30 December 2021: Clinical Research
Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home Within 3 Days or After 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021
Serafino Fazio1ABDEF, Paolo Bellavite2AE*, Elisabetta Zanolin3CD, Peter A. McCulloughDOI: 10.12659/MSM.935379
Med Sci Monit 2021; 27:e935379
Abstract
BACKGROUND: This retrospective study aimed to investigate outcomes and hospitalization rates in patients with a confirmed diagnosis of early COVID-19 treated at home with prescribed and non-prescribed treatments.
MATERIAL AND METHODS: The medical records of a cohort of 158 Italian patients with early COVID-19 treated at home were analyzed. Treatments consisted of indomethacin, low-dose aspirin, omeprazole, and a flavonoid-based food supplement, plus azithromycin, low-molecular-weight heparin, and betamethasone as needed. The association of treatment timeliness and of clinical variables with the duration of symptoms and with the risk of hospitalization was evaluated by logistic regression.
RESULTS: Patients were divided into 2 groups: group 1 (n=85) was treated at the earliest possible time (<72 h from onset of symptoms), and group 2 (n=73) was treated >72 h after the onset of symptoms. Clinical severity at the beginning of treatment was similar in the 2 groups. In group 1, symptom duration was shorter than in group 2 (median 6.0 days vs 13.0 days, P<0.001) and no hospitalizations occurred, compared with 19.18% hospitalizations in group 2. One patient in group 1 developed chest X-ray alterations and 2 patients experienced an increase in D-dimer levels, compared with 30 and 22 patients, respectively, in group 2. The main factor determining the duration of symptoms and the risk of hospitalization was the delay in starting therapy (P<0.001).
CONCLUSIONS: This real-world study of patients in the community showed that early diagnosis and early supportive patient management reduced the severity of COVID-19 and reduced the rate of hospitalization.
Keywords: Anti-Inflammatory Agents, Non-Steroidal, COVID-19, Drug Therapy, Combination, Flavonoids, Hospitalization, Indomethacin, Aged, Aged, 80 and over, Aspirin, Betamethasone, COVID-19, Cohort Studies, Dietary Supplements, Early Diagnosis, Female, Follow-Up Studies, Heparin, Low-Molecular-Weight, Humans, Italy, Male, Middle Aged, omeprazole, Patient Acuity, Risk Assessment, SARS-CoV-2, Time, Time-to-Treatment, Treatment Outcome
Background
Severe SARS-CoV-2 infection causes a broad spectrum of diseases, known as COVID-19. Some patients develop a condition of severe pneumonia with respiratory failure, which is associated with a pro-inflammatory state and a singular coagulation disorder with immune thrombosis [1]. This can be accompanied by an increase in D-dimer levels, whose sustained elevation positively correlates with disease exacerbation and the mortality rate in patients with COVID-19 [2].
We agree with the suggestion from Scotto di Vetta et al [3] that it should be required to promptly initiate a therapy at the earliest onset of symptoms to stop the progression of COVID-19. This would ultimately reduce the risk of cytokine storm, thrombosis, and, consequently, hospitalization overload. Furthermore, especially with the lack of a broadly approved therapeutic strategy and while waiting for evidence-based indications, it seems unethical to dismiss any intervention at least via therapies based on relevant pharmacodynamic- and pathophysiological-approved rationales [3].
In the most intense phases of the pandemic, it has often been the case that patients who fell ill at home found it difficult to get medical care, remained without treatment, or resorted to self-medication. For this reason, medical assistance and counseling groups based on knowledge and contacts through associations operating with internet communications have been self-organized. The present study retrospectively summarizes the results of the clinical experience of 1 doctor (SF), who operated in this situation and treated his patients with COVID-19 to the best of his ability. Patients were outpatients from the whole Italian territory who contacted the clinician (SF) through the Facebook group “TerapiadomiciliareCOVID19” (https://www.facebook.com/terapiadomiciliarecovid19). The committee that founded this group is composed of an informal group of citizens, physicians, pharmacists, psychologists, physiotherapists, biologists, nurses, and other caregivers and is aimed at providing support during the COVID-19 outbreak, sharing scientific information, and developing a home-care protocol, pending the absence of specific recommendations and agreed-upon directives or guidelines.
This study included a series of patients, some who contacted the doctor in the early days after the onset of symptoms, while others waited for the disease to evolve for several days without taking medication or taking antipyretics only (mainly paracetamol). Since the symptoms at the time of diagnosis were substantially similar in all patients, the same basic therapeutic approach was adopted, with changes tailored to individual patients as needed. We used a multi-therapy approach based on this rationale: 1) a non-steroidal anti-inflammatory drug (NSAID) with antiviral property (indomethacin); 2) an anti-aggregating drug (low-dose aspirin); 3) a gastric protector (omeprazole); and 4) a food supplement containing 2 flavonoids with antioxidant and possibly antiviral properties (hesperidin and quercetin) and vitamin C to support the immune system.
The importance of early treatment with NSAIDs in the treatment of patients with COVID-19 has been suggested by various authors [4–6]. Although there is no available conclusive evidence for or against the use of NSAIDs, observational studies suggest that the use of relatively selective cyclooxygenase (COX)-2 inhibitors together with other drugs could reduce the frequency of hospitalizations, although it does not reduce the duration of symptoms [7]. In particular, indomethacin is a well-known, powerful anti-inflammatory agent that non-selectively inhibits COX-1 and widely inhibits COX-2 enzymes and has been mostly used in the past to treat inflammatory conditions of the musculoskeletal system [8]. Since this drug inhibits COX-2 and viral protein synthesis, its antiviral activities against different viruses have been explored, including herpes virus 6, cytomegalovirus, and hepatitis B virus [9–11]. A recent network pharmacology approach identified 3 target proteins associated with the renin-angiotensin system imbalance caused by SARS-CoV-2 and indicated indomethacin as one of the most promising agents capable of inactivating them, thus reducing excessive inflammation [12]. The same drug, at a low micromolar range, was shown to have antiviral properties in vitro against human SARS-CoV-1, canine coronavirus, and recently against SARS-CoV-2, with no cytotoxic effects [13–16]. A molecular docking study suggested that indomethacin is a potential antagonist of COVID-19 main protease [4]. The drug also reduces the levels of cytokines that greatly increase during COVID-19 and are responsible for some of its detrimental effects [17] and counteracts bradykinin pro-inflammatory effects, thereby possibly reducing dry cough or other bradykinin-induced symptoms [18]. The use of indomethacin in association with resveratrol as an antioxidant to ameliorate disease burden has been suggested by others [19].
Low-dose aspirin was used for its clearly demonstrated anti-thrombotic action, as it could reduce or dampen platelet aggregation, with the aim of preventing micro- or macro-thrombosis during the initial stage of the disease [20,21]. The use of a gastro-protector was considered to avoid the cumulative dangerous effects of the anti-inflammatory agent and the anti-aggregating drug on the stomach, including stress ulceration and gastrointestinal bleeding [22]. Finally, the choice of an antioxidant formula in the therapy protocol was suggested, taking into account the crucial role of oxidative stress in the viral infection [23] and in the pro-thrombotic mechanisms resulting from the impaired endothelia-platelets crosstalk rapidly occurring during SARS-CoV-2 infection [24–28].
Many studies have highlighted the importance of the intracellular redox state as a new target for natural or synthetic drugs aimed at blocking viral replication and excess inflammation [23,29–31]. Hesperidin is a common flavone glycoside found in citrus fruits, such as lemons and sweet oranges. It activates cell antioxidant defenses [32] and suppresses pro-inflammatory cytokine production [33]. Hesperidin exhibits antiviral activity against the influenza virus [34,35] via a significant reduction of viral replication and together with quercetin has been recently indicated as a promising candidate for inhibition of SARS-CoV-2 virus replication and interaction with angiotensin-converting enzyme 2 receptors [36–39]. Recently, a synergistic effect of quercetin and vitamin C against SARS-CoV-2 has been suggested [40].
The choice of symptomatic drugs followed the Italian Medicines Agency (AIFA) guidelines [41], in which the use of paracetamol or NSAIDs is recommended in case of fever or joint or muscle pain (unless there is a clear contraindication for use), and the use of other drugs is recommended following clinical judgment.
This retrospective study from Italy aimed to investigate outcomes and hospitalization rates in 158 patients with a confirmed diagnosis of early COVID-19 who were treated at home with prescribed and non-prescribed treatments between November 2020 and August 2021. The main objective was to analyze the medical records of patients to identify whether a prompt pharmacologic intervention at the onset of COVID-19 (at the earliest), compared with a delayed treatment, could resolve symptoms more rapidly, prevent initial organ damage, and reduce hospitalizations and deaths.
Material and Methods
ETHICS STATEMENT:
All procedures performed in the study were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol was reviewed by the Ethics Committee of Azienda Sanitaria Locale of Lecce (I) and was approved by the tacit acceptance procedure, according to the Italian guidelines of retrospective observational studies (Gazzetta Ufficiale 31.03.2008, n 76, page 69). Informed consent was obtained from all patients after thorough information was provided as a pre-formulated written text that was submitted and discussed with each patient via telemedicine (telephone, Email, or WhatsApp). Because of the emergency conditions of the pandemic, oral consent was given in the presence of an impartial witness that signed in place of the patient, in conformity with the recommendations of the independent European advisory board on data protection and privacy [42] and of the ISS Bio-Ethics COVID-19 Working Group [43]. All data were fully anonymized before tabulation and statistical analysis. The pharmaceutical companies producing the drugs used had no role in the study design, data collection, analyses, interpretation, manuscript writing, or decision to publish the results.
PATIENT ENROLLMENT:
This was a retrospective observational cohort study conducted on a consecutive series of patients who spontaneously presented to the clinician (SF) from the whole Italian territory with the request to be treated for COVID-19. Non-hospitalized patients with a confirmed positive reverse transcription polymerase chain reaction (RT-PCR) swab and symptoms compatible with COVID-19, as indicated in Table 1, fulfilled the main eligibility criteria for the study. All patients were advised to start therapy immediately, and those with negative swabs were then excluded from the study. Patients who were hospitalized for COVID-19, had acute and severe respiratory distress syndrome following SARS-CoV-2 infection, or had critical reasons to be excluded from the study, were considered noneligible for our research. The diagnosis of COVID-19 was confirmed in authorized out-of-hospital laboratories by a nose-pharyngeal swab for a nucleic acid amplification test to detect SARS-CoV-2 genomes by using the RT-PCR technique. The kits used for the procedure were certified by the Italian Ministry of Health (Direzione Generale della Prevenzione sanitaria – DGPRE 11715-03/04/2020 annex 1).
This study included all patients treated from November 2020 to August 2021, with the exception of 2: a 45-year-old woman who took the drugs after different time intervals (some drugs were taken before and other were taken after 3 days), and an 81-year-old man who had been previously vaccinated against COVID-19.
TREATMENT:
The basic treatment for all patients consisted of indomethacin 75 to 100 mg daily (75 mg for people weighing ≤70 kg, and 100 mg for 71 kg and over), Cardioaspirin® (100 mg aspirin) 1 tablet daily, omeprazole 20 mg 1 tablet daily, and the food supplement Esperivit Q100® 2 tablets daily (corresponding to a total daily dose of 200 mg hesperidin, 200 mg quercetin, 100 mg vitamin C). Esperivit Q100® is a food supplement formulated to support the immune system, produced by Vanda s.r.l. (Frascati, Roma) and registered at the Italian Health Ministry in October 2020. The indicated multi-component treatment was administered for up to 4 days after symptoms subsided or until the day of hospitalization. In patients who saw their clinical condition worsen, azithromycin, and/or low-molecular-weight heparin at a prophylactic dose, and/or betamethasone were added to the basic treatment, according to clinical judgment.
Azithromycin was prescribed in patients with suspected bacterial superinfection and was preferred among other antibiotics because of its immunomodulatory and antiviral properties for SARS-CoV-2 infection [44,45]. Low-molecular-weight heparin was used for the prophylaxis of thrombo-embolic events in patients with acute respiratory infection and reduced mobility [45,46]. Betamethasone was used in patients with worsening of respiratory symptoms [47]. This treatment was also made in accordance with the AIFA recommendation on medicines to be used in home management of COVID-19 cases (version n. 3-updated 04/10/2021).
FOLLOW-UP:
Therapeutic prescriptions, monitoring, and follow-up were performed by means of telemedicine and telehealth services, using any multimedia in the available telematic service, and home checks by physicians. All patients were asked to daily monitor their body temperature and oxygen saturation at rest and after a 6-min walk, and to report any change, even slight, in symptoms, which was recorded by 2 doctors (SF and FA). The doctors were available every day to receive information on clinical progress and for patient follow-up. During follow-up, patients could contact the doctor by telephone, and this was even done up to 3 times a day. Patients were hospitalized if their oxygen saturation went lower than 92%. In case of hospital admission, the therapy of this protocol was interrupted, and the total number of symptom days was estimated on the basis of information received from the patient or the patient’s relatives.
To confirm complete recovery, chest X-ray and venous blood tests, including complete blood count, D-dimer, C-reactive protein (CRP), creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total protein electrophoresis were performed after at least 3 weeks from the first positive swab and 1 week without symptoms, after complete clinical recovery, or after the negativization of the molecular swab test. It was not possible to perform these analyses at home during the infectious stage of the disease; therefore, they were mainly used to monitor the permanence of lesions or complete healing over time.
OUTCOMES:
The following data were extracted from the patients’ records and analyzed: symptom duration after start of therapy, which was analyzed as a continuous variable as number of days and a binary variable by categorizing it into 2 classes: duration of symptoms less than or equal to or greater than the median of the whole population (8 days); time passed after the onset of symptoms and before calling the attending physician; symptom duration after start of therapy; total disease duration, including the time before start of therapy; need for hospitalization; death; chest X-ray findings of consequences of interstitial pneumonia; and D-dimer plasma levels. Furthermore, any possible serious and non-serious adverse events related to the administered treatments were collected during daily telephone conversations.
STATISTICAL ANALYSIS:
Clinical data were tabulated after coding and were transmitted between authors anonymously.
Statistical analyses were performed with SPSS version 24 (
The simultaneous effect of the variables of demographic and clinical interest (including independent variables of age, sex, weight, body mass index [BMI], and oxygen saturation at first visit) on symptom duration after start of therapy (dependent binary variable: duration of symptoms equal to or greater than the median of the whole population [8 days]) were assessed by multiple logistic regression (multivariate analysis). Also, the outcome of hospitalization (dependent binary variable) was analyzed by multiple regression analysis to study the effect of the independent variables, including age, sex, weight, BMI, and oxygen saturation at first visit of hospitalization.
The Pearson correlation coefficient (r) was used to calculate the correlation between the start of therapy and the duration of symptoms. An r between 0 and 0.25 shows an absent or low correlation, 0.25 to 0.5, a decent correlation, 0.5 to 0.75, a moderate to good correlation, and 0.75 to 1, a very good to excellent correlation. A
Results
CHARACTERISTICS OF STUDY GROUPS:
Patients’ major demographic and clinical characteristics are summarized in Table 1. The 2 study groups were similar for potential confounders, including age, sex, and concomitant diseases. Group 1 consisted of 48 women and 37 men with symptoms suggestive of COVID-19 and who had been treated with the multi-therapy starting within 3 days from the beginning of symptoms. Group 2 consisted of 36 women and 37 men who had presented to the clinician more than 3 days after the onset of symptoms. At the beginning of clinical observation, the main symptoms of COVID-19 were similar in the 2 groups. Among the variables considered, fever (temperature >37°C) affected the same percentage of patients in the 2 groups, but a slightly lower average temperature (37.29±0.43°C) was observed in group 2 than in group 1 (37.42±0.61°C).
Treatment in group 1 was started 2.05±0.68 (SD) days from the beginning of symptoms, and treatment in the group 2 was started 5.85±1.36 (SD) days after the beginning of symptoms. The patients were treated with the same drug combination, except for the added drugs in some cases as detailed in the
SYMPTOM DURATION AND HOSPITALIZATIONS:
On the day the first call was received from a patient, the prescription of the therapy was given, and the duration of the clinical symptoms was recorded. The patients were followed up daily by telemedicine, and the therapy was continued for up to 4 days after the symptoms disappeared. Even after hospitalization, the doctor continued to receive information from family members, so that it was possible to determine the total duration of the symptoms, including, if necessary, the days of hospitalization. Figure 1 shows the distribution of the duration of symptoms after the start of therapy in the patients in the 2 groups. Symptom duration in group 1 ranged from 3 to 16 days, with a peak around 5 days, while in group 2, COVID-19 symptoms persisted longer and with a markedly bimodal distribution: a first subgroup of patients showed a clinical recovery in 5 to 11 days after the start of therapy, while in a second subgroup, the symptoms lasted much longer, and 14 patients needed hospitalization due to acute respiratory failure.
Table 2 describes the major outcomes that occurred in the 2 groups during the therapeutic treatment. The median duration of COVID-19 symptoms in the whole study population was 8 days (range 3–34), but most outcomes were markedly different in the 2 groups. In group 1, COVID-19 symptoms disappeared within a median of 6 days (range 3–16, mean 6.34±2.18 [SD]) days from the beginning of the therapeutic combination. Furthermore, no hospitalizations and no deaths were registered in group 1. Chest X-ray and blood tests were performed after a median of 24 days from the first positive swab. None of the patients from group 1 developed pathological oxygen desaturation at rest or after a 6-min walk.
In group 2, COVID-19 symptoms persisted for a median of 13 days (range 5–34, mean 13.7±6.4 [SD] days). Fourteen patients (19.18%) needed hospitalization due to acute respiratory failure, 2 of whom died, with a lethality rate of 2.7% in group 2 and overall lethality of 1.26% in the whole study population. Of the 2 patients who died during hospitalization, 1 was a 53-year-old woman with first degree obesity and type 2 diabetes, and the other was a 53-year-old man, who also had first degree obesity and type 2 diabetes. Chest X-ray and blood tests were performed after a median of 35 days from the first positive swab. Except for the death of 2 patients, the number of which was too low to show a significant difference between the 2 groups, all the differences of outcome between the 2 groups were statistically significant.
EFFECT OF THE TIMELINESS OF TREATMENT:
The relation between the number of days passed before starting therapy and the duration of symptoms (with reference to home therapy only) is shown in Figure 2. The duration of symptoms was clearly shorter in group 1, in which only 13 and 2 out of 85 patients experienced a duration greater than 8 and 10 days, respectively. There was a moderate positive correlation between the start of therapy and the duration of symptoms (Pearson coefficient r=0.419). In group 2, the duration of illness at home showed a good correlation with the delay of treatment after the beginning of symptoms (Pearson coefficient r=0.681). However, even in group 2, the subgroup of patients who started treatment on the fourth day were all recovered at home in relatively few days. Out of 24 patients who delayed the start of therapy for more than 6 days, 11 worsened to the point of requiring hospitalization.
OTHER OUTCOMES:
Chest X-ray was performed in all patients but the 2 who died in hospital. Only 1 patient in group 1 had abnormal X-ray findings suggestive of outcomes of interstitial pneumonia, and 2 patients had a pathological increase of D-dimer level, which returned to a normal value in 3 weeks. CRP, creatinine, ALT, AST, GGT, and total protein electrophoresis resulted in a normal range. In group 2, 42% of patients had chest X-ray signs of outcomes of interstitial pneumonia.
As reported in Table 2, after clinical recovery and swab negativization, the D-dimer level was measured in all patients (except the 2 who died). Since the determinations were carried out in different laboratories, it was possible to encode only the possible abnormality of the parameter considered, when the value was higher than the normal range indicated by each laboratory. In group 1, only 2 of 85 patients (2.35%) had elevated D-dimer values, while in group 2, the value was off the scale (up to 4 times over the normal values) in 22 of 71 patients (30.98%). This suggests that early therapy promoted not only a faster healing of symptoms but also a more consistent normalization of this important laboratory parameter.
In the patients in group 2 who saw their clinical condition worsen between days 7 and 10 from the beginning of treatment, azithromycin 500 mg a day, low-molecular-weight heparin at a prophylactic dose, and betamethasone 3 to 4 mg a day were added according to patient weight and clinical status. This supplementary treatment was necessary in only 1 patient in group 1.
Finally, no adverse events were associated with the therapeutic combination in either group.
RISK OF DISEASE DURATION AND HOSPITALIZATION:
Table 3 shows the results of the logistic regression analysis performed to explore the risk of a duration of symptoms equal or greater than the median of the whole population (8 days). Most of the health and clinical variables considered at enrollment (see Table 1) did not influence symptom duration, while early treatment initiation was associated with a marked and statistically significant reduction of the risk. For each day of delay in starting therapy, there was a fourfold increase in the risk of reaching an unfavorable outcome. In addition to the delay in starting treatment, among all the variables considered, it was observed that the risk was significantly increased by the presence of pleural pain at the time of the first visit to the doctor.
A similar result was obtained when considering the outcome of hospitalization (Table 4). Even in this case, it was observed that the odds ratio for hospitalization was markedly influenced by the delay in starting therapy. In addition, a separate univariate analysis showed a slight increase in risk for the symptom of cough. More precisely, among the patients who were not hospitalized (n=144), 103 had cough, while 41 did not; among the patients ultimately hospitalized (n=14), all had cough (chi-square=0.020). In other words, none of the patients without cough at the start of therapy ended up in the hospital.
Discussion
Although it is well known that timely intervention can make the difference in the treatment of an infectious disease, most guidelines in Western countries have preferentially given instructions to treat COVID-19 at the onset of symptoms with only symptomatic drugs for fever, pain, and cough and to adopt a “watchful waiting” attitude, including prescription of paracetamol or other anti-inflammatory agents [45,48]. This modus operandi has produced an overwhelming of hospitals and has possibly increased mortality due to delayed treatment. The use of paracetamol as the only recommended drug for home therapy at the earliest appearance of fever has been highly criticized as well because it may cause COVID-19 exacerbation by dampening GSH levels in patients [7,48].
Our study results suggest that the early application of this multi-therapy approach may reduce the intensity, severity, and duration of COVID-19 symptoms and the risk of hospitalization.
Conversely, a delayed beginning of treatment was associated with a relevant rate of hospitalization (19.2% globally in group 2) and a significantly greater number of patients developing chest X-ray alterations as an outcome of interstitial pneumonia or other organ damage, which often cause permanent outcomes featuring organ dysfunction, impairment of quality of life, and economic burden on the health system.
This timely approach to therapy, based on common anti-inflammatory drugs and the use of nutraceuticals, has already been proposed by others [49,50]; however, experimental demonstrative studies are lacking, and there is no consensus on the several candidate drugs [51,52].
The number of patients followed in these groups (n=85 and n=73) is not very large, but it is comparable to that of other works and, in any case, the duration of the disease, from diagnosis to resolution of symptoms, is much lower than that reported by others with the use of anti-inflammatory drugs alone (about 18 days) [7].
Logistic regression analyses indicated that, in addition to delayed initiation of therapy, cough and pleural pain are associated with a greater risk of symptom duration and hospitalization. It could be interesting to confirm in further studies if these 2 symptoms are indicative of a more severe prognosis and therefore of the need for more intensive treatment.
The follow-up of our patients confirmed that the measurement of D-dimer was a useful parameter to evaluate the progress of COVID-19 and the complete recovery from the disease. As previously reported, about 20% of patients with COVID-19 after complete clinical recovery, restoration of a status of well-being, and molecular swab normalization still showed substantially elevated D-dimer levels [53]. In the present study, only 2 patients in group 1 showed slightly elevated D-dimer levels, compared with about 30% of patients in group 2, suggesting that when the treatment is given promptly after symptom development it may prevent coagulation disorders and excess inflammation. It is conceivable that very early intervention with agents that act synergistically by preventing oxidative stress, as associated with their anti-inflammatory and anti-platelet mechanisms, may avoid a negative evolution of the disease.
Apparently, many different drug combinations, as compared to no treatment at all, are associated with a reduction in morbidity and mortality. Alexander et al demonstrated that any one of a variety of medical regimens applied in nursing home residents was associated with an approximately 60% reduction in mortality compared with patients who received no medical therapy [51]. McCullough et al published a sequential, multi-drug regimen demonstrating that a combination of 4 to 6 pharmacological agents can reduce the risk of hospitalization and death by 85% [49,52,54]. This evidence is also supported by Derwand et al [55].
Ravichandran et al have reported (as preprint) the results of a randomized controlled clinical trial performed in a hospital setting to evaluate the efficacy and safety of indomethacin in RT-PCR-positive patients with COVID-19 [56]. The patients were randomized to treatment with either indomethacin (102 patients) or paracetamol (108 patients) along with the same background therapy for all the patients. The primary aim of the study was to verify the efficacy of indomethacin in preventing desaturation and deterioration in patients with mild and moderate COVID-19, while the secondary aim was to evaluate the symptomatic relief in patients on indomethacin, compared with patients on paracetamol. Both primary and secondary endpoints were achieved [56]. Other authors have also suggested that indomethacin could play a favorable role as an adjunct immunomodulatory therapy in COVID-19 [14,57,58]. Suter et al demonstrated that treatment regimens targeting early and mild symptoms of COVID-19 in the outpatient setting greatly reduced the risk of hospitalization and cumulative costs by >90% but failed to accelerate recovery from major symptoms of COVID-19 [7]. By contrast, our results showed that the treatment we used and its initiation within the first 3 days from the onset of symptoms produced not only a drastic reduction in hospitalization and a relevant reduction in the duration of the symptomatic disease but also a dramatic reduction of outcomes, as shown by the comparison of chest X-ray and D-dimer level alterations in the 2 groups. This could probably be due to the different treatment and, perhaps, to an additional antiviral effect provided by indomethacin, hesperidin, and quercetin, as indicated by laboratory studies. Recently, Consolaro et al reported (as preprint) the results of a matched cohort study that evaluated outcomes in 108 consecutive patients with mild COVID-19 managed at home by their family doctors according to a recommended algorithm [50]. This was based on 3 pillars: 1) intervene at the very onset of mild/moderate symptoms at home; 2) start therapy as early as possible after the family doctor has been contacted by the patient, without awaiting the results of a nasopharyngeal swab; and 3) rely on non-steroidal anti-inflammatory drugs, especially relatively selective COX-2 inhibitors. The results of this study show that the proposed outpatient treatment algorithm reduced the incidence of subsequent hospitalization and related costs [50].
Regarding the nutraceutical substances used, a prospective, randomized, controlled, and open-label study suggested the possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection [59]. A further study showed that quercetin phytosome, a novel bioavailable form of the flavonoid, produced a shorter time to virus clearance, milder symptomatology, and higher probabilities of benign earlier resolution of the disease in patients with mild COVID-19 [60]. Importantly, it has been shown that quercetin inhibits the coronavirus major protease [61] and NLRP3 inflammasome [62]. Furthermore, the flavonoids and vitamin C counteract oxidative stress, which is one of the major mechanisms of the direct cytotoxic effect of the virus [27,29,37,40,63–67] and of the activated NADPH oxidase of phagocytes involved in hyper-inflammatory syndromes [68,69].
Our study had some limitations. The main limitation was the study design, which was retrospective and observational and did not allow for direct causal inferences on the efficacy of the drugs used. Furthermore, the number of cases was not very large, and patients vaccinated for COVID-19 were intentionally excluded; therefore, we do not have information on patients with COVID-19 after vaccination. Larger studies with a prospective, multi-center, and controlled design are needed to confirm these results. In such a complex viral disease with characteristics of probable progressivity in the absence of an effective and resolving drug, there are obviously different therapeutic proposals, which mostly consist of various combinations of drugs. There are also hopes that the use of therapeutic monoclonal antibodies, which could represent an early favorable intervention, can be used in conjunction with other treatments, such as those here described. It is essential that the results of different approaches be compared so that the most suitable choices for each phase of the disease are progressively specified and clarified on the basis of solid evidence.
Conclusions
This real-world study of patients in the population of Italy has shown that early diagnosis and early supportive patient management started within 3 days of the onset of symptoms reduced the severity of COVID-19 and the rate of hospitalization.
Figures
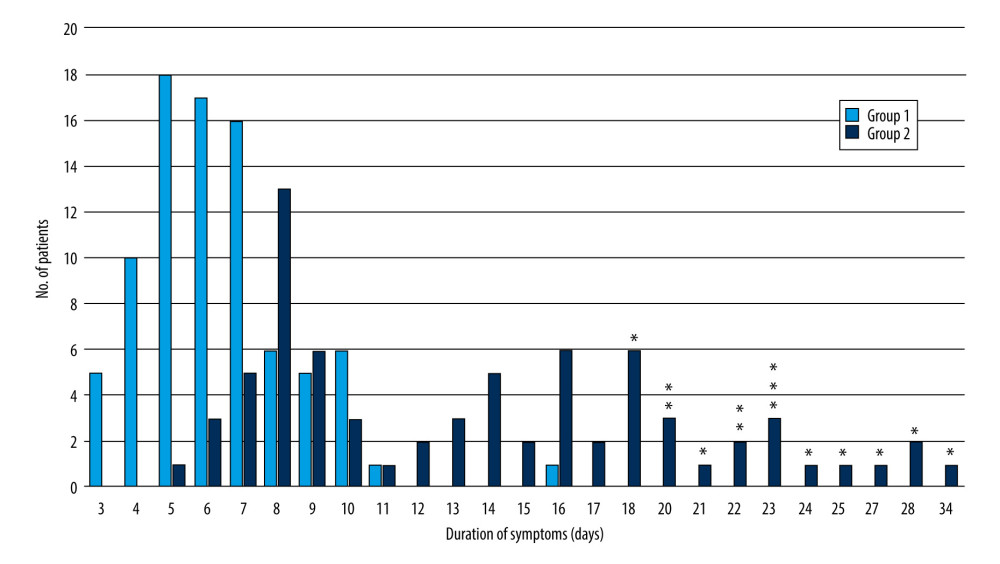 Figure 1. Distribution of symptom duration in the 2 groups. Group 1: start of multi-therapy ≤3 days of symptom appearance; group 2: start of multi-therapy >3 days after symptoms appearance. Asterisks (*) indicate the patients who were hospitalized. The figure was created with Excel software and asterisks were added where indicated with PowerPoint software (Microsoft Office 2019).
Figure 1. Distribution of symptom duration in the 2 groups. Group 1: start of multi-therapy ≤3 days of symptom appearance; group 2: start of multi-therapy >3 days after symptoms appearance. Asterisks (*) indicate the patients who were hospitalized. The figure was created with Excel software and asterisks were added where indicated with PowerPoint software (Microsoft Office 2019). 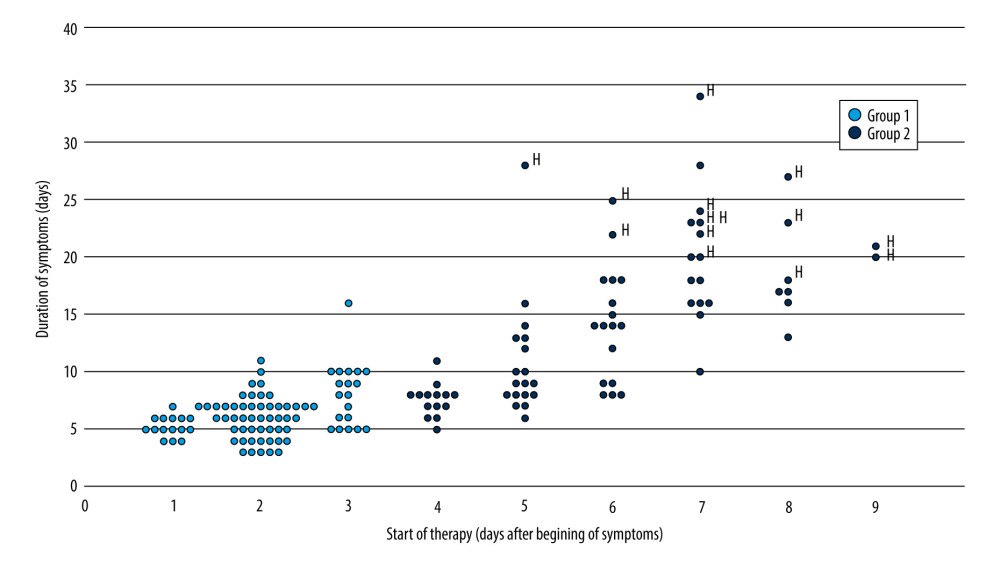 Figure 2. Duration of symptoms in relation to the delay in start of therapy. The symbol “H” specifies the patients who were hospitalized. The figure was created with Excel software and the “H” labels were added where indicated with PowerPoint software (Microsoft Office 2019).
Figure 2. Duration of symptoms in relation to the delay in start of therapy. The symbol “H” specifies the patients who were hospitalized. The figure was created with Excel software and the “H” labels were added where indicated with PowerPoint software (Microsoft Office 2019). Tables
Table 1. General characteristics of the study groups.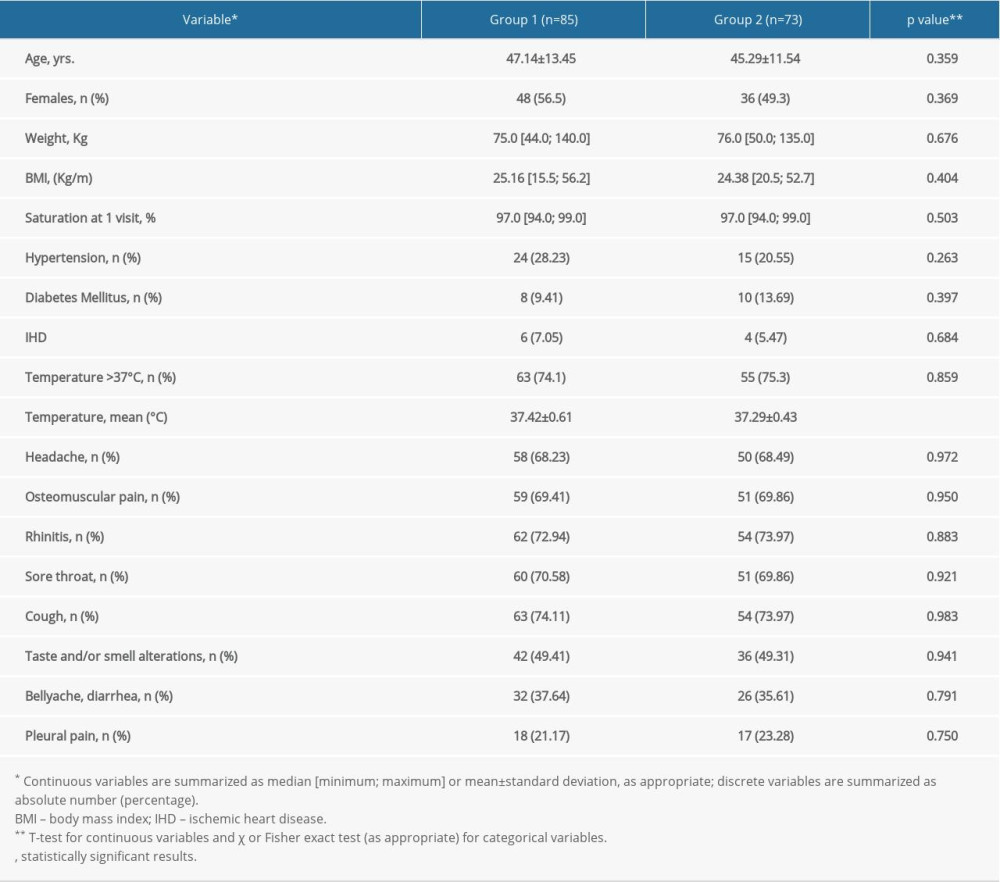 Table 2. Description of outcome occurrence in the study groups.
Table 2. Description of outcome occurrence in the study groups.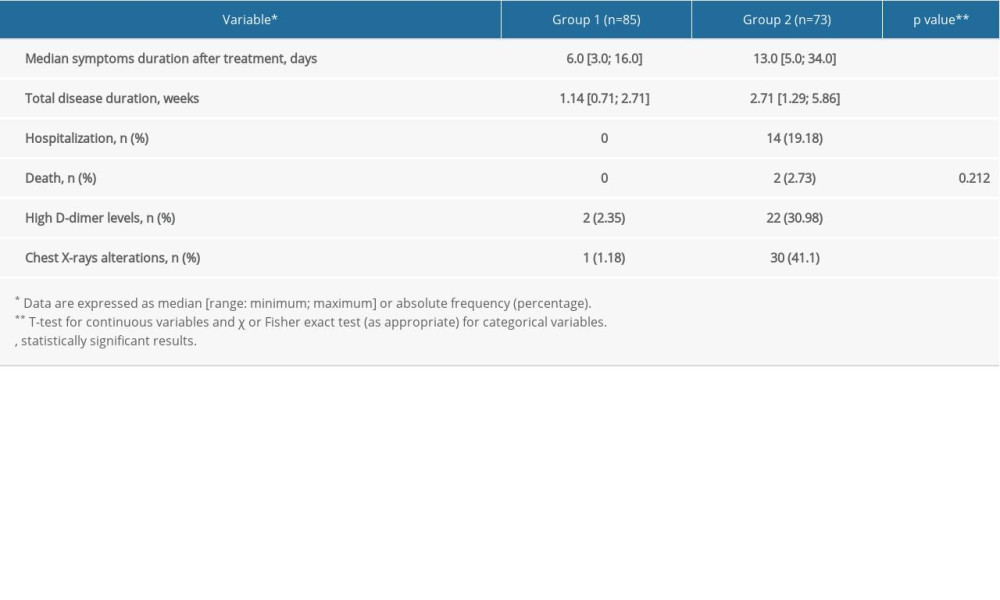 Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample.
Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample.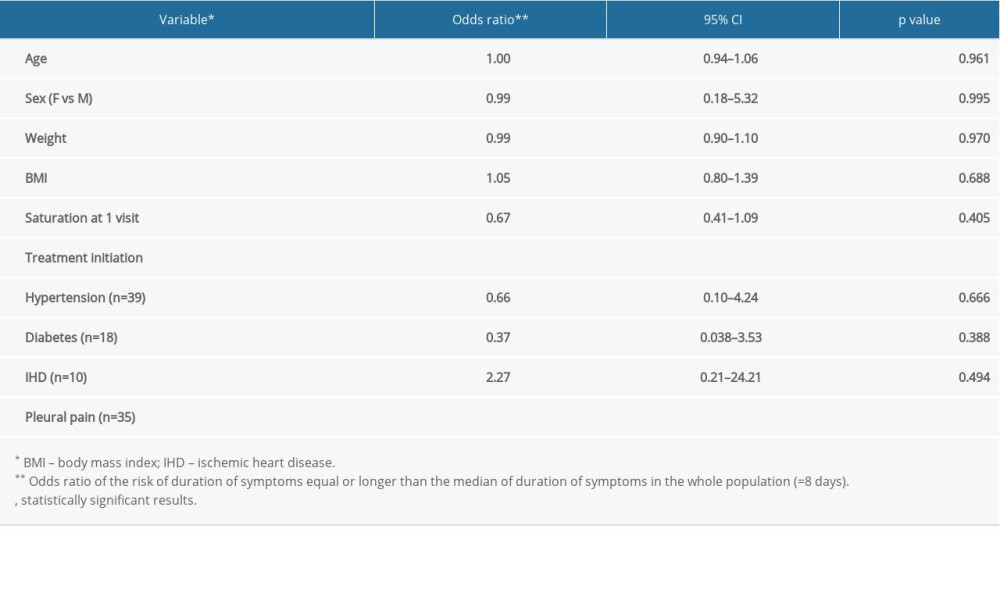 Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample.
Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample.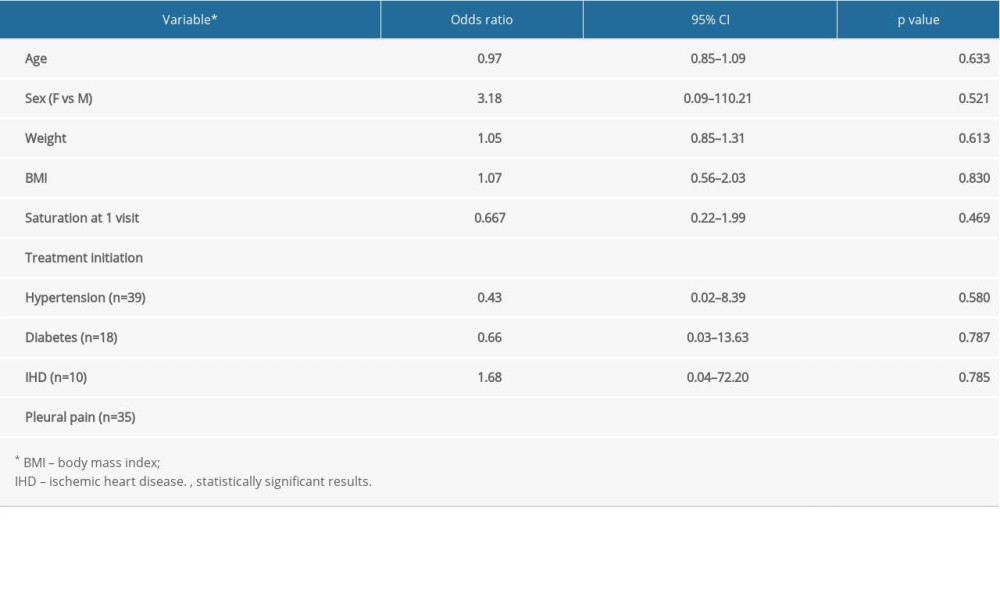
References
1. Bonaventura A, Vecchie A, Dagna L, Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19: Nat Rev Immunol, 2021; 21; 319-29
2. Yao Y, Cao J, Wang Q, D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study: J Intensive Care, 2020; 8; 49
3. Scotto Di Vetta M, Morrone M, Fazio S, COVID-19: Off-label therapies based on mechanism of action while waiting for evidence-based medicine recommendations: World J Meta-Anal, 2020; 8; 173-77
4. Abo Elmaaty A, Hamed MIA, Ismail MI, Computational insights on the potential of some NSAIDs for treating COVID-19: Priority set and lead optimization: Molecules, 2021; 26; 3772
5. Cabbab ILN, Manalo RVM, Anti-inflammatory drugs and the renin-angiotensin-aldosterone system: Current knowledge and potential effects on early SARS-CoV-2 infection: Virus Res, 2021; 291; 198190
6. Prasher P, Sharma M, Gunupuru R, Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy: Drug Dev Res, 2021; 82; 469-73
7. Suter F, Consolaro E, Pedroni S, A simple home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study: EClinical Medicine, 2021; 37; 100941
8. Lucas S, The pharmacology of indomethacin: Headache, 2016; 56; 436-46
9. Reynolds AE, Enquist LW, Biological interactions between herpesviruses and cyclooxygenase enzymes: Rev Med Virol, 2006; 16; 393-403
10. Schroer J, Shenk T, Inhibition of cyclooxygenase activity blocks cell-to-cell spread of human cytomegalovirus: Proc Natl Acad Sci USA, 2008; 105; 19468-73
11. Bahrami H, Daryani NE, Haghpanah B, Effects of indomethacin on viral replication markers in asymptomatic carriers of hepatitis B: A randomized placebo-controlled trial: Am J Gastroenterol, 2005; 100; 856-61
12. Oh KK, Adnan M, Cho DH, Network pharmacology approach to decipher signaling pathways associated with target proteins of NSAIDs against COVID-19: Sci Rep, 2021; 11; 9606
13. Amici C, Di CA, Ciucci A, Indomethacin has a potent antiviral activity against SARS coronavirus: Antivir Ther, 2006; 11; 1021-30
14. Gomeni R, Xu T, Gao X, Bressolle-Gomeni F, Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV-2: J Pharmacokinet Pharmacodyn, 2020; 47; 189-98
15. Kiani P, Scholey A, Dahl TA, McMann L, Iversen JM, Verster JC, In vitro assessment of the antiviral activity of ketotifen indomethacin and naproxen alone and in combination against SARS-CoV-2: Viruses, 2021; 3; 558
16. Xu T, Gao X, Wu Z, Indomethacin has a potent antiviral activity against SARS-COV-2 in vitro and canine coronavirus in vivo: BioRxiv, 2020; 2020; 017624
17. Russell B, Moss C, George G, Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence: Ecancermedicalscience, 2020; 14; 1022
18. Alkotaji M, Al-Zidan RN, Indomethacin: Can it counteract bradykinin effects in COVID-19 patients?: Curr Pharmacol Rep, 2021 [Online ahead of print]
19. Marinella MA, Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19: Int J Clin Pract, 2020; 74; e13535
20. Li G, Wei W, Suo L, Low-dose aspirin prevents kidney damage in LPS-induced preeclampsia by inhibiting the WNT5A and NF-kappaB signaling pathways: Front Endocrinol (Lausanne), 2021; 12; 639592
21. Liu Q, Huang N, Li A, Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19: Medicine (Baltimore), 2021; 100; e24544
22. Bianchi Porro G, Lazzaroni M, Petrillo M, Prevention of gastroduodenal damage with omeprazole in patients receiving continuous NSAIDs treatment. A double blind placebo controlled study: Ital J Gastroenterol Hepatol, 1998; 30; 43-47
23. Checconi P, De AM, Marcocci ME, Redox-modulating agents in the treatment of viral infections: Int J Mol Sci, 2020; 21; 4084
24. Delgado-Roche L, Mesta F, Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection: Arch Med Res, 2020; 51; 384-87
25. Mironova GD, Belosludtseva NV, Ananyan MA, Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease 2019 (COVID-19) and its complications: Eur Rev Med Pharmacol Sci, 2020; 24; 8585-91
26. Aviram M, Dornfeld L, Rosenblat M, Pomegranate juice consumption reduces oxidative stress atherogenic modifications to LDL and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice: Am J Clin Nutr, 2000; 71; 1062-76
27. Saleh J, Peyssonnaux C, Singh KK, Edeas M, Mitochondria and microbiota dysfunction in COVID-19 pathogenesis: Mitochondrion, 2020; 54; 1-7
28. Qiao J, Arthur JF, Gardiner EE, Regulation of platelet activation and thrombus formation by reactive oxygen species: Redox Biol, 2018; 14; 126-30
29. Suhail S, Zajac J, Fossum C, Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: A review: Protein J, 2020; 39; 644-56
30. Soto ME, Guarner-Lans V, Soria-Castro E, Is antioxidant therapy a useful complementary measure for COVID-19 treatment? An algorithm for its application: Medicina (Kaunas), 2020; 56; 386
31. Solnier J, Fladerer JP, Flavonoids: A complementary approach to conventional therapy of COVID-19?: Phytochem Rev, 2020 [Online ahead of print]
32. Parhiz H, Roohbakhsh A, Soltani F, Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models: Phytother Res, 2015; 29; 323-31
33. Haggag YA, El-Ashmawy NE, Okasha KM, Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection?: Med Hypotheses, 2020; 144; 109957
34. Saha RK, Takahashi T, Suzuki T, Glucosyl hesperidin prevents influenza a virus replication in vitro by inhibition of viral sialidase: Biol Pharm Bull, 2009; 32; 1188-92
35. Ding Z, Sun G, Zhu Z, Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect: Antivir Ther, 2018; 23; 611-15
36. Wu C, Liu Y, Yang Y, Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods: Acta Pharm Sin B, 2020; 10; 766-88
37. Bellavite P, Donzelli A, Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits: Antioxidants (Basel), 2020; 9; 742
38. Balmeh N, Mahmoudi S, Mohammadi N, Karabedianhajiabadi A, Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors: Inform Med Unlocked, 2020; 20; 100407
39. Derosa G, Maffioli P, D’Angelo A, Di Pierro F, A role for quercetin in coronavirus disease 2019 (COVID-19): Phytother Res, 2021; 35; 1230-36
40. Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE, Quercetin and vitamin C: An experimental synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19): Front Immunol, 2020; 11; 1451
41. Agenzia Italiana dei Farmaci: Raccomandazioni AIFA sui farmaci per la gestione domiciliare di COVID-19, 2021, Roma, AIFA publisher [in Italian]
42. Article 29 Working Party: Guidelines on consent under Regulation 2016/679 (last revised 10 April 2018) Directorate C (Fundamental Rights and Union Citizenship) of the European Commission, 2018, Brussels
43. ISS Bioethics COVID-19 Working Group, Research ethic during the COVID-19 pandemics: Consideration on observational and epidemiologic studies. Rapporto ISS COVID-19 n.47/2020, 2020, Istituto Superiore di Sanità Roma
44. Khezri MR, Zolbanin NM, Ghasemnejad-Berenji M, Jafari R, Azithromycin: Immunomodulatory and antiviral properties for SARS-CoV-2 infection: Eur J Pharmacol, 2021; 905; 174191
45. Ministero della Salute: Gestione domiciliare dei pazienti con infezione da SARS-CoV-2. Edition 26 aprile 2021, 2021, Ministero Salute Roma [in Italian]
46. Thachil J, The versatile heparin in COVID-19: J Thromb Haemost, 2020; 18(5); 1020-22
47. Siemieniuk RA, Bartoszko JJ, Ge L, Drug treatments for COVID-19: Living systematic review and network meta-analysis: BMJ, 2020; 370; m2980
48. Pandolfi S, Simonetti V, Ricevuti G, Chirumbolo S, Paracetamol in the home treatment of early COVID-19 symptoms: A possible foe rather than a friend for elderly patients?: J Med Virol, 2021; 93; 5704-6
49. McCullough PA, Kelly RJ, Ruocco G, Pathophysiological basis and rationale for early outpatient treatment of SARS-CoV-2 (COVID-19) infection: Am J Med, 2021; 134; 16-22
50. Consolaro E, Rubis N, Pedroni S, A home-treatment algorithm based on anti-inflammatory drugs to prevent hospitalization of patients with early COVID-19: A matched-cohort study (Cover 2): MedRxiv, 2021; 2021; 21264298
51. Alexander PE, Armstrong R, Fareed G, Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents: Med Hypotheses, 2021; 153; 110622
52. McCullough PA, Favipiravir and the need for early ambulatory treatment of SARS-CoV-2 infection (COVID-19): Antimicrob Agents Chemother, 2020; 64; e02017-20
53. Fazio S, Tufano A, de Simone G, Sustained high D-dimer in outpatients who have recovered from mild to moderate coronavirus disease 2019 (COVID-19): Semin Thromb Hemost, 2021 [Online ahead of print]
54. McCullough PA, Vijay K, SARS-CoV-2 infection and the COVID-19 pandemic: a call to action for therapy and interventions to resolve the crisis of hospitalization death and handle the aftermath: Rev Cardiovasc Med, 2021; 22; 9-10
55. Derwand R, Scholz M, Zelenko V, COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study: Int J Antimicrob Agents, 2020; 56; 106214
56. Ravichandran R, Mohan SK, Sukumaran SK, Use of indomethacin for mild and moderate COVID-19 patients. A randomized controlled trial: MedRxiv, 2021; 2021; 21261007
57. Ghareeb DA, Saleh SR, Nofal MS, Potential therapeutic and pharmacological strategies for SARS-CoV2: J Pharm Investig, 2021 [Online ahead of print]
58. Ho P, Zheng JQ, Wu CC, Perspective adjunctive therapies for COVID-19: Beyond antiviral therapy: Int J Med Sci, 2021; 18; 314-24
59. Di Pierro F, Derosa G, Maffioli P, Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective randomized controlled and open-label study: Int J Gen Med, 2021; 14; 2359-66
60. Di Pierro F, Iqtadar S, Khan A, Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second pilot randomized controlled and open-label clinical trial: Int J Gen Med, 2021; 14; 2807-16
61. Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening: Int J Biol Macromol, 2020; 164; 1693-703
62. Saeedi-Boroujeni A, Mahmoudian-Sani MR, Anti-inflammatory potential of Quercetin in COVID-19 treatment: J Inflamm (Lond), 2021; 18; 3
63. Shovlin CL, Vizcaychipi MP, Vascular inflammation and endothelial injury in SARS-CoV-2 infection: The overlooked regulatory cascades implicated by the ACE2 gene cluster: QJM, 2020 [Online ahead of print]
64. Potus F, Mai V, Lebret M, Novel insights on the pulmonary vascular consequences of COVID-19: Am J Physiol Lung Cell Mol Physiol, 2020; 319; L277-88
65. Polonikov A, Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients: ACS Infect Dis, 2020; 6; 1558-62
66. Iddir M, Brito A, Dingeo G, Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis: Nutrients, 2020; 12(6); 1562
67. Mrityunjaya M, Pavithra V, Neelam R, Immune-boosting antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19: Front Immunol, 2020; 11; 570122
68. Violi F, Oliva A, Cangemi R, Nox2 activation in COVID-19: Redox Biol, 2020; 36; 101655
69. Bellavite P, Reappraisal of dietary phytochemicals for coronavirus infection: Focus on hesperidin and quercetin: Antioxidants: Benefits, sources, mechanisms of action, 1995; 473-87, Londo, Intechopen
Figures
 Figure 1. Distribution of symptom duration in the 2 groups. Group 1: start of multi-therapy ≤3 days of symptom appearance; group 2: start of multi-therapy >3 days after symptoms appearance. Asterisks (*) indicate the patients who were hospitalized. The figure was created with Excel software and asterisks were added where indicated with PowerPoint software (Microsoft Office 2019).
Figure 1. Distribution of symptom duration in the 2 groups. Group 1: start of multi-therapy ≤3 days of symptom appearance; group 2: start of multi-therapy >3 days after symptoms appearance. Asterisks (*) indicate the patients who were hospitalized. The figure was created with Excel software and asterisks were added where indicated with PowerPoint software (Microsoft Office 2019). Figure 2. Duration of symptoms in relation to the delay in start of therapy. The symbol “H” specifies the patients who were hospitalized. The figure was created with Excel software and the “H” labels were added where indicated with PowerPoint software (Microsoft Office 2019).
Figure 2. Duration of symptoms in relation to the delay in start of therapy. The symbol “H” specifies the patients who were hospitalized. The figure was created with Excel software and the “H” labels were added where indicated with PowerPoint software (Microsoft Office 2019). Tables
 Table 1. General characteristics of the study groups.
Table 1. General characteristics of the study groups. Table 2. Description of outcome occurrence in the study groups.
Table 2. Description of outcome occurrence in the study groups. Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample.
Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample. Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample.
Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample. Table 1. General characteristics of the study groups.
Table 1. General characteristics of the study groups. Table 2. Description of outcome occurrence in the study groups.
Table 2. Description of outcome occurrence in the study groups. Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample.
Table 3. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with duration of symptoms in the whole sample. Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample.
Table 4. Mutually adjusted odds ratios and 95% confidence intervals for the association of considered variables with hospitalization in the whole sample. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








