10 January 2022: Clinical Research
Expression of Long Non-Coding RNA (lncRNA) SNHG5 in Patients with Refractory Diabetic Macular Edema and Its Regulatory Mechanism
Junwen He1ABEFG*, Zhang RuiDOI: 10.12659/MSM.932996
Med Sci Monit 2022; 28:e932996
Abstract
BACKGROUND: The aim of this study was to assess use of lncRNAs as biomarkers in serum and aqueous humor of patients with diabetic macular edema (DME).
MATERIAL AND METHODS: Optical coherence tomography and fundus photography were used to analyze the retinal features of the patients. RT-qPCR was used to analyze the differential expression of lncRNA snhg5 in patients who have idiopathic macular hole (MH), DME, or refractory DME. The relationship between SNHG5 and the clinical characteristics of the patients was analyzed. The effect of SNHG5 on the hyperplasia and apoptosis of human retino-microvascular endothelial cells (HRMECs) and its mechanism were analyzed in vitro.
RESULTS: Patients with idiopathic MH developed retinal nerve epithelium rupture and retinal fundus thickening, and patients with DME or refractory DME showed significant macular edema with hemorrhaging. The refractory DME patients improved after treatment but still showed significant macular edema and multiple laser scarring. SNHG5 expression was not only low in the atrial fluid and plasma in DME patients, but also lower in the refractory DME group compared to the idiopathic MH patients. SNHG5 expression in the aqueous humor and plasma was negatively correlated with disease duration, body mass index, and levels of fasting blood glucose, glycated hemoglobin, proteinuria, and glycosuria. In the in vitro experiments, SNHG5 expression was significantly downregulated in high glucose-induced HMECs. After SNHG5 overexpression, cell proliferation, angiogenesis, and VEGF-A protein levels were distinctly downregulated.
CONCLUSIONS: SNHG5 correlates with the development of DME and is a potential target for therapy.
Keywords: Diabetic Retinopathy, Long Non-Coding RNA SNHG5, Human, Retinal Neovascularization, Tomography, Optical Coherence, Aqueous Humor, endothelial cells, Female, Fluorescein Angiography, Gene Expression Profiling, Humans, Macular edema, Male, Retina, Retinal Vessels, Vascular Endothelial Growth Factor A, Visual Acuity
Background
Diabetic macular edema (DME) is one of the main causes of vision loss in diabetic patients [1], and the critical factor is the increase of protein level of vascular endothelial growth factor (VEGF)-A [2,3]. The upregulation of VEGF-A can disrupt the retinal barrier, causing chronic damage to the neurovascular structure of the retina, leading to vision loss [4,5]. Development of DME in patients with DME can be effectively controlled by focal/grid laser treatment, vitrectomy accompanied by anti-VEGF treatment, and other combined treatments [6,7], as well as by good blood glucose control, but with the complications of DME, the prognosis is worse [8]. Inhibition of VEGF-A expression is highly beneficial for treatment of DME [9,10]. Therefore, controlling blood glucose, blood pressure, and lipids are still the basic methods of reducing DME [11,12]. Refractory DME is a recurrent DME despite repeated nonsurgical treatments and surgical treatments [13]. Previously, triple therapy including vitrectomy, IVTA, and macular focal laser photocoagulation has been reported to be effective [14]. At present, anti-EGF treatments and anti-EGF combination treatments are the main therapies for refractory DME [15].
Long non-coding RNAs (lncRNAs) are non-coding RNAs with a length of more than 200 nt [16]. In recent years, there have been many studies of the molecular mechanism of MALAT1 [17], AK139328 [18], and NEAT1 [19] in the development of diabetes. Wei et al confirmed that lncRNA SNHG5 is involved in type 2 diabetes [20]. Knockdown of SNHG5 inhibits VSMC proliferation and migration and promotes apoptosis in abdominal aortic aneurysm [21]. Recent study have shown that lncRNA SNHG5 is underexpressed in patients with Kawasaki disease [22]. Although it is a critical regulatory gene in many diseases, such as colorectal cancer [23], gastric cancer [24], endometrial cancer [25], and acute myeloid leukemia [26], lncRNA SNHG5 has not been assessed in DME studies. In addition, in vitro molecular studies have demonstrated that lncRNA regulation of VEGF-A promotes apoptosis and angiogenesis in HRMECs cells induced by high glucose levels [27,28].
The aim of the study was to explore the biological mechanism of DME therapy by analyzing the clinical relationship between lncRNA SNHG5 and disease course in DME patients and to assess the molecular regulation mechanism of lncRNA SNHG5 in HRMECs caused by high glucose induction.
Material and Methods
MATERIALS:
Antibodies against VEGF and GAPDH were purchased from Abcam (Cambridge, UK). The overexpression of SNHG5 plasmid (ov-SNHG5), negative control (ov-NC), and primers for RT-qPCR were synthesized by Sangon Biotech (Shanghai, China). The small-molecule RNA (siRNA) sequences targeting SNHG5 were as follows:
The primer sequences for the RT-qPCR assay were:
STUDY SUBJECTS:
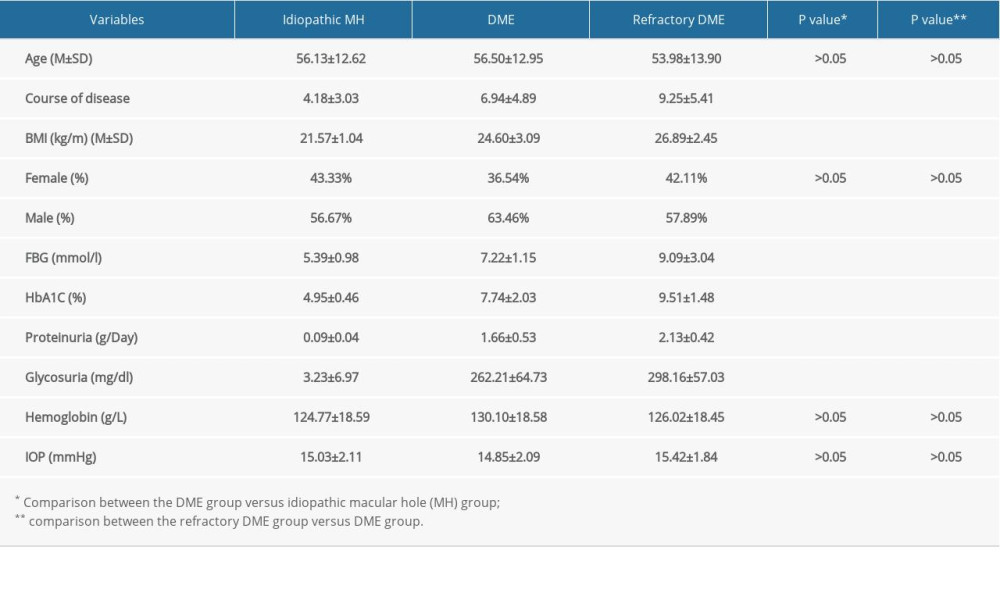
From April 2019 to March 2021, we selected 109 patients diagnosed with DME from the population of outpatients at Wuhan Aier Eye Hospital, which included 66 men and 43 women, aged 30–85 years, and the range of disease duration was 0.5–21 years. All patients underwent a detailed medical examination and ophthalmoscopy. According to the guidelines of the 2002 Academic Conference on Ocular Fundus Diseases [29], these 109 patients were divided into 2 groups. The DME group (n=52) included 33 males and 19 females, whose average age was 56.50±12.95 years and the average disease duration was 6.94±4.89 years. The refractory DME group (n=57) included 33 males and 24 females, whose average age was 53.98±13.90 years and the average duration of disease was 9.25±5.41 years. Exclusion criteria were: diabetic acute complications such as diabetic ketoacidosis and diabetic hyperosmolar coma, patients showing severe stress, patients with acute or chronic infections, and patients with liver disease. The control group (n=30) consisted of idiopathic MH patients (17 males and 13 females) matched with the study groups for age, sex ratio, and body mass index (BMI). The mean age was 56.13±12.62 years old. The examination of idiopathic MH patients excluded hypertension and endocrine and metabolic diseases, such as heart, liver, and kidney diseases. All the patients or their families signed the informed consent form, and the experiment was approved by Aier Eye Hospital, in accordance with the guidelines of the Helsinki Declaration [30].
ETHICS APPROVAL AND CONSENT TO PARTICIPATE:
The study was approved by the Ethics Committee of Wuhan Aier Eye Hospital (No. 2021IRBLW01). Written informed consent was obtained from all patients.
MEASUREMENTS:
We measured the height and weight of patients at admission, and calculated BMI as weight/height2 (kg/m2). We recorded patients’ age, medical history, sex, course of diabetes, intraocular pressure (IOP), blood glucose control, and other parameters. We used a Beckman automatic biochemical analyzer (Cobas c311; Roche, Germany) to measure fasting blood glucose (FBG), glycated hemoglobin (HbA1c), hemoglobin (HB), proteinuria, and glycosuria. We used optical coherence tomography (OCT; RTVue XR; Optovue, Inc; Fremont, USA) with RTVue XR software (version: 2018.1.0.43) to analyze retinal thickness, and we used the Optos PLC’s Panoramic Ophthalmoscope (200TX; Dunfermline, England) with the software Optos V2®Vantage Pro Review (version: 2.11.0.3) to take fundus photographs.
AQUEOUS HUMOR (AH):
Approximately 50–100 μL AH was collected by transparent corneal puncture from each patient. After the AH samples were collected, they were centrifuged for 30 min at 1200 g and 4°C to remove cell debris and prevent contamination, after which the RNA was extracted.
We used BD Vacutainer-EDTA tubes (BD Biosciences, San Jose, CA, USA) to collect a 10-mL sample of each patient’s whole blood, which was immediately inverted 5–8 times for mixing. After blood collection, the cells were centrifuged for 10 min at 1200 g and 4°C to remove impurities and cell fragments, after which the RNA was extracted. Hemolytic plasma samples were excluded from the study.
CELL CULTURE, TRANSFECTION, AND TREATMENTS:
HRMECs (CP-H130, Procell, Wuhan, China) were cultivated in endothelial cell culture medium (CM-H130, Procell) at 37°C with 5% CO2. Based on the instructions of manufacturer, Lipofectamine® 2000 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to transfect RNAs into HRMECs (37°C, 4 h). Taking HRMECs that pretreated with 5 mM/30 mM glucose as the normal glucose group (control)/high glucose group (HG) with a treatment time of 48 h. The glucose dosage was based on previous studies [31,32].
OVEREXPRESSION OF SNHG5:
The SNHG5 fragment was inserted into pcDNA3.1 vector, and the 2 enzymes were digested into BamH I and Not I.
INHIBITION OF SNHG5:
Three siRNA targeting SNHG5 were constructed, namely si-SNHG5-1, si-SNHG5-2, and si-SNHG5-3. Overexpression/inhibition of SNHG5 was transfected into the HRMECs. Total RNA was extracted and analyzed using RT-qPCR.
RT-QPCR ASSAY: AH or plasma samples were centrifuged for 5 min (3000 g, 23±2°C) to remove cell debris. Based on the manufacturer’s instructions, the AH and plasma samples were lysed by adding 3 times the volume of Trizol (Invitrogen). Then, they were dissolved in 10 μL RNA-free water. Extracted RNA was reverse-transcribed to cDNA with a SYBR Premix ExTaq II kit (Thermo Fisher Scientific) in conjunction with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) by a PrimeScript RT reagent kit (Thermo Fisher Scientific). Cycling conditions were as follows: 50°C (2 min), 95°C (10 min), 72°C (2 min), followed by 40 cycles of 95°C (15 s) and 60°C (32 s). Then, the relative expression of SNHG5 was calculated using the 2−ΔΔCt method [33].
WESTERN BLOTTING:
We used RIPA lysis buffer (Beyotime, Shanghai, China) to cleave the transfected HRMECs on ice, followed by BCA protein assay kit (Tiangen biotech co., Ltd.; Beijing, China) pyrolysis lysate protein concentration calculation. We used 10% SDS-polyacrylamide gel electrophoresis (Solarbio) to resolve equivalent of denatured proteins, and transferred protein bands to a polyvinylidene fluoride membrane (Solarbio). TBST (Beyotime) and 5% BSA (Solarbio) were used to wash and block the membrane, which was incubated with an anti-VEGF antibody (1: 1000) at 4°C overnight. After being rinsed, the membrane was incubated with secondary antibody (goat anti-rabbit; 1: 10,000, Abcam) at 23±2°C for 2 h. ELC-enhanced chemiluminescence reagent (Thermo Fisher Scientific) was added in conjunction with an imaging system (FluorChem R; ProteinSimple Co., Silicon Valley, CA, USA) to visualize the protein bands. Then using an anti-GAPDH antibody (1: 10,000) as the loading control.
CELL COUNTING KIT-8 (CCK8) ASSAY:
We used the trypsin method to prepare a single-cell suspension, then inoculated it in 96-well plates at a density of 2.5×103 cells/well, then added 10 μL of CCK8 reagent (Elabscience, Wuhan, China) into the cell suspension at 0, 24, 48, and 72 h and incubating for 60 min. An enzyme-labeling instrument (Multiscan MK3, Thermo Fisher Scientific) was used to measure the optical density at 490 nm.
ANGIOGENESIS ASSAY:
We coated HRMECs (2×104 cells) with Matrigel (BD Biosciences) in medium and incubated them in 24-well plates for 24 h (37°C, 5% CO2). An inverted microscope (Shanghai Yongke Optical Instrument Co., Shanghai, China) was used to examine the tubular structures of HRMECs in the Matrigel.
STATISTICAL ANALYSIS:
All experiments were performed in triplicate, and data are presented as mean±standard deviation (mean±SD). SPSS (version 21.0; IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Multiple groups were compared simultaneously by one-way ANOVA and Dunnett’s post hoc test. Two groups were compared using the
Results
OPTICAL COHERENCE TOMOGRAPHY:
The results showed that, compared with the control group, retinal interlaminar edema was more obvious in the DME group, and retinal interlaminar edema was more obvious in the refractory DME group. Retinal interlaminar edema was more obvious in the DME group and more obvious in the refractory DME group compared with the control group. In addition, marked edema and dark cavities were also present between the retinal layers in patients with refractory DME (Figure 1B).
DIFFERENCES IN AH AND SNHG5 EXPRESSION IN DME PATIENTS:
SNHG5 expression in AH and serum was lower in DME patients than in patients with idiopathic MH, and higher than in patients with refractory DME (Figure 2A, 2B).
CORRELATION BETWEEN SNHG5 EXPRESSION AND THE CLINICAL CHARACTERISTICS OF PATIENTS:
We analyzed the clinical characteristics of patient in all groups, which included BMI, FBG, HbA1c, Hb, IOP, proteinuria, and glycosuria. SNHG5 expression was negatively correlated with disease course, BMI, FBG, HbA1c, proteinuria, and glycosuria, but there were no obvious differences between groups in age, Hb, IOP, and sex (Figues 3–5; see also Table 1 for specific data). The above results suggest that low expression of SNHG5 is closely related with the development of DME.
HIGH GLUCOSE LEVELS INDUCED DOWNREGULATION OF SNHG5 EXPRESSION:
We then analyzed the potential mechanism of SNHG5 action from a molecular point of view. To this end, we treated HRMECs with high glucose levels in vitro. According to the results of RT-qPCR, SNHG5 expression was downregulated in HRMECs induced by high glucose (Figure 6A). This result is further evidence of the association between SNHG5 and DME.
OVEREXPRESSION/INHIBITION OF SNHG5 PLASMID WAS CONSTRUCTED:
We constructed the overexpressed SNHG5 plasmid and transfected 3 SNHG5-targeting siRNAs into HRMECs, which allowed us to analyze their effects on the function of HRMECs. RT-qPCR analysis showed that SNHG5 expression was obviously upregulated in the ov-SNHG5 group and downregulated in the si-SNHG5 group (Figure 6B), suggesting that the overexpression/inhibition of SNHG5 was successfully constructed. Among them, si-SNHG5-1 showed the lowest inhibition rate. In subsequent experiments, si-SNHG5-1 was used as an inhibitor of SNHG5 to further analyze the molecular mechanism of action in HRMECs.
EFFECT OF SNHG5 ON HRMECS:
To confirm the effect of SNHG5 in HRMECs, RT-qPCR was used to analyze SNHG5 expression in HRMECs after transfection with overexpression or inhibition of SNHG5 plasmid under the induction of high glucose. According to the results, SNHG5 overexpression led to a reversal of SNHG5 downregulation induced by high glucose. However, Si-SNHG5 transfection promoted the downregulation of SNHG5 induced by high glucose levels (Figure 6C). Based on the results, in the presence of high glucose, HRMEC proliferation (Figure 6D) and angiogenesis (Figure 7A, 7B) were both promoted, and VEGF-A protein levels (Figure 7C, 7D) were also upregulated. These effects were further enhanced by the inhibition of SNHG5 but were reversed by overexpression of SNHG5. The above results show that SNHG5 regulates HRMEC proliferation and angiogenesis and affects the protein level of VEGF-A.
Discussion
DME is a complication in diabetic patients. This study revealed for the first time the low expression of SNHG5 in patients with DME or refractory DME, and this expression was closely related to the clinical characteristics of DME patients. We also found that SNHG5 can regulate the apoptosis and angiogenesis of HRMECs in vitro.
Recent studies have shown that patients with long-term DME have a significantly increased probability of developing refractory DME [15,34], and one of the conclusions we have drawn from the present study – that patients with refractory DME have a longer disease course than do patients with DME – also supports this view. With the development of diagnostic and therapeutic techniques, the treatment of DME has improved significantly, but other high-risk ocular complications still occur [35,36]. Due to individual differences, the recognition of DME in diabetic patients before the clinical signs are obvious will facilitate the development of more targeted treatments. With the development of OCT and fundus photography, DME can be identified more accurately [37,38]. In this study, OCT and fundus images were performed on 109 DM patients and 30 idiopathic MH patients, in an attempt to discover whether the retinal lesions in these patients are present for targeted treatment. Currently, the treatment methods for DME mainly include laser photocoagulation [39,40], vitrectomy, or combination with VEGF or bevacizumab [6,41,42]. If patients can maintain good blood glucose control, postoperative adverse visual effects will be significantly reduced [43,44]. Anti-vascular endothelial growth factor (VEGF) therapy has gradually become one of the main treatments of DME. Additionally, conventional therapy combined with anti-VEGF therapy is becoming another effective treatment for DME [45]. In the Aier Hospital of Wuhan University, a multipronged treatment strategy focused on anti-VEGF therapy was established. The OCT results in this study revealed retinal thickening in patients with DME compared to those with idiopathic MH, with rupture of the retinal nerve epithelium. Fundus photography revealed that idiopathic MH patients developed macular holes in the retina, DME patients developed retinal hemorrhage, and refractory DME patients recovered from retinal bleeding after treatment but retained significant pre-treatment scars. These results are consistent with those of previous studies [1,15].
Prevention of DME, elucidation of the molecular mechanism of DME, complete prognosis, and regular follow-up will contribute to the treatment of DME [46–49]. Previous studies have shown that lncRNAs can act as biomarkers for DME and are regulated in DME [50]. In this study, we investigated the functions of lncRNA SNHG5 in AH and plasma of patients with DME. The main result was that SNHG5 expression in the DME group was lower than that in the idiopathic MH group and higher than that in the refractory DME group. Combined with SNHG5 and clinical data of patients, the role of SNHG5 in DME was further revealed. According to the results, SNHG5 had no obvious correlation with age, Hb, IOP, and sex of patients, but was negatively correlated with disease course, BMI, FBG, HbA1c, proteinuria, and glycosuria in patients with DME. Clinical data showed that the disease course, BMI, FBG, HbA1c, proteinuria, and glycosuria in DME patients were higher than those in refractory DME patients. Therefore, dysregulated lncRNA SNHG5 is closely related to the occurrence and development of DME, and long-term DME can promote the occurrence of refractory DME and increase the levels of BMI, FBG, HbA1c, proteinuria, and glycosuria in diabetic patients. Therefore, the regulation of SNHG5 expression may become an effective treatment for DME and refractory DME, and it is necessary to further explore the molecular mechanism of SNHG5 in DME.
Previous studies have explored the molecular mechanism of DME, and a hyperglycemic model was constructed by adding glucose to HRMECs [51,52]. Thus, in this study we investigated the relationship between SNHG5 overexpression/inhibition and high glucose induction by inducing HRMECs with high glucose, and assessed whether the cell proliferation and angiogenesis induced by high glucose was able to be reversed. Based on the data, under the induction of high glucose, VEGF protein level, HRMEC proliferation, and angiogenesis was increased, and SNHG5 expression was downregulated. However, all these effects were reversed by overexpression of SNHG5 and promoted by the inhibition of SNHG5. According to the results, dysregulated SNHG5 has a close relationship with HRMECs proliferation and angiogenesis under the action of high glucose.
This study had some limitations. First, we did not inject VEGF and SNHG5 expression vectors into the vitreous of type 2 diabetic animals to test the efficacy of SNHG5. Second, miRNAs downstream of SNHG5, as well as hypothesized target genes and associated miRNAs signaling pathways, were not studied in great depth. Finally, the small number of patients in this study was insufficient to elucidate the relationship between SNHG5 and DME or refractory DME. These deficiencies indicate the direction for future research. We plan to collect more samples and conduct more experiments to verify the correlation between the lncRNA-miRNA-mRNA signaling pathway and DME.
Conclusions
During DME development, lncRNA SNHG5 expression was downregulated in AH and plasma, and its molecular mechanisms was verified in the hyperglycemic model of HRMECs in vitro, which can serve as a potential target for treating this disease. SNHG5 expression was also negatively correlated with disease duration, BMI, FBG, HbA1c, proteinuria, and glycosuria. This study provides a valuable reference for the diagnosis and treatment of DME.
Figures
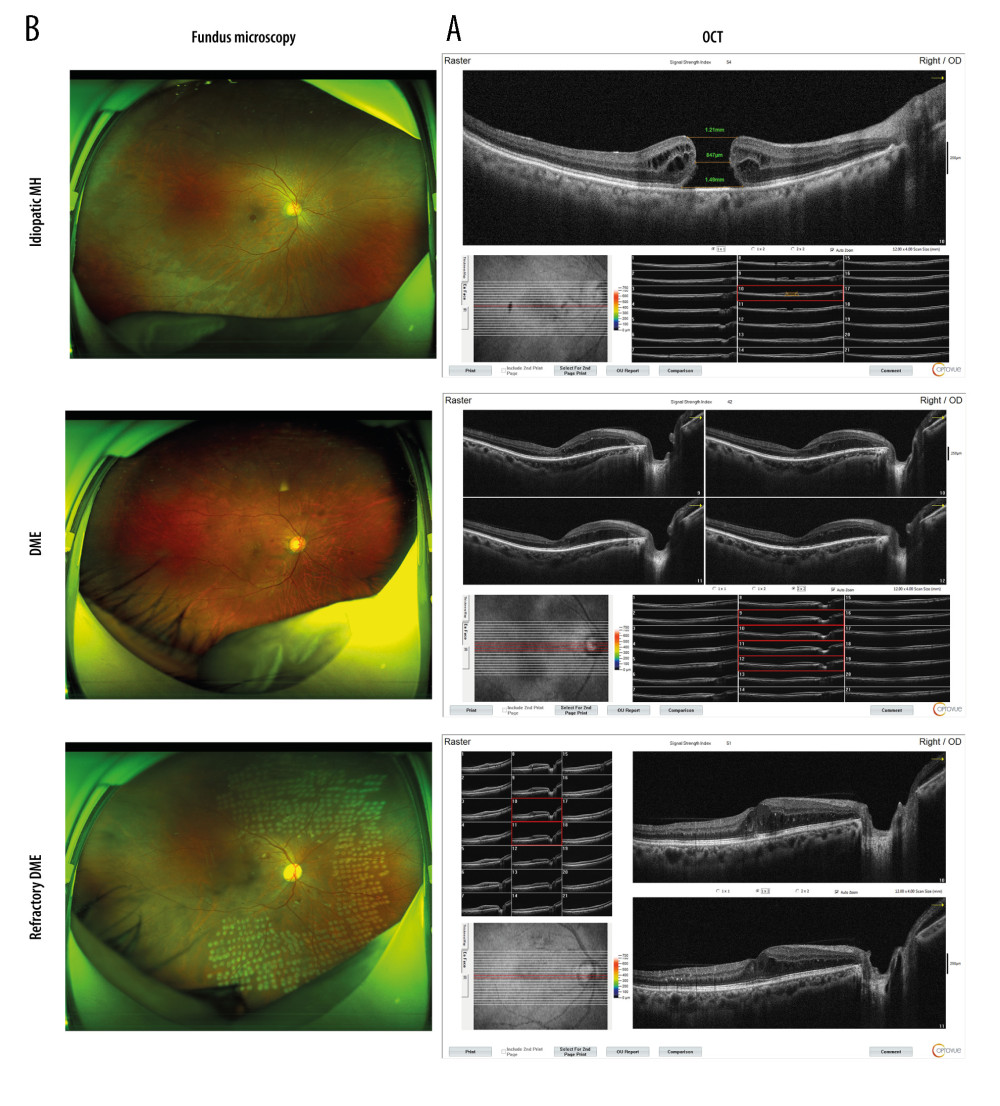 Figure 1. DME exacerbated the symptoms of macular edema. (A) OCT analyses of the retinal symptoms among patients in different groups; (B) fundus microscopy to analyze the differences in macular edema area and eyeball characteristics among patients in different groups. * P<0.05; OCT – optical coherence tomography; DME – diabetic macular edema; MH – macular hole. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 1. DME exacerbated the symptoms of macular edema. (A) OCT analyses of the retinal symptoms among patients in different groups; (B) fundus microscopy to analyze the differences in macular edema area and eyeball characteristics among patients in different groups. * P<0.05; OCT – optical coherence tomography; DME – diabetic macular edema; MH – macular hole. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 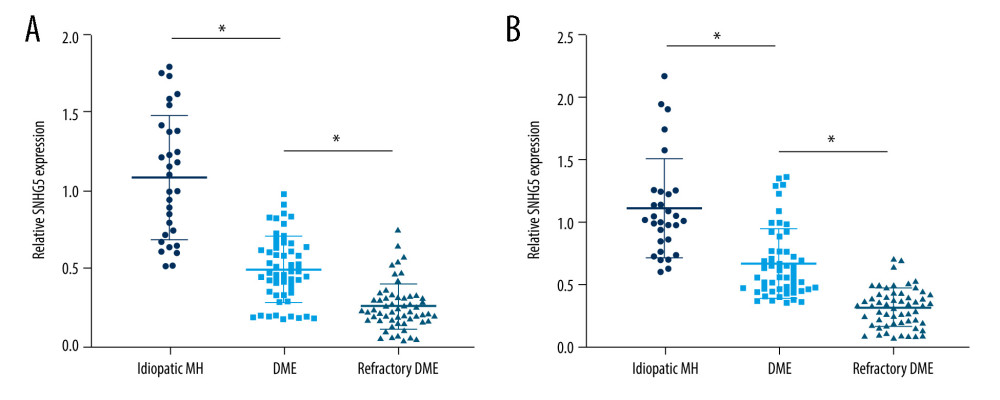 Figure 2. Expression differences in SNHG5 between the DME and refractory DME groups. RT-qPCR analysis of the differences in the expression of SNHG5 in the aqueous humor (A) and serum (B) of patients. * P<0.05; DME – diabetic macular edema; RT-qPCR – quantitative reverse transcription polymerase chain reaction; AH – aqueous humor. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 2. Expression differences in SNHG5 between the DME and refractory DME groups. RT-qPCR analysis of the differences in the expression of SNHG5 in the aqueous humor (A) and serum (B) of patients. * P<0.05; DME – diabetic macular edema; RT-qPCR – quantitative reverse transcription polymerase chain reaction; AH – aqueous humor. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 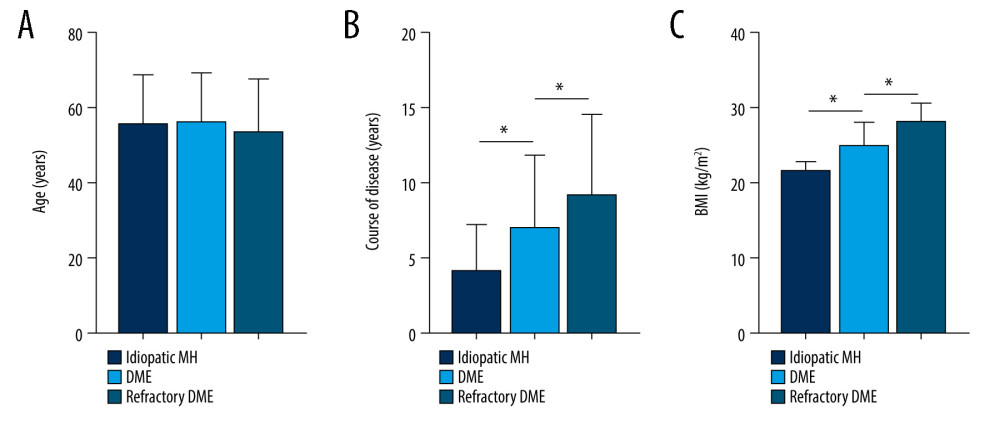 Figure 3. Correlation between SNHG5 and clinical symptoms of patients. Differences in age (A), course of disease (B), and BMI (C) in each group of patients are shown. * P<0.05; BMI – body mass index. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 3. Correlation between SNHG5 and clinical symptoms of patients. Differences in age (A), course of disease (B), and BMI (C) in each group of patients are shown. * P<0.05; BMI – body mass index. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 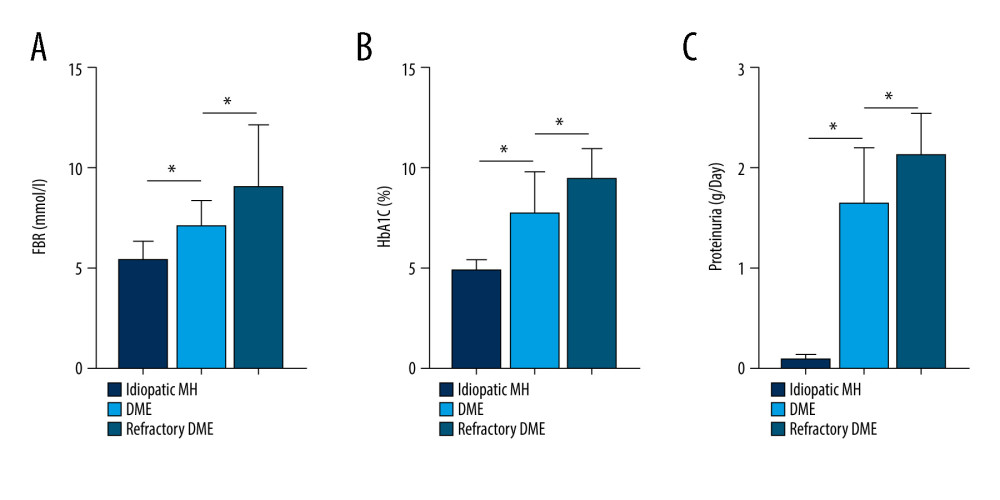 Figure 4. Correlation between SNHG5 and clinical symptoms of patients. The differences in FBG (A), HbA1C (B), and proteinuria (C) in each group of patients are shown. * P<0.05; FBG – fasting blood glucose; HbA1c – glycated hemoglobin. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 4. Correlation between SNHG5 and clinical symptoms of patients. The differences in FBG (A), HbA1C (B), and proteinuria (C) in each group of patients are shown. * P<0.05; FBG – fasting blood glucose; HbA1c – glycated hemoglobin. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 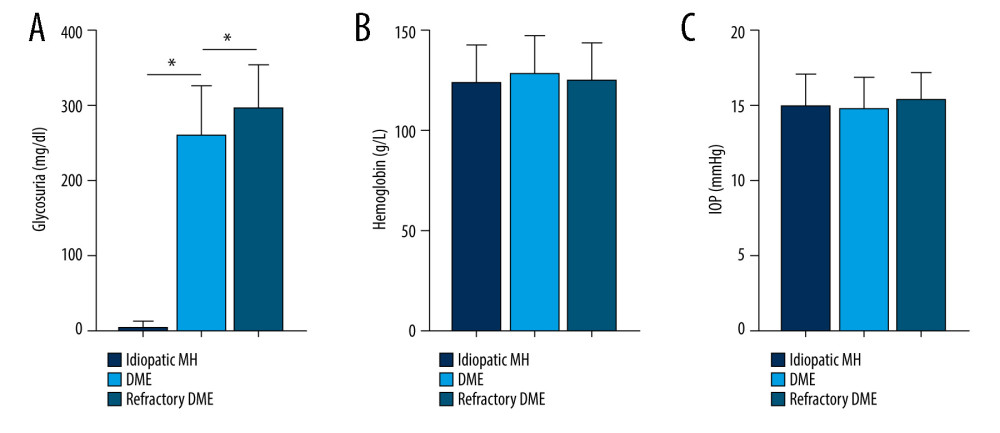 Figure 5. Correlation between SNHG5 and clinical symptoms of patients. The differences in the glycosuria (A), HB (B), and IOP (C) in each group of patients are shown. * P<0.05; HB – hemoglobin; IOP – intraocular pressure. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 5. Correlation between SNHG5 and clinical symptoms of patients. The differences in the glycosuria (A), HB (B), and IOP (C) in each group of patients are shown. * P<0.05; HB – hemoglobin; IOP – intraocular pressure. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 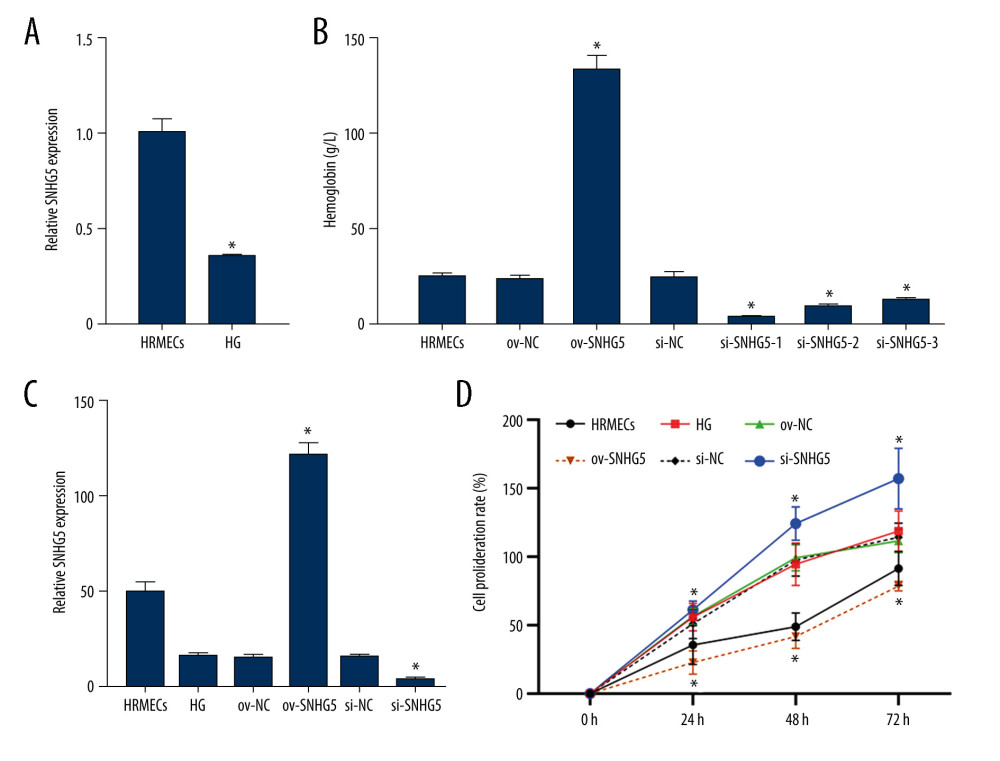 Figure 6. SNHG5 reversed the effect of high glucose induction. RT-qPCR (A) analysis showed that the expression of SNHG5 was different in HRMECs induced by high glucose. (B) RT-qPCR analyzed the expression of SNHG5 after transfecting HRMECs with the plasmid overexpressing lncRNA SNHG5 and 3 si-SNHG5. After transfection of ov-SNHG5 or si-SNHG5, RT-qPCR (C) assays and CCK8 (D) assays were performed to analyze the changes in expression of SNHG5 and cell proliferation in each group. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; CCK8 – Cell Counting Kit-8; HG – high glucose; NC – negative control; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 6. SNHG5 reversed the effect of high glucose induction. RT-qPCR (A) analysis showed that the expression of SNHG5 was different in HRMECs induced by high glucose. (B) RT-qPCR analyzed the expression of SNHG5 after transfecting HRMECs with the plasmid overexpressing lncRNA SNHG5 and 3 si-SNHG5. After transfection of ov-SNHG5 or si-SNHG5, RT-qPCR (C) assays and CCK8 (D) assays were performed to analyze the changes in expression of SNHG5 and cell proliferation in each group. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; CCK8 – Cell Counting Kit-8; HG – high glucose; NC – negative control; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. 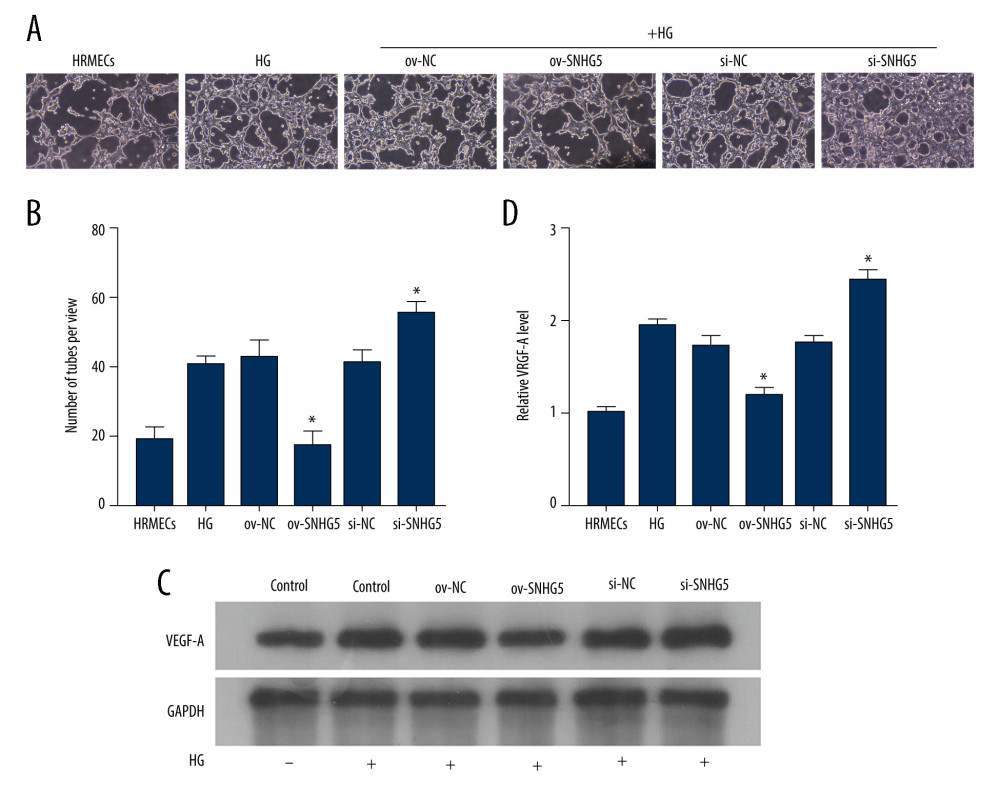 Figure 7. SNHG5 reverses the effect of high glucose induction. Angiogenesis (A, B) was analyzed in each group, and western blotting (C, D) was performed to analyze the changes in VEGF-A protein levels. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; HG – high glucose; NC – negative control; VEGF – vascular endothelial growth factor; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 7. SNHG5 reverses the effect of high glucose induction. Angiogenesis (A, B) was analyzed in each group, and western blotting (C, D) was performed to analyze the changes in VEGF-A protein levels. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; HG – high glucose; NC – negative control; VEGF – vascular endothelial growth factor; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. References
1. Browning DJ, Stewart MW, Lee C, Diabetic macular edema: Evidence-based management: Indian J Ophthalmol, 2018; 66(12); 1736-50
2. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Diabetic macular edema pathophysiology: Vasogenic versus inflammatory: J Diabetes Res, 2016; 2016; 2156273
3. Liu R, Liu CM, Cui LL, Expression and significance of MiR-126 and VEGF in proliferative diabetic retinopathy: Eur Rev Med Pharmacol Sci, 2019; 23(15); 6387-93
4. Cohen SR, Gardner TW, Diabetic retinopathy and diabetic macular edema: Dev Ophthalmol, 2016; 55; 137-46
5. Wong TY, Cheung CM, Larsen M, Sharma S, Simo R, Diabetic retinopathy: Nat Rev Dis Primers, 2016; 2; 16012
6. Kim EJ, Lin WV, Rodriguez SM, Treatment of diabetic macular edema: Curr Diab Rep, 2019; 19(9); 68
7. Sorour OA, Sabrosa AS, Yasin Alibhai A, Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy: Int Ophthalmol, 2019; 39(10); 2361-71
8. Lechner J, O’Leary OE, Stitt AW, The pathology associated with diabetic retinopathy: Vision Res, 2017; 139; 7-14
9. Urias EA, Urias GA, Monickaraj F, Novel therapeutic targets in diabetic macular edema: Beyond VEGF: Vision Res, 2017; 139; 221-27
10. Sodhi A, Ma T, Menon D, Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema: J Clin Invest, 2019; 129(11); 4593-608
11. Cheung N, Mitchell P, Wong TY, Diabetic retinopathy: Lancet, 2010; 376(9735); 124-36
12. Ebneter A, Zinkernagel MS, Novelties in diabetic retinopathy: Endocr Dev, 2016; 31; 84-96
13. Kim KT, Jang JW, Kang SW, Vitrectomy combined with intraoperative dexamethasone implant for the management of refractory diabetic macular edema: Korean J Ophthalmol, 2019; 33(3); 249-58
14. Kang SW, Park SC, Cho HY, Kang JH, Triple therapy of vitrectomy, intravitreal triamcinolone, and macular laser photocoagulation for intractable diabetic macular edema: Am J Ophthalmol, 2007; 144(6); 878-85
15. Hussain RM, Ciulla TA, Treatment strategies for refractory diabetic macular edema: Switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy: Expert Opin Biol Ther, 2016; 16(3); 365-74
16. Jathar S, Kumar V, Srivastava J, Tripathi V, Technological developments in lncRNA biology: Adv Exp Med Biol, 2017; 1008; 283-323
17. Abdulle LE, Hao JL, Pant OP, MALAT1 as a diagnostic and therapeutic target in diabetes-related complications: A promising long-noncoding RNA: Int J Med Sci, 2019; 16(4); 548-55
18. Yu SY, Dong B, Fang ZF, Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR-204-3p and inhibiting autophagy: J Cell Mol Med, 2018; 22(10); 4886-98
19. Li N, Jia T, Li YR, LncRNA NEAT1 accelerates the occurrence and development of diabetic nephropathy by sponging miR-23c: Eur Rev Med Pharmacol Sci, 2020; 24(3); 1325-37
20. Wei W, Tian H, Fu X, Long non-coding RNA (lncRNA) SNHG5 participates in vertical sleeve gastrectomy for type II diabetes mellitus by regulating TGR5: Med Sci Monit, 2020; 26; e920628
21. Nie H, Zhao W, Wang S, Zhou W, Based on bioinformatics analysis lncrna SNHG5 modulates the function of vascular smooth muscle cells through mir-205-5p/SMAD4 in abdominal aortic aneurysm: Immun Inflamm Dis, 2021 [Online ahead of print]
22. Guo C, Hua Y, Qian Z, Differentially expressed genes, lncRNAs, and competing endogenous RNAs in Kawasaki disease: Peer J, 2021; 9; e11169
23. Zhang M, Li Y, Wang H, LncRNA SNHG5 affects cell proliferation, metastasis and migration of colorectal cancer through regulating miR-132-3p/CREB5: Cancer Biol Ther, 2019; 20(4); 524-36
24. Zhao L, Han T, Li Y, The lncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4: FASEB J, 2017; 31(3); 893-903
25. Li S, Shan Y, Li X, lncRNA SNHG5 modulates endometrial cancer progression via the miR-25-3p/BTG2 axis: J Oncol, 2019; 2019; 7024675
26. Ying X, Zhang W, Fang M, LncRNA SNHG5 regulates SOX4 expression through competitive binding to miR-489-3p in acute myeloid leukemia: Inflamm Res, 2020; 69(6); 607-18
27. Liu P, Jia SB, Shi JM, LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis: Biosci Rep, 2019; 39(5) BSR20181469
28. Yu L, Fu J, Yu N, Long noncoding RNA MALAT1 participates in the pathological angiogenesis of diabetic retinopathy in an oxygen-induced retinopathy mouse model by sponging miR-203a-3p: Can J Physiol Pharmacol, 2020; 98(4); 219-27
29. Wilkinson CP, Ferris FL, Klein RE, Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales: Ophthalmology, 2003; 110(9); 1677-82
30. Mastroleo I, Post-trial obligations in the Declaration of Helsinki 2013: Classification, reconstruction and interpretation: Dev World Bioeth, 2016; 16(2); 80-90
31. Lu L, Lu Q, Chen W, Vitamin D3 Protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway: J Diabetes Res, 2018; 2018; 8193523
32. Gu C, Draga D, Zhou C, miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway: Invest Ophthalmol Vis Sci, 2019; 60(13); 4215-23
33. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method: Methods, 2001; 25(4); 402-8
34. Choi MY, Jee D, Kwon JW, Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant: PLoS One, 2019; 14(9); e0222364
35. Yamamoto M, Fujihara K, Ishizawa M, Overt proteinuria, moderately reduced eGFR and their combination are predictive of severe diabetic retinopathy or diabetic macular edema in diabetes: Invest Ophthalmol Vis Sci, 2019; 60(7); 2685-89
36. Pelzek C, Lim JI, Diabetic macular edema: Review and update: Ophthalmol Clin North Am, 2002; 15(4); 555-63
37. Kwan CC, Fawzi AA, Imaging and biomarkers in diabetic macular edema and diabetic retinopathy: Curr Diab Rep, 2019; 19(10); 95
38. Sun Z, Tang F, Wong R, OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: A prospective study: Ophthalmology, 2019; 126(12); 1675-84
39. Baker CW, Glassman AR, Beaulieu WT, Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial: JAMA, 2019; 321(19); 1880-94
40. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA): Ophthalmologica, 2017; 237(4); 185-222
41. Miller K, Fortun JA, Diabetic macular edema: Current understanding, pharmacologic treatment options, and developing therapies: Asia Pac J Ophthalmol (Phila), 2018; 7(1); 28-35
42. Cai S, Bressler NM, Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: Recent clinically relevant findings from DRCR.net Protocol T: Curr Opin Ophthalmol, 2017; 28(6); 636-43
43. Murphy RP, Management of diabetic retinopathy: Am Fam Physician, 1995; 51(4); 785-96
44. Wong JS, Aiello LP, Diabetic retinopathy: Ann Acad Med Singap, 2000; 29(6); 745-52
45. Terasaki H, Ogura Y, Kitano S, Management of diabetic macular edema in Japan: A review and expert opinion: Jpn J Ophthalmol, 2018; 62(1); 1-23
46. Safi SZ, Qvist R, Kumar S, Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets: Biomed Res Int, 2014; 2014; 801269
47. Patelli F, Radice P, Giacomotti E, Diabetic macular edema: Dev Ophthalmol, 2014; 54; 164-73
48. Gomes-Porras M, Cardenas-Salas J, Alvarez-Escola C, Somatostatin analogs in clinical practice: A review: Int J Mol Sci, 2020; 21(5); 1682
49. Iglicki M, Busch C, Zur D, Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: The international retina group real-life 24-month multicenter study. The IRGREL-DEX study: Retina, 2019; 39(1); 44-51
50. Toraih EA, Abdelghany AA, Abd El Fadeal NM, Deciphering the role of circulating lncRNAs: RNCR2, NEAT2, CDKN2B-AS1, and PVT1 and the possible prediction of anti-VEGF treatment outcomes in diabetic retinopathy patients: Graefes Arch Clin Exp Ophthalmol, 2019; 257(9); 1897-913
51. Li Y, Bai YJ, Jiang YR, Apelin-13 is an early promoter of cytoskeleton and tight junction in diabetic macular edema via PI-3K/Akt and MAPK/Erk signaling pathways: Biomed Res Int, 2018; 2018; 3242574
52. Imai S, Otsuka T, Naito A, Shimazawa M, Hara H, Triamcinolone acetonide suppresses inflammation and facilitates vascular barrier function in human retinal microvascular endothelial cells: Curr Neurovasc Res, 2017; 14(3); 232-41
Figures
 Figure 1. DME exacerbated the symptoms of macular edema. (A) OCT analyses of the retinal symptoms among patients in different groups; (B) fundus microscopy to analyze the differences in macular edema area and eyeball characteristics among patients in different groups. * P<0.05; OCT – optical coherence tomography; DME – diabetic macular edema; MH – macular hole. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 1. DME exacerbated the symptoms of macular edema. (A) OCT analyses of the retinal symptoms among patients in different groups; (B) fundus microscopy to analyze the differences in macular edema area and eyeball characteristics among patients in different groups. * P<0.05; OCT – optical coherence tomography; DME – diabetic macular edema; MH – macular hole. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 2. Expression differences in SNHG5 between the DME and refractory DME groups. RT-qPCR analysis of the differences in the expression of SNHG5 in the aqueous humor (A) and serum (B) of patients. * P<0.05; DME – diabetic macular edema; RT-qPCR – quantitative reverse transcription polymerase chain reaction; AH – aqueous humor. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 2. Expression differences in SNHG5 between the DME and refractory DME groups. RT-qPCR analysis of the differences in the expression of SNHG5 in the aqueous humor (A) and serum (B) of patients. * P<0.05; DME – diabetic macular edema; RT-qPCR – quantitative reverse transcription polymerase chain reaction; AH – aqueous humor. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 3. Correlation between SNHG5 and clinical symptoms of patients. Differences in age (A), course of disease (B), and BMI (C) in each group of patients are shown. * P<0.05; BMI – body mass index. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 3. Correlation between SNHG5 and clinical symptoms of patients. Differences in age (A), course of disease (B), and BMI (C) in each group of patients are shown. * P<0.05; BMI – body mass index. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 4. Correlation between SNHG5 and clinical symptoms of patients. The differences in FBG (A), HbA1C (B), and proteinuria (C) in each group of patients are shown. * P<0.05; FBG – fasting blood glucose; HbA1c – glycated hemoglobin. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 4. Correlation between SNHG5 and clinical symptoms of patients. The differences in FBG (A), HbA1C (B), and proteinuria (C) in each group of patients are shown. * P<0.05; FBG – fasting blood glucose; HbA1c – glycated hemoglobin. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 5. Correlation between SNHG5 and clinical symptoms of patients. The differences in the glycosuria (A), HB (B), and IOP (C) in each group of patients are shown. * P<0.05; HB – hemoglobin; IOP – intraocular pressure. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 5. Correlation between SNHG5 and clinical symptoms of patients. The differences in the glycosuria (A), HB (B), and IOP (C) in each group of patients are shown. * P<0.05; HB – hemoglobin; IOP – intraocular pressure. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 6. SNHG5 reversed the effect of high glucose induction. RT-qPCR (A) analysis showed that the expression of SNHG5 was different in HRMECs induced by high glucose. (B) RT-qPCR analyzed the expression of SNHG5 after transfecting HRMECs with the plasmid overexpressing lncRNA SNHG5 and 3 si-SNHG5. After transfection of ov-SNHG5 or si-SNHG5, RT-qPCR (C) assays and CCK8 (D) assays were performed to analyze the changes in expression of SNHG5 and cell proliferation in each group. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; CCK8 – Cell Counting Kit-8; HG – high glucose; NC – negative control; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 6. SNHG5 reversed the effect of high glucose induction. RT-qPCR (A) analysis showed that the expression of SNHG5 was different in HRMECs induced by high glucose. (B) RT-qPCR analyzed the expression of SNHG5 after transfecting HRMECs with the plasmid overexpressing lncRNA SNHG5 and 3 si-SNHG5. After transfection of ov-SNHG5 or si-SNHG5, RT-qPCR (C) assays and CCK8 (D) assays were performed to analyze the changes in expression of SNHG5 and cell proliferation in each group. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; CCK8 – Cell Counting Kit-8; HG – high glucose; NC – negative control; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. Figure 7. SNHG5 reverses the effect of high glucose induction. Angiogenesis (A, B) was analyzed in each group, and western blotting (C, D) was performed to analyze the changes in VEGF-A protein levels. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; HG – high glucose; NC – negative control; VEGF – vascular endothelial growth factor; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure.
Figure 7. SNHG5 reverses the effect of high glucose induction. Angiogenesis (A, B) was analyzed in each group, and western blotting (C, D) was performed to analyze the changes in VEGF-A protein levels. Transfection of ov-SNHG5 or si-SNHG5 was divided into the control group, HG group, HG+ov-NC group, HG+ov-SNHG5 group, HG+si-NC group, and HG+si-SNHG5 group. * P<0.05; HG – high glucose; NC – negative control; VEGF – vascular endothelial growth factor; HRMECs – Human Retinal Microvascular Endothelial Cells. Photoshop software (version 21.2.4; Adobe, Inc; San Jose, USA) was used for creation of the figure. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952