPC cell lines were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). CNE2 cells are more highly malignant then CNE1 cells. They were used as comparisons with each other in the present study. Both cell lines were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA). The 293T cell line was purchased from Invitrogen and grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin (Hyclone, South Logan, UT, USA). All cells were maintained in an incubator with a humidified atmosphere of 5% CO2 at 37°C.
Thiostrepton (ALX-380-261-G001; ENZO Lifescience, Farmingdale, NY, USA), a FoxM1-selective inhibitor [25], was dissolved in dimethyl sulfoxide (DMSO).
ASSESSMENT OF CELL GROWTH INHIBITION:
Cells were plated in 96-well plates at a density of 4000 cells/well and kept in culture for 24 h before treatment with vehicle or thiostrepton at various concentrations ranging from 1 to 16 μmol/L. Cell viability was measured 48 and 72 h after the treatment using CCK-8 (Cell Counting Kit-8, Beyotime Biotechnology, Zhejiang, China) in a microplate reader at 450 nm according to the manufacturer’s directions. Each experiment was performed 3 times with 5 samples each time.
ASSESSMENT OF CELL CYCLE PROGRESSION AND APOPTOSIS:
The NPC cells were exposed to 8 μM thiostrepton for 72 h in culture in 6-well plates. After this treatment, the cells were washed with phosphate-buffered saline (PBS) 3 times, resuspended in binding buffer, and then stained with annexin-V/PI solution (Sungene Biotech, Tianjin, China) in the dark at room temperature. Cell cycle distribution and apoptosis were assessed using a FACScan system (BD Biosciences, San Jose, CA, USA) as previously described [26].
NUCLEAR MORPHOLOGICAL ANALYSIS:
Morphological changes in apoptotic cells were evaluated by Hoechst 33258 staining. Briefly, NPC cells were plated at 5×104 cells/ml, cultured overnight, and then treated with 8 μM thiostrepton for 72 h, followed by fixation in 4% formaldehyde for 10 min. The cells were washed twice in PBS and stained with 0.5 ml of Hoechst 33258 solution (Sigma-Aldrich, St. Louis, MO, USA) for 5 min. The staining was visualized under an inverted fluorescence microscope and imaged using a camera attached to the microscope.
SMALL INTERFERING RNA (SIRNA) TRANSFECTION:
For the siRNA-knockdown experiment, double-stranded RNA duplexes were targeted from the human FoxM1 gene (5′-GGUCCUGGACACAAUGAA UTT-3′, 5′-AUUCAUUGUGUCCAGGACCTT-3′). Scrambled siRNA (control) and FoxM1 siRNA (oligoDNA: TGCTGAGT AGATGCTGTTTCCTCCGAGTTTTGGCCACTGACTGACTCGGAG GACAGCATCTACT; R: CCTGAGTAGATGCTGTCCTCCGAG TCAGTCAGTGGCCAAAACTCGGAGGAAACAGCATCTACTC) were obtained from Invitrogen. NPC cells were plated in 6-cm dishes, cultured overnight, and then transfected with siRNAs using Lipofectamine 2000 reagent (Invitrogen) as previously described [27]. Upon reaching 90% confluency, the cells were treated with 8 μM thiostrepton for 72 h.
WESTERN BLOT ASSAY:
NPC cells were collected and lysed in ice-cold lysis buffer (TaKaRa) to obtain cellular lysates. The BCA method was applied to measure the total protein concentrations. For western blot analysis, 5–10 μg of protein was subjected to 10% SDS-PAGE before transfer to nitrocellulose membranes (Millipore Biotechnology, Billerica, MA, USA). After blocking in 5% skim milk in Tris-buffered saline containing Tween-20 (TBST), the membranes were incubated in solutions of primary antibodies against FoxM1 (AT2098A; Abgent, San Diego, CA, USA), GAPDH (M121107; Huaan Biotechnology Company, Gansu, China), cyclin D1 (YT1173), p27 (YT3499), caspase-3 (YT0656), caspase-9 (YT0662, ImmunoWay Biotechnology Company, Plano, TX, USA) and SKP2 (sc-74477, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. The membranes were then washed 3 times with TBST before incubation with solutions of horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature, followed by an additional 3 washes with TBST. Protein bands were detected using Hybond-enhanced chemiluminescence. GAPDH was used as an internal control.
ANIMAL STUDIES:
BALB/CA nude mice (male, 4–5 weeks old) were obtained from Shanghai Institute of Material Medicine (Chinese Academy of Science, Shanghai, China). To induce tumor growth in vivo, the mice were anesthetized with isoflurane, and 2×106 NPC cells suspended in 200 μl PBS were injected into a flank of each mouse. One week later, the mice were randomly assigned to the thiostrepton group (n=6), in which DMSO-dissolved thiostrepton (200 mg/kg) was administered intraperitoneally every 7 days as previously described, or the vehicle group (n=6), in which the same volume of DMSO was administered every 7 days [23]. The dosage of thiostrepton was determined based on previous reports [23,28] and our preliminary research. Mouse tumor xenografts were harvested 42 days after the inoculation, and tumor volumes were calculated as: tumor volume=(length×width2)/2 [28]. All experiments involving animals were carried out in accordance with the institutional guidelines and approved by University’s Animal Use and Care Committee.
STATISTICAL ANALYSIS:
All data were collected from at least 3 experiments and are presented as mean±standard deviation (SD). SPSS version 17.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses, with
Results
INHIBITION OF FOXM1 SUPPRESSES NPC CELL VIABILITY IN VITRO:
To examine the possible role of FoxM1 in NPC development, we examined the effect of thiostrepton, a transcriptional inhibitor of FoxM1 [29], on NPC development. As shown in Figure 1A, inhibition of FoxM1 by thiostrepton markedly reduced CNE-1 cell viability in a dose- and time-dependent manner, with a growth inhibition rate of up to 85% at the dose of 16 μM. The IC50 at 48 h and 72 h in CNE-1 were 6.6 μM and 4.2 μM, respectively. Similar results were also found in CNE-2 cells (Figure 1B). The IC50 at 48 h and 72 h in CNE-2 were 7.3 μM and 5.1 μM, respectively. These findings indicate that FoxM1 may play a promotive role in NPC cell proliferation and growth.
INHIBITION OF FOXM1 EXPRESSION INDUCES NPC CELL APOPTOSIS AND NUCLEAR MORPHOLOGICAL IMPAIRMENT IN VITRO:
We next sought to determine the role of thiostrepton in NPC cell apoptosis, a key process in blocking the development and progression of NPC. As shown in Figure 2A and 2B, inhibition of FoxM1 by thiostrepton significantly increased apoptotic rates in CNE-1 and CNE-2 cells, compared to vehicle-treated cells (4.70%+13.48%=18.18% vs 1.93%+3.98%=5.91%; 3.86%+12.01%=15.87% vs 1.74%+3.62%=5.36%, respectively). The proapoptotic function of thiostrepton was further confirmed by thiostrepton-induced upregulation of cleaved caspase-3, an important pro-apoptotic marker (Figure 2C). Consistently, treatment with thiostrepton appreciably impaired the nuclear morphology in CNE-1 and CNE-2 cells, as evidenced by thiostrepton-caused nuclear condensation and fragmentation (Figure 2D, 2E), a characteristic feature of apoptotic cells. Collectively, our data suggest that inhibition of FoxM1 can induce alterations in the nuclear structure of NPC cells, thus leading to increased cell apoptosis.
INHIBITION OF FOXM1 INCREASES NPC CELL CYCLE ARREST IN VITRO:
To investigate whether thiostrepton has an effect on NPC cell cycle progression that is essential for cell division and proliferation, we performed a flow cytometry-based cell cycle analysis. As shown in Figure 3A–3C, thiostrepton treatment dramatically increased the population of CNE-1 and CNE-2 cells in G1 phase while it significantly decreased the population of both cell lines in S phase, suggesting that NPC cell cycle progression was retarded at G1/S transition by thiostrepton. These data indicate that inhibition of FoxM1 by thiostrepton induces NPC cell cycle arrest, thus attenuating NPC cell growth and proliferation.
INHIBITION OF FOXM1 DIMINISHES NPC CELL-DERIVED TUMOR GROWTH IN VIVO:
For further in vivo investigation of the role of FoxM1 in NPC development and progression, we established a mouse xenograft model of NPC treated with thiostrepton. The results revealed that administration of thiostrepton significantly diminished CNE-2 cell-derived tumor growth compared to the vehicle-treated group (Figure 4A), as evidenced by time-dependent reductions in tumor size and weight following thiostrepton treatment (Figure 4B, 4C). These findings strongly suggest that upregulation of FoxM1 plays a key role in NPC development and progression.
INHIBITION OF FOXM1 INACTIVATES THE WNT/β-CATENIN SIGNALING PATHWAY:
Studies have shown that FoxM1 functions through Wnt/beta-catenin signaling in several cancer types [30–32]. Moreover, overactivation of Wnt/β-catenin signaling has been shown to contribute to cancer development [33]. To explore the regulatory mechanism underlying the role of FoxM1 in NPC development, we examined the effect of FoxM1 inhibition using thiostrepton or FoxM1 knockdown on Wnt/β-catenin signaling activity. As shown in Figure 5A–5C, inhibition of FoxM1 by thiostrepton or silencing of FoxM1 by FoxM1 siRNA in CNE-1 and CNE-2 cells remarkably decreased protein expression of downstream target genes of Wnt/β-catenin signaling like Skp2 and cyclin D1 while enhancing the expression of p27, which has been reported to be negatively correlated with Wnt/β-catenin signaling activity [34]. These findings were further confirmed by our in vivo study, as evidenced by downregulation of Skp2 and cyclin D1 and upregulation of p27 in NPC xenograft tissues with FoxM1 inhibition or knockdown (Figure 5D, 5E). Collectively, these data suggest that FoxM1 promotes NPC development, at least partially through activating the Wnt/β-catenin signaling pathway.
Discussion
In the present study, we found that inhibition of FoxM1 significantly suppressed NPC cell viability, induced apoptosis, increased cell cycle arrest, impaired nuclear morphology, and reduced NPC cell-derived tumor xenograft growth. Moreover, inhibition or knockdown of FoxM1 downregulated the expression of Skp2 and cyclin D1 and upregulated the expression of p27, which are Wnt/β-catenin signaling-related proteins.
It is well established that FoxM1 is upregulated during early cancer development [35–37]. The involvement of FOXM1 in initiation of tumorigenesis is closely associated with its role in cell cycle progression, proliferation, and apoptosis. Overexpression of FoxM1 has been shown to be a contributing factor to the pathogenesis of a variety of cancers [38,39]. Our results showed that thiostrepton treatment significantly inhibited NPC cell tumorigenesis by reducing NPC cell viability, inducing apoptosis among NPC cells, and causing arrest of cell cycle progression. Moreover, thiostrepton reduced NPC xenograft growth. These data were consistent with previous reports about the effects of FoxM1 on cancers [21,22,25], suggesting a key role for FoxM1 in NPC development.
The Wnt/beta-catenin signaling pathway is a main regulator of development in all animals and has been shown to have roles in many diseases, including cancer [40]. Studies have shown that FoxM1 functions through Wnt/beta-catenin signaling in cancers [30–32]. FoxM1 can be activated by Wnt and promotes beta-catenin nuclear localization in tumor cells, thus regulating the expression of downstream genes. FoxM1 knockdown accelerates cellular senescence in gastric cancer partially via p27Kip1 [41]. In addition, FoxM1 mediates astrocyte transformation in glioblastoma through activating Akt and promoting expression of survivin, Skp2, and cyclin D1 [42–44]. p27Kip1, survivin, Skp2, and cyclin D1 are all downstream genes of the Wnt/beta-catenin signaling pathway. We also found that thiostrepton- or siRNA-mediated silencing of FoxM1 markedly altered the protein expression of important genes of the Wnt/beta-catenin signaling pathway, including cyclin D1, p27, and SKP2. These data are highly consistent with the findings of previous studies [17,41–44], suggesting that the underlying molecular mechanism of the effects of FoxM1 on NPC cells are similar to those in other tumor cells.
The present study has some limitations. First, only 2 EBV-negative cell lines were used, and whether FoxM1 shows similar function in EBV-positive cells or whether EBV infection will affect the functions of FoxM1 in NPC remains unclear. Second, we did not examine the effect of thiostrepton on other NPC cell behaviors, such as cell migration or invasion and colony-forming ability. Third, we also did not determine whether FoxM1 is sufficient to promote NPC development and progression. Therefore, further investigation is needed to address these issues by examining the role of FoxM1 overexpression in the regulation of the tumorigenic behavior of NPC cells, including cell viability, apoptosis, cell cycle, migration, and colony formation.
Conclusions
We identified FoxM1 as a novel regulator of NPC cell tumorigenesis in vitro and in vivo. As an inhibitor of FoxM1, thiostrepton appears to be an effective therapeutic agent for treatment of NPC. Preclinical and clinical data are required to further verify the therapeutic role of thiostrepton in NPC.
Figures
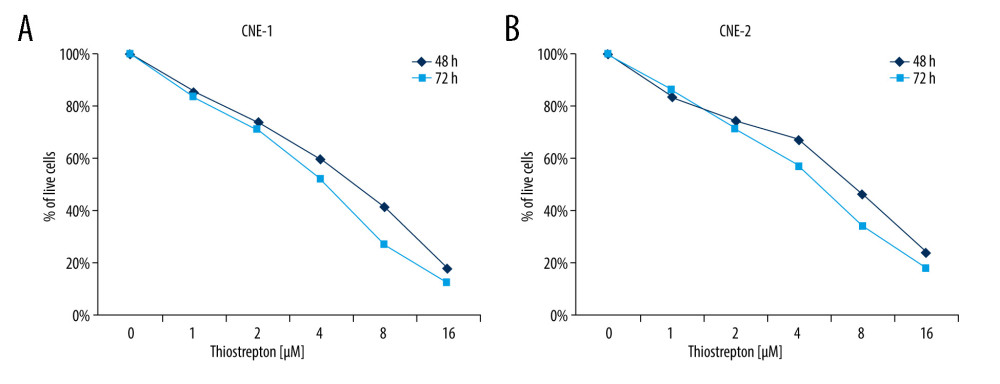 Figure 1. Effect of inhibition of FoxM1 on NPC cell viabilityCNE-1 (A) and CNE-2 cells (B) were exposed to thiostrepton at various concentrations as indicated for 48 and 72 h. Cell viability was examined using the CK-8 assay.
Figure 1. Effect of inhibition of FoxM1 on NPC cell viabilityCNE-1 (A) and CNE-2 cells (B) were exposed to thiostrepton at various concentrations as indicated for 48 and 72 h. Cell viability was examined using the CK-8 assay. 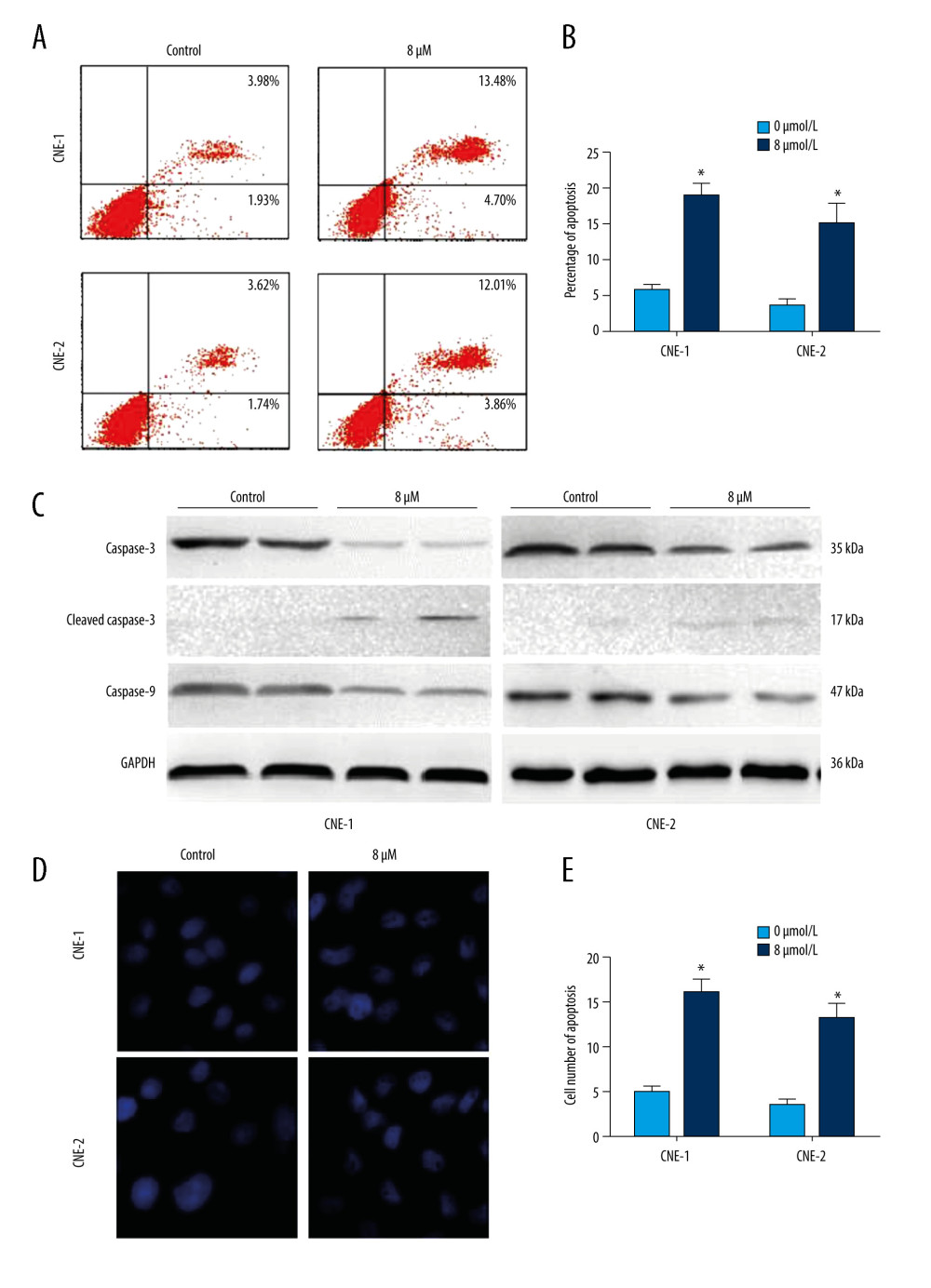 Figure 2. Effect of inhibition of FoxM1 on NPC cell apoptosis and nuclear morphology(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM of thiostrepton for 72 h, and then labeled with annexin-V and propidium iodide (PI) for an apoptosis assay using flow cytometry. (B) Quantification of data in A. (C) The cells were treated as in A for analysis by a western blot assay for protein expression of apoptotic markers caspases-3 and -9 in CNE-1 and CNE-2 cells. GAPDH was used as an internal control. (D) Thiostrepton-treated cells were stained with Hoechst 33258, and their nuclear morphology was observed under an immunofluorescence microscope. (E) Quantification of nuclear morphological impairment shown in D. Quantitative data are expressed as the means±SD for individual groups of cells from 3 separate experiments. * P<0.05 compared to the controls. NPC – nasopharyngeal carcinoma; FoxM1 – forkhead box M1.
Figure 2. Effect of inhibition of FoxM1 on NPC cell apoptosis and nuclear morphology(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM of thiostrepton for 72 h, and then labeled with annexin-V and propidium iodide (PI) for an apoptosis assay using flow cytometry. (B) Quantification of data in A. (C) The cells were treated as in A for analysis by a western blot assay for protein expression of apoptotic markers caspases-3 and -9 in CNE-1 and CNE-2 cells. GAPDH was used as an internal control. (D) Thiostrepton-treated cells were stained with Hoechst 33258, and their nuclear morphology was observed under an immunofluorescence microscope. (E) Quantification of nuclear morphological impairment shown in D. Quantitative data are expressed as the means±SD for individual groups of cells from 3 separate experiments. * P<0.05 compared to the controls. NPC – nasopharyngeal carcinoma; FoxM1 – forkhead box M1. 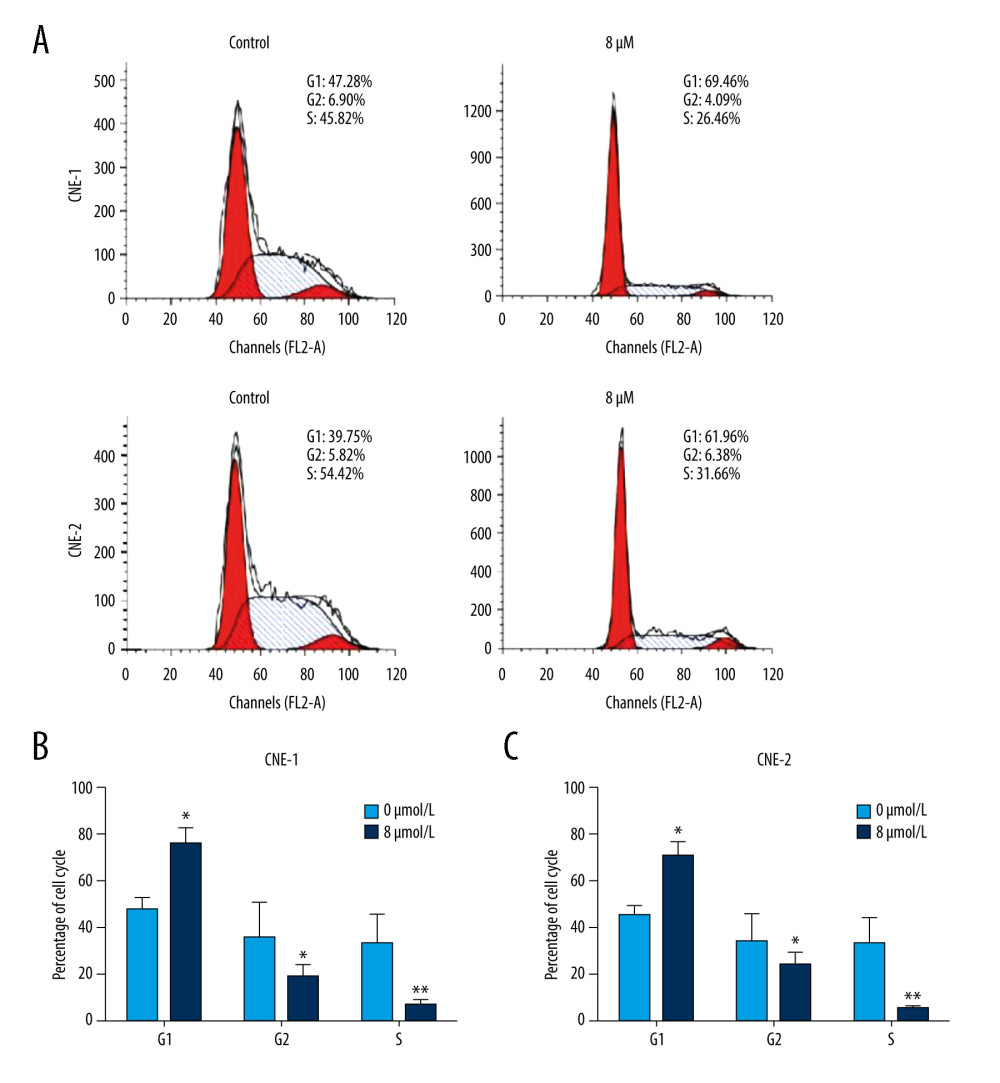 Figure 3. Effect of inhibition of FoxM1 on NPC cell cycle progression(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then a flow cytometric assay was performed to analyze the cell cycle distribution based on the DNA content in the cells. (B) Quantification of data for CNE-1 cells in A (n=3). (C) Quantification of data for CNE-2 cells in A (n=3). * P<0.05, ** P<0.01 compared to the control.
Figure 3. Effect of inhibition of FoxM1 on NPC cell cycle progression(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then a flow cytometric assay was performed to analyze the cell cycle distribution based on the DNA content in the cells. (B) Quantification of data for CNE-1 cells in A (n=3). (C) Quantification of data for CNE-2 cells in A (n=3). * P<0.05, ** P<0.01 compared to the control. 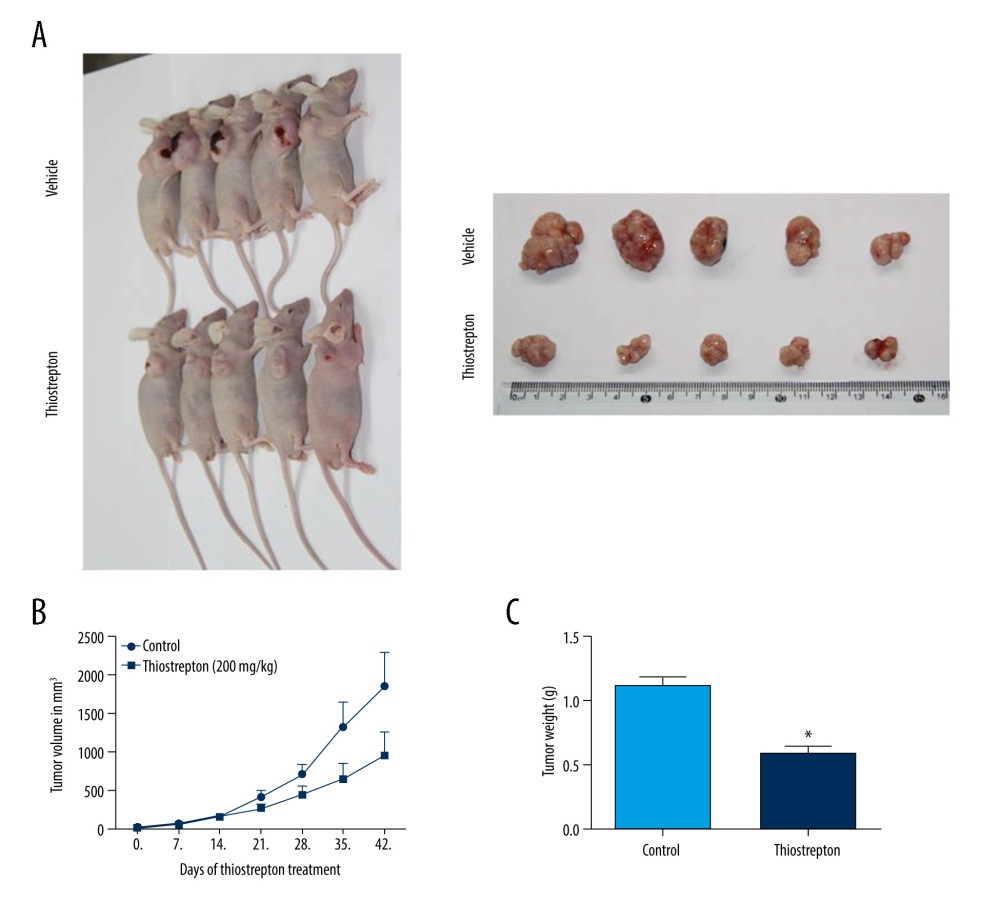 Figure 4. Effect of inhibition of FoxM1 on NPC cell-derived tumor growth(A) Xenografts were established by subcutaneous injection of 5×106 CNE-2 cells into the right flank of nude mice. At 42 days after intraperitoneal administration of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the xenografts were harvested. (B) Tumor volume was measured every 7 days. (C) Tumor xenografts were weighed immediately after being excised. * P<0.05 compared to the control.
Figure 4. Effect of inhibition of FoxM1 on NPC cell-derived tumor growth(A) Xenografts were established by subcutaneous injection of 5×106 CNE-2 cells into the right flank of nude mice. At 42 days after intraperitoneal administration of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the xenografts were harvested. (B) Tumor volume was measured every 7 days. (C) Tumor xenografts were weighed immediately after being excised. * P<0.05 compared to the control. 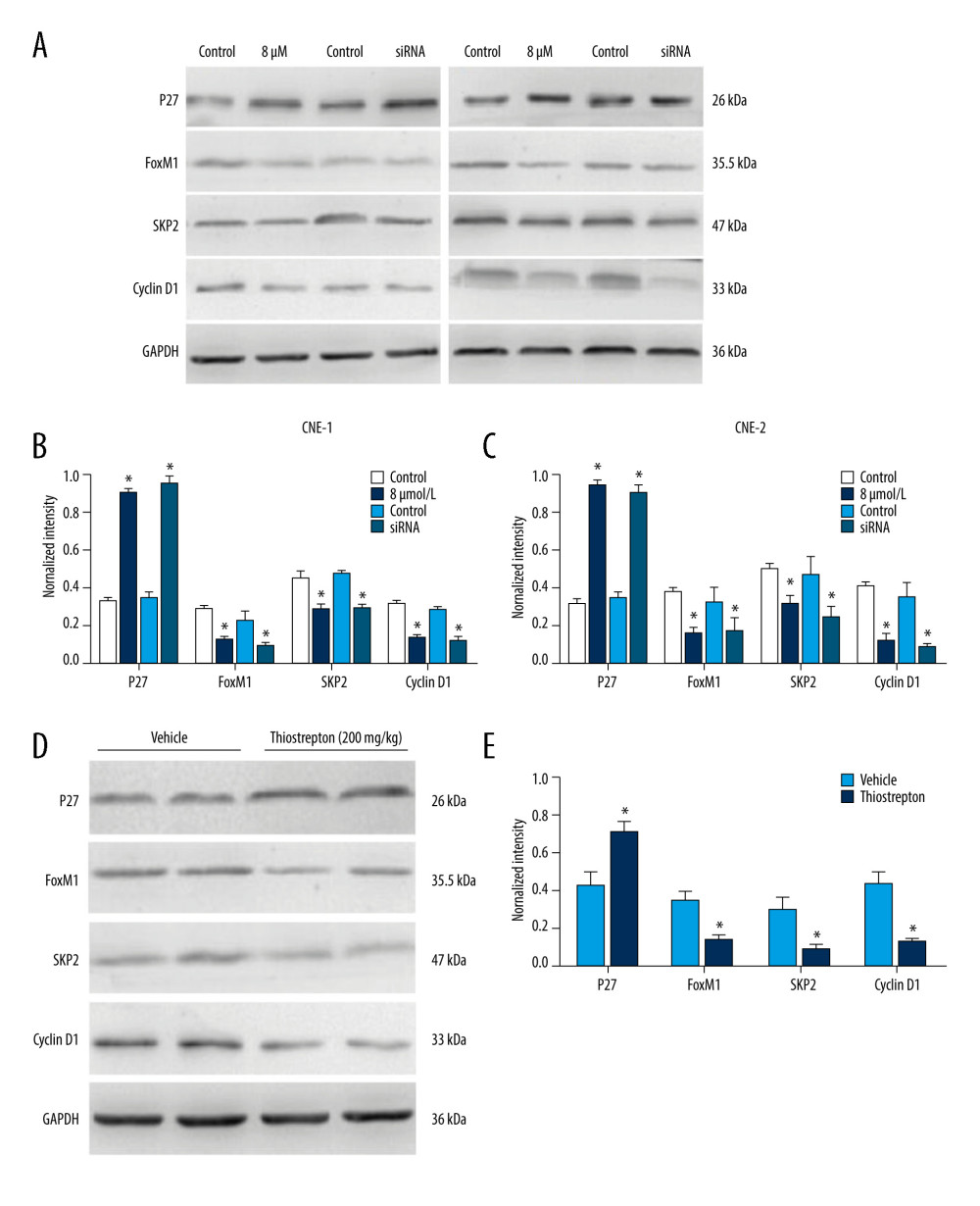 Figure 5. Effect of inhibition of FoxM1 on Wnt/β-catenin signaling activity in NPC cells and NPC xenograft tissues(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. (B) Quantitative data for relative protein expression in CNE-1 cells. (C) Quantitative data for relative protein expression in CNE-2 cells. (D) At 42 days after intraperitoneal injection of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the NPC xenografts were harvested. Tissue lysates were collected, and the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. Protein expression levels were normalized to the expression level of GAPDH, and the results were then further quantified. (E) Quantitative data for relative protein expression in D. * P<0.05 compared to the control.
Figure 5. Effect of inhibition of FoxM1 on Wnt/β-catenin signaling activity in NPC cells and NPC xenograft tissues(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. (B) Quantitative data for relative protein expression in CNE-1 cells. (C) Quantitative data for relative protein expression in CNE-2 cells. (D) At 42 days after intraperitoneal injection of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the NPC xenografts were harvested. Tissue lysates were collected, and the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. Protein expression levels were normalized to the expression level of GAPDH, and the results were then further quantified. (E) Quantitative data for relative protein expression in D. * P<0.05 compared to the control. References
1. Wei WI, Sham JS, Nasopharyngeal carcinoma: Lancet, 2005; 365; 2041-54
2. Liu Z, Luo W, Zhou Y, Potential tumor suppressor NESG1 as an unfavorable prognosis factor in nasopharyngeal carcinoma: PLoS One, 2011; 6; e27887
3. Alajez NM, Shi W, Hui AB, Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98: Cell Death Dis, 2010; 1; e85
4. Wang S, Fang W, Increased expression of hepatoma-derived growth factor correlates with poor prognosis in human nasopharyngeal carcinoma: Histopathology, 2011; 58; 217-24
5. Fang W, Li X, Jiang Q, Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of Southern China: J Transl Med, 2008; 6; 32
6. Huang Y, Qiu QH, Endoscopic surgery for early-stage nasopharyngeal carcinoma: A justified initial option: Acta Otolaryngol, 2017; 137; 1194-98
7. Galletti B, Gazia F, Freni F, Endoscopic sinus surgery with and without computer assisted navigation: A retrospective study: Auris Nasus Larynx, 2019; 46; 520-25
8. Chua DT, Ma J, Sham JS, Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: A pooled data analysis of two phase III trials: J Clin Oncol, 2005; 23; 1118-24
9. Razak AR, Siu LL, Liu FF, Nasopharyngeal carcinoma: The next challenges: Eur J Cancer, 2010; 46; 1967-78
10. Costa RH, Kalinichenko VV, Holterman AX, Wang X, Transcription factors in liver development, differentiation, and regeneration: Hepatology, 2003; 38; 1331-47
11. Ye H, Kelly TF, Samadani U, Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues: Mol Cell Biol, 1997; 17; 1626-41
12. Wierstra I, Alves J, FOXM1, a typical proliferation-associated transcription factor: Biol Chem, 2007; 388; 1257-74
13. Fu Z, Malureanu L, Huang J, Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression: Nat Cell Biol, 2008; 10; 1076-82
14. Chen YJ, Dominguez-Brauer C, Wang Z, A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1: J Biol Chem, 2009; 284; 30695-707
15. Anders L, Ke N, Hydbring P, A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells: Cancer Cell, 2011; 20; 620-34
16. Chan DW, Yu SY, Chiu PM, Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis: J Pathol, 2008; 215; 245-52
17. Liu M, Dai B, Kang SH, FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells: Cancer Res, 2006; 66; 3593-602
18. Hui MK, Chan KW, Luk JM, Cytoplasmic Forkhead box M1 (FoxM1) in esophageal squamous cell carcinoma significantly correlates with pathological disease stage: World J Surg, 2012; 36; 90-97
19. Jiang LZ, Wang P, Deng B, Overexpression of Forkhead Box M1 transcription factor and nuclear factor-kappaB in laryngeal squamous cell carcinoma: A potential indicator for poor prognosis: Hum Pathol, 2011; 42; 1185-93
20. Yu J, Deshmukh H, Payton JE, Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor: Clin Cancer Res, 2011; 17; 1924-34
21. Priller M, Poschl J, Abrao L, Expression of FoxM1 is required for the proliferation of medulloblastoma cells and indicates worse survival of patients: Clin Cancer Res, 2011; 17; 6791-801
22. Chen H, Yang C, Yu L, Adenovirus-mediated RNA interference targeting FOXM1 transcription factor suppresses cell proliferation and tumor growth of nasopharyngeal carcinoma: J Gene Med, 2012; 14; 231-40
23. Ahmed M, Uddin S, Hussain AR, FoxM1 and its association with matrix metalloproteinases (MMP) signaling pathway in papillary thyroid carcinoma: J Clin Endocrinol Metab, 2012; 97; E1-13
24. Huang PY, Li Y, Luo DH, Expression of Aurora-B and FOXM1 predict poor survival in patients with nasopharyngeal carcinoma: Strahlenther Onkol, 2015; 191; 649-55
25. Kwok JM, Myatt SS, Marson CM, Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression: Mol Cancer Ther, 2008; 7; 2022-32
26. Wlodkowic D, Skommer J, Darzynkiewicz Z, Flow cytometry-based apoptosis detection: Methods Mol Biol, 2009; 559; 19-32
27. Hussain AR, Al-Jomah NA, Siraj AK, Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells: Cancer Res, 2007; 67; 3888-97
28. Chiu WT, Huang YF, Tsai HY, FOXM1 confers to epithelial-mesenchymal transition, stemness and chemoresistance in epithelial ovarian carcinoma cells: Oncotarget, 2015; 6; 2349-65
29. Uddin S, Bavi P, Siraj AK, Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma: Endocr Relat Cancer, 2010; 17; 191-202
30. Zhang N, Wei P, Gong A, FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis: Cancer Cell, 2011; 20; 427-42
31. Hwang SM, Lee HJ, Jung JH, Inhibition of Wnt3a/FOXM1/beta-catenin axis and activation of GSK3beta and caspases are critically involved in apoptotic effect of Moracin D in breast cancers: Int J Mol Sci, 2018; 19; 2681
32. Pratheeshkumar P, Divya SP, Parvathareddy SK, FoxM1 and beta-catenin predicts aggressiveness in Middle Eastern ovarian cancer and their co-targeting impairs the growth of ovarian cancer cells: Oncotarget, 2018; 9; 3590-604
33. Krishnamurthy N, Kurzrock R, Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors: Cancer Treat Rev, 2018; 62; 50-60
34. Gustin SE, Hogg K, Stringer JM, WNT/beta-catenin and p27/FOXL2 differentially regulate supporting cell proliferation in the developing ovary: Dev Biol, 2016; 412; 250-60
35. Francis RE, Myatt SS, Krol J, FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer: Int J Oncol, 2009; 35; 57-68
36. Costa RH, Kalinichenko VV, Major ML, Raychaudhuri P, New and unexpected: forkhead meets ARF: Curr Opin Genet Dev, 2005; 15; 42-48
37. Kretschmer C, Sterner-Kock A, Siedentopf F, Identification of early molecular markers for breast cancer: Mol Cancer, 2011; 10; 15
38. Li Q, Zhang N, Jia Z, Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression: Cancer Res, 2009; 69; 3501-9
39. Nakamura S, Hirano I, Okinaka K, The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia: Carcinogenesis, 2010; 31; 2012-21
40. Nusse R, Clevers H, Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities: Cell, 2017; 169; 985-99
41. Zeng J, Wang L, Li Q, FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1: J Pathol, 2009; 218; 419-27
42. Dai B, Pieper RO, Li D, FoxM1B regulates NEDD4-1 expression, leading to cellular transformation and full malignant phenotype in immortalized human astrocytes: Cancer Res, 2010; 70; 2951-61
43. Zhang Y, Zhang N, Dai B, FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells: Cancer Res, 2008; 68; 8733-42
44. Dai B, Kang SH, Gong W, Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells: Oncogene, 2007; 26; 6212-19
Figures
 Figure 1. Effect of inhibition of FoxM1 on NPC cell viabilityCNE-1 (A) and CNE-2 cells (B) were exposed to thiostrepton at various concentrations as indicated for 48 and 72 h. Cell viability was examined using the CK-8 assay.
Figure 1. Effect of inhibition of FoxM1 on NPC cell viabilityCNE-1 (A) and CNE-2 cells (B) were exposed to thiostrepton at various concentrations as indicated for 48 and 72 h. Cell viability was examined using the CK-8 assay. Figure 2. Effect of inhibition of FoxM1 on NPC cell apoptosis and nuclear morphology(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM of thiostrepton for 72 h, and then labeled with annexin-V and propidium iodide (PI) for an apoptosis assay using flow cytometry. (B) Quantification of data in A. (C) The cells were treated as in A for analysis by a western blot assay for protein expression of apoptotic markers caspases-3 and -9 in CNE-1 and CNE-2 cells. GAPDH was used as an internal control. (D) Thiostrepton-treated cells were stained with Hoechst 33258, and their nuclear morphology was observed under an immunofluorescence microscope. (E) Quantification of nuclear morphological impairment shown in D. Quantitative data are expressed as the means±SD for individual groups of cells from 3 separate experiments. * P<0.05 compared to the controls. NPC – nasopharyngeal carcinoma; FoxM1 – forkhead box M1.
Figure 2. Effect of inhibition of FoxM1 on NPC cell apoptosis and nuclear morphology(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM of thiostrepton for 72 h, and then labeled with annexin-V and propidium iodide (PI) for an apoptosis assay using flow cytometry. (B) Quantification of data in A. (C) The cells were treated as in A for analysis by a western blot assay for protein expression of apoptotic markers caspases-3 and -9 in CNE-1 and CNE-2 cells. GAPDH was used as an internal control. (D) Thiostrepton-treated cells were stained with Hoechst 33258, and their nuclear morphology was observed under an immunofluorescence microscope. (E) Quantification of nuclear morphological impairment shown in D. Quantitative data are expressed as the means±SD for individual groups of cells from 3 separate experiments. * P<0.05 compared to the controls. NPC – nasopharyngeal carcinoma; FoxM1 – forkhead box M1. Figure 3. Effect of inhibition of FoxM1 on NPC cell cycle progression(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then a flow cytometric assay was performed to analyze the cell cycle distribution based on the DNA content in the cells. (B) Quantification of data for CNE-1 cells in A (n=3). (C) Quantification of data for CNE-2 cells in A (n=3). * P<0.05, ** P<0.01 compared to the control.
Figure 3. Effect of inhibition of FoxM1 on NPC cell cycle progression(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then a flow cytometric assay was performed to analyze the cell cycle distribution based on the DNA content in the cells. (B) Quantification of data for CNE-1 cells in A (n=3). (C) Quantification of data for CNE-2 cells in A (n=3). * P<0.05, ** P<0.01 compared to the control. Figure 4. Effect of inhibition of FoxM1 on NPC cell-derived tumor growth(A) Xenografts were established by subcutaneous injection of 5×106 CNE-2 cells into the right flank of nude mice. At 42 days after intraperitoneal administration of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the xenografts were harvested. (B) Tumor volume was measured every 7 days. (C) Tumor xenografts were weighed immediately after being excised. * P<0.05 compared to the control.
Figure 4. Effect of inhibition of FoxM1 on NPC cell-derived tumor growth(A) Xenografts were established by subcutaneous injection of 5×106 CNE-2 cells into the right flank of nude mice. At 42 days after intraperitoneal administration of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the xenografts were harvested. (B) Tumor volume was measured every 7 days. (C) Tumor xenografts were weighed immediately after being excised. * P<0.05 compared to the control. Figure 5. Effect of inhibition of FoxM1 on Wnt/β-catenin signaling activity in NPC cells and NPC xenograft tissues(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. (B) Quantitative data for relative protein expression in CNE-1 cells. (C) Quantitative data for relative protein expression in CNE-2 cells. (D) At 42 days after intraperitoneal injection of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the NPC xenografts were harvested. Tissue lysates were collected, and the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. Protein expression levels were normalized to the expression level of GAPDH, and the results were then further quantified. (E) Quantitative data for relative protein expression in D. * P<0.05 compared to the control.
Figure 5. Effect of inhibition of FoxM1 on Wnt/β-catenin signaling activity in NPC cells and NPC xenograft tissues(A) CNE-1 and CNE-2 cells were exposed to vehicle or 8 μM thiostrepton for 72 h, and then the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. (B) Quantitative data for relative protein expression in CNE-1 cells. (C) Quantitative data for relative protein expression in CNE-2 cells. (D) At 42 days after intraperitoneal injection of vehicle or thiostrepton at a dose of 200 mg/kg body weight, the NPC xenografts were harvested. Tissue lysates were collected, and the protein expression of Wnt/β-catenin signaling-related proteins was evaluated by western blotting, as indicated. Protein expression levels were normalized to the expression level of GAPDH, and the results were then further quantified. (E) Quantitative data for relative protein expression in D. * P<0.05 compared to the control. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








