27 May 2022: Clinical Research
Factors Associated with Falls During Hospitalization for Coronavirus Disease 2019 (COVID-19)
Marcin Mikos1ABCDE*, Błażej Szydło2BCE, Ivan SzergyukDOI: 10.12659/MSM.936547
Med Sci Monit 2022; 28:e936547
Abstract
BACKGROUND: During the current Coronavirus Disease 2019 (COVID-19) pandemic, falls have been identified as a potential presenting symptom in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; however, data on factors increasing fall risk in this patient population are limited. This study aimed to examine the factors that may predispose hospitalized COVID-19 disease patients to falls.
MATERIAL AND METHODS: In this retrospective observational study, hospitalized COVID-19 disease patients were examined for fall incidence, as well as demographics, comorbidities, and clinical and laboratory data. Patients were stratified according to their fall status and their characteristics were compared using Fisher’s exact test or Mann-Whitney U test. A total of 312 hospitalized COVID-19 disease patients were enrolled (median age, 75 years; males, 51.3%), of whom 11 (3.5%) fell.
RESULTS: There was a greater prevalence of falls among patients who experienced arrhythmias than those that did not (28.6% vs 1.7%; P<0.001). Additionally, a significantly greater proportion of those that were discharged to the internal ward and to the intensive care unit fell (10.3% and 10.0%, respectively) compared to those that were discharged home (1.6%, P=0.008). Thyroid-stimulating hormone (TSH) was significantly elevated in patients who fell (5.3 vs 0.97 µIU/mL, P=0.013), while alanine aminotransferase (ALT) was significantly lower in those who fell (17.1 vs 33.5 IU/L, P=0.041).
CONCLUSIONS: Arrhythmias may be an important predisposing factor for falls in COVID-19 disease patients and fall prevention programs should prioritize interventions directed at this vulnerable patient population.
Keywords: Accidental Falls, Arrhythmias, Cardiac, COVID-19, Risk Factors, Syncope, Aged, COVID-19, Hospitalization, Humans, Male, SARS-CoV-2
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) classically presents with fever and respiratory symptoms [1,2]. However, throughout the ongoing Coronavirus Disease 2019 (COVID-19) pandemic, atypical symptoms of this infectious illness have garnered increasing attention. A recent meta-analysis identified falls as an important manifestation of COVID-19 disease, particularly among the geriatric population [3]. Falls may occur secondary to an array of symptoms such as dizziness, impaired consciousness [4,5], syncope [6], delirium, confusion [7], fatigue [3], and impaired balance [8], which are all well described in COVID-19 disease. Chen et al found that around 24% of patients that tested positive for SARS-CoV-2 infection initially presented themselves to the hospital with syncope, pre-syncope, or a non-mechanical fall [6]. Additional intrinsic risk factors which could play a role include age, gender, comorbid conditions (particularly cardiovascular disease), and medication use [9–11], in part explaining the increased tendency for falls in elderly patients [12]. Furthermore, slippery flooring, hospital equipment failures, staff shortages, and insufficient training and teamwork are extrinsic factors which may be implicated in some cases as well [13].
Falls are associated with significant disability and reduced patient independence, reduced quality of life, prolonged hospitalization, increased economic burden [12,14], and, importantly, increased rates of mortality [15], particularly when fractures are involved [16]. Annually, falls cause injuries severe enough to require medical attention in 37.3 million individuals, while fatality occurs in about 684 000 cases, making it a significant public health issue [12]. Some sources estimate that fall-related healthcare costs amount to over $14 000 per fall [14], which may further strain the already high financial burden of the COVID-19 disease pandemic on the healthcare system. Furthermore, a recent review reported a considerable (4-fold) increase in 30-day post-surgery mortality rate in COVID-19 disease patients treated for femoral fracture compared to pre-COVID-19 disease rates, stressing the importance of fall prevention measures in SARS-CoV-2-infected patients [17]. Identifying risk factors for falls is essential for addressing this issue. Therefore, this study aimed to explore factors associated with falls in hospitalized COVID-19 disease patients.
Material and Methods
ETHICS STATEMENT:
To ensure patient privacy and maintain good practice, all patient personal data were removed before statistical analysis. Patient consent was waived due to the retroactive design. This study was approved by the Bioethics Committee of Andrzej Frycz Modrzewski Cracow University. This study was conducted in compliance with the Declaration of Helsinki, under the terms of relevant local and national legislation.
STUDY DESIGN AND PARTICIPANTS:
In this retrospective observational cohort study, COVID-19 disease patients, over 18 years old, admitted to the special “COVID-19 Disease Treatment” ward in J. Dietl Specialist Hospital in Cracow over the period of October 2020 to May 2021 were enrolled. A total of 312 hospitalized COVID-19 disease patients were analyzed, of whom 11 (3.5%) fell and 301 (96.5%) did not. Since our team aimed to investigate all potential effects of SARS-CoV-2 infection on patients, there were no exclusion criteria.
DATA COLLECTION:
To ensure the multidisciplinary character of the study, our team included various types of data, including demographics, comorbidities, and clinical and laboratory data. Demographic data were collected during patient interviews. Laboratory tests were based on blood samples taken as part of routine blood draws and performed at an ISO-certified laboratory of J. Dietl Specialist Hospital. Data were extracted from patients’ medical records and put together to provide material for statistical analysis by Błażej Szydło and Michał Kuboń. Patients with missing data were omitted from the analysis.
VARIABLES:
The main outcome of interest was falls, defined as loss of stability followed by touching the ground with a minimum of 3 limbs, resulting in injury or not, noticed by ward staff or reported by the patients themselves.
Demographic data included basic information from patients such as age, sex, weight, height, and body mass index (BMI). Clinical data measured included comorbidities (history of arrhythmia, smoking habit, hypertension, obesity, diabetes, chronic obstructive pulmonary disease (COPD)/asthma, rheumatological conditions, vascular incidents, and neoplastic disease), given drugs, type of oxygen therapy applied, patients’ state of mobility, hospitalization duration, and hospitalization outcome (death or discharge). Laboratory variables measured included levels of C-reactive protein (CRP, mg/L), procalcitonin (ng/mL), d-dimers (μgFEU/nL), white blood cell (WBC) count (103/μL), lymphocyte count (103/μL), thyroid-stimulating hormone (TSH, μIU/mL), creatinine kinase (CK, μmol/L), alanine aminotransferase (ALT, IU/L), and aspartate aminotransferase (AST, IU/L).
STATISTICAL ANALYSIS:
Patients were stratified according to their fall status, and their characteristics were compared. Qualitative variables were described using absolute and relative frequencies, and their significance was assessed using Fisher’s exact test. Quantitative variables were described through median and interquartile ranges and their significance was assessed using the Mann-Whitney’s U test. A
Results
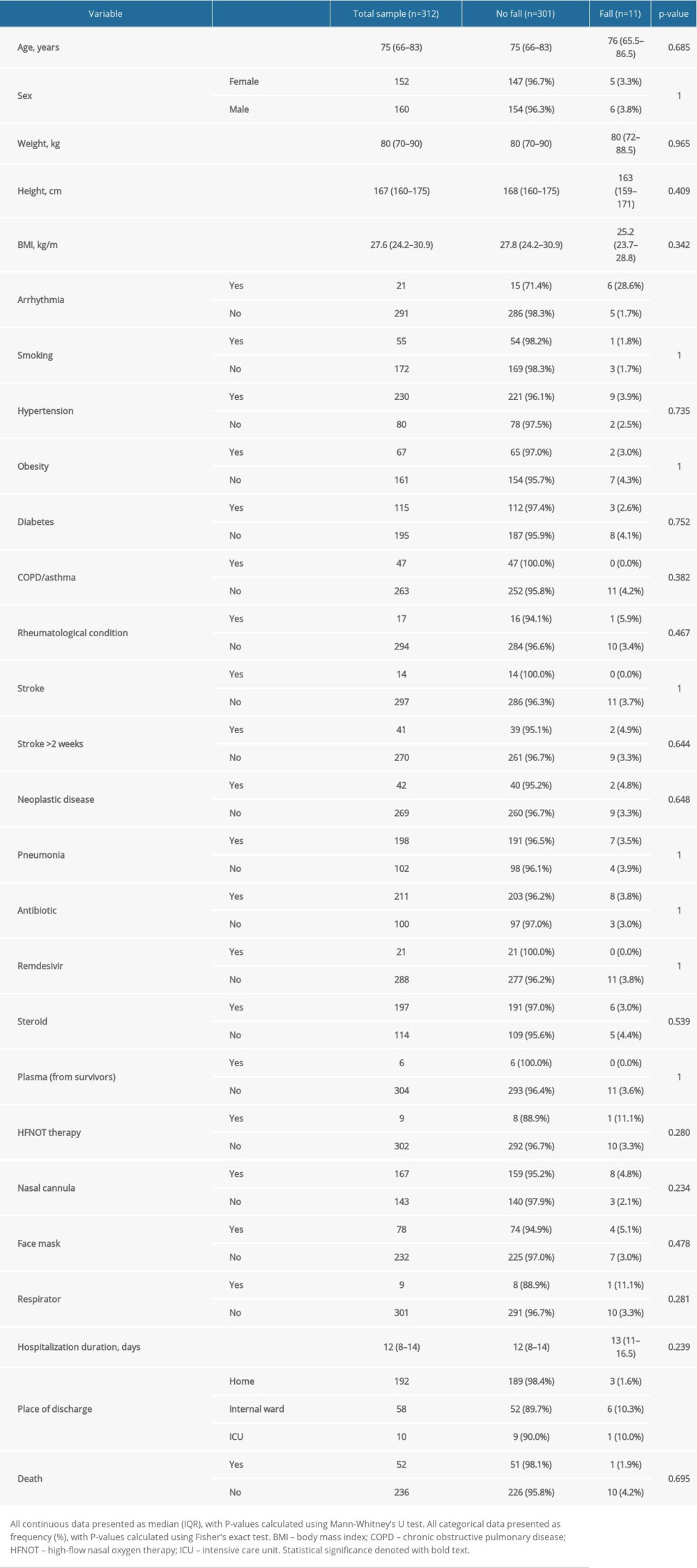
Table 1 presents demographic variables, comorbidities, and clinical variables for the whole sample and separately for patients that fell and those that did not (Table 1). Overall, the median age was 75 (IQR, 66–83) years, and males comprised 51.3% (n=160) of the sample. There were no significant differences in age nor sex distribution between those that fell and those that did not.
Among comorbidities, hypertension (73.7%, n=230), diabetes (36.9%, n=115), and pneumonia (63.5%, n=198) were the most prevalent, but only the arrhythmia group showed a difference in fall incidence. Patients who experienced arrhythmias had a greater incidence of falls that those without arrhythmias (28.6% vs 1.7%;
The average hospital stay was 12 (IQR, 8–14) days, with no difference between groups. Overall, 16.7% (n=52) of the patients died, and there was no significant difference between those that fell and those that did not fall with respect to mortality. Most patients were discharged home from the COVID-19 ward (61.5%, n=192), but a significantly greater proportion of those that were discharged to the internal ward and the intensive care unit (ICU) fell (10.3% and 10.0%) compared to those that were discharged home (1.6%,
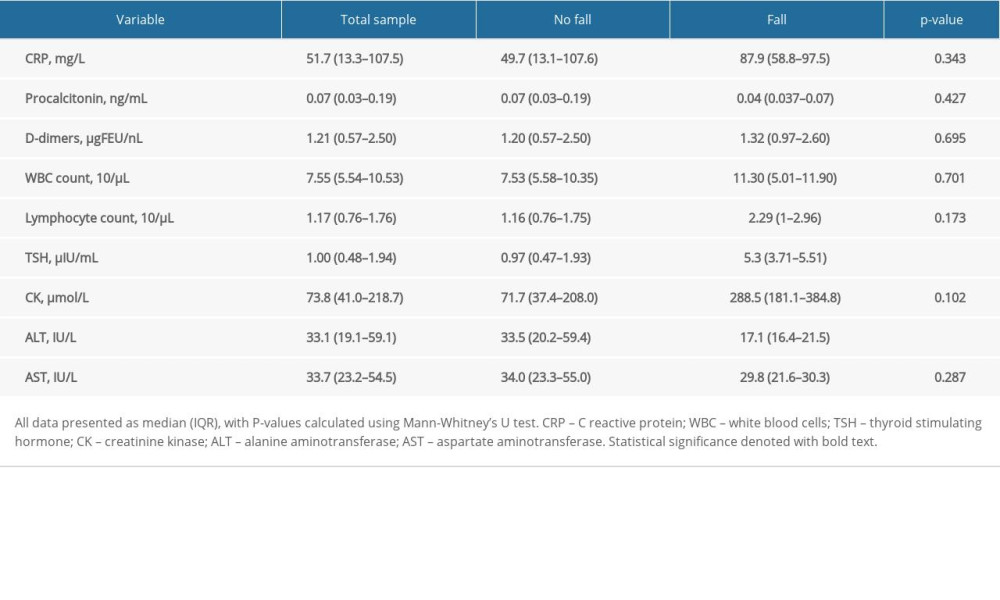
With regard to circulating biomarkers, only TSH and ALT differed significantly between groups (Table 2). TSH was significantly elevated in patients that fell (5.3 vs 0.97 μIU/mL,
Discussion
In this observational cohort study, our primary objective was to identify factors that may explain falls, an atypical presenting symptom, in COVID-19 disease patients. In summary, we demonstrated that there was a greater incidence of falls among patients that experienced arrhythmias, as well as in those that were discharged from the COVID-19 disease ward to the ICU and the internal ward versus to patients’ home, and in patients with higher TSH and lower ALT levels. These findings are essential for assessing an individual patient’s fall risk such that appropriate fall prevention strategies can be applied.
Although there has been no direct evidence linking arrythmias to falls in COVID-19 disease patients, our study found that COVID-19 disease patients with arrhythmia have an increased propensity to falling. In a recent study, Wang et al reported that arrhythmias occur in up to 16.7% of hospitalized and 44.4% of ICU-admitted COVID-19 disease patients [18]. Chen and colleagues observed that around 24% of SARS-CoV-2-infected patients present to the hospital with syncope, pre-syncope, and/or an associated fall [6], a finding which may in part be explained by cardiac rhythm disturbances in COVID-19 disease patients. Extensive research over the past decade has shown that arrhythmias are an important cause of cerebral hypoperfusion, syncope, and subsequent falls [19–21]. A systematic review and meta-analysis by Malik and colleagues on a total of 43 213 older adults found that atrial fibrillation was independently associated with syncope (odds ratio [OR], 1.19) and falls (OR, 1.88) [20]. Jansen et al found an even stronger association (OR, 4.4 for syncope and OR, 2.0 for falls) [21]. Cardiovascular disease in general has been associated with moderate to high fall risk in up to 60% of patients [22]. It is hence conceivable that arrythmias in COVID-19 disease patients may be responsible for falls.
Several studies have proposed a link between COVID-19 disease and arrhythmias, induced by a combination of direct cardiomyocyte injury and systemic inflammation [23,24]. Turagam et al attributed cases of malignant ventricular arrhythmia in COVID-19 disease patients to metabolic derangements caused by the illness [25]. Cardiac arrhythmias have also been reported in cases where COVID-19 disease patients were treated with hydroxychloroquine or azithromycin, both of which prolong the QT interval [26]. In the hyperinflammatory state of COVID-19 disease, rhythm disturbances and endotheliopathy may act in synergy to promote thromboembolic complications [27], potentially leading to impaired cerebral blood flow and falls. These observations not only propose potential mechanisms for falls in COVID-19 disease patients, but also stress the importance of long-term anticoagulation therapy in cases when there is a high risk of arrhythmia and thromboembolism [5]. Khurshid and colleagues have demonstrated an association between delayed anticoagulation for atrial fibrillation and falls (hazard ratio, 1.53) [28]. Although fall prevention strategies may benefit from incorporating rhythm management and anticoagulation in susceptible patients, these interventions may equally be detrimental in this patient population. Antiarrhythmics are arrhythmogenic and have been associated with both syncope and traumatic falls [29], while anticoagulants can increase a patient’s bleeding risk if a fall does occur [19,30]. The evidence is mixed, however, and this risk may not be significant enough to outweigh the benefits of anticoagulation. In a prospective cohort study, the use of oral anticoagulants in patients with an increased propensity for falling was not significantly associated with increased risk of hemorrhage in multivariate analysis [31].
Alternatively, syncope in COVID-19 disease may develop due to hypovolemia and circulatory failure. A subset of COVID-19 disease patients present with vomiting and diarrhea, making them prone to developing volume depletion and orthostatic hypotension [32], necessitating fluid resuscitation. Additionally, an impaired baroreflex response caused by SARS-CoV-2 affinity to angiotensin-converting enzyme 2 (ACE-2) receptors in midbrain nuclei may compromise compensatory tachycardia to hypoxia, in part explaining the postural hypotension and syncope [33,34]. Canetta et al observed that a lower heart rate was specific to COVID-19 disease patients who presented with syncope [34].
In a recent systematic review of 37 studies, syncope in COVID-19 disease patients was attributable to cardiac disease in only 2.2% of cases, while orthostatic hypotension was responsible for another 2.2% of the cases, and reflex syncope accounted for 7.8% [35]. The vast majority of the episodes (87.9%) were unexplained [35] and may have been related to some poorly elucidated mechanism triggered by SARS-CoV-2 infection. Early case series on COVID-19 disease patients with syncope as the sole presenting symptom proposed autonomic failure as a potential mechanism, secondary to either direct viral effects or autoimmune/inflammatory autonomic neuropathy [36]. Neurological and psychiatric manifestations, attributed to neuroinflammation, autoimmunity, and gut-brain axis dysregulation caused by SARS-CoV-2 infection, have been increasingly reported during the COVID-19 disease pandemic [37].
We also found that falls were more frequent in patients discharged from the COVID-19 disease ward to the ICU or internal ward than those discharged to their home. Those requiring further attention in another hospital ward for COVID-19 disease or non-COVID-19 disease-related complications likely had comorbidities predisposing to falls as opposed to the healthier patients who were discharged home.
As for the laboratory markers, we observed that patients who fell had higher TSH and lower ALT levels compared to those that did not fall. Research has shown that COVID-19 disease patients with more severe illness have lower TSH and higher ALT levels than those with milder illness [38–40]. Thus, higher TSH and lower ALT in patients who fell may simply be attributable to the fact that they had a milder illness and fell because they were ambulatory. A systematic review by de Freitas et al found that elevation in C-reactive protein (CRP) and D-dimer were among the most frequently observed laboratory finding in COVID-19 disease patients who presented with syncope, suggesting a possible role of hypercoagulability in syncope during SARS-CoV-2 infection [35]. Troponins were also elevated, but less frequently, in those experiencing syncope, underpinning the potential mechanism for syncope by the viropathic effects of SARS-CoV-2 infection on the heart [35]. Although we did observe a slight elevation of CRP and D-dimer in fallers, the differences were statistically non-significant.
Besides the effects of COVID-19 disease on the cardiovascular and neurological systems, derangements in the musculoskeletal system may also play a substantial role in falls. Disability of the lower extremities, including poor muscle strength, has been associated with increased odds of falls, especially in older adults (OR, 3.8) [41]. In a systematic review including 12 046 COVID-19 disease patients, the prevalence of fatigue was 25.6%, while myalgia and/or arthralgia was reported in 15.5% of patients [42]. These manifestations may be mediated by direct viral invasion and toxicity, systemic inflammation, and a dysregulated immune response and immune complex deposition [43–45]. A biochemical signaling study by Disser and colleagues found that human skeletal muscle, smooth muscle, synovial, cortical bone, and articular cartilage cells express either ACE-2 receptors, TMPRSS2 (transmembrane protease, serine 2), or both, which may allow for direct SARS-CoV-2 infection [46]. That study did not, however, investigate whether SARS-CoV-2 was present in these tissues, and it is not yet clear which mechanism is most responsible for musculoskeletal involvement in COVID-19 disease. Musculoskeletal symptoms are concerning not only because they may predispose patients to falls, but also the main treatment, NSAIDs (nonsteroidal anti-inflammatory drugs), is one of the major ‘fall risk-increasing’ drugs as defined by Woolcott et al [47]. Additionally, muscle stiffness and atrophy may be a manifestation of prolonged hospitalization for COVID-19 disease and must be countered by exercise-based rehabilitation regimens [43]. Interestingly, a number of reports have found that compared to non-infected individuals, COVID-19 disease patients were more likely to present with vitamin D deficiency [48], a finding which has been extensively associated with an increased propensity for falling [49]. Supplementation of 800 IU of vitamin D per day together with calcium has been shown to improve muscle strength and postural equilibrium, in turn reducing fall risk [50,51], and may therefore prove useful as a fall prevention intervention in COVID-19 disease patients. Although we did not observe any difference in fall incidence in patients taking steroids, prolonged use of steroids for COVID-19 illness may exacerbate osteoporosis in older patients and put them at risk of fall-related fractures. Given that this was a pilot investigation, we were unable to analyze many of these factors potentially influencing falls, which would require much larger cohort sizes. Therefore, the extent to which musculoskeletal manifestations increase fall risk in COVID-19 disease patients needs to be further investigated.
We did not find any significant associations between falls and drugs commonly prescribed to COVID-19 disease patients, such as antibiotics, remdesivir, and steroids. Generally, the major ‘fall risk-increasing’ drug classes are antidepressants, antihypertensives, NSAIDs, benzodiazepines, sedatives and hypnotics, and antipsychotics [47]. In a recent systematic review, the use of beta-blockers against hypertension, but not drugs targeting the angiotensin system, has been associated with syncope in COVID-19 disease patients [35], a consequence of which may be falls. Beta-blockers may be deleterious for the cardiac reflex response to hypoxia and shock in COVID-19 disease [35]; therefore, the latter antihypertensive class may be the preferable option in this patient population. In general, healthcare providers should remain vigilant for the use of these fall risk-increasing drugs in all patients, including those with COVID-19 disease, and periodic drug review must remain a vital component of fall prevention programs [10]. Nevertheless, removing certain drugs to reduce fall risk or finding an equally efficacious alternative may not always be a viable option [52]. Additionally, in patients with SARS-CoV-2 infection, the increased risk of falls should be carefully considered when prescribing arrhythmogenic drugs such as azithromycin and hydroxychloroquine, although these have now been largely discontinued for COVID-19 disease [53].
One may expect that the shift in focus toward treating SARS-CoV-2 infection during the current pandemic may have been associated with an increased rate of falls, but there are limited data reporting fall incidence rates during the pandemic. One recent retrospective study from Taiwan identified a 3-fold increase in the fall incidence rate in hospitalized patients during the pandemic in 2020 compared to same period in the previous year, and attributed this to restrictions imposed on the number of caregivers and visitors allowed in hospitals [54]. Additionally, these restrictions have likely contributed to decreased physical exercise, particularly among elderly people [5,55], regardless of infection status. Physical inactivity is an important risk factor for falls [56], and according to World Health Organization (WHO), exercise significantly reduces this risk [57]. However, to date there is no research reporting fall incidence rates in COVID-19 disease patients specifically, both as an acute symptom and a long-term complication after recovery from SARS-CoV-2 infection, and should therefore be the focus of future investigations.
Falls impose a significant burden on both the patient and the healthcare system. The most common complications associated with falls are superficial injuries (21%), open wounds (8%), fractures (60%), and head trauma (8.7%) [10,58]. Additionally, people who fall often experience depression/anxiety (28%), mobility issues (70%), decreased independence (41%), reduced quality of life, and in severe cases the falls are fatal [10,12,58]. Globally, the incidence of falls is estimated at 2.2 per 1000 patients in a year [59], while in Poland, the annual fall incidence rate was 6.48 per 1000 patients during the 2013–2019 period [13]. Every year, approximately 37.3 million falls are severe enough to require immediate medical attention [12]. A third of those presenting to the emergency department following a fall require hospitalization [10], and an estimated 684 000 people die as a result of the fall [12]. It is thus not difficult to conceive that there are significant healthcare costs associated with falls. Florence and colleagues estimated that the total cost associated with falls in the US in the year 2015 was approximately 50 billion USD [60]. It is not yet known to what extent COVID-19 disease-associated falls contribute to the total medical costs, but this input should not be minimized given the already high economic burden of COVID-19 disease on the healthcare system [61].
Despite the limitations pertaining to its retrospective nature, this study may form a basis on which prospective studies are devised. The study period was relatively short, although this is largely limited by the duration of the ongoing pandemic. Additionally, the sample size was limited due to inclusion criteria and number of patients hospitalized due to COVID-19 disease. However, it was possible to reach the statistical significance for important clinical risk factors. We encourage future well-designed studies to extend this important topic. Many patients had to be excluded from the analysis given that they died soon after admission and thus some of their parameters were missing. Lastly, we did not quantify the odds of falling, but merely the rate. Nonetheless, to the best of our knowledge, this study is one of the first to investigate falls in COVID-19 disease patients, and hence should be followed with studies with larger cohort size and analyzing for association to further validate our findings.
Conclusions
In conclusion, COVID-19 disease patients who experience arrhythmias may be at increased risk for falls, and fall prevention strategies should be aimed at this vulnerable patient population to reduce fall-related injuries and the associated costs. A holistic approach involving both hospital staff training and patient education, with a focus on screening for multiple potential risk factors, should be effective in helping minimize fall risk.
References
1. CDC: Coronavirus Disease 2019 (COVID-19) – Symptoms, 2021, Centers for Disease Control and Prevention [Internet] [cited 2021 Jun 4]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
2. Oliveira BA, de Oliveira LC, Sabino EC, Okay TS, SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods: Rev Inst Med Trop Sao Paulo, 2020; 62; e44
3. Gawronska K, Lorkowski J, Falls as one of the atypical presentations of COVID-19 in older population: Geriatr Orthop Surg Rehabil, 2021; 12; 2151459321996619
4. Mao L, Jin H, Wang M, Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China: JAMA Neurol, 2020; 77; 683-90
5. Li Q, Zhao C, A review of the current status of clinical management of COVID-19 in the elderly: Med Sci Monit, 2021; 27; e930278
6. Chen T, Hanna J, Walsh EE, Syncope, near syncope, or nonmechanical falls as a presenting feature of COVID-19: Ann Emerg Med, 2020; 76; 115-17
7. Aknin LB, Neve JED, Dunn EW, The neurological consequences of contracting COVID-19. The Lancet’s COVID-19 Commission Mental Health Task Force: Acta Neuropsychologica, 2021; 19(3); 301-5
8. Norman RE, Stall NM, Sinha SK, Typically atypical: COVID-19 presenting as a fall in an older adult: J Am Geriatr Soc, 2020; 68; E36-37
9. Nugraha S, Hapsari I, Sabarinah , Multimorbidity increases the risk of falling among indonesian elderly living in community dwelling and elderly home: A cross sectional study: Indian J Public Health Res Dev, 2019; 10(11); 2263
10. de Jong MR, Van der Elst M, Hartholt KA, Drug-related falls in older patients: Implicated drugs, consequences, and possible prevention strategies: Ther Adv Drug Saf, 2013; 4(4); 147-54
11. Stel VS, Smit JH, Pluijm SMF, Lips P, Consequences of falling in older men and women and risk factors for health service use and functional decline: Age Ageing, 2004; 33(1); 58-65
12. World Health Organization: Falls [Internet] [cited 2021 Sep 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/falls
13. Mikos M, Banas T, Czerw A, Hospital inpatient falls across clinical departments: Int J Environ Res Public Health, 2021; 18(15); 8167
14. Joint Commission: Preventing Falls| Center for Transforming Healthcare [Internet] [cited 2021 Sep 19]. Available from: https://www.centerfortransforminghealthcare.org/improvement-topics/preventing-falls/
15. Ambrose AF, Paul G, Hausdorff JM, Risk factors for falls among older adults: A review of the literature: Maturitas, 2013; 75(1); 51-61
16. Muñoz Vives JM, Jornet-Gibert M, Cámara-Cabrera J, Mortality rates of patients with proximal femoral fracture in a worldwide pandemic: Preliminary results of the Spanish HIP-COVID observational study: J Bone Jt Surg, 2020; 102(13); e69
17. Dupley L, Oputa TJ, Bourne JTNorth West COVID NOF Study Group, 30-day mortality for fractured neck of femur patients with concurrent COVID-19 infection: Eur J Orthop Surg Traumatol, 2021; 31(2); 341-47
18. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323(11); 1061-69
19. Mikos M, Winnicki K, Henry BM, Sanchis-Gomar F, Link between cardiovascular disease and the risk of falling: A comprehensive review of the evidence: Pol Arch Intern Med, 2021; 131; 369-76
20. Malik V, Gallagher C, Linz D, Atrial fibrillation is associated with syncope and falls in older adults: Mayo Clin Proc, 2020; 95(4); 676-87
21. Jansen S, Frewen J, Finucane C, AF is associated with self-reported syncope and falls in a general population cohort: Age Ageing, 2015; 44(4); 598-603
22. Manemann SM, Chamberlain AM, Boyd CM, Fall risk and outcomes among patients hospitalized with cardiovascular disease in the community: Circ Cardiovasc Qual Outcomes, 2018; 11(8); e004199
23. Bhatla A, Mayer MM, Adusumalli S, COVID-19 and cardiac arrhythmias: Heart Rhythm, 2020; 17(9); 1439-44
24. Babapoor-Farrokhran S, Gill D, Walker J, Myocardial injury and COVID-19: Possible mechanisms: Life Sci, 2020; 253; 117723
25. Turagam MK, Musikantow D, Goldman ME, Malignant arrhythmias in patients with COVID-19: Incidence, mechanisms, and outcomes: Circ Arrhythm Electrophysiol, 2020; 13(11); e008920
26. Rosenberg ES, Dufort EM, Udo T, Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State: JAMA, 2020; 323(24); 2493-502
27. Lippi G, Sanchis-Gomar F, Favaloro EJ, Coronavirus disease 2019 – associated coagulopathy: Mayo Clin Proc, 2021; 96(1); 203-17
28. Khurshid S, Weng L-C, Hulme OL, Factors associated with anticoagulation delay following new-onset atrial fibrillation: Am J Cardiol, 2017; 120(8); 1316-21
29. Dalgaard F, Pallisgaard JL, Numé A, Rate or rhythm control in older atrial fibrillation patients: Risk of fall-related injuries and syncope: J Am Geriatr Soc, 2019; 67(10); 2023-30
30. Brook R, Aswapanyawongse O, Tacey M, Real-world direct oral anticoagulant experience in atrial fibrillation: Falls risk and low dose anticoagulation are predictive of both bleeding and stroke risk: Intern Med J, 2020; 50(11); 1359-66
31. Donzé J, Clair C, Hug B, Risk of falls and major bleeds in patients on oral anticoagulation therapy: Am J Med, 2012; 125(8); 773-78
32. Guan W, Ni Z, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
33. Xia H, Lazartigues E, Angiotensin-converting enzyme 2: Central regulator for cardiovascular function: Curr Hypertens Rep, 2010; 12(3); 170-75
34. Canetta C, Accordino S, Buscarini E, Syncope at SARS-CoV-2 onset: Auton Neurosci, 2020; 229; 102734
35. de Freitas RF, Torres SC, Martín-Sánchez FJ, Syncope and COVID-19 disease – a systematic review: Auton Neurosci Basic Clin, 2021; 235; 102872
36. Ebrille E, Lucciola MT, Amellone C, Syncope as the presenting symptom of COVID-19 infection: Hear Case Rep, 2020; 6(7); 363-66
37. Bliźniewska-Kowalska KM, Halaris A, Wang S-C, A review of the global impact of the COVID-19 pandemic on public mental health, with a comparison between the USA, Australia, and Poland with Taiwan and Thailand: Med Sci Monit, 2021; 27; e932220
38. Xu W, Huang C, Fei L, Li Q, Chen L, Dynamic changes in liver function tests and their correlation with illness severity and mortality in patients with COVID-19: A retrospective cohort study: Clin Interv Aging, 2021; 16; 675-85
39. Chen M, Zhou W, Xu W, Thyroid function analysis in 50 patients with COVID-19: A retrospective study: Thyroid, 2021; 31(1); 8-11
40. Henry BM, de Oliveira MHS, Benoit S, Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis: Clin Chem Lab Med, 2020; 58(7); 1021-28
41. Tinetti ME, Speechley M, Ginter SF, Risk factors for falls among elderly persons living in the community: N Engl J Med, 1988; 319(26); 1701-7
42. Cipollaro L, Giordano L, Padulo J, Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients: J Orthop Surg, 2020; 15; 178
43. Vaishya R, Jain VK, Iyengar KP, Musculoskeletal manifestations of COVID-19: J Clin Orthop Trauma, 2021; 17; 280-81
44. Ferrandi PJ, Alway SE, Mohamed JS, The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies: J Appl Physiol, 2020; 129(4); 864-67
45. Paliwal VK, Garg RK, Gupta A, Tejan N, Neuromuscular presentations in patients with COVID-19: Neurol Sci, 2020; 41(11); 3039-56
46. Disser NP, De Micheli AJ, Schonk MM, Musculoskeletal consequences of COVID-19: J Bone Jt Surg, 2020; 102(14); 1197-204
47. Woolcott JC, Meta-analysis of the impact of 9 medication classes on falls in elderly persons: Arch Intern Med, 2009; 169(21); 1952-60
48. Das P, Samad N, Ahinkorah BO, Effect of vitamin D deficiency on COVID-19 status: A systematic review: COVID, 2021; 1(1); 97-104
49. Janssen HC, Samson MM, Verhaar HJ, Vitamin D deficiency, muscle function, and falls in elderly people: Am J Clin Nutr, 2002; 75(4); 611-15
50. Dhesi JK, Bearne LM, Moniz C, Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status: J Bone Miner Res, 2002; 17(5); 891-97
51. Pfeifer M, Begerow B, Minne HW, Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals: Osteoporos Int, 2009; 20(2); 315-22
52. Boyé NDA, van der Velde N, de Vries OJ, Effectiveness of medication withdrawal in older fallers: Results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial: Age Ageing, 2017; 46(1); 142-46
53. Johnston C, Brown ER, Stewart J, Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: A randomized clinical trial: EClinicalMedicine, 2021; 33; 100773
54. Liang S-C, Wei P-C, Ma H-L, Hsiao S-H, Higher fall rate of admitted patients during the ongoing COVID-19 epidemic: Is it coincidence or not?: J Patient Saf, 2021; 17(1); e45-46
55. Yamada M, Kimura Y, Ishiyama D, Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in Japan: A cross-sectional online survey: J Nutr Health Aging, 2020; 24(9); 948-50
56. Klenk J, Kerse N, Rapp K, Physical activity and different concepts of fall risk estimation in older people – results of the ActiFE-Ulm study: PLoS One, 2015; 10(6); e0129098
57. World Health Organization: WHO guidelines on physical activity and sedentary behaviour [Internet] [cited 2021 Sep 18]. Available from:https://www.who.int/publications-detail-redirect/9789240015128
58. Hartholt KA, van Beeck EF, Polinder S, Societal consequences of falls in the older population: Injuries, healthcare costs, and long-term reduced quality of life: J Trauma Inj Infect Crit Care, 2011; 71(3); 748-53
59. James SL, Lucchesi LR, Bisignano C, The global burden of falls: Global, regional and national estimates of morbidity and mortality from the Global Burden of Disease Study 2017: Inj Prev, 2020; 26(Supp 1); i3-11
60. Florence CS, Bergen G, Atherly A, Medical costs of fatal and nonfatal falls in older adults: Medical costs of falls: J Am Geriatr Soc, 2018; 66(4); 693-98
61. Chen S, Prettner K, Kuhn M, Bloom DE, The economic burden of COVID-19 in the United States: Estimates and projections under an infection-based herd immunity approach: J Econ Ageing, 2021; 20; 100328
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952