18 November 2023: Lab/In Vitro Research
Enhanced Proliferation and Adhesion Marker Gene Expression in Fibroblast Cells: Evaluating the Efficacy of a Non-Surgical Treatment for Urogenital Fistula
Efriyan ImantikaDOI: 10.12659/MSM.941641
Med Sci Monit 2023; 29:e941641
Abstract
BACKGROUND: Vesicovaginal fistula (VVF) due to posterior bladder wall and/or anterior vaginal wall necrosis is a condition that leads to urinary incontinence. Both microscopic and macroscopic VVFs severely impact quality of life. They are also associated with frequent recurrence after surgery. A non-surgical intervention for VVF is urgently required. A membrane bilayer could act as a mechanical tamponade and stimulate defect closure.
MATERIAL AND METHODS: This is an experimental study that explored the characteristics of mucoadhesive bilayer membrane complexes for non-operative treatment of VVF in vitro. We synthesized a mucoadhesive bilayer membrane, and inoculated it with cultured fibroblast cells. The mucoadhesive bilayer membrane was prepared with 3 different treatments: (1) estrogen; (2) lyophilized radiation-sterilized amnion (ALSR), a prepared amniotic membrane; and (3) arginine and glutamine (arginine+glutamine), 2 amino acids associated with wound repair. Expression levels of 3 genes, namely tumor growth factor beta (TGF-β), lysil oxidase (LOX), and junctional adhesion molecules (JAMs), were measured using the Livak method and polymerase chain reaction (PCR).
RESULTS: On the fifth day after inoculation, there was no statistically significant difference in expression of the genes in the 3 conditions. However, on the tenth day, gene expression of the LOX and JAMs genes in the fibroblast cells inoculated onto the mucoadhesive bilayer membrane with arginine+glutamine was significantly higher than the expression in the fibroblast cells inoculated onto the mucoadhesive bilayer membrane with estrogen or with ALSR.
CONCLUSIONS: The mucoadhesive bilayer membrane complex with arginine+glutamine gave rise to the highest expression of the LOX and JAMs genes, indicating that the highest proliferation and cell adhesion were found in cells inoculated with the mucoadhesive bilayer membrane complex with arginine+glutamine.
Keywords: Cell Proliferation, Urinary Fistula, Vaginal Fistula
Background
Vesicovaginal fistula (VVF) is an important gynecological problem in developing countries. According to the WHO, there are 3 million women who experience obstetrical fistulas, with as many as 50–130 000 cases per year [1]. Surgery, including both abdominal and vaginal approaches, is the most common preferred technique for the repair of VVF in most patients [2]. Recent data shows that 80% of 6000 cases of VVF were due to obstetric complications, and 13% were iatrogenic following benign or malignant tumor intervention [3]. In developed countries, the government, through health system-based strategies, has successfully prevented obstetric fistulas by providing access to professional health personnel. However, in developing countries, the problem is complex due to limited facilities and infrastructure, fewer professional experts, and low socioeconomic status. The present research was conducted to find practical and economical alternative approaches to fistula treatment [4].
Many factors play roles in the optimal defect closure of fistulas and prevention of their recurrence. The primary treatment goal is to restore continence, and there are 2 methods to achieve this: operative and non-operative approaches [5,6].The operative approach lacks time efficiency and has high cost due to the length of hospital stay (about 14 days), limitation of mobility (urinary catheter attached), and absence from work [7,8]. The most common urogenital fistula that occurs in urogynecology is VVF, which most often is brought about by obstetric anal sphincter injuries (OASIS) [9,10]. Fistula repair (fistuloraphy) in the case of VVF is mostly performed with a vaginal approach rather than an abdominal approach. Current non-operative approaches are still limited to small fistulas, and have a high recurrence rate. It is necessary to find a better non-operative solution.
For fistuloraphy, autologous slings, rather than synthetic ones, should be used, and a combined transabdominal and transvaginal approach may be necessary for larger, complex, or recurrent cases. Laparoscopic surgery using a robot and a peritoneal flap technique have also been described [11,12]. Research has been underway to find alternative curative efforts, including mucoadhesive bilayer membranes. The technique of using these membranes for repair had been previously developed by Anggraeni et al, in 2019, who showed that carbonated apatite has the ability to act as an adjuvant in transmucosal administration in the buccal mucosa region [12–14].
Compounds with demonstrated potential in the repair of female reproductive and genital organs are lyophilized radiation-sterilized amnion (ALSR), synthetic estradiol, and the amino acids arginine+glutamine [15–18]. Mucoadhesive bilayer compounds are inorganic materials that function as carrier nanoparticles of active agents that regulate particle synthesis, which can be highly efficient in transfecting various types of mammalian cells [19].
The wound-healing process in urogenital fistula is different from the wound-healing process in general, due to the location of the defect in a wet area, which prolongs the wound-healing process and increases the risk of infection [9,20,21]. Therefore, the present study considered 3 conditions: mucoadhesive bilayer membrane complex with ALSR, mucoadhesive bilayer membrane complex with estradiol, and mucoadhesive bilayer membrane complex with arginine+glutamine; and analyzed the following 2 issues: (1) Which combination of mucoadhesive bilayer membrane and active substance is most effective in inducing the process of proliferation and cell adhesion in fibroblast cell cultures? (2) How does the expression of transforming growth factor beta (TGF-β), lysil oxidase (LOX), and junctional adhesion molecules (JAMs) genes in fibroblast cell cultures differ in the presence of a mucoadhesive bilayer membrane complex with ALSR, mucoadhesive bilayer complex with estradiol, and mucoadhesive bilayer membrane complex with arginine+glutamine?
Material and Methods
STEP 1: SYNTHESIS OF THE MUCOADHESIVE BILAYER MEMBRANE COMPLEX AND PREPARATION FOR USE:
In previous research, complex membranes made of a mucoadhesive bilayer have been used as carrier substances or transgenic agents to enhance intracellular protein absorption or transfer non-viral genetic materials into the cells [14,22]. Research by Anggraeni et al (2018) used a membrane that consisted of 2 sides with a mucoadhesive surface that can stick to the inside of a mucosal surface (buccal) to enhance the absorption of ovalbumin for buccal vaccination. This concept can also be applied to the mucosa of the vagina as a “leak plug” on VVFs and promote re-epithelialization and remodeling, combined with ALSR as an active substance [1]. We prepared the mucoadhesive bilayer membrane used in the present study based on modification of the Nagahama et al (2008) and Anggraeni et al (2018) procedure [20,23]. The procedure is explained diagrammatically in Figure 1.
The mucoadhesive membrane was made from gelatin and chitosan, with weight ratios of 4/6 (0.4 grams of gelatin and 0.6 grams of chitosan dissolved in 20 mL of polybutylene succinate [PBS]). This formulation is denoted as F-04. The mucoadhesive bilayer membrane based on the Nagahama procedure is a bilayer membrane consisting of a mucoadhesive layer and a backing layer, but in our study, we used the membrane without the backing layer due to its toxicity risk. The mucoadhesive layer is a hydrogel mixture of chitosan with gelatin, which is a modification of that used by Nagahama et al [23].
The chitosan hydrogel was first made by dissolving 1 gram of chitosan in 1000 mL of 2% acetic acid on a magnetic stirrer unit until the chitosan was completely dissolved. The pH was adjusted by slowly adding 10% NaOH into the chitosan solution until it reached pH 10.0. Next, the chitosan hydrogel was separated by centrifugation. The water content of the chitosan hydrogel was 97.3% (w/w). The chitosan hydrogel was filtered using filter paper and stored in a refrigerator. At the same time, the gelatin was dissolved in 20 mL of distilled water at 40°C. Then, the chitosan hydrogel was mixed with the gelatin solution and stirred at 50°C. The gelatin/chitosan mixture was filtered using filter paper to remove water. The resulting chitosan/gelatin membrane was freeze-dried for a week.
The ratio of gelatin to chitosan in the hydrogel used in this study was chosen from among several formulations that produced the best physical mucoadhesive bilayer characteristics during a preliminary study (Imantika et al, unpublished data). Formulation F-04 was selected because it consistently produced intact membranes without any damage when removed from the filter paper. The membrane was sterilized using γ-irradiation with a dose of 15 kGy for 7.5 hours (rate, 2 kGy/h).
17β-ESTRADIOL:
We added 2.5 g of 17β-estradiol in the last step after the gelatin and chitosan were dissolved. Then, the mixture was stirred at 50° C for 5 minutes. We added estradiol for its role in inhibiting cutaneous aging and intensifying wound repair or wound healing through its receptors [18]. The proliferative process of wound healing involves estrogen to increase the number of fibroblasts, keratinocytes, and endothelial cells, resulting in an accumulation of extracellular matrix (ECM) [24].
ARGININE AND GLUTAMINE:
We added 1 g of 10% arginine and 0.1 g of 1% glutamine after the gelatin and chitosan were dissolved. Then, the mixture was stirred at 50° C for 5 minutes.
Arginine is a semi-essential amino acid that is required in adults for wound healing and repair. Arginine plays an important role in the survival and multiplication of mammalian cell cultures in vitro [15]. While glutamine is not an essential amino acid in humans, it is required as a source of energy and carbon by many mammals, insects, and cells in culture. In vitro and in vivo studies have shown that glutamine is an essential nutrient for lymphocyte proliferation and cytokine production, phagocytosis by macrophages, secretory activity, neutrophil killing of bacteria, and the proliferation phase of wound healing through the arginase pathway and the inducible nitric oxide synthetase pathway [16,17].
LYOPHILIZED RADIATION-STERILIZED AMNION MEMBRANE:
An ALSR membrane is an amnion that is dried by lyophilization and then sterilized using γ radiation. To create an ALSR for the present study, the amnion layer was separated from the chorion layer through mechanical dissection and then submerged in a sterile saline solution. This process involved removing water through sublimation, which converts liquid into vapor while simultaneously inhibiting the growth of microorganisms and minimizing tissue damage. The resulting lyophilized tissue contained 5–7% water content. It was then packaged in polyethylene plastic. The lyophilized amniotic membrane preserves the structure and appearance of fresh tissue, including a simple cuboidal epithelium, multiple layers of stratification, and edematous stroma, when observed under the microscope, and Periodic Acid Schiff staining can reveal the presence of a limiting membrane beneath the stroma [17]. In addition to type II and IV collagen, the lyophilized amniotic membrane also includes growth factors such as TGF-β, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor binding protein (IGFBP), platelet derived growth factor (PDGF), and bone morphogenetic protein (BMP), all of which play roles in wound healing [24,25,26].
In the present study, we lined the mucoadhesive bilayer membrane with the smaller ALSR membrane (±10 mm) on the inside (epithelial side).
STEP 2: SEEDING AND MAINTAINING THE FIBROBLAST CELL CULTURE:
The fibroblast cells used in this study were BJ CRL 2522 cells from the American Type Culture Collection (ATCC, Manassas, VA, USA), derived from neonate foreskin. Fibroblast cells were chosen to match the histological structure of the cells that make up the vaginal wall, and because they are high-survival cells in a variety of environments. It was expected that the mucoadhesive bilayer membrane complex combined with selected active compounds would exhibit different cell proliferation and adhesion processes compared with cultures of cells that were not treated with the mucoadhesive bilayer-active compound complex. Our study also used fibroblast cells because they make up the histological structure of the vaginal wall in the lamina propria layer, which is a connective tissue. A total of 10 000 fibroblast cells per well were cultured, in a 6-well plate. The 3 treatments (mucoadhesive bilayer complex with estradiol, ALSR, or arginine+glutamine) were carried out and cell cultivation was performed on the fifth and tenth days.
STEP 3: RT-PCR FOR GENE EXPRESSION:
RNA extraction was performed on each well of cultured fibroblast cells, including each control. We used the ΔCt method using a reference gene, which is a variation of the Livak method that is simpler to perform and gives essentially the same results. This method uses the difference between the reference and target Ct values for each sample.
The primers for the TGF-β gene were:
The primers for the JAMs gene were:
The primers for the LOX gene were:
The RT-PCR was done using a BioLine My Taq One-Step Kit (BioLine Reagents Ltd, London, UK), according to the procedure described below:
Into each PCR tube, we added a mixture consisting of 10 μl 2× SensiFast, a reagent from the SYBR One-Step kit (BioLine Reagents Ltd., UK); 0.8 μl forward primer (10 mM), 0.8 μl reverse primer (10 mM); 0.4 μl RiboSafe RNase inhibitor (BioLine Reagents Ltd., UK); 0.2 μl reverse transcriptase, 5.8 μl ddH2O; and 2 μl template RNA. Then, the tube was inserted into the Rotor-gene real time PCR thermocycler (Qiagen, Venlo, Netherlands) with the following settings: reverse transcription, 45°C for 5 minutes and polymerase activation at 95°C for 2 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds and annealing/extension at 60°C for 30 seconds.
The Ct results were calculated to get a ratio of gene expression using the following steps: Calculation of relative expression of the Ct target gene was normalized to the Ct of the reference gene for both the test sample and the calibration sample according to the formula (ΔΔCt=Δ Ct (target) – Δ Ct (control)). The expression ratio was then calculated as 2-ΔΔCt.
Results
Of the 3 treatments, the mucoadhesive bilayer membrane complex with arginine+glutamine had the highest expression of the LOX and JAMs genes. The fact that this condition exhibited higher expression than the mucoadhesive membrane with the other active substances (estradiol and ALSR) indicated that the arginine+glutamine treatment induced optimum cell proliferation and cell adhesion. There is existing research suggesting that the mucoadhesive bilayer membrane can adhere to the inside of a mucosal surface (buccal) to enhance the absorption of active substances. This finding served as the basic proof of concept in our search to find an alternative treatment for defect closure in VVF.
The macroscopic and microscopic appearance of the mucoadhesive bilayer membrane exhibited variation (Table 1). Color and elasticity of the membrane varied between transparent, yellowish, and greyish. The most elastic membrane was the mucoadhesive bilayer membrane combined with estrogen. The number of cells in the mucoadhesive bilayer membrane complex with ALSR and the mucoadhesive bilayer membrane complex with arginine+glutamine was almost the same as that in the mucoadhesive bilayer membrane complex with estrogen and the control mucoadhesive bilayer membrane, as shown in Figure 2A–2C. The fibroblast cells in the mucoadhesive bilayer membrane complex with arginine+glutamine were spindle-shaped; long and with a branched spindle, as shown in Figure 2D. The largest number of cells is indicative of best proliferation and the long and branched spindle appearance is indicative of best adhesion of the cells inoculated with the mucoadhesive bilayer membrane complex with arginine+glutamine. This was associated with high expressions of the LOX and JAMs gene markers, even though the expression of TGF-β was similar in the 3 separate cell cultures.
Ct is defined as the number of PCR cycles required for the fluorescent signal to cross the threshold value, and Ct levels are inversely related to the number of target genes in the sample. From Table 1, we can see that the Ct value from the 3 gene markers is less than or equal to 29, which means that they showed a strong positive reaction. This indicates abundant target nucleic acid in the sample.
Table 2 shows the result from one-way ANOVA followed by post hoc Tukey to determine whether there was a difference in mean gene expression between the 4 groups (the 3 experimental groups and a control group consisting of cultured fibroblast cells without any treatment). A one-way ANOVA was performed to compare the effect of the 3 interventions in the fibroblast cell culture. This one-way ANOVA revealed that there was a statistically significant difference in mean JAMs gene expression in 10 days (F (3,8)=[27.2],
The mean±standard deviation of TGF-β gene expression was not significantly different between the 4 groups, with a
In Table 3, we show the results from 4 groups of treated or untreated cultured fibroblast cells, based on the following treatments: Group 1: mucoadhesive bilayer membrane complex with ALSR; Group 2: mucoadhesive bilayer membrane complex with estradiol; Group 3: mucoadhesive bilayer membrane complex with arginine+glutamine; Group 4: control group; cultured fibroblasts without treatment. Tukey’s honestly significant difference (HSD) test for multiple comparisons found that the mean value of JAMs gene expression after 10 days was significantly different between group 1 and group 2 (
From our study, we found that the mucoadhesive bilayer membrane complex with arginine+glutamine treatment provided the highest LOX and JAMs gene expression results among all of the treatments. In the mucoadhesive bilayer membrane complex with arginine+glutamine group, the expression of JAMs reached 5.47-fold higher than that in group 2 and 6-fold higher than that in group 4 (control). The expression of LOX reached 2.85-fold higher than that in group 3, 3.9-fold higher than that in group 2, and 4.08-fold higher than that in group 4 (control). Even though Barski et al, in 2015, repaired VVF using an amniotic membrane as a material (used during the stitching of the fistula in a 64-year-old woman who had successfully undergone radiation treatment), in our research we did not find a significant difference between the treatment with ALSR vs 17β estradiol [26].
Table 4 shows that there was no significant difference in mean±standard deviation of the expression levels of the 3 different genes considered, in the mucoadhesive bilayer membrane complex with ALSR treatment, on day 5 and day 10 (
Discussion
The wound-healing process is regulated by various important factors, one of which is the presence of a rich ECM in the wound environment [28,29]. The present research was conducted to find the expression level of 3 genes (TGF-β, LOX, and JAMs) that play important roles in ECM and collagen production pathways related to cell proliferation and adhesion. TGF-β is expressed in the nucleus and the cytoplasm of the cell; LOX is expressed in the nucleus; and JAMs is expressed in the cell membrane. A mucoadhesive bilayer membrane is expected to act as a mechanical tamponade and induce cell proliferation and adhesion in fistula to promote defect closure.
The ECM is made up of different types of large molecules, including fibrous components like collagen and elastin, and glycoprotein components like fibronectin, proteoglycans, and laminate. These molecules work together to support tissue growth, function, and repair [30,31]. Wound-healing can be broken down into 4 phases, which happen in a specific order but overlap in time: hemostasis, inflammation, proliferation, angiogenesis-reepithelization, and maturation or remodeling. Critical stages of the wound-healing process, such as hemostasis, inflammation, and angiogenesis, are all responsive to the ECM, collagen, and their compounds [32]. Collagen triggers platelet activation and aggregation, forming a blood clot at the injury site. During inflammation, immune cells secrete proinflammatory cytokines that affect the migration of fibroblasts, epithelial cells, and endothelial cells. Fibroblasts also contribute to collagen deposition and degradation, releasing fragments that promote fibroblast proliferation and growth factor synthesis, which leads to angiogenesis and re-epithelialization. Finally, the balance of ECM remodeling (new matrix synthesis and matrix metalloproteinase degradation activity) determines the increase in tensile strength [33]
The wound-healing process related to fistula closure is highly complex and involves multiple signalling pathways and cell types (Figure 3). The proliferation and remodeling process of closure started in the 5th day. A mucoadhesive bilayer membrane can act as a mechanical tamponade and stimulate defect closure, depending on its active substance. Several growth factors play a role in wound healing, with TGF-β being essential for all stages. TGF-β regulates cell growth, differentiation, ECM production, and the immune response, but its effects vary depending on the context of the wound [32]. Understanding the TGF-β signalling pathway in chronic wounds is a major challenge for gaining comprehensive knowledge of wound-healing therapy. TGF-β exerts a pleiotropic effect by regulating cell growth, differentiation, ECM production, and the immune response. The effects of TGF-β have been studied in animal models by manipulating TGF-β signalling using exogenous TGF-β protein, anti-TGF neutralizing antibodies, or genetic alterations in the signalling pathway [34].
The process of breaking down and rebuilding the ECM is crucial, particularly when it comes to collagen. LOX and related enzymes can catalyze the cross-linking of collagen and elastin, leading to covalent bonds that strengthen the ECM. This helps to stabilize the ECM and plays a role in tissue repair and regeneration [34]. Current research indicates an essential role for LOX expression in expediting healing processes in various phases. Studies have shown that LOX plays a vital role in tissue repair, such as healing of tendons, ligaments, and skin wounds, as well as remodeling cartilage. It also plays a role in the different phases of fistula repair [35–38].
LOX, an enzyme that requires copper, starts the cross-linking of elastin and collagen by catalyzing the oxidation of lysine and hydroxylysine amino groups and ultimately breaking down extracellular proteins resistant to degradation [35,36]. To a large extent, the collagen network is stabilized through a cross-linking reaction that is dependent on LOX. Hence, LOX is a critical intermediary responsible for remodeling during the process of healing. LOX expression gives rise to. a collagen network with highly favorable biological properties that requires sufficient cross-links to stabilize the matrix and provide it with mechanical support. Therefore, the expression of LOX is essential to the maturation of the ECM and to favorable properties of the engineered tissue [37,38].
If the use of a mucoadhesive bilayer membrane complex with ALSR can aid in the proliferation and remodeling phase in the wound-healing process, then the closure of the defect can occur without surgical intervention. This process is regulated by various important factors, particularly a rich ECM in the wound environment. The critical stages of wound healing, namely hemostasis, inflammation, proliferation, angiogenesis-reepithelization, and maturation all take place simultaneously and overlap each other to a large degree. Several important growth factors play a role [32,33]. TGF-β helps in regulating cell growth, differentiation, ECM production and the immune response [32,37]. LOX is crucial in breaking down and rebuilding the ECM, particularly when it comes to collagen [33,34]. JAMs play a role in re-epithelization as they allow proper proliferation and orientation of the new epithelial cells [33,34]. The use of a synthetic membrane that contains all of the above growth factors is supposedly crucial in allowing proper tissue repair and wound remodeling, such as in a fistula [33,34].
An ALSR is an amnion that is dried by lyophilization and separated from the chorion through a mechanical dissection process and then submerged in a sterile saline solution. The lyophilized membrane preserves the structure and appearance of fresh tissue, including a simple cuboidal epithelium, multiple layers of stratification, and stroma [23].The membrane is expected to act as a mechanical tamponade, preventing leakage in the vaginal wall while also increasing the production of growth factors that play an important role in the process of re-epithelialization and adhesion of already-formed cells. It has been found that the ALSR has high levels of TGF-β expression that can promote the migration and differentiation of stem cells [23].
It is known that the microenvironment components in the process of skin wound repair and regeneration form the ECM, which plays an important role in cell proliferation and adhesion. Previous research found higher expression of epidermal growth factor (EGF) and PDGF in scratch wounds in mice given amniotic stem cells, resulting in accelerated wound healing and epithelialization [35].
The expression of JAMs in this study was found to be very high in cells treated with the mucoadhesive bilayer membrane complex with ALSR. The JAMs gene triggers the expression of claudin, which is the main component of tight junctions and epithelial structures in the mammalian bladder, and is the densest structure in uroepithelial cells [37,38].
Generally, TGF-β and LOX genes play a role in the proliferation phase of wound healing by converting type I collagen, a key component of the extracellular matrix, to type III collagen, which makes up only 10% of the matrix. However, this proportion changes after injury, with a decrease in collagen I and an increase in collagen III. Research has shown that LOX is produced before collagen synthesis in the early stages of healing to prepare for cross-linking. Type III fibers provide less strength to the wound than type I fibers, and they will be replaced with type I fibers during the repair process. The switch from type III to type I collagen provides greater mechanical strength. The ratio of type I to type III fibers and the degree of their maturity significantly impact the rate of tissue remodeling and the strength of the newly formed wound [35,36,38].
Above all, fibroblasts synthesize collagen, which precipitates after a cross-linking reaction catalyzed by LOX, driving the healing process in tissues such as cardiovascular, skin, and bone tissue. To sum up, different extracellular matrix components, like collagen, fibroblasts, and various chemical mediators, are essential for maintaining tissue homeostasis [35,38,39].
JAMs are immunoglobulins found at the tight junctions of polarized cells and on the surface of white blood cells. Multiple members exist within the JAM family. They influence cell polarity, endothelial permeability, and white blood cell migration through interactions with other JAM family members and non-family members [23,40,41]. JAM-A, a specific member of the JAM family, is mainly found in the tight junctions of epithelial and endothelial cells. Re-epithelialization, a critical step in wound healing, relies on the proper proliferation and migration of skin epithelial cells. Researchers have studied the effect of JAM-A on cell proliferation and migration, to understand its role in the normal healing process [40–42].
Limitations of this research include the long time required to seed the fibroblast cells and their susceptibility to yeast contamination (which increases the number of dead cells). To minimize this, the cell culture procedure was done 3 times, and during the third repetition, we used gamma irradiation for the sterilization process.
A future scope of this research is related to the effectiveness test of the mucoadhesive bilayer membrane complex with arginine+glutamine in tissue models of fistula (ex vivo study). In these ex vivo studies, histological examination will be done to identify the quality of the defect closure in terms of tissue characteristics.
Conclusions
The mucoadhesive bilayer membrane complex with arginine+glutamine showed the highest expression of the LOX and JAMs genes, indicating that the highest proliferation and cell adhesion were conferred by this treatment. The mucoadhesive bilayer membrane complex with arginine+glutamine offers a new potential for non-operative treatment of VVF, as it serves as a mechanical barrier while simultaneously promoting the growth and remodeling of the fistula. This approach shows promise for a more effective and efficient closure of fistula defects, but needs further exploration.
Figures
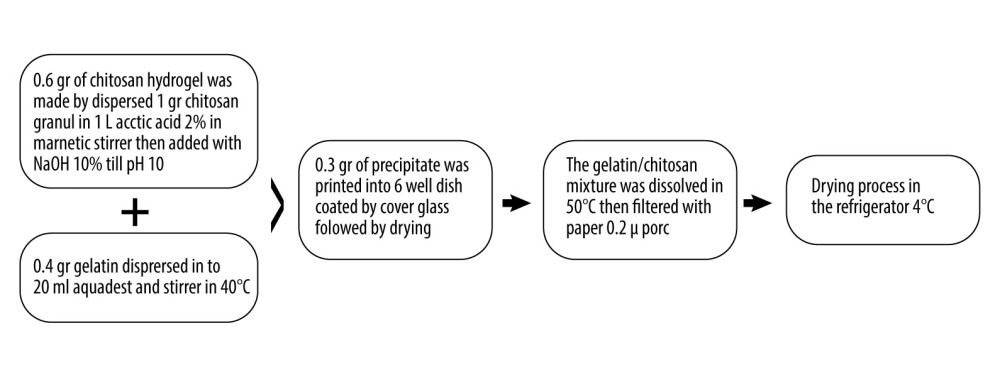 Figure 1. Mucoadhesive bilayer membrane preparation. (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 1. Mucoadhesive bilayer membrane preparation. (Figure created using Microsoft Word for Mac, version 16.69.1). 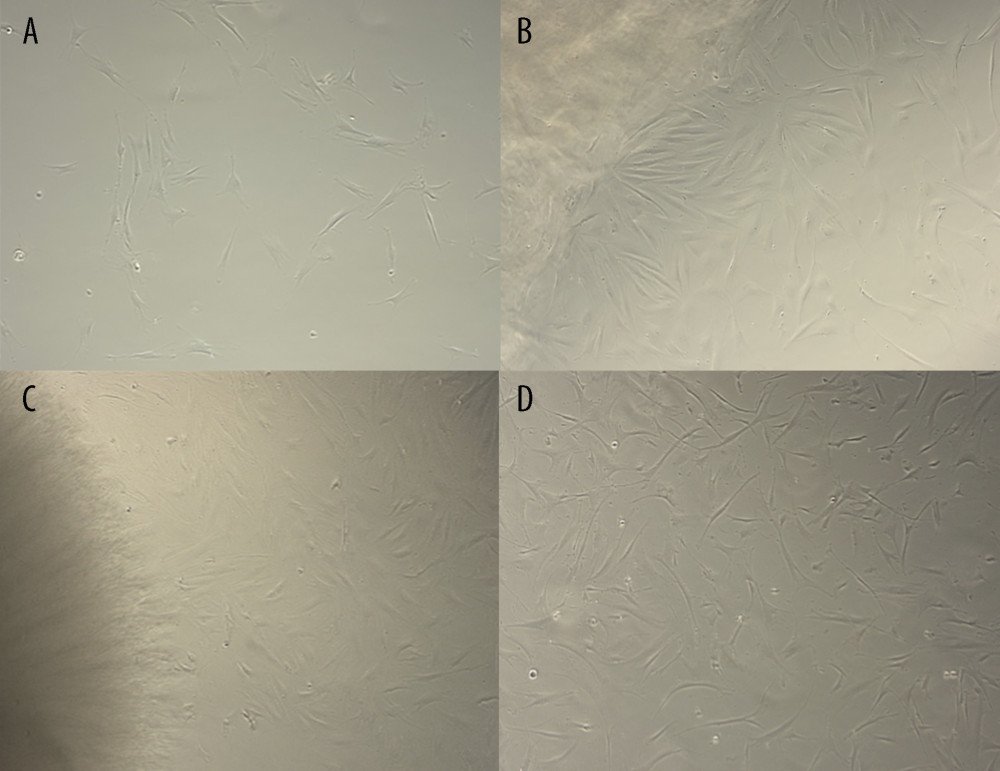 Figure 2. Microscopic view of fibroblast cell culture inoculated with mucoadhesive bilayer membrane-Active Substance complex. In Figure A, control cells were evenly distributed. The black arrow in Figure B shows clusters of wide, spindle-shaped cells. The black arrow in Figure C shows a smaller group of medium-sized spindle-shaped cells. The black arrow in Figure D shows a cluster of long spindle-shaped cells. Details: all slides are magnified to 40×. (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 2. Microscopic view of fibroblast cell culture inoculated with mucoadhesive bilayer membrane-Active Substance complex. In Figure A, control cells were evenly distributed. The black arrow in Figure B shows clusters of wide, spindle-shaped cells. The black arrow in Figure C shows a smaller group of medium-sized spindle-shaped cells. The black arrow in Figure D shows a cluster of long spindle-shaped cells. Details: all slides are magnified to 40×. (Figure created using Microsoft Word for Mac, version 16.69.1). ![The role of the mucoadhesive bilayer membrane-ASLR complex in wound-healing stage as a basic concept of fistula closure [21,25,30,31,34,36,37]. (Black bold square shows a research-related gene). (Figure created using Microsoft Word for Mac, version 16.69.1).](https://jours.isi-science.com/imageXml.php?i=medscimonit-29-e941641-g003.jpg&idArt=941641&w=1000) Figure 3. The role of the mucoadhesive bilayer membrane-ASLR complex in wound-healing stage as a basic concept of fistula closure [21,25,30,31,34,36,37]. (Black bold square shows a research-related gene). (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 3. The role of the mucoadhesive bilayer membrane-ASLR complex in wound-healing stage as a basic concept of fistula closure [21,25,30,31,34,36,37]. (Black bold square shows a research-related gene). (Figure created using Microsoft Word for Mac, version 16.69.1). Tables
Table 1. Characteristics of research variables.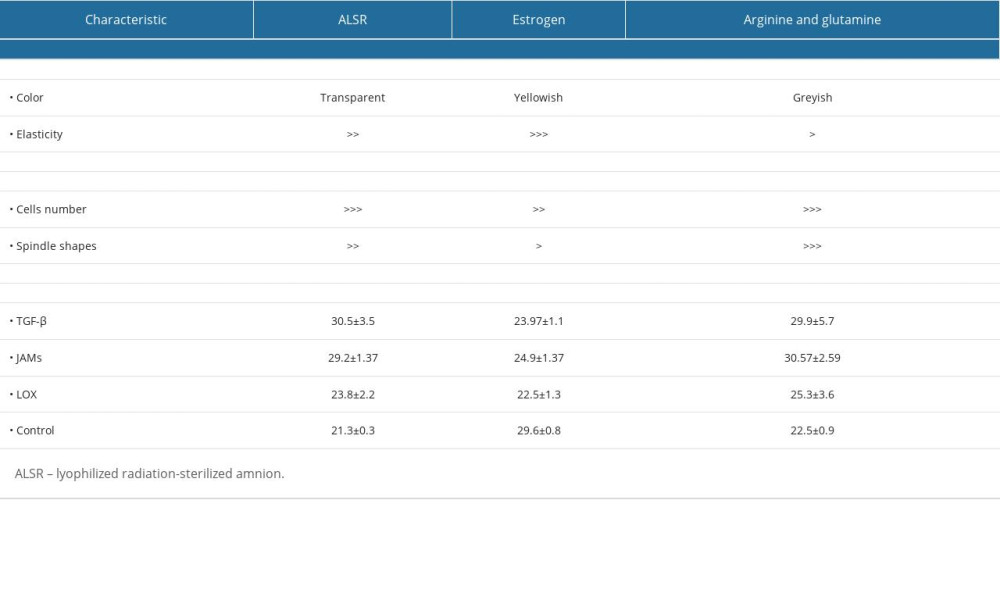 Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only).
Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only). Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine.
Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine.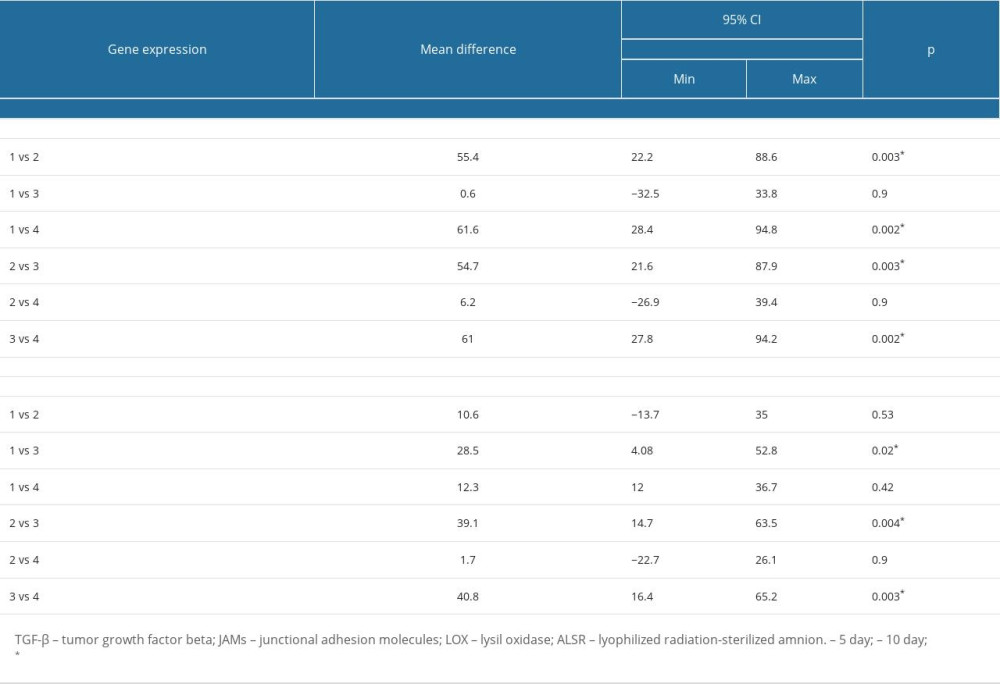 Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days.
Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days.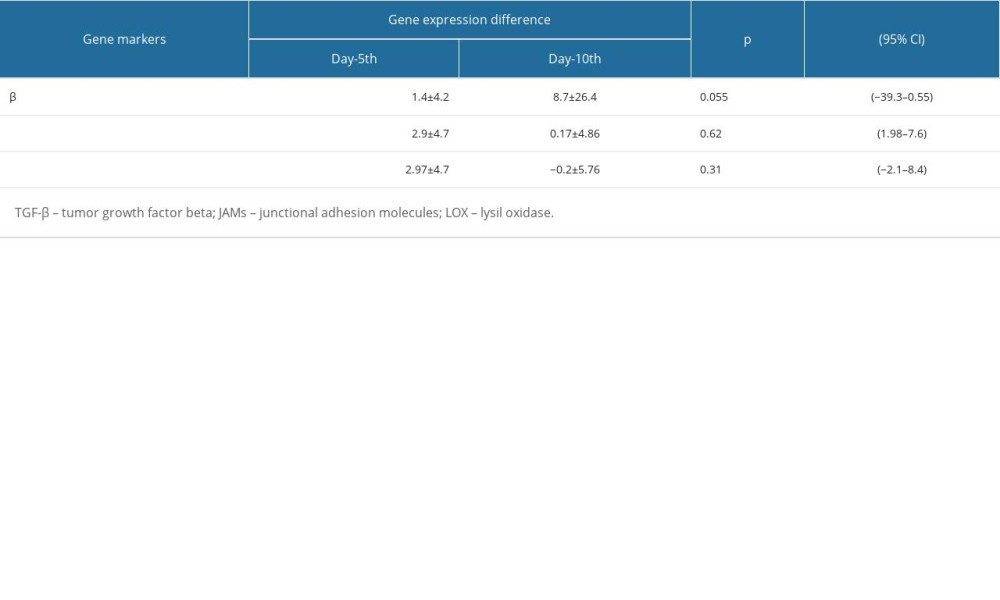
References
1. Diallo AB, Diallo TMO, Bah I, Vesicovaginal fistula: Anatomical clinical and surgical aspect in Conakry University Hospital Centre: Open J Urol, 2015; 5; 224-30
2. Bodner-Adler B, Hanzal E, Pablik E, Management of vesicovaginal fistulas (VVFs) in women following benign gynaecologic surgery: A systematic review and meta-analysis: PLoS One, 2017; 12(2); e0171554
3. Bazi T, Spontaneous closure of vesicovaginal fistula after bladder drainage alone: Review of the evidence: Int Urogynecol J pelvic Floor Dysfunct, 2007; 18(4); 475
4. Banke-Thomas AO, Wilton-Waddell OE, Kouraogo SF, Mueller JE, Current evidence supporting obstetric fistula prevention strategies in Sub Saharan Africa: A systematic review of the literature: Afr J Reprod Health, 2014; 18(3); 118-27
5. Lewis G, de Bernis L: Obstetric fistula: Guiding principles for clinical management and programme development, 2006, Publication of the World Health Organization
6. Waaldijk K, The immediate management of fresh obstetric fistulas: Am J Obstet Gynecol, 2004; 191; 795-99
7. Wall LL, Overcoming phase 1 delays: The critical component of obstetric fistula prevention programs in resource-poor countries: BMC Pregnancy Childbirth, 2012; 12(68); 1-13
8. Harrison MS, Mabeya H, Goldenberg RL, McClure EM, Urogenital fistula reviewed: A marker of severe maternal morbidity and an indicator of the quality of maternal healthcare delivery: Matern Health Neonatol Perinatol, 2015; 1; 20
9. Marsh F, Lynne R, Christine L, Alison W, Obstetric anal sphincter injury in the UK and its effect on bowel, bladder and sexual function: Eur J Obstet Gynecol Reprod Biol, 2011; 154(2); 223-27
10. Linneberg S, Leenskjold S, Glavind K, A five-year follow-up of women with obstetric anal sphincter rupture at their first delivery: Eur J Obstet Gynecol Reprod Biol, 2016; 203; 315-19
11. El-Azab AS, Abolella HA, Farouk M, Update on vesicovaginal fistula: A systematic review: Arab J Urol, 2019; 17(1); 61-68
12. Miklos JR, Moore RD, Chinthakanan O, Laparoscopic and robotic assisted vesicovaginal fistula repair: A systematic review of the literature: J Minim Invasive Gynecol, 2015; 22(5); 727-36
13. Kraan H, Vrieling H, Czerkinsky C, Buccal and sublingual vaccine delivery: J Control Release, 2014; 190; 580-92
14. Anggraeni R, Ika DA, Dewi A, Ronny M, Induction of protein specific antibody by carbonate apatite (MUCOADHESIVE BILAYER) as a candidate for mucosal vaccine adjuvant: Disertasi, 2019, Yogyakarta, FKG Universitas Gadjah Mada
15. Stechmiller JK, Childress B, Cowan L, Arginine supplementation and wound healing: Nutr Clin Pract, 2005; 20(1); 52-61
16. Cruzat V, Macedo Rogero M, Noel Keane K, Glutamine: Metabolism and immune function, supplementation and clinical translation: Nutrients, 2018; 10(11); 1564
17. Arribas-López E, Zand N, Ojo O, The effect of amino acids on wound healing: A systematic review and meta-analysis on arginine and glutamine: Nutrients, 2021; 13(8); 2498
18. Basril A, Febrid A, Hilmy N, Validation of amniotic membrane washing for amnion grafts, Agriculture, Animal Husbandry, Industry, Hydrology and Environment
19. Prossnitz ER, Barton M, Estrogen biology: New insights into GPER function and clinical opportunities: Mol Cell Endocrinol, 2014; 389(1–2); 71-83
20. Anggraeni R, Martien R, Agustina D, Ana ID, Incorporation of ovalbumin into carbonate apatite as a candidate for protein delivery: Key Eng Mater, 2018; 782; 27-31
21. Umeora O, Vesico-vaginal fistula in developing countries-time to turn off the tap: J Pregnancy Child Health, 2015; 02, doi: 10.4172/2376-127X.1000e120
22. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method: Methods, 2001; 25(4); 402-8
23. Nagahama H, Hideaki , Maeda H, Preparation and characterization of novel chitosan/gelatin membranes using chitosan hydrogel: J Carb Pol, 2008; 76; 255-60
24. Horng HC, Chang WH, Yeh CC, Estrogen effects on wound healing: Int J Mol Sci, 2017; 18(11); 2325
25. Wallace HA, Basehore BM, Zito PM, Wound healing phases [Updated 2022 Aug 25]: StatPearls [Internet], 2022, Treasure Island (FL), Stat Pearls Publishing
26. Wang Y, Chen X, Yin Y, Li S, Human amnion-derived mesenchymal stem cells induced osteogenesis and angiogenesis in human adipose-derived stem cells via ERK1/2 MAPK signaling pathway: BMB Rep, 2018; 51(4); 194-99
27. Barski D, Gerullis H, Ecke T, Repair of a vesico-vaginal fistula with amniotic membrane – Step 1 of the IDEAL recommendations of surgical innovation: Cent European J Urol, 2015; 68(4); 459-61
28. Horng HC, Chang WH, Yeh CC, Estrogen effects on wound healing: Int J Mol Sci, 2017; 18(11); 2325
29. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC, Wound healing: A cellular perspective: Physiol Rev, 2019; 99(1); 665-706
30. Murphy PS, Evans GRD, Advances in wound healing: A review of current wound healing products: Plast Surg Int, 2012; 2012; 190436
31. Mouw JK, Ou G, Weaver VM, Extracellular matrix assembly: A multiscale deconstruction: Nat Rev Mol Cell Biol, 2014; 15; 771-85
32. Hynes RO, Stretching the boundaries of extracellular matrix research: Nat Rev Mol Cell Biol, 2014; 15; 761-63
33. Mathew-Steiner SS, Roy S, Sen CK, Collagen in wound healing: Bioengineering (Basel), 2021; 8(5); 63
34. Schultz GS, Chin GA, Moldawer L, Principles of wound healing: Mechanisms of vascular disease: A reference book for vascular specialists [Internet], 2011; 23, Adelaide (AU), University of Adelaide Press
35. Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, Contribution of collagen network features to functional properties of engineered cartilage: Osteoarthritis Cartilage, 2008; 16(3); 359-66
36. Li J, Chen J, Kirsner R, Pathophysiology of acute wound healing: Clin Dermatol, 2007; 25(1); 9-18
37. Finnson KW, McLean S, Di Guglielmo GM, Philip A, Dynamics of transforming growth factor beta signaling in wound healing and scarring: Adv Wound Care (New Rochelle), 2013; 2(5); 195-214
38. Cai L, Xiong X, Kong X, Xie J, The role of the lysyl oxidases in tissue repair and remodeling: A concise review: Tissue Eng Regen Med, 2017; 14(1); 15-30
39. Wang G, Zhao F, Yang D, Human amniotic epithelial cells regulate osteoblast differentiation through the secretion of TGFβ1 and microRNA-34a-5p: Int J Mol Med, 2018; 41(2); 791-99
40. Gonzalez AC, Costa TF, Andrade ZA, Medrado AR, Wound healing – a literature review: An Bras Dermatol, 2016; 91(5); 614-20
41. Shi J, Barakat M, Chen D, Chen L, Bicellular tight junctions and wound healing: Int J Mol Sci, 2018; 19(12); 3862
42. Ebnet K, Kummer D, Steinbacher T, Regulation of cell polarity by cell adhesion receptors: Semin Cell Dev Biol, 2018; 81; 2-12
Figures
 Figure 1. Mucoadhesive bilayer membrane preparation. (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 1. Mucoadhesive bilayer membrane preparation. (Figure created using Microsoft Word for Mac, version 16.69.1). Figure 2. Microscopic view of fibroblast cell culture inoculated with mucoadhesive bilayer membrane-Active Substance complex. In Figure A, control cells were evenly distributed. The black arrow in Figure B shows clusters of wide, spindle-shaped cells. The black arrow in Figure C shows a smaller group of medium-sized spindle-shaped cells. The black arrow in Figure D shows a cluster of long spindle-shaped cells. Details: all slides are magnified to 40×. (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 2. Microscopic view of fibroblast cell culture inoculated with mucoadhesive bilayer membrane-Active Substance complex. In Figure A, control cells were evenly distributed. The black arrow in Figure B shows clusters of wide, spindle-shaped cells. The black arrow in Figure C shows a smaller group of medium-sized spindle-shaped cells. The black arrow in Figure D shows a cluster of long spindle-shaped cells. Details: all slides are magnified to 40×. (Figure created using Microsoft Word for Mac, version 16.69.1).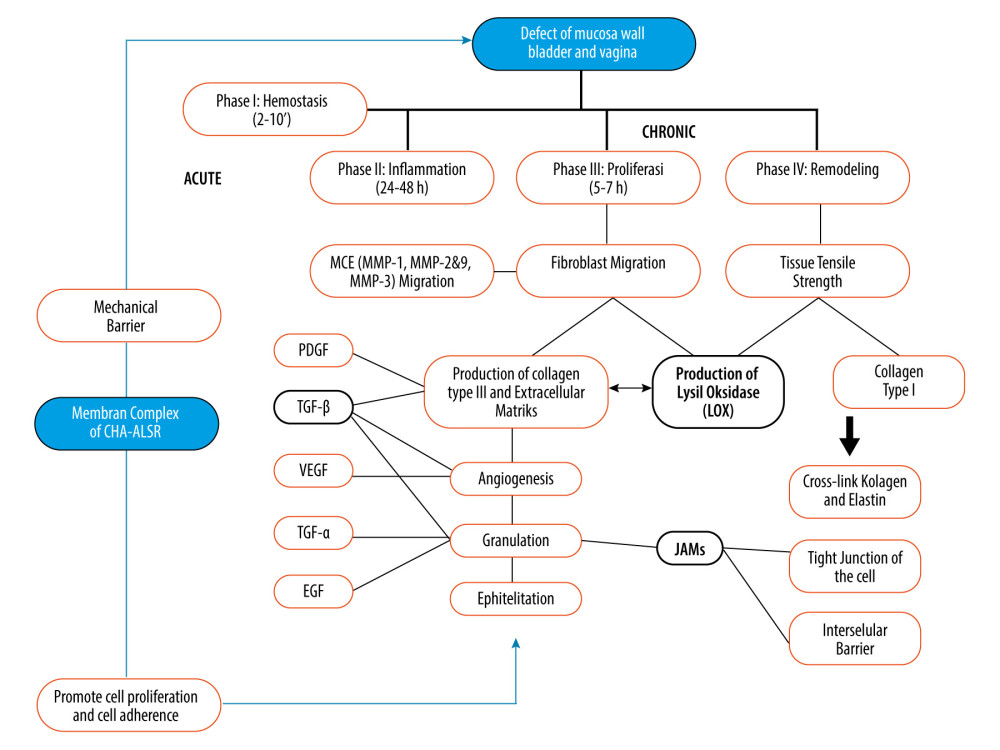 Figure 3. The role of the mucoadhesive bilayer membrane-ASLR complex in wound-healing stage as a basic concept of fistula closure [21,25,30,31,34,36,37]. (Black bold square shows a research-related gene). (Figure created using Microsoft Word for Mac, version 16.69.1).
Figure 3. The role of the mucoadhesive bilayer membrane-ASLR complex in wound-healing stage as a basic concept of fistula closure [21,25,30,31,34,36,37]. (Black bold square shows a research-related gene). (Figure created using Microsoft Word for Mac, version 16.69.1). Tables
 Table 1. Characteristics of research variables.
Table 1. Characteristics of research variables. Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only).
Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only). Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine.
Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine. Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days.
Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days. Table 1. Characteristics of research variables.
Table 1. Characteristics of research variables. Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only).
Table 2. One-way ANOVA analysis of TGF-β, JAMs, and LOX gene expression between 4 groups treated with a mucoadhesive bilayer membrane combined with: (1) ALSR; (2) estradiol; (3) arginine+glutamine; (4) control (cultured cells only). Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine.
Table 3. Tukey’s Post Hoc Analysis of TGF-β, JAMs, and LOX expression in fibroblast cells treated with mucoadhesive bilayer membrane combined with ALSR, estrogen, or arginine+glutamine. Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days.
Table 4. Gene expression of TGF-β, JAMs, and LOX in fibroblast cells treated with mucoadhesive bilayer membrane complex on the 5th and 10th days. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








