04 June 2022: Clinical Research
Screening for SARS-CoV-2 Infection in Students at the Medical University of Warsaw, Poland Between November 15 and December 10, 2021 Using a Single Lateral Flow Test, the Panbio™ COVID-19 Ag Rapid Test
Mariusz Gujski1ABCDEG, Paulina Mularczyk-Tomczewska1BCDEF*, Filip Raciborski2ABCDE, Piotr Samel-Kowalik2ABCDE, Łukasz SamolińskiDOI: 10.12659/MSM.936962
Med Sci Monit 2022; 28:e936962
Abstract
BACKGROUND: Education was significantly affected by the coronavirus disease 2019 (COVID-19) pandemic, caused by the SARS-CoV-2 virus. Online learning affects the quality of learning as well as the mental health status of students. Regular screening for COVID-19 may be crucial to provide practical classes during the pandemic. The present study aimed to analyze the usefulness of rapid antigen tests for on-campus COVID-19 screening in real-life conditions at a medical university in Poland.
MATERIAL AND METHODS: This screening study was carried out among students attending practical classes at the Medical University of Warsaw, Poland between November 15 and December 10, 2021, during which a series of rapid antigen tests (Panbio™ COVID-19 Ag Rapid Test Device, nasal) were performed by healthcare professionals (nurses). Out of 104 student groups selected for the study (n=1847 students), 423 individuals from 63 student groups were tested at least once (22.9% response rate). A total of 2295 samples were collected.
RESULTS: Among the participants, 3.4% (n=15) had positive test results. Out of 15 COVID-19 cases, 14 were vaccinated. At least 1 positive COVID-19 case was detected in 8 student groups. In 3 student groups, we observed ≥2 infections that occurred at intervals, which may suggest student-to-student SARS-CoV-2 transmission.
CONCLUSIONS: This study produced real-world data from a COVID-19 screening study and confirmed the usefulness of the rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device nasal) for on-campus COVID-19 screening prior to practical classes. Maintaining a high percentage of participants is crucial to ensuring the effectiveness of on-campus COVID-19 screening.
Keywords: COVID-19, COVID-19 Testing, Point-of-Care Testing, Poland, Students, Medical, COVID-19, Humans, SARS-CoV-2, Students, Universities
Background
The coronavirus disease 2019 (COVID-19) pandemic has significantly affected global health [1,2]. Key areas of everyday life such as education, health, culture, and entertainment have been significantly limited due to anti-epidemic measures worldwide [1–3]. In 2020, most governments decided to temporarily close educational institutions to mitigate the spread of COVID-19 [3,4]. According to United Nations Children’s Fund (UNICEF) estimates, more than 1 billion learners are at risk of falling behind due to school closures in response to the COVID-19 pandemic [3]. Lockdown measures, such as closure of public premises, limiting gatherings, and travel restrictions have also had a marked impact on higher education [5,6]. In response to the anti-epidemic measures, higher education institutions replaced face-to-face learning with online learning [4].
Medical universities are an example of educational facilities that were significantly affected by the COVID-19 pandemic [7]. Practical classes were suspended, and most of the classes during the academic year 2020/2021 were online [3,4]. Practical education is a crucial part of medical education. Consequently, for practical classes, online learning should be applied with caution.
Poland is one of the most populous countries in the European Union (EU) [8]. According to the Central Statistical Office, there are approximately 1.2 million students enrolled in Poland, of which 100 000 are international students [9]. The incidence of COVID-19 and death toll from COVID-19 in Poland are among the highest in the EU [8,10]. The first laboratory-confirmed COVID-19 case was reported on March 4, 2020 [10]. On March 12, 2020, educational institutions in Poland (including universities) were obligated to implement online learning [10]. On May 21, 2020, based on the amendment to the law, universities had the possibility of stationary education in selected practical fields [10,11]. However, most of the practical classes remained suspended [11]. In the academic year 2020/2021, most of the universities had implemented online learning or hybrid education (a mix of stationary classes and e-learning, but most of the classes were online). On October 1, 2021, stationary classes (including practical classes) were resumed [11].
Numerous studies showed that online learning has a negative impact on the quality of learning, examinations, and experiential learning of international students, as well as lifestyle habits and mental health status of university students [12–14]. It is estimated that approximately 40% of students in Poland developed emotional distress during COVID-19 lockdown [12]. Findings from a study carried out among medical students in Poland showed that 70% of students indicated the lack of interactions with patients as the main disadvantages of online learning [13]. Moreover, online learning was considered significantly less effective than face-to-face learning in terms of increasing skills and social competencies [13]. Evidence-based strategies to provide practical classes during the COVID-19 pandemic are needed to maintain a high level of medical education [7].
To provide safe re-opening of educational institutions, different anti-epidemic strategies were implemented worldwide [15]. Most of the strategies are based on basic preventative measures such as mask wearing, sanitization, and enforcement of social distancing [5,6,15]. However, some universities oblige students to show proof of vaccination, recovery, or testing to attend in-person classes (eg, an EU COVID Certificate) [16]. Previously published data showed that screening testing can be an important prevention strategy to limit the spread of COVID-19 in in-person education settings [16–18]. COVID-19 Campus Testing Programs (including point-of-care rapid antigen screening) were launched in numerous universities in the EU as well as in the United States [16,17,19,20].
As of March 2022, according to Polish law, universities or employers do not have a right to verify the COVID-19 vaccination status (eg, EU COVID Certificate) of students or employees (including academic teachers, assistant staff) [6,10,11]. Moreover, COVID-19 vaccination is voluntary, even among healthcare professionals [21]. Therefore, anti-epidemic strategies at the universities in Poland are mostly based on hand hygiene, face mask wearing, and social distancing [6,10].
Medical students can be considered as a high-risk group and may be a source of numerous epidemic clusters in different hospital wards. Infected student can transmit SARS-CoV-2 infection to patients, healthcare workers, education sector workers, or other students [22]. Real-life data on SARS-CoV-2 transmission among medical students are needed to assess the potential risk of SARS-CoV-2 transmission among medical students.
Universal COVID-19 screening of both symptomatic and asymptomatic healthcare workers was implemented in numerous countries (including Poland) [23–25]. However, there are limited data on COVID-19 screening in medical students attending practical classes. Regular screening for COVID-19 may be crucial to provide practical classes during the COVID-19 pandemic. During the pandemic, none of the medical universities in Poland had introduced universal access to COVID-19 tests. Public acceptance and effectiveness of using rapid antigen tests as a method of screening medical students has not been sufficiently investigated.
The aim of this study was to analyze the usefulness of rapid antigen tests such as the Panbio™ COVID-19 Ag Rapid Test Device (nasal) for on-campus COVID-19 screening in real-life conditions at the Medical University of Warsaw, Poland. Particular attention was paid to analyzing the willingness of medical students to participate in voluntary COVID-19 screening, as well as the occurrence of infections within individual groups attending practical classes.
Material and Methods
ETHICS:
The study protocol was approved by the Ethics Board at the Medical University of Warsaw, Warsaw, Poland (approval number: KB/165/2021). Participation in the study was voluntary, anonymous, and free of charge. All participants gave written informed consent before participation in the study.
STUDY DESIGN AND PARTICIPANTS:
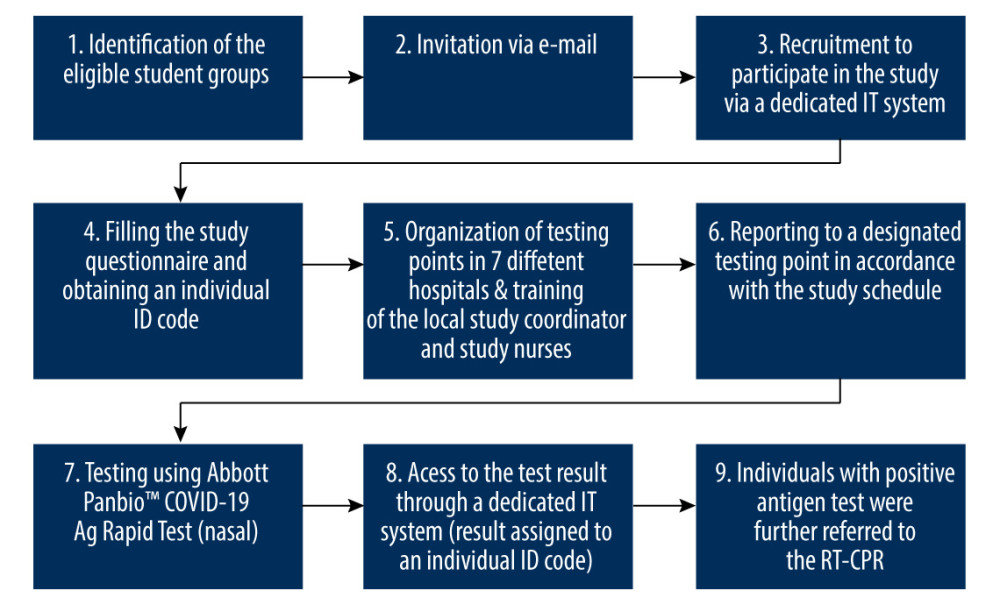
This screening study was carried out among students attending practical classes at the Medical University of Warsaw, between November 15 and December 10 2021.
The list of eligible students (entire student groups) was determined based on the timetables published by the university. Out of all student groups at the Medical University of Warsaw, 104 student groups with a total number of 1847 students were eligible to participate in the study. On average, there were 20–22 subjects in each medical or dental student group, 8 subjects in the nursing student group, and 10–12 in the dental hygiene student group.
Inclusion criteria: All students attending practical classes between November 15 and December 10, 2021, were eligible (for at least 3 weeks).
Exclusion criteria: refusal to participate in the study or lack of written informed consent.
All student groups eligible for the study received an invitation to participate in the study, with basic information about the study procedures provided by e-mail.
The timeframe was based on previous experience from the COVID-19 pandemic in Poland (number of COVID-19 cases in the same period in 2020) as well as epidemic forecasting that suggested the fourth wave of the COVID-19 pandemic in Poland will be observed during the last 2–3 months of 2021 [8]. This study was carried out 1 month after re-opening of universities in Poland. Due to the high COVID-19 incidence rate in Poland, from November 24, 2021, additional swabs were offered to students who presented clinical symptoms of COVID-19/suspected COVID-19 and had classes at the Medical Campus (Banacha Street). Except the 104 student groups selected to take part in the study, 25 students (11 dietetics students; 10 pharmacy students; 2 students of medical analytics, and 1 public health student) who presented clinical symptoms of COVID-19/suspected COVID-19 and had classes at the Medical Campus were included in the study.
SETTINGS:
The study was carried out at 7 hospitals carrying out practical classes for students at the Medical University of Warsaw. Initially, 9 hospitals were planned to be included, but in 2 hospitals the director of the unit did not agree to conduct the study on the premises of the hospital. In total, 7 testing points were launched. The working hours were established in consultation with the students and hospital staff to ensure availability of the testing point for the participants. Students who had classes in 2 hospitals where it was not possible to designate a testing point could be tested at 2 other sites participating in the study. The working hours of the points in these 2 hospitals were extended to allow students from other hospitals to travel to the point. Out of 1847 students invited to take part in the study, 83% (n=1538) had access to testing points at the hospital where they were taking practical classes for the duration of the study. The testing points were located in places easily accessible to students, and each testing point was appropriately marked.
A local coordinator (head nurse of the hospital or the chief epidemiological nurse) was appointed for each of the 7 sites included in the study. The local coordinator was responsible for the organization of the study at a given hospital including designating a testing point in the facility, recruiting nurses responsible for collecting material (nasal swabs), and recruiting study assistants responsible for entering the results into the study database. Before the start of the study, coordinators and study nurses were trained in the study protocol, rules for obtaining informed consent, requirements of sample collection, and test result interpretation.
RECRUITMENT – SCREENING ORGANIZATION MODEL:
Participation in the study was voluntary. According to Polish law, a mandatory COVID-19 test at the university may be carried out as a part of epidemiological surveillance (eg, testing the entire group of students in which a positive case was detected) [10,11]. However, due to the lack of legislative means for mandatory screening at the universities (except epidemiological surveillance), participation of students in screening COVID-19 tests was voluntary. Recruitment to participate in the study took place via a dedicated study portal developed for the purpose of the study [26]. All students invited to participate in this screening study received a link to the online questionnaire. The questionnaire included 25 questions related to the attitudes towards the COVID-19 pandemic, anti-epidemic measures, history of SARS-CoV-2 infection, vaccination status, and sociodemographic characteristics. After completing all the required fields in the questionnaire, the student was given an individual identification (ID) code to ensure anonymity. The ID code was presented by the student to the study nurse at the testing point. The ID code was used to label the samples and to identify the result in a dedicated IT system.
All the eligible students had the opportunity to register and participate in the study throughout the entire study period (ie, the student could complete the registration questionnaire in the last week of the study and visit the testing point on December 8 or December 10).
As this study was voluntary, numerous promotional activities were undertaken to encourage students to participate in the study, including having 2 online meetings with question and answer sessions. In total, individuals (including chairmen of the student groups) from more than 60 student groups took part in the meeting with the study team. Moreover, each student had the opportunity to ask a question about the research via the contact details available on the project’s website. In addition, follow-up e-mails were sent to the chairmen of individual student groups twice a week, reminding them about the date and place of planned swabs. If the student group started classes in the new place (clinics located in different hospitals within the study period), information about the location and working hours of the testing point was provided. Moreover, the students’ self-government of the Medical University of Warsaw (local students’ council) was actively involved in project promotion among the students. Eight posts promoting the project were published on the student council Facebook page (with more than 15 000 followers).
STUDY PROCEDURES:
A series of Abbott Panbio™ COVID-19 Ag Rapid Tests (nasal) were carried out. Panbio™ COVID-19 Ag Rapid Tests (nasal) are listed on the EU Common list of COVID-19 rapid antigen tests and approved by the Health Security Committee [27]. Previously published data showed that the Panbio™ COVID-19 Ag Rapid Test is an effective tool that may be used for rapid testing at the point-of-care in community-based testing centers [28], as well as among asymptomatic individuals [29].
A single nasal swab was collected from each participant and used for the Panbio™ COVID-19 Ag Rapid Test (nasal) performed according to the product Instructions for Use (IFU) [30]. Samples were collected on the premises of the Hospitals conducting practical classes for medical students (testing points) by nursing staff and supervised by a medical team. Rapid antigen tests are widely used and well-recognized among healthcare providers in Poland. The result of the Abbott Panbio™ COVID-19 Ag Rapid Test (nasal) is available within 15 min, which allows for fast identification of infected individuals and justifies the use of this test for screening purposes.
Each student group was given a test schedule with an indication of the date and place of testing. Depending on the schedule (organization of the practical classes), the tests were performed 2 or 3 times a week with 48–72 h intervals (according to the epidemiological measures in place to reduce viral transmission), mostly on Monday, Wednesday, and Friday. Each subject had an opportunity to have 9–12 swabs.
The results were available in the study portal within 60 min from swab collection (on average 30 min). Participants could check their results by entering an individual ID code (the same as the one initially allocated upon registration into the study) in a dedicated field on the project website (identification using an individual ID code).
All individuals who had a positive Panbio™ COVID-19 Ag Rapid Test result were further referred to be tested by reverse-transcription polymerase chain reaction (RT-PCR) to confirm the initial result. RT-PCR test were performed in accredited laboratories in accordance with the SARS-CoV-2 molecular diagnostic guidelines developed by the National Institute of Public Health-National Institute of Hygiene (NIPH-NIH) [31].
Epidemiological surveillance procedures were implemented according to local laws and internal procedures in the hospital. The study flow chart is presented in Figure 1.
STATISTICAL ANALYSIS:
The data were analyzed with SPSS version 28 (IBM, Armonk, NY, USA). The distribution of categorical variables was shown by frequencies and proportions. Statistical significance of differences between continuous variables was analyzed by the independent-samples
Results
Out of 1847 eligible students, 423 (22.9% response rate) reported to the testing point and had at least 1 swab (Table 1). Out of 104 student groups selected for the study, individuals from 63 student groups (60.6% of invited student groups) were tested at least once. During the study period, a total of 2295 samples were collected (Table 1). Out of 423 participants, 15 (3.5%) had a positive Panbio™ COVID-19 Ag Rapid Test result during the study period. Characteristics of the study population are presented in Table 2. The highest percentage of participants with positive test results (15.4%) was observed among the nursing students (Table 3).
Participants with positive Panbio™ COVID-19 Ag Rapid Test were significantly younger (
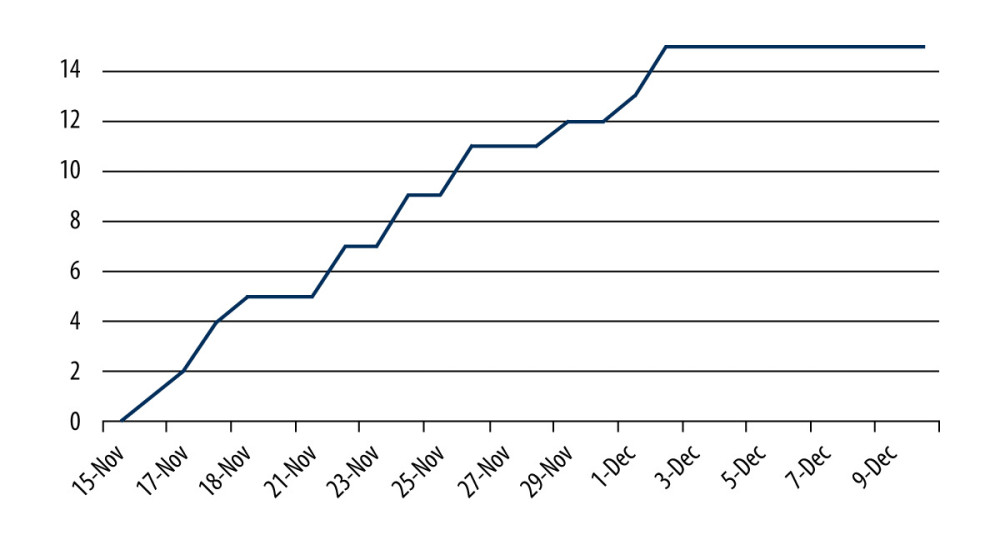
All the participants with positive Panbio™ COVID-19 Ag Rapid Test (n=15) results were referred for the RT-PCR test and 11 participants submitted the RT-PCR results to the study team. All 11 positive Panbio™ COVID-19 Ag Rapid Test results were confirmed in the RT-PCR. Four participants did not inform the study team about the RT-PCR test result. All positive COVID-19 cases were detected between November 16 and December 2, 2021, when the peak of the fourth (IV) wave of the COVID-19 pandemic was reported in Poland (Figure 2).
Out of 63 student groups participating in the study, at least 1 positive COVID-19 case was detected in 8 student groups. Moreover, 1 positive COVID-19 case (dietetics student) was detected in a group of 25 participants who presented clinical symptoms of COVID-19 or had suspected COVID-19 and had classes (but without direct contact with the patients) at the Medical Campus (Banacha Street).
Out of 15 positive COVID-19 cases, 6 were from nursing students. Almost all participants (93.3%) with positive Panbio™ COVID-19 Ag Rapid Test results had been vaccinated. One of the participants had COVID-19 in the past (before participation in the study). Details are presented in Table 4.
Of 15 participants with positive Panbio™ COVID-19 Ag Rapid Test results, 7 participants had positive test results when they were tested for the first time (6 participants who have practical classes, and 1 dietetics student with suspected COVID-19 and who got tested at the testing point). Dentistry students (group D3-N1) who had positive Panbio™ COVID-19 Ag Rapid Test result during the first week of the study (November 15–19, 2021) came back for testing after the end of isolation. Details are presented in Table 5.
In 3 student groups (Table 5), there was more than 1 COVID-19 case diagnosed because of possible COVID-19 transmission within the same student group. In a group of dentistry students (ID Code: D3-N1), 4 COVID-19 cases were detected, of which the first one was detected on November 16, 2021 (ID Code: D3-N1-B), the second one was detected on November 17, 2021 (ID Code: D3-N1-C), and 2 more cases were detected on November 18, 2021, of which D3-N1-D was positive during the first test, but D3-N1-A was negative on November 15, 2021, but positive on November 18, 2021 (infection transmission within the student group). A similar COVID-19 transmission was observed among the nursing students – group I3-03B. In total, 3 COVID-19 cases were detected in this group. The first COVID-19 case (ID Code: I3-03B-C) was detected on November 26, 2021 (during the first test), the second COVID-19 case (ID Code: I3-03B-B) was detected on November 29, 2021 in a participant who was previously negative on November 26, 2021, and the third COVID-19 case (ID Code: I3-03B-A) was detected on December 2, 2021 and it was the third swab of this participant. Moreover, 2 COVID-19 cases were detected in another nursing student group (ID Code I3-08A-B and ID Code I3-08A-A). Details are presented in Table 5.
Discussion
In this study, real-world data from a COVID-19 screening study with rapid antigen tests among medical students in Poland – one of the biggest Central and Eastern Europe countries – have been presented. Findings from this study confirmed the usefulness of the rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device nasal) for on-campus COVID-19 screening. Among the medical students attending practical classes, 3.5% had positive Panbio™ COVID-19 Ag Rapid Test results, which indicated an acute phase of COVID-19 detection. Moreover, in 3 student groups, we observed numerous infections that occurred at intervals, which may suggest student-to-student transmission.
The potential role of medical students as vectors for COVID-19 transmission in clinical hospitals has not been thoroughly investigated [32,33]. However, some authors suggest that medical students acting solely as learners introduce unnecessary risks for patients and other clinicians (eg, consuming personal protective equipment and placing an additional burden on teaching healthcare workers) [22].
The level of knowledge about COVID-19 and infection prevention skills among students of medical faculties are considered higher compared to students of non-medical faculties [34–36]. The COVID-19 vaccination coverage rate among healthcare workers and students of medical faculties is higher compared to the general population [37]. This study showed that students of medical faculties are a high-risk group for COVID-19, despite the high COVID-19 vaccination coverage rate (98.3%) and uptake of third doses of COVID-19 by almost two-thirds of participants (63.5%). Moreover, we observed a significant proportion of participants who did not comply with the facemask use guidelines (35.6% of participants did not change the facemask during the working day). In this study, only 39% of participants declared that they had used FFP2 or FPP3 facemasks. Furthermore, more than two-thirds of participants had traveled by public transport during the work days, which implies exposure to numerous interpersonal contacts.
In this study, participants with positive Panbio™ COVID-19 Ag Rapid Test results were significantly younger than negative subjects. We hypothesize that older students had a higher level of medical knowledge, which also influences anti-epidemic behaviors [38]. Moreover, the percentage of respondents who used FFP2 or FFP3 masks was significantly higher among those who had a negative test result. FFP2 or FFP3 masks are considered more effective and offer a greater degree of protection than surgical masks [39,40]. FFP2 or FFP3 facemask should be considered obligatory equipment for students attending practical classes during the COVID-19 pandemic.
In September 2021, public health institutions in Poland published the general rules for the organization of full-time (stationary) classes at universities [11,13]. Wearing masks, disinfecting hands, keeping a distance, and walking only along designated paths in an educational institution constituted the basic anti-pediatric activities at universities in Poland. In addition, hospital infection control departments may have defined additional anti-epidemic policies within the unit. In this study, only 40% of participants used FFP2 or FFP3 masks and one-third of participants used 1 facemask all day, which may increase the risk of transmission between the students. Moreover, the use of shared changing rooms and eating areas in hospitals can also increase the risk of transmission between students. This hypothesis requires further investigation.
Almost half of participants with positive Panbio™ COVID-19 Ag Rapid Test results and only every-tenth of negative subjects had received AstraZeneca vaccine as the first dose. The effectiveness of COVID-19 vaccines differs among manufacturers [41] and should be considered during the preparation of the recommendations on immunization of medical students.
This study was carried out between November and December 2021, when the peak of the fourth (IV) wave of the COVID-19 pandemic in Poland was observed. All positive COVID-19 cases were detected during the first 3 weeks of the study (November 15 to December 3, 2021). We hypothesize that the transmission of COVID-19 infections among students of medical faculties was parallel to the transmission of COVID-19 in the general population. Moreover, the ongoing COVID-19 pandemic and daily number of laboratory-confirmed COVID-19 cases announced by the public health authorities may also influence students’ attitudes towards participation in voluntary COVID-19 screening (increasing the number of participants).
Findings from the studies carried out among medical students confirmed that online learning has a negative impact on medical education [7,12,13]. Due to this fact, maintaining practical classes even during the COVID-19 lockdowns seems to be an important element of health policy in many countries due to shortages of medical staff and the need to provide qualified medical professionals. Moreover, in some countries, students of medical faculties served as volunteers supporting healthcare professionals during the COVID-19 pandemic [42,43]. In this study, the potential student-to-student transmission was observed. Dates of positive Panbio™ COVID-19 Ag Rapid Test results in a group of dentistry students (group D3-N1, 4 cases detected) and among nursing students (group I3-03B, 3 cases detected; group I3-08A, 2 cases detected) showed that students attending practical classes may be a vector for COVID-19 transmission within the student group. We hypothesize that these students can also transmit COVID-19 to patients and healthcare workers, but data from in-hospital epidemiological surveillance were unavailable.
Rapid diagnosis of COVID-19 infection and subsequent contact tracing are essential in the containment of the pandemic. Rapid antigen tests are used in healthcare facilities for screening healthcare workers [44,45]. Universal screening programs in healthcare facilities are essential to detect asymptomatic infections [45]. Findings from the baseline voluntary screening of 4040 healthcare workers in 12 public hospitals and medical centers in Egypt showed that 4.2% of healthcare workers were COVID-19-positive [23]. Out of 170 infected healthcare workers, 68.2% were asymptomatic [23]. In a study among 1032 asymptomatic healthcare workers in a large UK teaching hospital, 3% of healthcare workers tested positive for COVID-19 [24]. In this study, 3.5% of students of medical faculties tested positive for COVID-19. This percentage is comparable to those observed among healthcare professionals attending COVID-19 screening.
This study showed that willingness to participate in voluntary and free-of-charge COVID-19 screening before/after practical classes at the medical university differed by faculty. Moreover, the model of the organization of screening on the medical campus presented in this study can be used by other research teams. Further research should assess the factors associated with willingness to participate in on-campus screening programs among medical students.
According to Polish law, the COVID-19 testing at the university may be carried out as a part of epidemiological surveillance (eg, testing the entire group of students in which a positive case was detected). However, due to the lack of legislative means for screening at the universities (except epidemiological surveillance), the participation of students in screening tests is voluntary. Despite the extensive informative activities, engagement of the student council and the university authorities, the participation rate was 23%. We observed a lack of interest in COVID-19 screening among students of obstetrics, dental hygiene, and emergency medical services. However, more than 85% of participants had at least 2 swabs, which may indicate that nasal swabs were well-appreciated by the participants and should be considered as the first choice in the screening studies.
This study has several practical implications. Our study provides real-life data on COVID-19 screening at the largest medical university in Poland. This study confirmed that students of medical faculties are at higher risk of COVID-19 infection, even despite full COVID-19 vaccination. On-campus, COVID-19 screening should be considered as a standard of medical education during the COVID-19 pandemic. A moderate response rate presented in this study suggests that the introduction of an on-campus COVID-19 screening test should be supported by the legislative framework. Moreover, rapid antigen tests with nasal swabs that minimalize discomfort during sample collection may have an impact on the willingness of students to undergo regular COVID-19 screening. Moreover, the on-campus screening model presented in this study can be implemented by other countries (especially in Eastern Europe) with a similar legislative framework on COVID-19.
This study has several limitations. First, out of all invited individuals, only 22.9% had at least 1 swab. The real number of COVID-19 cases may be higher due to the lack of testing of all students attending practical classes during the study period, especially those individuals from student groups with at least 1 confirmed COVID-19 case. Secondly, due to lack of access to the epidemiological surveillance data, a detailed analysis of epidemic outbreaks (including data on patients and healthcare workers infected, because of close contact with COVID-19-positive students) was not carried out. Thirdly, other factors, such as using public transport, practicing infection control in the class, and number of COVID-19-positive among household members were not considered in this study.
Conclusions
This study produced real-world data from a COVID-19 screening study and confirmed the usefulness of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device nasal) for on-campus COVID-19 screening prior to practical classes. Maintaining a high percentage of participants is crucial to ensuring the effectiveness of on-campus COVID-19 screening. Despite the extensive informative activities, we observed a lack of interest in COVID-19 screening among students of obstetrics, dental hygiene, and emergency medical services. An effective on-campus COVID-19 screening program requires the provision of legal standards requiring testing in high-risk groups.
Tables
Table 1. Number of samples collected during the study period (November 15 and December 10, 2021).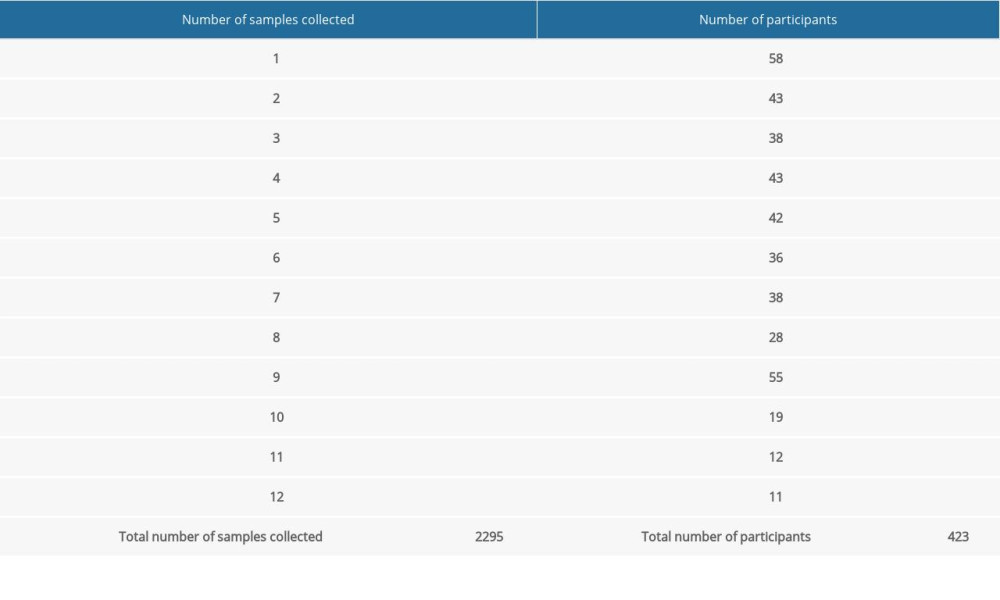 Table 2. Characteristics of the study population by rapid antigen test result (n=423).
Table 2. Characteristics of the study population by rapid antigen test result (n=423).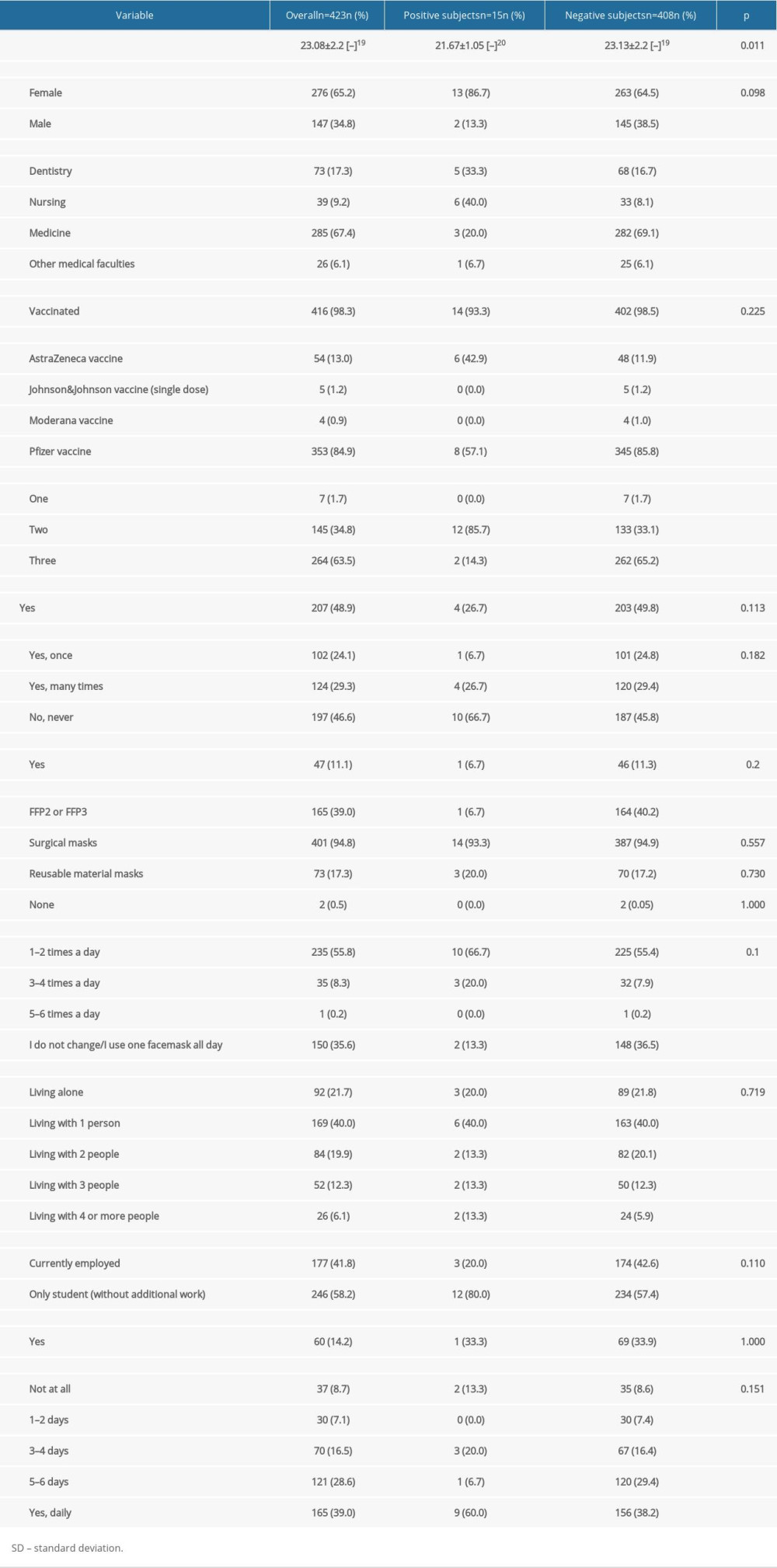 Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test.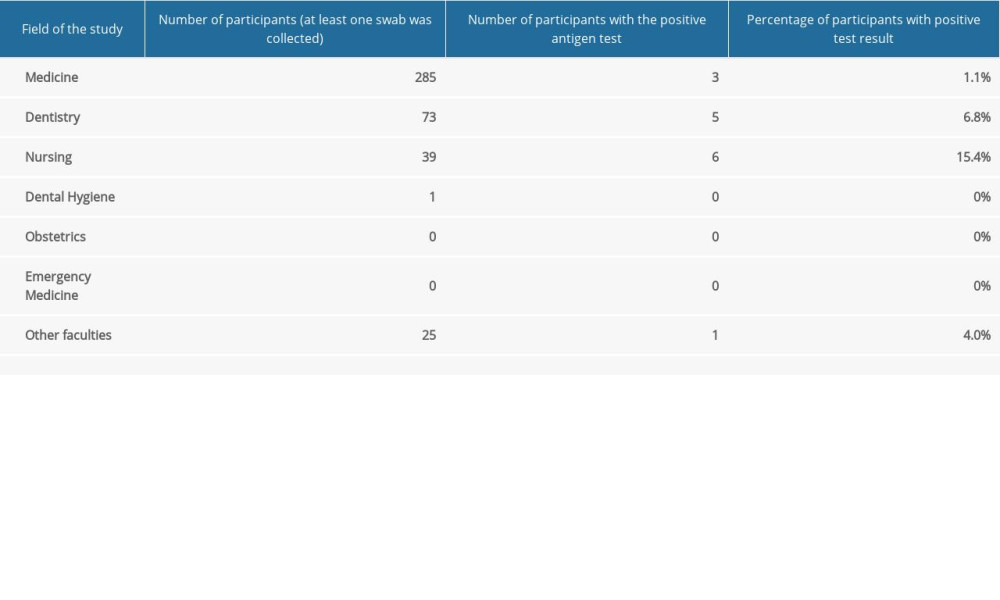 Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test.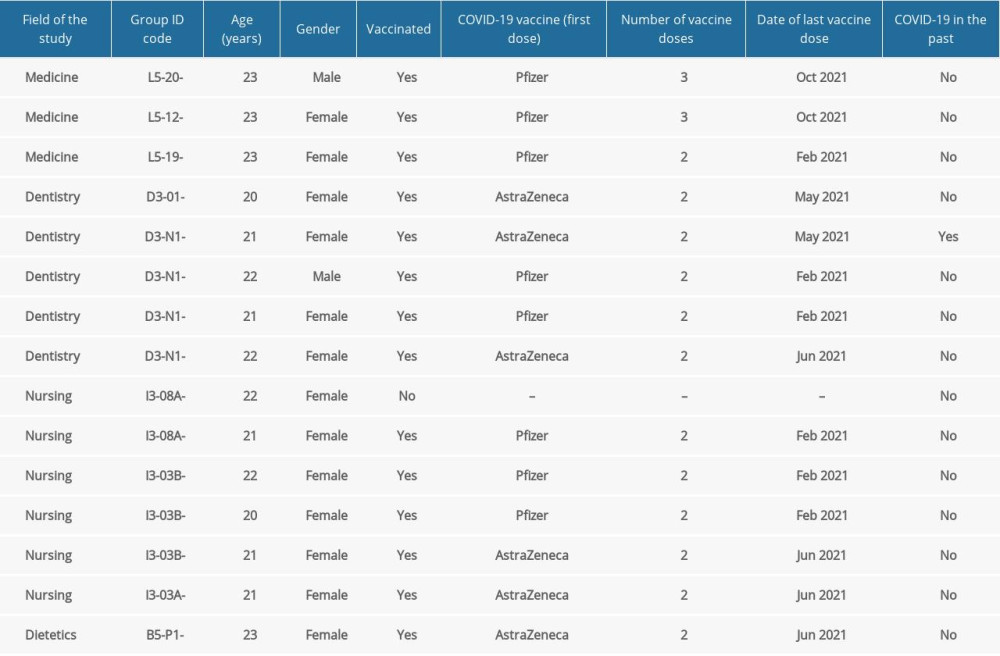 Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results.
Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results.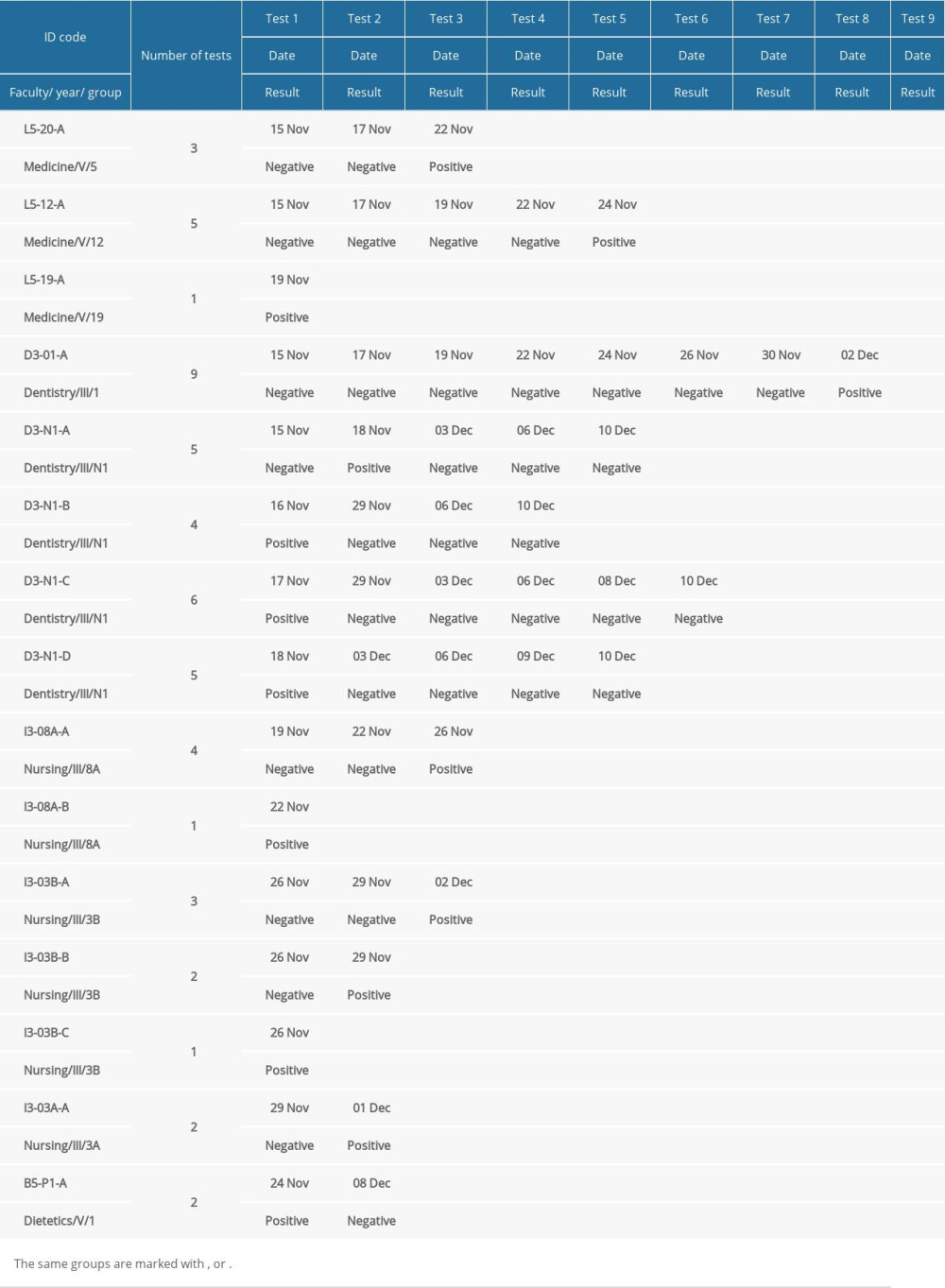
References
1. Kolahchi Z, De Domenico M, Uddin LQ, COVID-19 and its global economic impact: Adv Exp Med Biol, 2021; 1318; 825-37
2. Nicola M, Alsafi Z, Sohrabi C, The socio-economic implications of the coronavirus pandemic (COVID-19): A review: Int J Surg, 2020; 78; 185-93
3. United Nations Children’s Fund (UNICEF): Education and COVID-19 [Internet] September, 2020 Available from; https://data.unicef.org/topic/education/covid-19/
4. Hammerstein S, König C, Dreisörner T, Effects of COVID-19-related school closures on student achievement – a systematic review: Front Psychol, 2021; 12; 746289
5. Iezadi S, Gholipour K, Azami-Aghdash S, Effectiveness of non-pharmaceutical public health interventions against COVID-19: A systematic review and meta-analysis: PLoS One, 2021; 16(11); e0260371
6. Ayouni I, Maatoug J, Dhouib W, Effective public health measures to mitigate the spread of COVID-19: A systematic review: BMC Public Health, 2021; 21(1); 1015
7. Ferrel MN, Ryan JJ, The impact of COVID-19 on medical education: Cureus, 2020; 12(3); e7492
8. Worldometer: COVID-19 coronavirus pandemic [Internet] Continually updated. Available from: https://www.worldometers.info/coronavirus/
9. Central Statistical Office (GUS): Higher education in the 2018/2019 academic year (preliminary results) [Internet] June 14, 2019 Available from: [in Polish]https://stat.gov.pl/obszary-tematyczne/edukacja/edukacja/szkolnictwo-wyzsze-w-roku-akademickim-20182019-wyniki-wstepne,8,6.html
10. Pinkas J, Jankowski M, Szumowski Ł, Public health interventions to mitigate early spread of SARS-CoV-2 in Poland: Med Sci Monit, 2020; 26; e924730
11. Polish Ministry of Education and Science: Organization of education in the new academic year. Recommendations for university authorities [Internet] September 2, 2020 Available from[in Polish]https://www.gov.pl/web/edukacja-i-nauka/organizacja-ksztalcenia-w-nowym-roku-akademickim-rekomendacje-dla-wladz-uczelni
12. Juchnowicz D, Baj J, Forma A, The outbreak of SARS-CoV-2 pandemic and the well-being of polish students: The risk factors of the emotional distress during COVID-19 lockdown: J Clin Med, 2021; 10(5); 944
13. Bączek M, Zagańczyk-Bączek M, Szpringer M, Students’ perception of online learning during the COVID-19 pandemic: A survey study of Polish medical students: Medicine (Baltimore), 2021; 100(7); e24821
14. Stojan J, Haas M, Thammasitboon S, Online learning developments in undergraduate medical education in response to the COVID-19 pandemic: A BEME systematic review: BEME Guide No. 69: Med Teach, 2022; 44(2); 109-29
15. Mukherjee UK, Bose S, Ivanov A, Evaluation of reopening strategies for educational institutions during COVID-19 through agent based simulation: Sci Rep, 2021; 11(1); 6264
16. Erudera College News: More German Universities Oblige Students to Show Proof of Vaccination, Recovery or Testing to Attend In-Person Classes [Internet] November 14, 2021 Available from: https://collegenews.org/more-german-universities-oblige-students-to-show-proof-of-vaccination-recovery-or-testing-to-attend-in-person-classes/
17. University of Arizona: COVID-19 Testing [Internet] April, 2022 Available from: https://covid.arizona.edu/covid19-testing
18. Centers for Disease Control and Prevention (CDC): Guidance for Institutions of Higher Education (IHEs) [Internet] February 7, 2022 Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universities/considerations.html
19. Pollock BH, Kilpatrick AM, Eisenman DP, Safe reopening of college campuses during COVID-19: The University of California experience in Fall 2020: PLoS One, 2021; 16(11); e0258738
20. Nerhood KJ, James ER, Hardin A, Screening programs for SARS-CoV-2 infections on a University Campus – Austin, Texas, September 30–November 30, 2020: MMWR Morb Mortal Wkly Rep, 2021; 70(35); 1201-5
21. Raciborski F, Jankowski M, Gujski M, Changes in attitudes towards the COVID-19 vaccine and the willingness to get vaccinated among adults in Poland: Analysis of serial, cross-sectional, representative surveys, January–April 2021: Vaccines (Basel), 2021; 9(8); 832
22. Hill EM, Atkins BD, Keeling MJ, Modelling SARS-CoV-2 transmission in a UK university setting: Epidemics, 2021; 36; 100476
23. Mostafa A, Kandil S, El-Sayed MH, Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: An Egyptian pilot model in a university with 12 public hospitals and medical centers: Int J Epidemiol, 2021; 50(1); 50-61
24. Rivett L, Sridhar S, Sparkes D, Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission: Elife, 2020; 9; e58728
25. Black JRM, Bailey C, Przewrocka J, COVID-19: The case for health-care worker screening to prevent hospital transmission: Lancet, 2020; 395(10234); 1418-20
26. Medical University of Warsaw: Official website of the research project entitled: The prevalence of SARS-CoV-2 infections among students at the Medical University of Warsaw [Internet] April 14, 2022 Available from: [in Polish[https://przesiewcovid.wum.edu.pl/
27. European Commission: Directorate-General for Health and Food Safety. EU Health Preparedness: A Common List of COVID-19 Rapid Antigen Tests. Agreed by the Health Security Committee [Internet], 2022 Available online: https://ec.europa.eu/health/system/files/2022-05/covid-19_rat_common-list_en.pdf
28. Berger A, Nsoga MTN, Perez-Rodriguez FJ, Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers: PLoS One, 2021; 16(3); e0248921
29. Shaw JLV, Deslandes V, Smith J, Desjardins M, Evaluation of the Abbott Panbio™ COVID-19 Ag rapid antigen test for the detection of SARS-CoV-2 in asymptomatic Canadians: Diagn Microbiol Infect Dis, 2021; 101(4); 115514
30. Abbott: The Panbio™ COVID-19 Ag Rapid Test Device (nasal) [Internet], 2022 Available online: https://dam.abbott.com/en-gb/panbio/120007883-v1-Panbio-COVID-19-Ag-Nasal-AsymptomaticSe.pdf
31. National Institute of Public Health-National Institute of Hygiene: Recommendations of the NIPH-NIH in the field of SARS-CoV-2 molecular diagnostics update on May 31, 2021 [Internet] May 31, 2021 Available online: [in Polish]https://www.pzh.gov.pl/wp-content/uploads/2021/05/Rekomendacje-dot.-interpretacji-testow-rtPCR-z-31.05.2021.pdf?msclkid=034cfd63d14211ec9ce3d8a94b6f125a
32. Rennert L, McMahan C, Kalbaugh CA, Surveillance-based informative testing for detection and containment of SARS-CoV-2 outbreaks on a public university campus: An observational and modelling study: Lancet Child Adolesc Health, 2021; 5(6); 428-36
33. O’Byrne L, Gavin B, McNicholas F, Medical students and COVID-19: The need for pandemic preparedness: J Med Ethics, 2020; 46(9); 623-26
34. Khasawneh AI, Humeidan AA, Alsulaiman JW, Medical students and COVID-19: Knowledge, attitudes, and precautionary measures. A descriptive study from Jordan: Front Public Health, 2020; 8; 253
35. Stefanowicz-Bielska A, Słomion M, Stefanowicz J, COVID-19 pandemic-knowledge, attitudes, behaviours, and actions among faculty of health sciences students: Int J Environ Res Public Health, 2021; 18(22); 12137
36. Gao Z, Ying S, Liu J, A cross-sectional study: Comparing the attitude and knowledge of medical and non-medical students toward 2019 novel coronavirus: J Infect Public Health, 2020; 13(10); 1419-23
37. European Centre for Disease Prevention and Control (ECDC): COVID-19 vaccination [Internet], 2022 Available from: https://www.ecdc.europa.eu/en/covid-19/prevention-and-control/vaccines
38. Raupach T, Vogel D, Schiekirka S, Increase in medical knowledge during the final year of undergraduate medical education in Germany: GMS Z Med Ausbild, 2013; 30(3); Doc33
39. Kim MC, Bae S, Kim JY, Effectiveness of surgical, KF94, and N95 respirator masks in blocking SARS-CoV-2: A controlled comparison in 7 patients: Infect Dis (Lond), 2020; 52(12); 908-12
40. Chou R, Dana T, Jungbauer R, Update alert 6: Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings: Ann Intern Med, 2021; 174(9); W68
41. Mayo Clinic: Comparing the differences between COVID-19 vaccines [Internet] April 1, 2022 Available from: https://www.mayoclinic.org/coronavirus-covid-19/vaccine/comparing-vaccines
42. Ali A, Staunton M, Quinn A, Exploring medical students’ perceptions of the challenges and benefits of volunteering in the intensive care unit during the COVID-19 pandemic: A qualitative study: BMJ Open, 2021; 11(12); e055001
43. Domaradzki J, Walkowiak D, Medical students’ voluntary service during the COVID-19 pandemic in Poland: Front Public Health, 2021; 9; 618608
44. Kolwijck E, Brouwers-Boers M, Broertjes J, Validation and implementation of the Panbio COVID-19 Ag rapid test for the diagnosis of SARS-CoV-2 infection in symptomatic hospital healthcare workers: Infect Prev Pract, 2021; 3(2); 100142
45. Aguilar-Shea AL, Vera-García M, Güerri-Fernández R, Rapid antigen tests for the detection of SARS-CoV-2: A narrative review: Aten Primaria, 2021; 53(9); 102127
Figures
Tables
 Table 1. Number of samples collected during the study period (November 15 and December 10, 2021).
Table 1. Number of samples collected during the study period (November 15 and December 10, 2021). Table 2. Characteristics of the study population by rapid antigen test result (n=423).
Table 2. Characteristics of the study population by rapid antigen test result (n=423). Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test. Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test. Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results.
Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results. Table 1. Number of samples collected during the study period (November 15 and December 10, 2021).
Table 1. Number of samples collected during the study period (November 15 and December 10, 2021). Table 2. Characteristics of the study population by rapid antigen test result (n=423).
Table 2. Characteristics of the study population by rapid antigen test result (n=423). Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 3. Number of participants with the positive Panbio™ COVID-19 Ag Rapid Test. Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test.
Table 4. Detailed characteristics of the participants with the positive Panbio™ COVID-19 Ag Rapid Test. Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results.
Table 5. Dates of Panbio™ COVID-19 Ag Rapid Tests and the test results. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952