24 August 2023: Clinical Research
Assessing the Impact of Hepatitis B Elimination Program on Maternal-Infant Health in West Java, Indonesia: A Cross-Sectional Study
Anita Deborah AnwarDOI: 10.12659/MSM.941639
Med Sci Monit 2023; 29:e941639
Abstract
BACKGROUND: Hepatitis B virus (HBV) infection during pregnancy is a significant concern due to the risk of vertical transmission to the newborn, posing serious health complications. Understanding the effectiveness of intervention programs is paramount, especially in regions where comprehensive research is sparse. This study delves into the efficacy of the HBV elimination program in Garut Regency, West Java, Indonesia, targeting pregnant women and their newborns.
MATERIAL AND METHODS: This cross-sectional research encompassed 100 HBsAg-positive pregnant women who delivered at a singular facility in Garut Regency and their 62 offspring. Clinical data collection was rigorous, and HBsAg status was determined using rapid test kits, employing the precision of the 2-sided sandwich assay immunochromatography method. Data interpretation was multifaceted, involving univariate, bivariate, and multiple regression logistic analyses.
RESULTS: Notably, 16.95% of women, previously diagnosed as HBsAg-negative by initial health assessments, were subsequently diagnosed as positive at the specialized referral hospital. A noteworthy finding was that children administered with the HBV vaccine manifested a significantly diminished Positive-HBsAg status (P=0.029). Intriguingly, a majority of the maternal variables displayed a direct correlation with the HBsAg status of their offspring. The protective role of the HBV vaccine against HBV infection stood out distinctly (OR=0.326; CI 0.019-5.554; P=0.029).
CONCLUSIONS: While our center successfully met the desired HBsAg testing coverage in pregnant women, the administration of the hepatitis B vaccine to infants born to HBsAg-positive mothers lags behind the intended target. Emphasizing the vaccination's vital role, our study underscores its significance as a frontline defense for such infants.
Keywords: Hepatitis B, Hepatitis B Surface Antigen Receptor, Infant, Newborn, Pregnancy, Child, Infant, Humans, Female, Indonesia, Cross-Sectional Studies, Hepatitis B Surface Antigens, infant health, Hepatitis B virus, Mothers, Vaccines
Background
Hepatitis B (HBV) is often transmitted vertically from mother to baby during delivery. Pregnant women have a 90% chance of vertical transmission of HBV infection to their children, which can result in childhood morbidity and mortality [1,2]. Pregnancy-related complications of HBV include the possibility of miscarriage, low birth weight in newborns, preterm labor, and maternal mortality due to hemorrhage. From the maternal aspect, mothers with HBV are advised to have an abortion, sterilization, or liver transplant because of the negative long-term effects of HBV infection. However, children with HBV infection can experience liver damage, and in the worst scenarios, this disease can lead to death. HBV infection remains difficult to treat in children and can become chronic, giving them the ability to spread the virus widely [1–3].
Therefore, as a form of state responsibility to ensure the survival of children in Indonesia, it is necessary to make efforts to break the chain of HBV transmission through the Triple Elimination Program, based on the regulation of the Minister of Health Republic of Indonesia No. 52 of 2017, along with efforts to prevent the transmission of HIV infection and syphilis [1]. The indicators of the Triple Elimination Program are HIV, syphilis, and HBV. The target of the program an amount of new infections of HIV, syphilis, and/or HBV in children in less than or equal to 50/100 000 live births. Moreover, in terms of public health importance, the World Health Organization (WHO) has adopted the first 2016–2020 Global Health Sector Strategy on viral hepatitis. The current definition of HBV elimination in absolute terms is ≤0.1% HBsAg prevalence in children aged 5 years or younger, incidence of 2 per 100 persons in people who inject drugs, and an annual mortality rate of ≤6 per 100 000 persons. These are comparable to the initial relative reduction targets set forth in the 2016 Global Health Sector Strategy [4].
The preferred method for determining whether a person has HBV infection is serologic testing for HBsAg. An active infection, either acute or chronic, is indicated by a positive HBsAg test. To distinguish between an acute, chronic, or resolving infection, other serological markers of HBV infection, such as the presence of HBV core IgM antibody, and the clinical setting are used. The screening method should solely use HBsAg to identify people who are at risk for chronic infection. Testing for HBsAg, HBV core antibody (anti-HBc), and HBV surface antibody (anti-HBs) should be included in screening in order to identify vulnerable individuals who should be offered HBV vaccination or patients who are at risk of reactivation or transmission of HBV [5]. According to the WHO, the precise binding of monoclonal antibodies to hepatitis B antigens has made the enzyme-linked immunosorbent test (ELISA) method the criterion standard, with a high degree of sensitivity. However, ELISA is relatively expensive and requires specialized expertise. Therefore, diagnostic tests for HBV in underdeveloped nations such as Indonesia can be performed quickly using a 2-sided sandwich assay immunochromatography with monoclonal and polyclonal antibodies [6] to be more accessible to all sections of society.
The HBV infection elimination program is being conducted starting with antenatal care, maternal-fetal screening, diagnosis, and treatment, as well as the administration of hepatitis B immunoglobulin (HBIG) and HBV Vaccine (HBV Vaccine) in children born to mothers with reactive HBsAg. Evaluation of children’s HBsAg status should be performed when the children are at least 9 months old [3,7]. The indicators of the HBV elimination program success rate include (1) coverage of pregnant women who have the HBV test ≥95%; (2) percentage of deliveries in pregnant women with reactive HBsAg assisted by skilled birth attendants ≥95%; and (3) coverage of newborns who receive HBV vaccine ≥95% [7,8].
To the best of our knowledge, although the Triple Elimination Program has been used for a long time, studies assessing the effectiveness of the HBV elimination program worldwide are insufficient. Previously, a study conducted by Purnomo et al [9] aimed to analyze the prevalence and significance of HBV variants, including emerging vaccine-escape mutants, among children in several districts in Indonesia based on HBV subtype and genotype zones. That previous study did not consider whether the child was born to a mother with positive HBsAg status. This is different from our study, which emphasizes the efficacy of the HBV Triple Elimination Program, which includes administering the HBV vaccine and HBIG and analyzing other associated factors in children born to HBsAg-positive mothers; therefore, the success rate of the Triple Elimination Program, according to the WHO roadmap and in Indonesia, could be measured. Nonetheless, the data regarding the HBV elimination program have been limited, especially in Indonesia and our region, Garut Regency, which is the regency with the highest maternal mortality rate and infant mortality rate in West Java Province, Indonesia [10]. Therefore, this study aimed to investigate the effectiveness of the HBV infection elimination program in 100 pregnant women and 62 infants in Garut Regency, West Java, Indonesia, between September 2022 and March 2023 by detection of HBsAg.
Material and Methods
ETHICS STATEMENT:
This observational study using a cross-sectional method was conducted between September 2022 and March 2023 to assess the effectiveness of the HBV infection elimination program in children with mothers with reactive HBsAg in Garut Regency, Indonesia. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board/Ethics Committee Review Board of Hasan Sadikin General Hospital – Faculty of Medicine, Universitas Padjadjaran (protocol date LB.02.01/X.6.5/349/2022 and date of approval June 6, 2022). The study was also approved by the regency’s Department of Health Officers, the primary healthcare units, and the sub-district and village levels. Verbal and written informed consent was obtained from parents or legal guardians. The study was conducted in Garut Regency General Hospital, with patients referred from 42 districts in Garut Regency.
STUDY DESIGN:
This was an observational study using a cross-sectional method conducted between September 2022 and March 2023 to assess the effectiveness of the HBV infection elimination program in children with mothers with reactive HBsAg in Garut Regency, Indonesia.
RESEARCH PARTICIPANTS:
Pregnant women diagnosed with reactive HBsAg who gave birth at Garut Regency General Hospital between January and December 2021, as well as their children, were included in this study. Patients with incomplete medical record data, pregnant women with uncontrolled comorbidities (chronic hypertension, diabetes mellitus, gestational diabetes, and immunosuppression), and those who refused to be evaluated were excluded from the study.
SAMPLING SIZES AND PROCEDURES:
The sample size for this study was calculated using a cross-sectional minimum sample formula and calculated with G*Power 3.1.9.4 for Windows, with the difference between 2 groups statistical test and assumptions having a 95% confidence level (CI) and a 5% margin of error. The minimum sample size in this study was 24 samples, but the size was set to a minimum of 30 samples (for each mother group or children group) to increase validity and accuracy [11]. This study used a consecutive sampling method to select participants.
CLINICAL DATA:
Maternal age, type of pregnancy (singleton or multiple), gestational age (at the time of delivery), type of delivery, HBsAg status during pregnancy, and postpartum HBsAg status were evaluated. HBIG administration status, HBV vaccine status, exclusive breastfeeding status, and HBsAg status were the infant variables evaluated. All sociodemographic and clinical data were obtained from the medical records.
DATA COLLECTING AND LABORATORY EXAMINATION OF HBSAG STATUS:
HBV antigen procedures were examined using a 2-sided sandwich assay immunochromatography method with the One Step brand from Standard Diagnostics, Inc. The results were measured using relative light units. The absence of a colored test line indicates the absence or presence of HBsAg, but at a quantity below the detection limit of the test. For reagent stability and test functionality, each test strip also had an integrated procedural control. If the control line was missing, the test was invalid.
STATISTICAL ANALYSIS:
Data were checked, coded, and analyzed using IBM SPSS version 24.00 statistical software. Frequencies, proportions, and standard deviations were calculated using descriptive statistics. In bivariate analysis, for numerical data, the
Results
OVERALL MATERNAL CHARACTERISTICS:
In Garut Regency, the HBsAg status of all pregnant women should be evaluated during antenatal care or at least no later than at admission to healthcare during labor observation of the patients. Thus, the coverage of pregnant women who underwent HBV testing was 100%. This finding shows that the coverage of HBsAg testing in pregnant women in Garut Regency has already met the target of the HBV elimination program, which is ≥95%. As shown in Table 1, of the 100 pregnant women who tested positive (reactive) for HBsAg and gave birth at hospitals in Garut Regency in 2021, 59 pregnant women and 62 of their children met the inclusion and exclusion criteria of this study. Pregnant women in this study had an average age of 30.08±6.29 years. A total of 59 mothers (94.91%) had single pregnancies and 3 (5.09%) had multiple pregnancies. The majority of mothers (91.52%) gave birth at term, using the spontaneous delivery method (81.35%). As many as 16.95% of pregnant women who were initially diagnosed with HBsAg-negative status at the primary health care unit became HBsAg positive when they were referred and examined at the Garut Regency General Hospital. This finding allows for further evaluation of currently available diagnostic tests for HBV infection.
CHARACTERISTICS OF INFANTS:
Regarding the characteristics of the infants (Table 2), 62 were born to HBsAg-reactive mothers throughout 2021 at the Garut Regency General Hospital. Only 40 infants (64.51%) were administered HBIG, and only 45 infants (72.58%) were administered the HBV vaccine. A total of 59 (95.16%) children were exclusively breastfed for 6 months. Only 2 infants (3.22%) were confirmed to have a reactive HBsAg status.
SUBGROUP ANALYSIS OF MATERNAL CHARACTERISTICS:
From a comparative analysis of the characteristics of the 2 groups (Table 3), it could be concluded that they were equal, or homogeneous. In the subgroup analysis of each variable of maternal age, type of pregnancy, gestational age, delivery method, and maternal HBsAg status at the General Hospital, there were no significant differences in reactive-HBsAg and negative-HBsAg status of the infants.
SUBGROUP ANALYSIS OF INFANT CHARACTERISTICS:
There was a significant difference in HBsAg status in children who received the HBV vaccine (P=0.029; 95% CI 0.01–1.10), based on subgroup analysis, as shown in Table 4. However, HBsAg status did not differ significantly among HBIG recipients (P=0.144; 95% CI 0.01–1.10) and exclusively breastfed children (P=1.000; 95% CI 0.01–1.10).
FACTORS ASSOCIATED WITH INFANTS’ HBSAG STATUS:
Based on the correlation test shown in Table 5, all variables except maternal HBsAg status at primary healthcare were weakly correlated with HBsAg status of children. The type of pregnancy and HBV vaccine recipient status had a weak positive correlation with the HBsAg status of children, with P values of 0.100 and 0.029, respectively. These 2 variables had P values <0.25; hence, they were included in the multivariate analysis (Table 6).
MULTIPLE REGRESSION LOGISTIC ANALYSIS:
Based on the results of the multiple regression logistic analysis, the HBV vaccine was a significant protective factor for children born to HBsAg-reactive mothers with HBV infection (OR=0.326; CI 95% (0.019–5.554;
Discussion
EFFECTIVENESS OF THE HBV ELIMINATION PROGRAM AND DIAGNOSTIC TOOL IN PREGNANT WOMEN:
We found that the coverage of pregnant women who underwent HBV testing was 100%. This finding shows that the coverage of HBsAg testing in pregnant women in Garut Regency has already met the target of the HBV elimination program, which is ≥95%. However, only 40 children (64.51%) were administered HBIG, and only 45 children (72.58%) were administered the HBV vaccine. This finding reveals that the coverage of the HBV vaccine among children born to HBsAg-reactive women was still below the target of the HBV elimination program (<95%) [4,5]. This is in line with the study conducted by Purnomo et al [9], which evaluated the efficacy of the HBV vaccine among children (not only focused on children born to HBsAg-reactive women). The percentage of children receiving 3 doses of the HBV vaccine is high (73.9–94.1%), although the coverage of the birth dose is less than 50%, according to the Local Health Office data in 5 locations. The seropositive rate for HBsAg in preschool- and school-aged children in those localities varied among 967 children, from 2.1% to 4.2% and 0% to 5.9%, respectively [9].
The Triple Elimination Program has emerged as a strategic opportunity to detect HIV, syphilis, and HBV in pregnant women to decrease maternal and fetal morbidity and mortality. The examination could be conducted at the nearest first-level health facility during the first antenatal care visit, ideally before 20 weeks of gestation. For pregnant women more than 20 weeks of gestation, screening tests and treatment should be conducted as soon as possible [1–10,12–14]. All children born to mothers with reactive HBsAg should undergo HBsAg and anti-HBs status evaluation at 9 to 12 months of age (at least 3 months after the final dose of HBV vaccine [13].
Moreover, as many as 16.95% of pregnant women who initially had HBsAg-negative status at the primary health care unit became positive for HBsAg when they were referred and examined at the Garut Regency General Hospital. According to the WHO, the precise binding of monoclonal antibodies to HBV antigens has made the ELISA method the criterion standard, with a high degree of sensitivity. However, this examination is relatively expensive and requires specialized expertise. Therefore, to be more accessible to all parts of society, diagnostic tests for HBV in developing countries such as Indonesia can be performed using a rapid test, based on the principle of 2-sided sandwich assay immunochromatography, with monoclonal and polyclonal antibodies [6]. A study in developing countries showed that the sensitivity of HBsAg detection using a rapid test for HBsAg screening was 91.43%, with a specificity of 98.28% and accuracy of 96.69% [15]. A meta-analysis of 33 studies [16] has shown combined sensitivity and specificity of 90.0% (95% CI 89.1–90.8) and 99.5% (95% CI 99.4–99.5), respectively. In comparison, in a meta-analysis from Korea, a country with a high burden of HBV infection, the aggregate sensitivity and specificity of serum HBsAg on the rapid test were 98.07% (95% CI 97.67–98.47%) and 99.56% (95% CI 99.21–99.91%), respectively [13]. Studies in Indonesia have shown that the HBsAg rapid screening test has a sensitivity of 84%, specificity of 100%, positive predictive value of 100%, negative predictive value of 89%, and accuracy of 93% [17].
The exact mechanism by which false positives and negatives can occur for HBsAg, as observed in this study, has not been highlighted in previous studies. This raises the question of whether sampling the process of reading the data using the tool has been standardized for accuracy and validated. It has been postulated that differences in tissue uptake, body composition, and muscle blood flow may affect the plasma vaccine antigen concentrations in patients previously vaccinated against HBV [18]. In addition, transient HBsAg-positive results can result from early, late, and variable uptake of vaccine antigens from the muscle. Moreover, other studies have suggested that heterophilic antibodies have been known to induce false-positive and false-negative results (eg, HIV infection). These naturally occurring human antibodies bind to various chemical structures, including animal antibodies frequently used in immunochemical assays, and can be neutralized by reagents consisting of specific inactivating binders [19–21]. These findings provide a basis for advocacy regarding the best diagnostic tool for HBV infection in Indonesia. Transforming rapid tests into ELISA method-based diagnostic tests or continuing to use rapid tests with further validation standards should consider the medical, social, and economic factors.
MATERNAL CHARACTERISTICS AND ASSOCIATED FACTORS:
There is a considerable likelihood that HBV infection during pregnancy will be transmitted vertically from the mother to the child, owing to several factors. Immunological adjustment, which results in T helper 1 suppression and T helper 2 induction, is the primary contributing factor. The primary mechanism is decreased CD8+ T cell levels that would interfere with the immune response to the HBV virus and increase viral activity [22,23].
In the present study, we found that reactive-HBsAg status of pregnant women was more common in pregnant women with singleton pregnancies, term gestational weeks, and spontaneous deliveries. This was in contrast to a study conducted by Carlo et al [23] in a developing country, in which HBsAg-positive patients tended to be older than HBsAg-negative patients (P=0.016) and had multiple pregnancies (P=0.0157). There are no adequate studies that show a detailed connection between the type of pregnancy (singleton or multiple) and maternal HBsAg status. However, it is postulated to be related to immunogenicity and intrauterine transmission, which allows it to occur more frequently in multiple pregnancies. However, this hypothesis requires further investigation. Although pregnant women with reactive HBsAg have an 18% risk of preterm birth and a higher risk of passing it to their children, HBsAg-reactive mothers in this study gave birth more frequently at term. This is possibly because HBeAg protein is also thought to cross the placenta and reach the fetus. HBeAg exposure to the immature fetal immune system can trigger helper T-cell tolerance to HBeAg and HbcAg, owing to cross-reaction, increased regulatory T cells, and dysfunction of CD8+ T cells [25–28].
CHILDREN’S CHARACTERISTICS AND ASSOCIATED FACTORS: ROLE OF HBIG AND HBV VACCINE:
Our study showed that children who were administered HBIG at birth to HBsAg-reactive mothers were less likely to have reactive HBsAg results. Based on this theory, newborns of HBsAg-reactive mothers should be given both active and passive immunizations, with the first dose of the HBV vaccine series (HB0, the dose at birth) and 1 dose of HBIG administered within 12 h after delivery. at a different location or maximally within <24 h. The baby should then complete the HBV vaccination, as scheduled. Delays in active and passive immunoprophylaxis can cause the transmission of the virus to the fetus [26,27]. However, the cost-effectiveness of HBIG supplementation for HBV remains controversial. The use of a combination of vaccine and HBIG within 24 h of birth has been reported to reduce the risk of chronic HBV infection by 10% to 15% and <1% for infants born to mothers with HBeAg and HBeAg, respectively [28]. However, because of the high cost of HBeAg screening and administration of HBIG, some countries with insufficient resources use only the HBV vaccine for immunization of infants born to HBsAg-reactive mothers, whereas most do not screen for the mother’s HBeAg status [28–30]. Additionally, the present study showed that HBIG administration and HBV vaccine immunization coverage were still insufficient and significantly below the target, allowing for HBsAg-reactive results in children. This can serve as the foundation for recommendations for the routine delivery of HBIG to children whose mothers have HBsAg-reactive status. Additionally, HBsAg, HBeAg, and HBV DNA are excreted in the milk of infected mothers. However, the WHO states that there is no additional risk of transmission through breast milk, even in the absence of immunization. Breastfeeding should be avoided if there are cracks or bleeding in the nipples, as this can result in mixing of the serous exudate with breast milk, resulting in viral transmission [26,27].
IMPLICATIONS FOR CLINICAL PRACTICE AND FURTHER RESEARCH:
The results of this study suggest that the type of pregnancy and HBV vaccine recipient status had a weak positive correlation with HBsAg status, while the HBV vaccine became a significant protective factor for children born to HBsAg-reactive women with HBV infection. These findings suggest that the HBV vaccine should be routinely administered to children born to HBsAg-reactive women, and further evaluation is needed to specifically evaluate the effectiveness of the HBV infection diagnostic test kit (whether to transform a rapid test into an ELISA method-based diagnostic test or to keep using a rapid test, with further validation standards). Additionally, future studies can benefit from exploring the factors that may influence the evaluation process of HBV infection transmission elimination programs, such as HBIG, which is still minimally used. By identifying the most important factors associated with HBsAg status in children, researchers and practitioners can develop more effective strategies for addressing HBV infection in pregnant women and their children.
STUDY STRENGTHS AND LIMITATIONS:
This was an observational study without any intervention. The number of samples in this study was limited, and the study was conducted using secondary data from a single center (regency) in Indonesia. In addition, some participants who met the research criteria could not be contacted for obtaining information regarding the characteristics of the mother and her child. Participants with comorbidities were excluded from the study. Also, we used the rapid test with the immunochromatography method to detect HBsAg (not ELISA, the criterion standard)
Conclusions
The coverage of HBsAg testing in pregnant women in Garut Regency had already met the target of the HBV elimination program, which is ≥95%, while the coverage of the HBV vaccine among children born to HBsAg-reactive women was still below the target of the HBV elimination program (<95%). Administration of the HBV vaccine has become a significant protective factor for children born to HBsAg-reactive women with HBV infection. Further evaluations regarding the efficacy of diagnostic tools and the most important factors associated with HBsAg status in children will play pivotal roles in increasing the effectiveness of HBV triple elimination strategies worldwide.
Tables
Table 1. Overall maternal characteristics.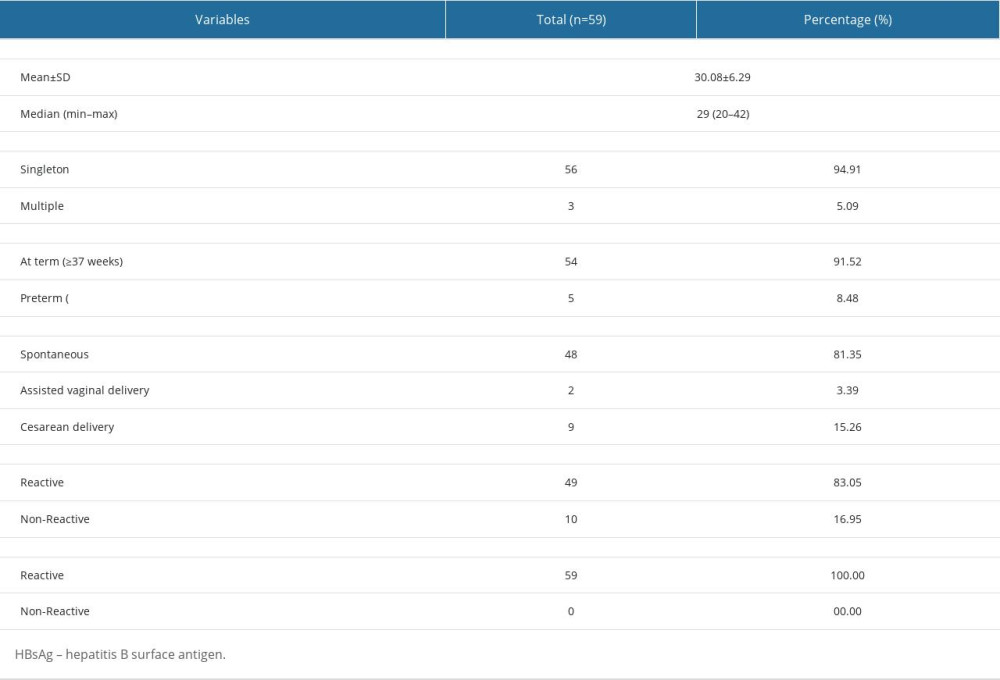 Table 2. Characteristics of the infants.
Table 2. Characteristics of the infants.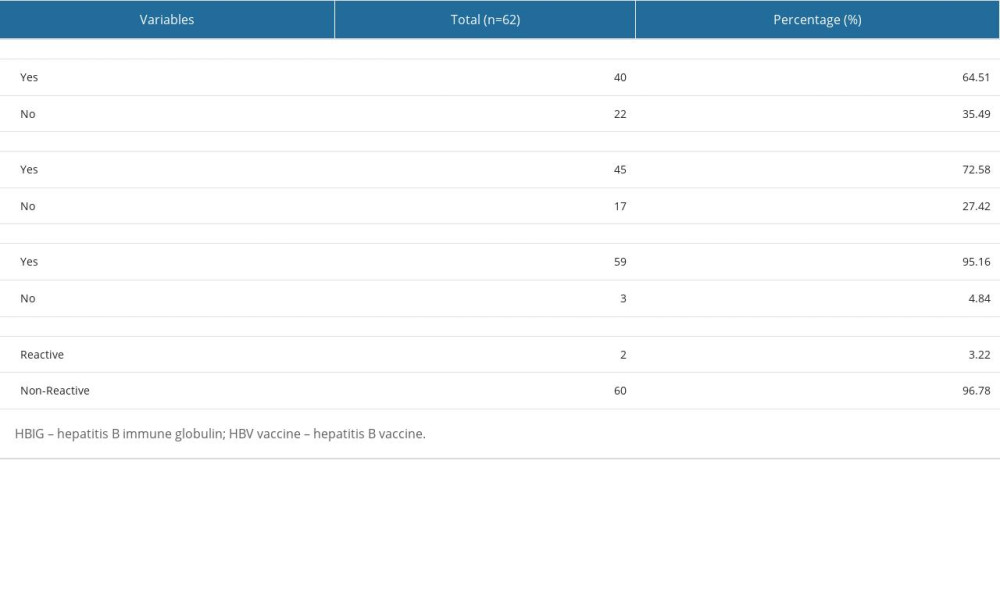 Table 3. Subgroup analysis of maternal characteristics.
Table 3. Subgroup analysis of maternal characteristics.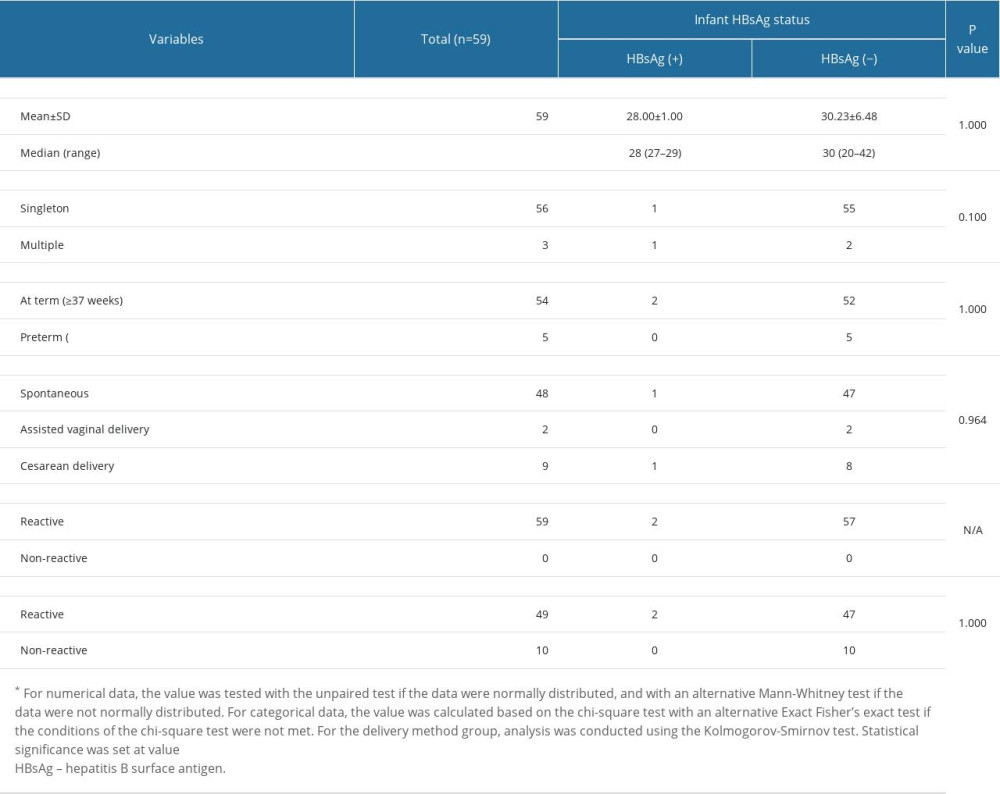 Table 4. Subgroup analysis of infant characteristics.
Table 4. Subgroup analysis of infant characteristics.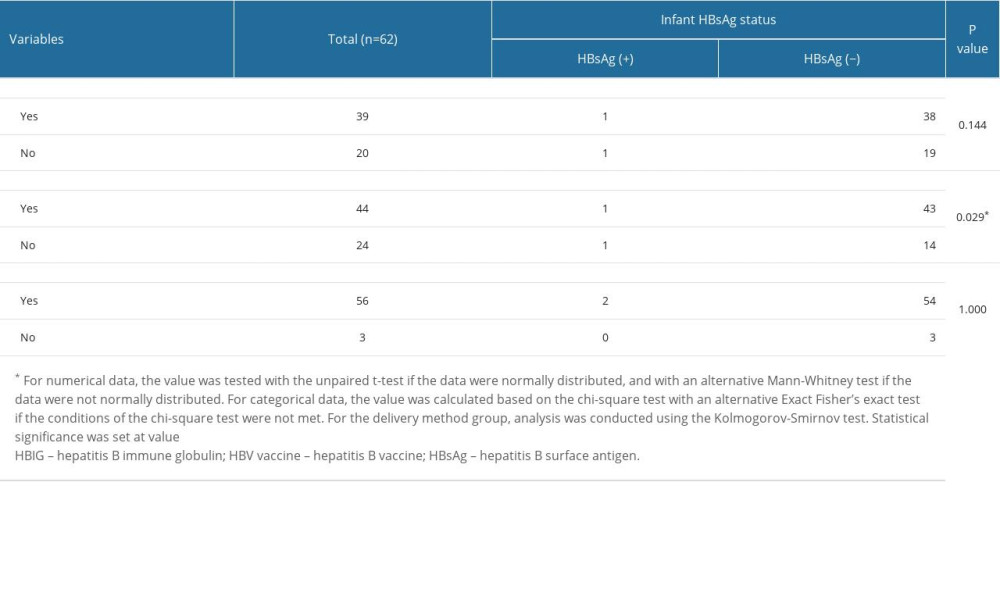 Table 5. Factors associated with infants’ HBsAg status.
Table 5. Factors associated with infants’ HBsAg status.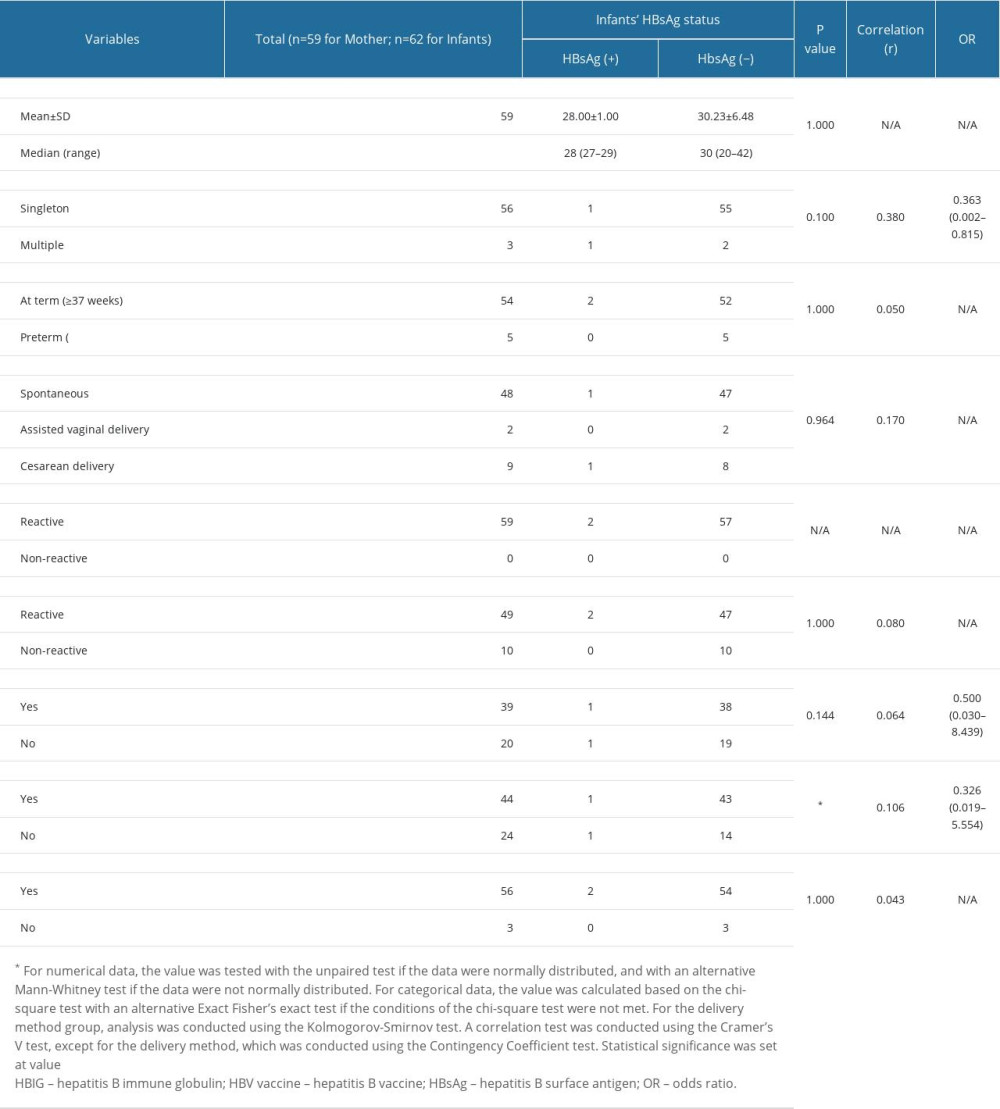 Table 6. Multiple regression logistic analysis.
Table 6. Multiple regression logistic analysis.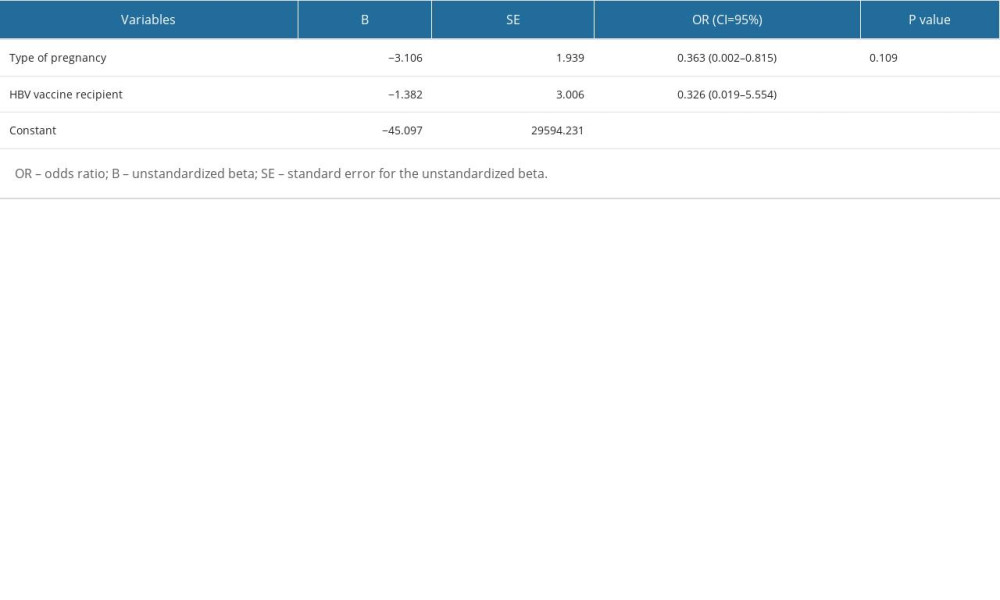
References
1. Indonesia Ministry of Health 2017: Minister of Health Regulations Number 52, 2017 Regarding Elimination of Human Immunodeficiency Virus, Syphilis, and Hepatitis B from maternal to children, 2017, (Indonesia) Jakarta Available from: [cited at 13 February 2023]https://peraturan.bpk.go.id/Home/Details/112155/permenkes-no-52-tahun-2017
2. United Nations. Sustainable Development Goals (SDGs): 2030 Target, 2017, Geneva [Internet]Available from: [cited February 13, 2023]https://www.sdg2030indonesia.org/
3. World Health Organization: Hepatitis B, 2019, Geneva [cited Feb 13, 2023]. Available from: [cited February 13, 2023]https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
4. World Health Organization (WHO): Hepatitis B, 2023 Available from: [cited July 25, 2023]https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#
5. Lingala S, Ghany MG, Hepatitis B: Screening, awareness, and the need to treat: Fed Pract, 2016; 33(Suppl 3); 19S-23S
6. Shimabukuro TT, Kim SY, Myers TR, Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons: New Engl J Med, 2021; 384(24); 2273-82
7. Cohn J, Owiredu MN, Taylor MM, Eliminating mother-to-child transmission of human immunodeficiency virus, syphilis and hepatitis B in sub-Saharan Africa: Bull World Health Organ, 2021; 99(4); 287-95
8. World Health Organization: Regional framework for the triple elimination of mother-to-child transmission of HIV, hepatitis B and syphilis in Asia and the Pacific 2018–2030, 2018, Geneva Available from: [cited February 13, 2023]https://apps.who.int/iris/handle/10665/274111
9. Purwono PB, Juniastuti , Amin M, Bramanthi R, Hepatitis B virus infection in indonesia 15 years after adoption of a universal infant vaccination program: Possible impacts of low birth dose coverage and a vaccine-escape mutant: Am J Trop Med Hyg, 2016; 95(3); 674-79
10. West Java Provincial Health Office: Garut Regency Health Profile 2017, 2017, (Indonesia) Garut Available from: [cited February 12, 2023]https://diskes.jabarprov.go.id/assets/unduhan/5.%20Profil%20Garut%202017.pdf
11. Smith S, Harmanci H, Hutin Y, Global progress on the elimination of viral hepatitis as a major public health threat: an analysis of WHO Member State responses 2017: JHEP Rep, 2019; 1(2); 81-89
12. Ishizaki A, Bouscaillou J, Luhmann N, Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries: BMC Infect Dis, 2017; 17(Suppl 1); 696
13. Wu Y, Gao J, Qin J, Mother-to-child transmission prevention of human immunodeficiency virus, syphilis and hepatitis B virus: Women Birth, 2019; 32(6); 570-78
14. Saboor Soomro R, Shah IA, Saboor A, Sensitivity and specificity of hepatitis B virus screening via rapid immunoassay chromatographic test: Cureus 25; 13(1); e12909
15. Amini A, Varsaneux O, Kelly H, Diagnostic accuracy of tests to detect hepatitis B surface antigen: A systematic review of the literature and meta-analysis: BMC Infect Dis, 2017; 17; 698
16. Amini A, Varsaneux O, Kelly H, Diagnostic accuracy of tests to detect hepatitis B surface antigen: A systematic review of the literature and meta-analysis: BMC Infect Dis, 2017; 17(S1); 698
17. Putri GSA, Santosa B, Comparison of sensitivity and specificity of the Anti-Hbs rapid test and ELISA. (Indonesia): Bali Medika Jurnal, 2022; 9(3); 309-16
18. Anjum Q, False positive hepatitis B surface antigen due to recent vaccination: Int J Health Sci (Qassim), 2014; 8(2); 189-93
19. Tang DM, Heller T, Koh C, The many faces of positive hepatitis B surface antigen: Hepatology; 64(4); 1379-81 201
20. Terrault NA, Bzowej NH, Chang KMAmerican Association for the Study of Liver Diseases, AASLD guidelines for treatment of chronic hepatitis B: Hepatology, 2016; 63(1); 261-83, doi: 10.1002/hep.28156
21. Klingen J, Yibirin M, Hwang JP, Torres HA, 919. Rates of false-positive hepatitis B surface antigen is low in cancer patients: Open Forum Infect Dis, 2021; 8(Suppl 1); 551
22. Sirilert S, Tongsong T, Hepatitis B virus infection in pregnancy: Immunological response, natural course and pregnancy outcomes: J Clin Med, 2021; 10(13); 2926
23. Wassner C, Bradley N, Lee Y, A Review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide: J Int Assoc Provid AIDS Care, 2020; 19; 2325958220919231
24. Carpio GC, Taguba AQ, Tan LC, Prevalence and risk factors of hepatitis B infection in pregnant women at the prenatal clinic of the University of the Philippines – Philippine General Hospital: Clin Gastroenterol Hepatol, 2015; 13(7); E83
25. Liu J, Zhang S, Liu M, Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: A population-based cohort study: Lancet Glob Health, 2017; 5(6); e624-e32
26. Ayoub WS, Cohen E, Hepatitis B management in the pregnant patient: An update: J Clin Transl Hepatol, 2016; 4(3); 241-47
27. Yuen MF, Chen DS, Dusheiko GM, Hepatitis B virus infection: Nat Rev Dis Primers, 2018; 4; 18035
28. Machaira M, Papaevangelou V, Vouloumanou EK, Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg− mothers: A systematic review and meta-analysis: J Antimicrob Chemother, 2015; 70(2); 396-404
29. Zhang L, Gui X-en, Teter C, Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine: Vaccine, 2014; 32(46); 6091
30. Zhang W, Xu C, Rui Y, Efficacy of the hepatitis B vaccine alone in the prevention of hepatitis B perinatal transmission in infants born to hepatitis B e antigen-negative carrier mothers: J Virus Erad, 2022; 8(2); 100076
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








