30 August 2023: Clinical Research
An Examination of Infection Incidence and Management in Systemic Lupus Erythematosus Patients: A Five-Year Review from a Saudi Arabian Center
Noha K. Khalil1ABDF, Fahidah AlenziDOI: 10.12659/MSM.941277
Med Sci Monit 2023; 29:e941277
Abstract
BACKGROUND: Systemic lupus erythematosus (SLE) is a chronic autoimmune condition often associated with an increased susceptibility to infections. The infections in patients with SLE, primarily involving the skin, respiratory tract, and urinary tract, can significantly complicate disease management. This study aimed to evaluate the occurrence, management, and patient outcomes associated with infections in a group of 74 SLE patients at a single center in Saudi Arabia, spanning a 5-year period.
MATERIAL AND METHODS: An observational, retrospective study was conducted at the King Khalid University Hospital, Riyadh, Saudi Arabia. Patient medical records from January 2016 to December 2020 were examined. All adult SLE patients (age >14 years, as per hospital policy), confirmed by SLICC criteria, and admitted due to infections (determined by quick Sequential Organ Failure Assessment or qSOFA scores) were included in the study.
RESULTS: Of the 74 SLE patients studied, 79.7% were administered hydroxychloroquine. A majority (83.8%) were classified as low-risk for sepsis-associated mortality based on qSOFA scores (0-1), a fact noted by 41.9% of rheumatology fellows. The sputum cultures most frequently identified were Klebsiella pneumoniae, yeast, and Haemophilus influenzae (each accounting for 33.3% of cases). Furthermore, 4.1% of patients had extended-spectrum beta-lactamases infections, and 2.7% tested positive for COVID-19. A history of sepsis was more commonly observed among non-survivors (P=0.010).
CONCLUSIONS: The majority of patients were classified as low-risk for sepsis-associated mortality based on qSOFA scores, with two-thirds prescribed antibiotics within 1 h. The primary causes of death were multiorgan failure and cardiac arrest.
Keywords: Autoimmune Diseases, Bacterial Infections, Immunosuppressive Agents, Lupus Erythematosus, Systemic, Saudi Arabia, Adolescent, Adult, Humans, COVID-19, Hospitals, University, Incidence, Retrospective Studies
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple organs. SLE has numerous phenotypes and clinical manifestations, ranging from mild mucocutaneous to severe central nervous system and multiple organ involvement manifestations [1–3]. Patients with SLE are at high risk for infection owing to multiple factors, such as the disease itself with high disease activity, renal involvement, and treatment-related risk [4,5]. Infections can resemble SLE exacerbations, making a diagnosis and adequate treatment difficult to determine. Patients with SLE are more susceptible than the general population to acquire bacterial, viral, or fungal infections, as well as common pathogens that typically show an aggressive course in SLE patients [6]. Clinically differentiating lupus flare-ups from infections in SLE patients who experience fevers requires consideration of C-reactive protein levels and disease activity markers in addition to clinical symptoms and physical examination [7,8].
Infections are the primary cause of hospitalization in patients with SLE and among the leading causes of mortality [9–12]. This is due to both the immunosuppressives used to treat SLE and the disease itself [13,14]. The risk of developing SLE is 14 times higher in individuals with Klinefelter syndrome, and women are 10 times more likely to develop SLE than are men. This is due to its correlation with X-chromosome genes. However, despite numerous investigations, the specific genes implicated in the development of SLE have not been discovered [15–17].
Infections can mimic SLE exacerbations, rendering their diagnosis and recommended course of action unclear. Although this problem has received increasing attention, infections remain the primary factor that drives morbidity and mortality in patients with SLE, particularly in the first year after SLE onset. Some regions have reported an increase in infection-associated SLE mortality, particularly with pneumonia and septicemia [18]. In developing countries, the survival rates of patients with SLE are low, with early death common due to infection and active disease [19]. Certain aspects of infection management have been proven to improve morbidity and mortality. The Surviving Sepsis Campaign guidelines recommend early broad-spectrum antibiotic administration within 1 h and immediate resuscitation in case of shock, with specific targets within the first 6 h [20]. In a local single-center study, implementing a sepsis protocol in an emergency department led to the quicker identification of sepsis and earlier introduction of antibiotic therapy [21] using the Qsofa score (systolic blood pressure ≤100 mmHg, respiratory rate ≥22 breaths/min, altered mental state, with Glasgow coma scale <15) [4]. Owing to the impact of adequate infection management and the high risk of infection in patients with SLE, studying infection management flow in hospitals can help identify any shortcomings and enable the development of protocols to correct and prevent these shortcomings. Therefore, in this observational and retrospective study, we aimed to evaluate infections and their management in 74 patients with SLE between January 2016 and December 2020 at a single center in Saudi Arabia.
Material and Methods
STUDY DESIGN:
This retrospective study was approved by the Ethics Committee of College of Medicine, King Saud University (E18-3582) and conducted at the King Khalid University Hospital in Riyadh, Saudi Arabia. Data were collected from medical records between January 2016 and December 2020. As this was a retrospective study using anonymized data, written consent was not required.
PATIENTS:
The study population included all adult patients with SLE (aged >14 years, as specified by the hospital policy) who were admitted with infections. We reviewed patient files and used the SLICC criteria to confirm each SLE diagnosis [22]. Data on age, sex, disease duration, vital signs upon admission, body mass index (BMI), other associated diseases (such as antiphospholipid syndrome, Sjogren syndrome, and COVID-19), blood, urine, sputum culture, lactate levels, drugs (such as immunosuppressants), biologics, and patient presentation outcomes (including length of hospital stay, critical care unit admission, and death) were collected. Further data on time to emergency department (ED) physician evaluation, internal medicine physician evaluation, rheumatology physician evaluation, and empirical antibiotic administration were calculated. The SLICC index and qSOFA scores were calculated for each patient. Pediatric patients (<14 years) and those who did not meet the SLICC criteria [22] were excluded.
STATISTICAL ANALYSIS:
We used the Statistical Package for Social Sciences (SPSS) version 26 (IBM Corp, Armonk, NY, USA) to analyze the data after it had been entered into an Excel spreadsheet. Descriptive statistics were presented as numbers, percentages, means, and standard deviations. We used Fisher’s exact test in a univariate analysis to identify factors linked to death. Statistical collinearity was measured using the Shapiro-Wilk test. Statistical significance was set at
Results
BASELINE CHARACTERISTICS:
This study analyzed 74 patients diagnosed with SLE. The mean age was 37.2±14.5 years, with most being men (85.1%). Regarding BMI, 43.2% had a normal BMI, 24.3% had overweight, and 16.2% had class I obesity. The mean disease duration was 8.27±7.75 years. For associated diseases, 5.4%, 1.4%, 14.9%, and 31.1% of patients had associated malignancies, secondary Sjogren syndrome, secondary antiphospholipid syndrome, and previous sepsis, respectively. Overall, 75.7% of patients had used prednisolone, with low-dose prednisolone being the most common (55.4%). Similarly, 79.7% had received hydroxychloroquine, with the most commonly prescribed dose being 200 mg daily (76.3%). Other prescribed medications included mycophenolic acid (40.5%), cyclophosphamide (8.4%), rituximab (5.4%), and tacrolimus (1.4%) (Table 1).
CLINICAL AND LABORATORY CHARACTERISTICS:
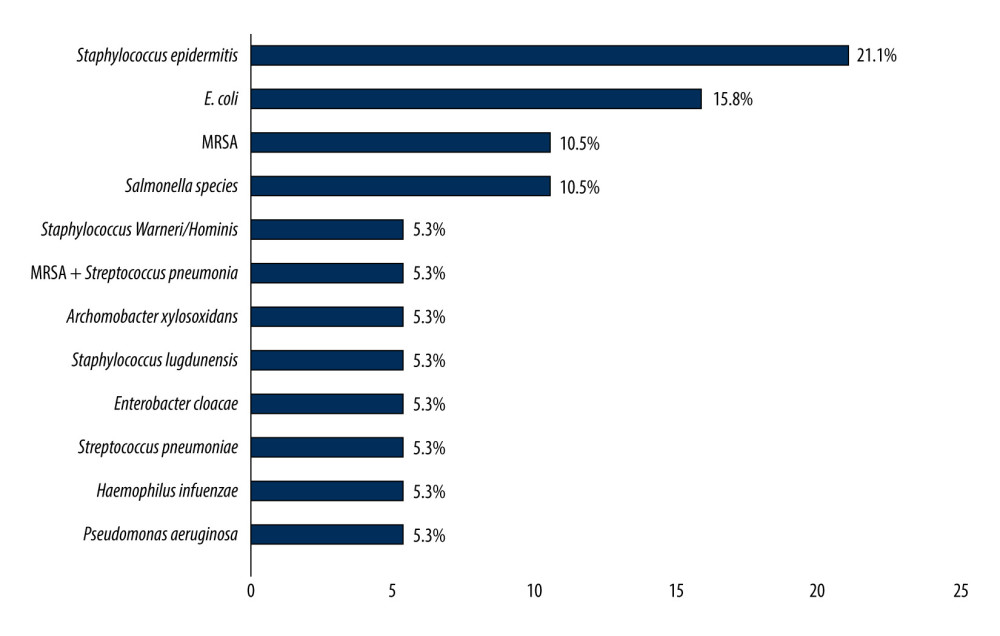
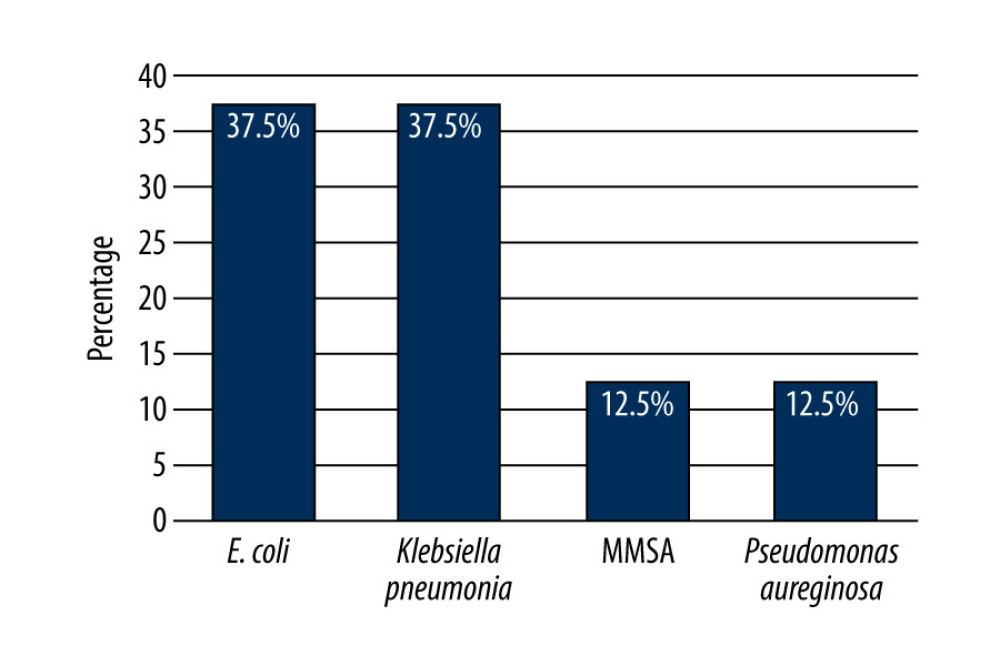
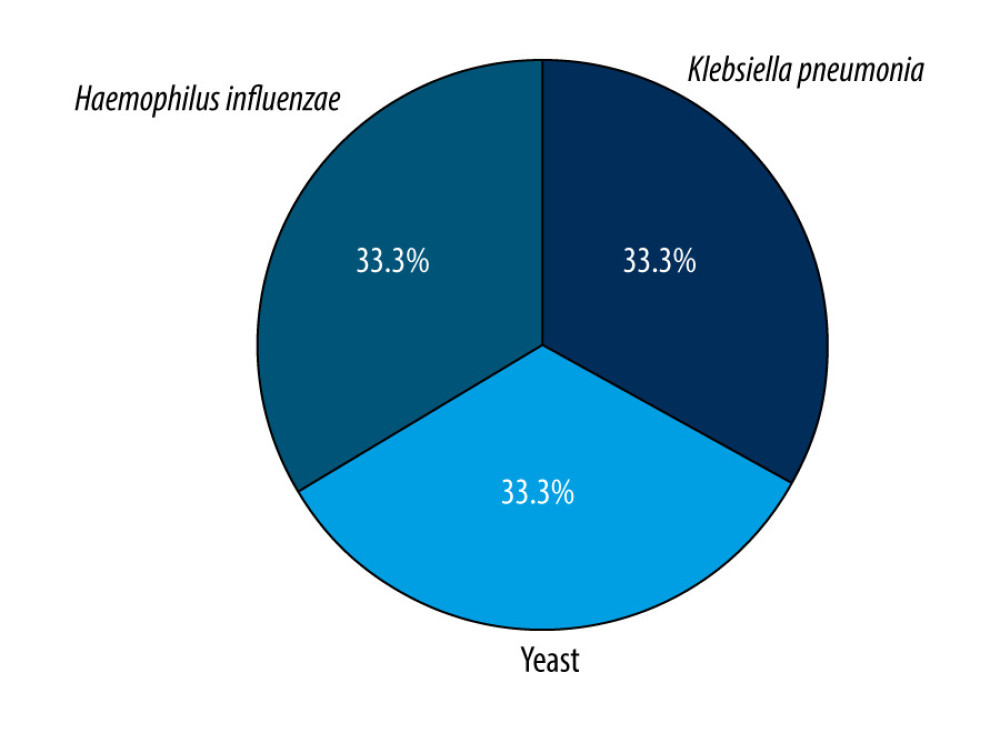
Upon presentation, patients’ mean vital measurements were as follows: temperature, 37.3 °C; systolic blood pressure, 120.4 mmHg; diastolic blood pressure, 70.4; heart rate, 100.7 beats/min; and respiratory rate, 20.3 breaths/min. Overall, 25.7%, 10.8%, and 4.1% of patients returned positive blood, urine, and sputum cultures for different types of bacteria, respectively. The most commonly detected bacteria in blood cultures were Staphylococcus epidermidis (21.1%), Escherichia coli (15.8%), methicillin-resistant Staphylococcus aureus (MRSA) (10.5%), Salmonella spp. (10.5%), and extended-spectrum beta-lactamase (4.1%) (Figure 1). The most commonly detected in urinary cultures were E.Coli (37.5%) and Klebsiella pneumoniae (37.5%) (Figure 2). The most common infectious agents identified in sputum cultures were K. pneumoniae (33.3%), yeast (33.3%), and Haemophilus influenzae (33.3%) (Figure 3). Additionally, 2.7% of patients were positive for COVID-19. Regarding lactate levels, 48.6% of patients did not have these measured, 6.8% had levels >4 mmol/L, and 35.1% had normal levels (Table 2).
FACTORS RELATED TO THE MONITORING AND MANAGEMENT FLOW OF INFECTIONS:
The median hours of triage to the ED physician, internal medicine physician, rheumatology physician, and culture drawing were 2, 5, 19, and 6 h, respectively. Fifteen (20.3%) patients waited over 6 h to be transferred to the Intensive Care Unit (ICU) after ED presentation, 47 (63.5%) did not require ICU admission, and 7 (9.5%) were transferred to the ICU after admission to the medical ward. Based on the qSOFA scores, 62 (83.8%) patients were categorized as low risk for sepsis-associated mortality, which was noted by 41.9% of the rheumatology fellows. Two-thirds (63.5%) of the patients were prescribed antibiotics after the first hour. The most commonly prescribed antibiotics were tazocin (20%), vancomycin + tazocin (10%), and carbapenems (8%). The non-survival rate was more common among patients with a history of sepsis (P=0.010), patients transferred to the ICU more than 6 h after admission, patients transferred to the ICU during admission to the medical ward (P=0.023), patients with lactate levels between 2 and 4 mmol/L (P<0.001), and patients with significantly higher SLICC index values (P=0.007) (Table 3). Other patient characteristics during presentation did not significantly influence non-survival rates (P>0.05). Overall, 8.1% of patients did not survive, and the most common causes of death were multiorgan failure (50%) and cardiac arrest (50%). We observed 6 cases of readmission (8.1%). The median hospital stay was 10 days, with a median ICU stay of 2.0 days. The median SLICC index was 2 (Table 4).
Discussion
SLE is a systemic autoimmune disease characterized by abnormal autoantibody production and clearance. It has been hypothesized that SLE patients’ immunological backgrounds contribute to their vulnerability to infections [1]. We analyzed 74 patients diagnosed with SLE. Most patients were men. The mean disease duration was 8.27±7.75 years, and the mean patient age was 37.2±14.5 years. Prednisolone was prescribed in 75.7% of the patients, while 79.7% received hydroxychloroquine, with the most commonly prescribed dose being 200 mg once daily (76.3%). A study conducted among British patients demonstrated that high-dose prednisolone was associated with a higher risk of infection-related death [23]. Immunosuppressive therapy has proven to be beneficial in patients with SLE; however, the associated decrease in immunity and increase in infection is a major concern [24]. Multiple studies have found that high-dose steroid treatment is a risk factor for infection, while several studies have found that antimalarial drugs decrease the risk of infection [25]. In addition to immunosuppressive treatments, disease activity, high anti-DNA titers, low complement levels, nephritis, leukopenia, and antiphospholipid antibodies are associated with a high risk of infection. In this study, most patients were found to not be at high risk for sepsis-associated mortality, based on their qSOFA scores. Positive blood and urine cultures were returned by 25.7% and 10.8% of patients, respectively. Nearly one-quarter (22%) of patients in the study by Minejima et al had qSOFA scores below 2 and thus were classed as being at high risk. Extended-spectrum beta-lactamase was isolated in 4.1% of patients in the present study, and nearly two-thirds (63.5%) were prescribed antibiotics after 1 h, which was similar to a previous study by Jeong et al [26]. A study conducted in a security force hospital found that
This study has some limitations. As this was an observational retrospective study involving a limited sample size, there is the possibility that some data were missing or patient records were incomplete.
Conclusions
The findings from this study showed that most patients were categorized as being at a low risk of sepsis-associated mortality, based on qSOFA scores. Two-thirds of patients were prescribed antibiotics after 1 h. The causes of death were multiorgan failure and cardiac arrest. To investigate the infection risk among Saudi Arabian SLE patients, additional prospective studies involving larger sample sizes and longer follow-up periods are required.
Tables
Table 1. Patients’ baseline characteristics in evaluation and treatment flow of 74 SLE patients with infections.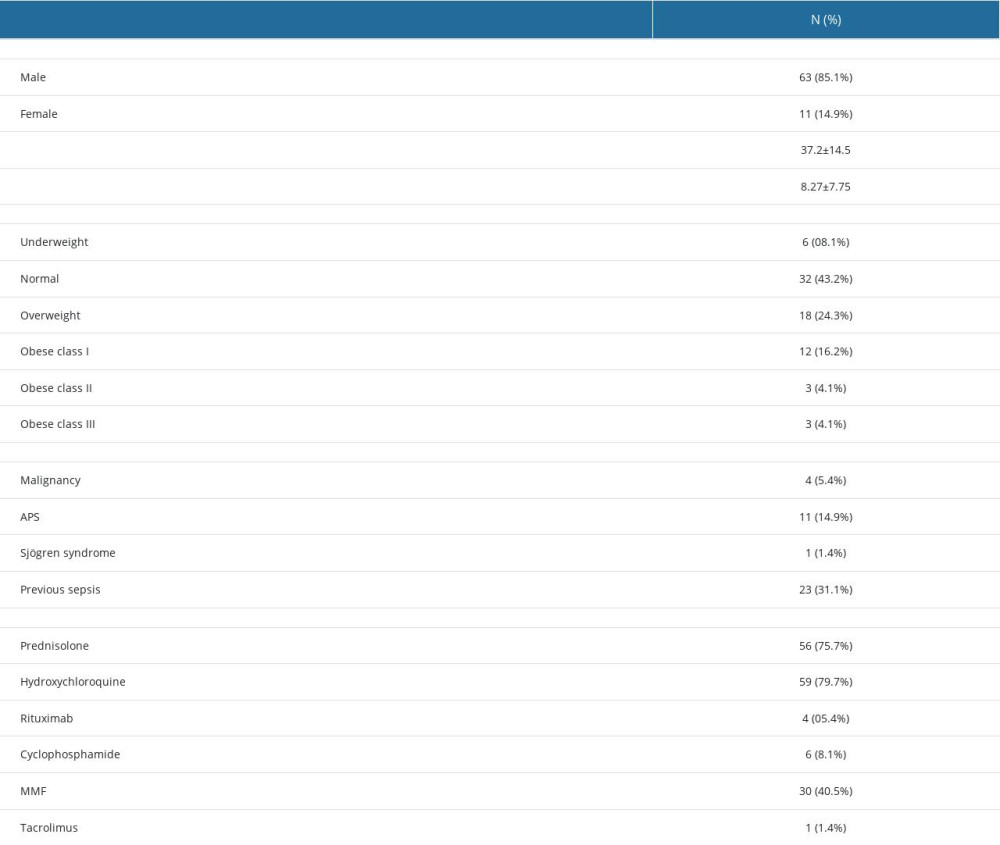 Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections.
Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections.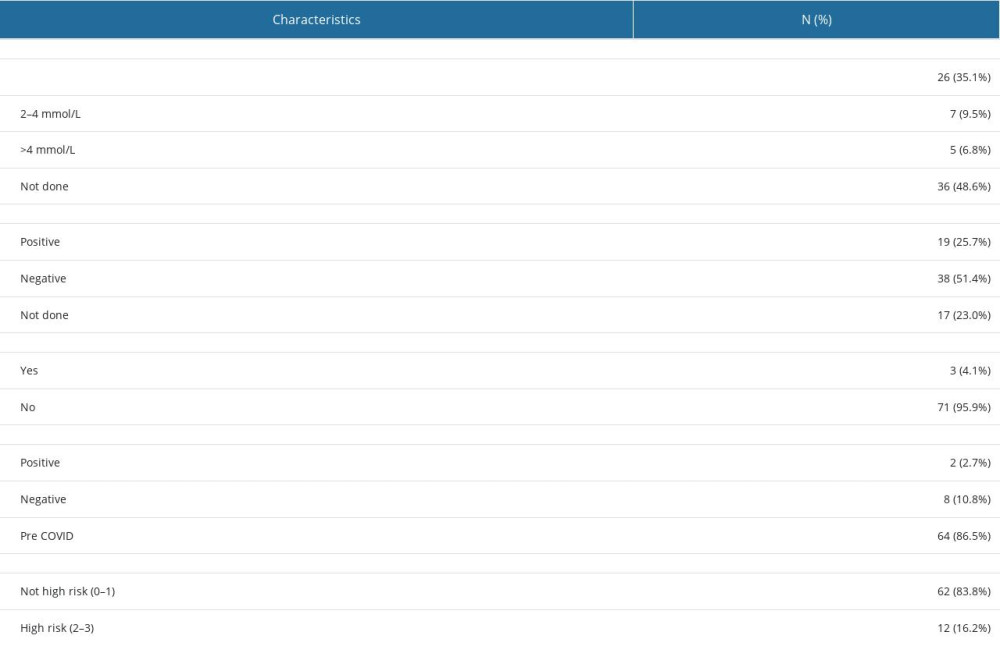 Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients.
Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients.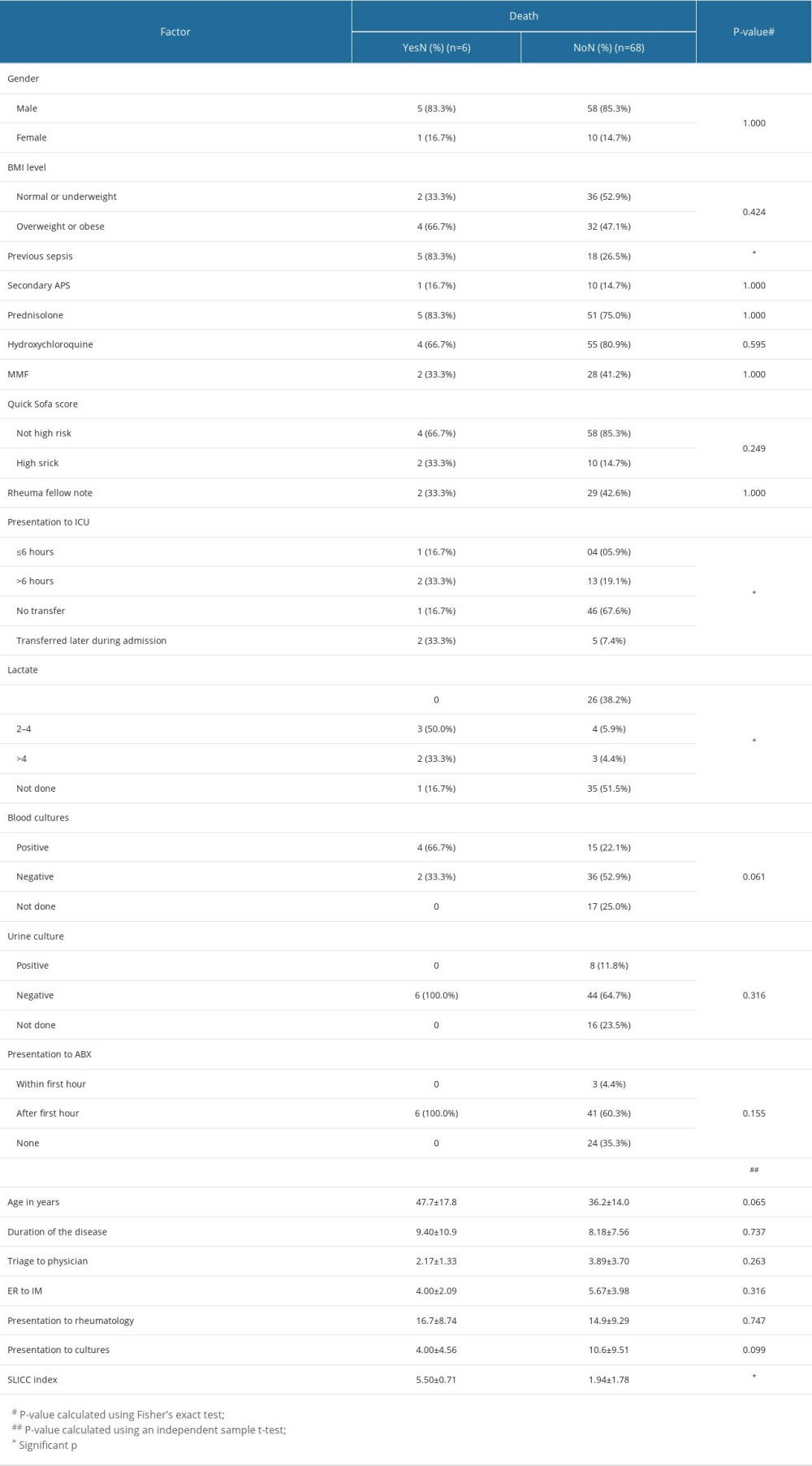 Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections.
Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections.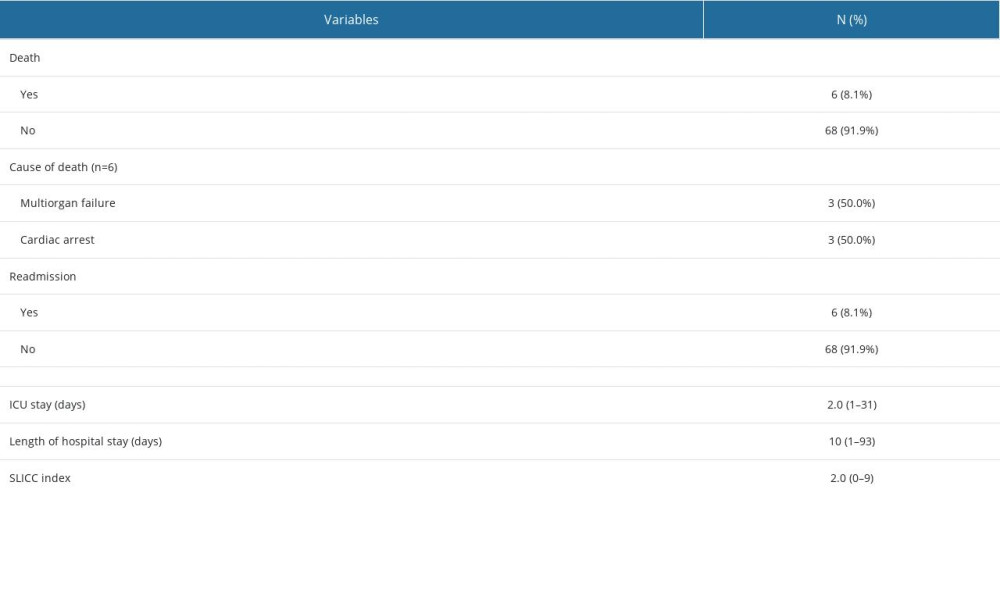
References
1. Sciascia S, Cuadrado MJ, Karim MY, Management of infection in systemic lupus erythematosus: Best Pract Res Clin Rheumatol, 2013; 27(3); 377-89
2. Tsokos GC, Autoimmunity and organ damage in systemic lupus erythematosus: Nat Immunol, 2020; 21(6); 605-14
3. Rhodes B, Vyse TJ, Systemic lupus erythematosus’ in genomic and personalized medicine, 2012; 970-82
4. Fanouriakis A, Kostopoulou M, Alunno A, 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus: Ann Rheum Dis, 2019; 78(6); 736-45
5. Cuchacovich R, Gedalia A, Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus: Rheum Dis Clin North Am, 2009; 35(1); 75-93
6. Sciascia S, Cuadrado MJ, Karim MY, Management of infection in systemic lupus erythematosus: Best Pract Res Clin Rheumatol, 2013; 27(3); 377-89
7. Jung JY, Suh CH, Infection in systemic lupus erythematosus, similarities, and differences with lupus flare: Korean J Intern Med, 2017; 32(3); 429-38
8. Sciascia S, Ceberio L, Garcia-Fernandez C, Systemic lupus erythematosus and infections: Clinical importance of conventional and upcoming biomarkers: Autoimmun Rev, 2012; 12(2); 157-63
9. Devlin A, Shmerling R: Systemic lupus erythematosus and infections, systemic lupus erythematosus: basic, applied and clinical aspects, 2016, Elsevier
10. Edwards CJ, Lian TY, Badsha H, Hospitalization of individuals with systemic lupus erythematosus: Characteristics and predictors of outcome: Lupus, 2003; 12(9); 672-76
11. Cervera R, Khamashta MA, Font J, Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients: Medicine (Baltimore), 2003; 82(5); 299-308
12. Hernández-Cruz B, Tapia N, Villa-Romero AR, Risk factors associated with mortality in systemic lupus erythematosus. A case-control study in a tertiary care center in Mexico City: Clin Exp Rheumatol, 2001; 19(4); 395-401
13. Wang X, Zuo X, Xie X, Risk factors for serious infections in inpatients with systemic lupus erythematosus: Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2021; 46(7); 704-10
14. Ross SC, Densen P, Complement deficiency states and infection: Epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency: Medicine (Baltimore), 1984; 63(5); 243-73
15. Kim JW, Kim HA, Suh CH, Jung JY, Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus: Front Med (Lausanne), 2022; 9; 906475
16. Fei Y, Liu Q, Peng N, Exosomes as crucial players in pathogenesis of systemic lupus erythematosus: J Immunol Res, 2022; 2022; 8286498
17. Tsokos GC, Lo MS, Costa Reis P, Sullivan KE, New insights into the immunopathogenesis of systemic lupus erythematosus: Nat Rev Rheumatol, 2016; 12(12); 716-30
18. Souza DC, Santo AH, Sato EI, Mortality profile related to systemic lupus erythematosus: A multiple cause-of-death analysis: J Rheumatol, 2012; 39(3); 496-503
19. Navarra SV, Leynes MS, Infections in systemic lupus erythematosus: Lupus, 2010; 19(12); 1419-24
20. De Backer D, Dorman T, Surviving sepsis guidelines: A continuous move toward better care of patients with sepsis: JAMA, 2017; 317(8); 807-8
21. Rehmani RS, Memon JI, Al-Gammal A, Implementing a collaborative sepsis protocol on the time to antibiotics in an Emergency Department of a Saudi hospital: Quasi randomized study: Crit Care Res Pract, 2014; 2014; 410430
22. Petri M, Orbai AM, Alarcón GS, Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus: Arthritis Rheum, 2012; 64(8); 2677-86
23. Goldblatt F, Chambers S, Rahman A, Isenberg DA, Serious infections in British patients with systemic lupus erythematosus: Hospitalisations and mortality: Lupus, 2009; 18(8); 682-89
24. Danza A, Ruiz-Irastorza G, Infection risk in systemic lupus erythematosus patients: Susceptibility factors and preventive strategies: Lupus, 2013; 22(12); 1286-94
25. Minejima E, Delayo V, Lou M: BMC Infect Dis, 2019; 19(1); 149
26. Jeong SJ, Choi H, Lee HS, Incidence and risk factors of infection in a single cohort of 110 adults with systemic lupus erythematosus: Scand J Infect Dis, 2009; 41(4); 268-74
27. Al-Rayes H, Al-Swailem R, Arfin M, Systemic lupus erythematosus and infections: A retrospective study in Saudis: Lupus, 2007; 16(9); 755-63
Figures
Tables
 Table 1. Patients’ baseline characteristics in evaluation and treatment flow of 74 SLE patients with infections.
Table 1. Patients’ baseline characteristics in evaluation and treatment flow of 74 SLE patients with infections. Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections.
Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections. Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients.
Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients. Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections.
Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections. Table 1. Patients’ baseline characteristics in evaluation and treatment flow of 74 SLE patients with infections.
Table 1. Patients’ baseline characteristics in evaluation and treatment flow of 74 SLE patients with infections. Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections.
Table 2. Patients’ clinical and laboratory characteristics in the evaluation and management flow in 74 SLE patients with infections. Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients.
Table 3. Univariate analysis to determine the factors associated with death in 74 Saudi SLE patients. Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections.
Table 4. Outcome of 74 SLE patients in the evaluation and management flow of infections. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952