17 December 2023: Clinical Research
Preoperative Systemic Immune-Inflammatory Index as a Predictive Biomarker for Lower Extremity Deep Vein Thrombosis after Breast Cancer Surgery: Stratified Nursing Intervention to Reduce Incidence
Lu Tian12345ABCDEF, Jun Zhu1ABDF, Xia Li1ACD, Lei HuangDOI: 10.12659/MSM.942087
Med Sci Monit 2023; 29:e942087
Abstract
BACKGROUND: Lower extremity deep vein thrombosis (LEDVT), a common postoperative breast cancer complication, depends on multiple factors, such as systemic inflammation and immune status. We assessed the preoperative systemic immune-inflammatory index (SII) as a LEDVT risk predictor and did stratified nursing intervention based on it.
MATERIAL AND METHODS: A retrospective analysis was conducted on 552 patients who underwent breast cancer surgery from January 2019 to May 2020. Univariate and multivariate methods were used to screen independent risk factors for postoperative LEDVT, and effects of risk stratification and nursing intervention on the validation cohort were observed.
RESULTS: A total of 46 patients (8.3%) developed postoperative LEDVT. Compared with the control group, the LEDVT group had older age, higher proportion of menopause, higher preoperative Autar score, SII, and plasma D-dimer level, lower albumin level, and later postoperative pathological stage, with statistically significant differences (P<0.05). Multivariate logistic regression showed SII, plasma D-dimer, and menopause were independent predictors of postoperative LEDVT. ROC curve analysis showed SII had the strongest predictive ability, with an AUC of 0.714. Subsequently, 126 patients in the validation set were stratified according to the preoperative SII score, and intensified nursing intervention was implemented for high-risk patients, resulting in a significant reduction in the incidence of LEDVT (3.3% vs 8.3%, P=0.046).
CONCLUSIONS: Preoperative SII level can be used as an independent risk predictor of postoperative LEDVT in breast cancer patients. Applying it for risk stratification and implementing intensified nursing intervention for high-risk patients can significantly reduce the incidence of postoperative LEDVT.
Keywords: Breast Neoplasms, Venous Thrombosis, Inflammation, Prognosis, Predictive Value of Tests
Background
Deep vein thrombosis (DVT) is a serious complication that can occur after surgery, especially in patients with cancer [1]. DVT can lead to pulmonary embolism, which is a life-threatening condition that causes sudden death in about 10% of cases [2]. Lower extremity deep vein thrombosis (LEDVT) is the most common type of DVT, accounting for about 80% of all cases [3]. LEDVT can impair the mobility and quality of life of patients, and increase the risk of post-thrombotic syndrome, which is characterized by chronic pain, swelling, and skin ulcers in the affected limb.
Breast cancer is the most common cancer among women worldwide, with an estimated 2.3 million new cases and 685 000 deaths in 2020 [4]. Breast cancer patients have a lower risk of thrombosis than do patients with most other malignant tumors; the incidence was measured to be approximately 0.3% to 2.3% [5]. However, owing to the high prevalence of breast cancer and many risk factors, such as radical surgery, chemotherapy, and endocrine therapy, the absolute number of patients with LEDVT remains high [6] Therefore, it is important to identify the risk factors and predictors of LEDVT in breast cancer patients and to implement appropriate preventive measures.
Several risk assessment models have been developed to estimate the probability of DVT in different populations, such as the Autar scale [7], Caprini score [8], and Khorana score [9]. However, these models have some limitations for patients with breast cancer, such as low sensitivity, specificity, or applicability [10]. Moreover, these models are based on clinical or demographic variables that may not reflect the underlying pathophysiology of DVT. One of the most widely used biomarkers for predicting DVT is D-dimer, which is a degradation product of cross-linked fibrin. D-dimer reflects the activation of coagulation and fibrinolysis and has a high negative predictive value for excluding DVT [11]. However, D-dimer also has several drawbacks as a predictor of LEDVT after breast cancer surgery. First, D-dimer levels are influenced by many factors, such as age, tumor stage, and neoadjuvant chemotherapy. Second, D-dimer levels vary depending on the assay method and cut-off point used. Third, D-dimer levels peak 1 to 2 days after surgery but decrease to normal range by 1 week, which limits its usefulness for detecting delayed-onset LEDVT. Therefore, there is a need for more reliable and specific biomarkers for predicting LEDVT after breast cancer surgery.
Recent studies have suggested that inflammation plays a key role in the initiation and progression of DVT, as well as in its association with cancer [12,13]. Inflammation can induce a hypercoagulable state by activating platelets, neutrophils, and endothelial cells and by modulating the expression and function of coagulation factors and inhibitors. Therefore, inflammation-related biomarkers may be useful for predicting the risk of DVT in patients with cancer [12]. One such biomarker is the systemic immune-inflammation index (SII), which is calculated as the platelet count multiplied by neutrophil count divided by lymphocyte count [14]. The SII reflects the balance between host inflammatory response and immune status and has been shown to be associated with the prognosis and drug resistance in a variety of tumors, including breast cancer [14–16]. The rationale for using SII as a predictive tool for LEDVT after breast cancer surgery is based on several lines of evidence. First, SII has been reported to be correlated with D-dimer levels in patients with colorectal cancer and gastric cancer [17], suggesting a link between SII and coagulation activation. Second, SII has been found to be an independent prognostic factor for venous thromboembolism in patients with gastrointestinal cancer [18] and lung cancer [19], indicating its potential value for predicting thrombotic events in cancer patients. Third, SII has been demonstrated to be associated with tumor angiogenesis, lymph node metastasis, tumor-infiltrating lymphocytes, and tumor microenvironment [20], which are all relevant factors for the development and progression of breast cancer and LEDVT. However, the predictive ability of SII for the risk of LEDVT after breast cancer surgery has not been reported in the literature.
In this study, we aimed to explore the independent risk factors of LEDVT after radical mastectomy for breast cancer. We hypothesized that preoperative levels of SII would be a valuable predictor of postoperative morbidity in LEDVT and did stratified nursing intervention based on it.
Material and Methods
PATIENTS:
We retrospectively analyzed the clinical data of patients undergoing radical mastectomy in our department from January 2018 to May 2021. The inclusion criteria were as follows: (1) breast cancer was diagnosed by pathology (including core needle biopsy, intraoperative frozen-section, and postoperative paraffin section); and (2) the medical records were complete. The exclusive criteria were as follows: (1) breast cancer patients with preoperative LEDVT; (2) metastatic or recurrent breast cancer; (3) pregnant patients; (4) patients with systemic active infection, major trauma or surgery and autoimmune diseases within 1 month before operation; (5) severe hepatic and renal insufficiency; (6) the medical records were incomplete; (7) history of malignant tumors other than breast cancer; and (8) preoperative neoadjuvant chemotherapy or radiotherapy.
DETERMINATION OF SAMPLE SIZE:
Sample size was calculated using a free online tool (http://powerandsamplesize.com/Calculators/). According to the observation of small sample size in the early stage, the average value of the SII in the non-LEDVT group was 460, while the average value in the LEDVT group was 760. Group sizes were calculated to achieve statistical power of 0.80 and type 1 error rate of 5%. Referring to the previous literature of our institute, the incidence of LEDVT after breast cancer surgery was estimated to be 5.2% [18]; 28 cases with LEDVT needed to be included, and the total sample size was estimated as at least 539.
METHODS:
Clinical data of patients were collected retrospectively, including demographic data; comorbidities; vital signs at admission; Autar scale score; tumor-related information (including pathology type, stage, lymph node involvement); treatment options (including surgical method, postoperative bed time, and whether to accept perioperative chemotherapy); preoperative laboratory test results (including complete blood count, liver and kidney function, coagulation function); echocardiography (including left ventricular ejection fraction, left ventricular end diastolic diameter); and clinical outcomes (postoperative hospital stay, complications, and outcomes). Univariate and multivariate methods were used to identify the independent risk factors for postoperative LEDVT.
In the validation cohort, patients were grouped based on the SII cutoff, and the high-risk group was administered the following intensive nursing care measures: (1) preoperative education on the risks and prevention of DVT, with emphasis on the elderly population; (2) postoperative reinforcement of active and passive exercises of both lower limbs; (3) advising patients to drink more water appropriately after anesthesia recovery, and providing adequate fluid replacement on the day of surgery; (4) recommending a low-fat and high-fiber diet; (5) monitoring postoperative coagulation, D-Dimer, lymph node pathological staging; (6) observing daily for any signs of pain, swelling, skin temperature and color changes in both lower limbs. Transferring suspected LEDVT or pulmonary embolism patients to undergo lower extremity ultrasound or chest computed tomography; (7) applying anti-thrombotic elastic stockings or pneumatic compression therapy to both lower limbs, until the patient was able to get out of bed; and (8) consulting with physicians about the treatment plan for high-risk patients, using anticoagulants if possible, and enhancing the surveillance of wound drainage.
STATISTICAL ANALYSIS:
The Shapiro-Wilk test was used for the normal distribution test. For quantitative data conforming to normal distribution, mean±standard deviation was used, and the
ETHICS:
This study was conducted in accordance with the ethical standards of our responsible ethics committee on human experimentation and according to the Helsinki Declaration of 1975, as revised in 2000.
Results
INFORMATION ON SURGERY-RELATED LEDVT:
A total of 552 patients who met the admission criteria were enrolled, including 46 patients (8.3%) with LEDVT. Among them, embolic sites were in the left leg in 19 cases (41.3%), right leg in 12 cases (26.1%), and both legs in 15 cases (32.6%). The time of morbidity was 3 (2, 4) days after operation. All patients experienced intermuscular venous thrombosis, clinical signs of morbidity side of the leg pain and soreness, as well as varying degrees of limited activity. Symptoms improved and all patients survived and were discharged after subcutaneous injection of low-molecular-weight heparin 1 mg/kg every 12 h. None of the patients had pulmonary embolism or anticoagulation-related bleeding.
UNIVARIATE ANALYSIS OF CLINICAL DATA BETWEEN LEDVT AND NON-LEDVT GROUPS:
Univariate analysis showed that patients with postoperative LEDVT were older, had higher rates of menopause, and had higher values of preoperative Autar score, SII, and plasma D-dimer levels. The difference between the groups was statistically significant (P<0.05). The body mass index and aspartate aminotransferase level in the LEDVT group tended to be higher (P=0.053 and P=0.063, respectively). There were no significant differences in other common comorbidities, vital signs, or cardiac function indexes at admission (Tables 1, 2).
The univariate analysis of surgical indicators showed that the LEDVT group had a higher trend of the proportion of pathological-confirmed lymph node involvement and postoperative chemotherapy, but this was not statistically significant (P=0.066 and P=0.061, respectively). There were no significant differences in lesion location (left or right), operation type, pathological stage, and pathological type between groups (Table 3).
UNIVARIATE AND MULTIVARIATE LOGISTIC REGRESSION ANALYSIS OF PREDICTORS OF POSTOPERATIVE LEDVT:
Potential predictors screened in univariate analysis were analyzed by univariate and multivariate logistic regression (Table 4). Age, body mass index, serum levels of albumin, aspartate aminotransferase, and plasma D-dimer, and SII values were all continuous quantitative data. Grouping cutoff values of age, body mass index, and serum albumin and aminotransferase levels were set at 60 years, 25 kg/m2, 40 g/L, and 40U/L, respectively, according to the clinical significance. Grouping for D-dimer level and SII value was defined by the cutoff values 397 ng/mL and 653×109/L, obtained from the ROC curves. Multivariate logistic regression analysis showed that SII, D-dimer, menopause, and N3 stage were independent predictors of postoperative DVT, with odd ratios (ORs) of 6.24 (95% CI 2.78–14.00, P<0.001), 3.26 (95% CI 1.49–7.10, P<0.001), 2.97 (95% CI 1.20–7.33, P=0.018), and 3.89 (95% CI 1.37–11.10, P=0.011) respectively.
ASSESSMENT OF THE PREDICTIVE ABILITY OF THE INDEPENDENT RISK FACTORS FOR LEDVT BY ROC CURVES:
The ROC curve was used to comparatively analyze the predictive ability of the SII, D-dimer level, menopause status, and Autar score (Table 5). The results showed that the SII had the strongest predictive ability; the area under the ROC curve (AUC) was 0.714 (P=0.01). The cut-off value was 653×109/L, and the sensitivity and specificity were 0.50 and 0.83, respectively. The second was plasma D-dimer level (AUC 0.682), and the predictive power of the Autar score was the lowest, with an AUC of 0.608 (P=0.021).
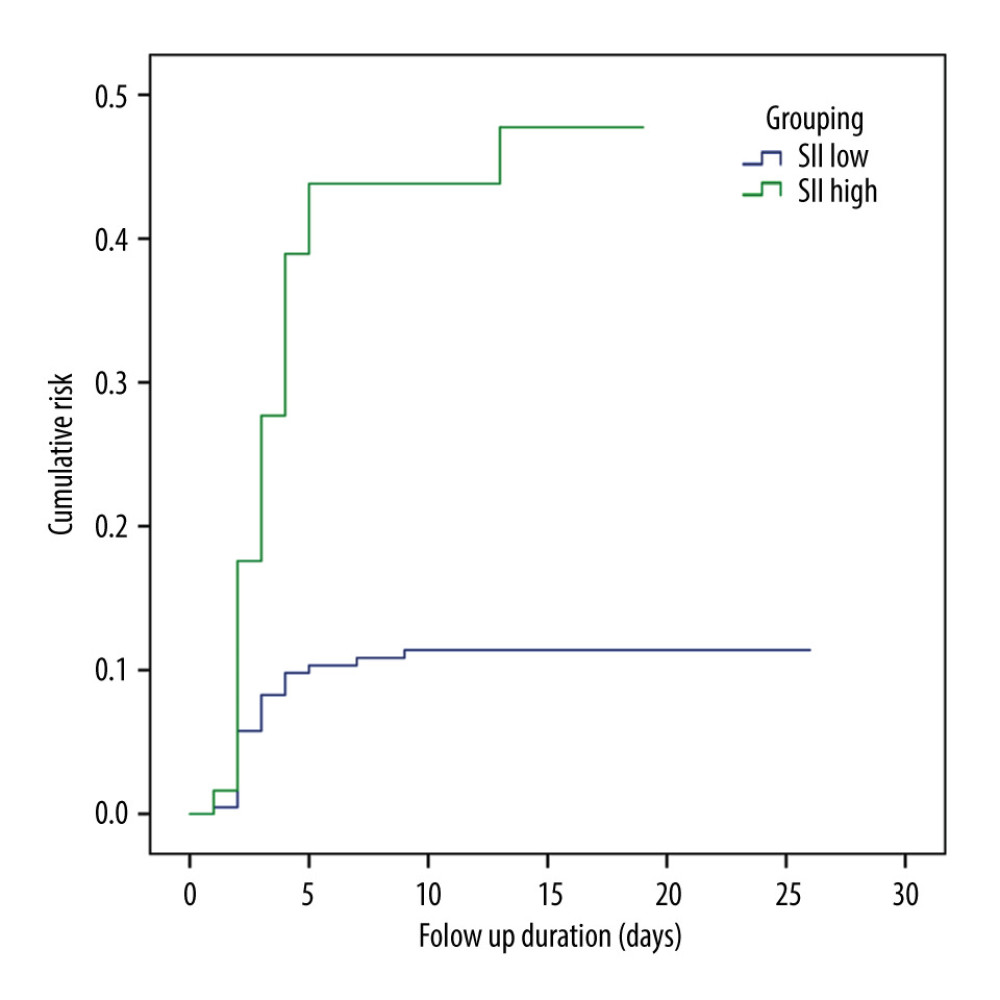
ASSESSMENT OF RISK STRATIFICATION OF SII TO POSTOPERATIVE LEDVT BY KAPLAN-MEIER PLOTS:
According to the cutoff value of the SII, the population was divided into 2 groups: SIIhigh (SII>653×109/L) and SIIlow (SII<653×109/L). The predictive ability of this cutoff value to postoperative LEDVT was evaluated by Kaplan-Meier plots. The results showed that the incidence of LEDVT in the SIIhigh group was significantly higher than that in the SIIlow group (log-rank test. χ2=25.039, P<0.001; Figure 1).
EFFECT OF A DIFFERENTIATED NURSING MODE BASED ON SII SCORE ON POSTOPERATIVE LEDVT:
From June to December 2020, we enrolled another 126 patients who were planned to undergo breast cancer surgery in our department, using the same inclusion and exclusion criteria as before. The patients were classified by their risk of postoperative LEDVT based on the cutoff value of the SII score. Intensified nursing intervention was implemented for high-risk patients. The results indicated that the differentiated nursing interventions could significantly lower the incidence of postoperative LEDVT, compared with the conventional nursing model (3.3% vs 8.5%,
Discussion
DVT is a common complication and the main cause of death in patients with cancer, with an incidence rate of approximately 5%, which is 6 times higher than that of the general population [21]. Hospitalization, surgery, and adjuvant therapy (eg, chemotherapy) can further increase morbidity risk [22]. With the aging of the population and the increasing incidence rates of cancer, the incidence of DVT after tumor surgery is increasing, which seriously threatens the prognosis and rehabilitation process of patients and brings great challenges for clinical care [23]. The results of a survey on the awareness of DVT awareness among medical personnel in China are not satisfactory, especially when considering the low proportion of risk assessments conducted of DVT for patients in surgical surgery department [24]. The attitude toward and knowledge of DVT prevention will directly affect the implementation of the protective measures. Therefore, it is important to improve the early recognition of the high-risk DVT group after tumor surgery to improve the quality of nursing care.
Activation of the coagulation system is closely related to inflammation, such as polymorphonuclear neutrophils that can produce neutrophil extracellular traps, which have high prothrombin activity [25]. Activated platelets can also play a pivotal role in the secretion of proinflammatory mediators and in the activation of the contact system [26]. Therefore, the ratios based on the combination of different types of inflammatory cell counts, such as the monocyte/lymphocyte ratio and neutrophil to lymphocyte ratio, are of increasing importance and have recently attracted the attention of clinicians [27,28]. This study focuses on the analysis of a recently proposed new biomarker, the SII, which reflects the state of systemic immune inflammation. The SII incorporates inflammatory cells with 3 distinct roles – neutrophils, platelets, and lymphocytes – better reflecting the immune status and serious balance of the host. A recent study has found that the SII was related to the long-term prognosis of elderly patients with acute myocardial infarction after coronary intervention [29], suggesting that the SII may be involved in acute thrombotic events and affect their prognosis. To the best of our knowledge, this is the first case-control study on the prediction of LEDVT after breast cancer surgery. The results showed that the SII was an independent predictor of postoperative LEDVT and had the strongest predictive power in all single indicators. Risk stratification of patients based on the cut-off value of the SII has proved to be effective in distinguishing high-risk groups, thus providing an effective early warning for follow-up preventive nursing intervention. Another advantage of the SII is its low cost and easy accessibility. The SII is readily obtained from the complete blood count calculation routinely performed at the time of admission, so it also has a strong clinical application value in primary medical institutions.
The Autar scale is a widely used risk assessment scale for DVT. A study has shown that the scale helps to avoid the risk of LEDVT after breast cancer surgery and to improve the quality of nursing care [30]. In the present study, we also found that the patients with postoperative LEDVT had significantly higher preoperative Autar scores than patients in the non-LEDVT group, but their early risk prediction ability was not strong (AUC was 0.608). We analyzed the statistical characteristics of the Autar score of the study population and found that the score ranged from 13 to 24, of which the number of patients with scores of 13 and 14 accounted for 89.8%. The overall distribution showed an obvious right skewness (skewness value 3.529), indicating that the Autar scale is not strong in risk stratification for patients at low and medium risk with scores ≤14. This phenomenon may be related to the characteristics of patients with breast cancer. None of the patients included in this study were bedridden, pregnant, on oral contraceptives, or affected by trauma. In addition, the center of this study is a tertiary cancer hospital affiliated with a university in China, and patients with a variety of comorbidities are rare, which weakens the risk prediction ability of the scale to a certain extent. In the observational study on the risk of DVT after breast cancer surgery, as carried out by Chen et al, only 11% of the patients were at moderate to low risk on the Autar scale [30], which was similar to the results of this study. The researchers adopted a differentiated nursing intervention strategy for patients with different risk stratification, and no patients in any risk stratification group developed LEDVT [30]. However, the overall sample size of the study was too small (n = 79), which limited its statistical power. In the present study, ROC curve analyses showed that of all the univariate factors included in the analysis, the SII had the strongest predictive ability for postoperative LEDVT (AUC 0.714), with good specificity (83%) and slightly poor sensitivity (50%). Then, we further constructed a joint prediction index containing the SII, and its prediction ability (AUC 0.798) and sensitivity (76%) were improved. According to the cut-off value of the SII, we divided the study population into a high-risk group and a low-risk group. The results also confirmed that this indicator had a strong predictive ability for risk stratification of postoperative LEDVT.
When analyzing the distribution of leukocyte subsets in preoperative blood cell count, we found that there was no significant difference in total leukocyte, monocyte, and platelet counts between the LEDVT group and non-LEDVT group, but the SII in LEDVT group was significantly higher than that in the non-LEDVT group, suggesting that there is a closer pathophysiological relationship between the composition of subgroups of cells and acute thrombotic events compared with the overall white blood cell count. Mechanically, neutrophil extracellular traps are released after the activation of neutrophils in blood, producing strong pro-inflammatory and pro-thrombotic effects [31]. Lymphocytes, as part of the adaptive immune response, are important elements of the immunoregulatory pathway. Recently, several studies have found that an elevated neutrophil count and low lymphocyte count are closely related to the progression of atherosclerosis, or ischemia-reperfusion injury after revascularization of acute myocardial infarction [32,33]. Platelets are the initiating factors of thrombosis. Persistent inflammatory stimulation can lead to a pro-thrombotic state in the presence of a relative increase of platelets [31]. In this study, we found that the preoperative platelet count of the LEDVT group was slightly higher than that of the control group; the difference did not reach statistical significance but had a higher baseline inflammatory level, as shown by the significant increase of the ratio of neutrophils to lymphocytes. The SII demonstrated better diagnostic efficacy, probably due to its comprehensive consideration of the role of the various inflammatory cells mentioned above.
LEDVT is a disease of multiple etiologies. In univariate and multivariate analysis, we also found significant inter-group differences in preoperative serum albumin levels. The level of albumin in the LEDVT group was significantly lower than that in the control group, which may be attributed to the later pathological stage and more significant tumor consumption in the LEDVT group. Lower albumin levels cause the effective blood volume to be vulnerable to intraoperative blood and fluid loss and postoperative plasma colloid osmotic pressure to decrease, leading to hemoconcentration and pro-thrombogenic effects. The analysis also showed that plasma D-dimer levels and menopause status were independent risk predictors for postoperative LEDVT morbidity and had a certain predictive value for postoperative LEDVT (the AUCs were 0.682 and 0.625, respectively), and the cutoff value of the D-dimer level was 392 ng/mL. D-dimer is a routine preoperative blood test item, which is generally considered to be one of the predictive markers of DVT risk [11]. Menopausal status often suggests that advanced age and significant changes in estrogen and progesterone levels, which affect the function of vascular endothelial cells and blood viscosity to a certain extent, result in the morbidity tendency of DVT.
Another finding of interest in this study was that the postoperative pathology stage was more advanced in the LEDVT group than in the non-LEDVT group. Lymph node involvement with N3 disease was an independent predictor of postoperative LEDVT regardless of the type of surgery. Several studies have shown that metastatic cancer is a powerful predictor of venous thromboembolism, compared with localized tumors [34,35]. Although the cause of the close relationship between the two is not clear, it may be related to the biological invasion of the tumor, which leads to the hypercoagulable state of the blood and the increased adhesion between platelets and endothelium. Lymph nodes are the sites of lymphopoiesis and functional maturation. Higher stage of lymph node involvement means more extensive lymph node involvement around the breast, and there may be a more significant imbalance of systemic inflammatory response. This may explain the greater predictive value of the “N” stage than of the “T” stage, the latter of which only measures the size of the tumor.
In this study, we used a retrospective case-control design to evaluate the predictive value of the SII for LEDVT after radical mastectomy for breast cancer. We estimated the sample size based on the previous data from our center and obtained a relatively large sample size. We also collected detailed clinical data of the patients. Moreover, from a nursing perspective, we validated the clinical intervention value of the SII. The validation results showed that the nursing practice mode based on preoperative SII cut-off could significantly reduce the incidence of postoperative LEDVT in the overall population and improve the prognosis of patients. To the best of our knowledge, this is the first study to explore the application of the SII for nursing intervention of LEDVT after breast cancer surgery.
The limitations of this study mainly included selection bias and cofounding factors. Since this was a single-center study, it might not be applicable to other clinical settings, and the extrapolation of conclusions should be considered with caution, with the results requiring validation by more multi-center research cohorts. Also, this was an observational study, which might have had confounding factors, and there might be other unconsidered or uncontrolled factors associated with LEDVT affecting the interpretation and inference of the data.
Conclusions
In this single-center retrospective case-control study, we found that the SII was an independent predictor of postoperative LEDVT in patients with breast cancer, having the strongest predictive ability and risk stratification value. Based on the SII cutoff value, risk stratification and differentiated nursing interventions helped to identify high-risk patients early, to initiate corresponding nursing interventions as soon as possible, and to improve postoperative prognosis. The conclusions of this study need to be confirmed by external cohorts or further randomized controlled trials.
Tables
Table 1. Univariate analysis of general clinical data between the LEDVT and non-LEDVT groups.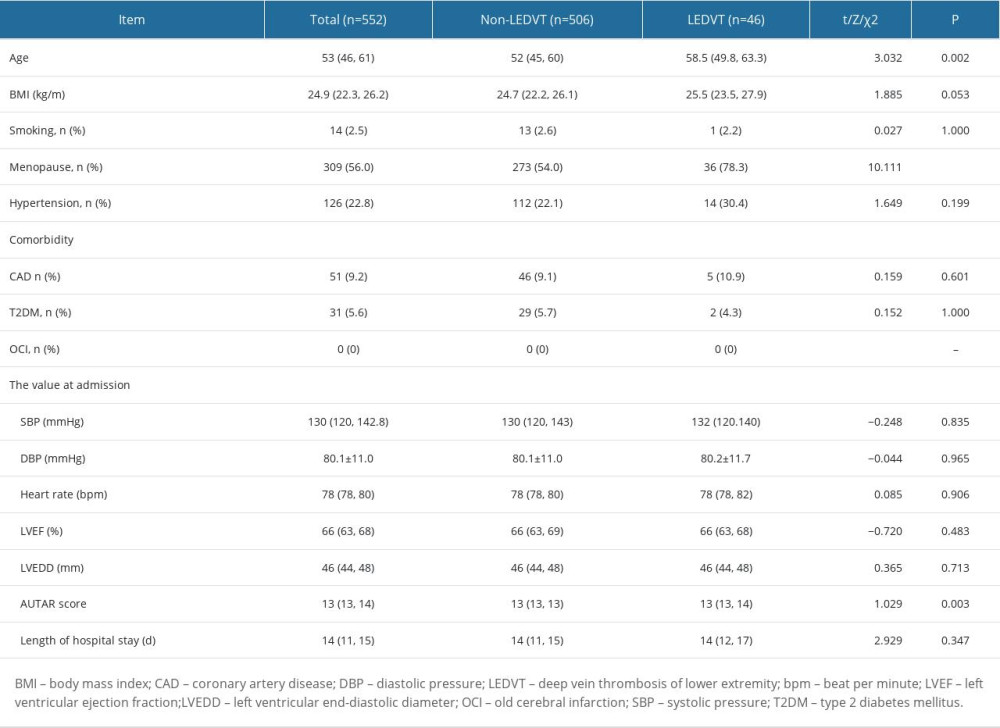 Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups.
Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups.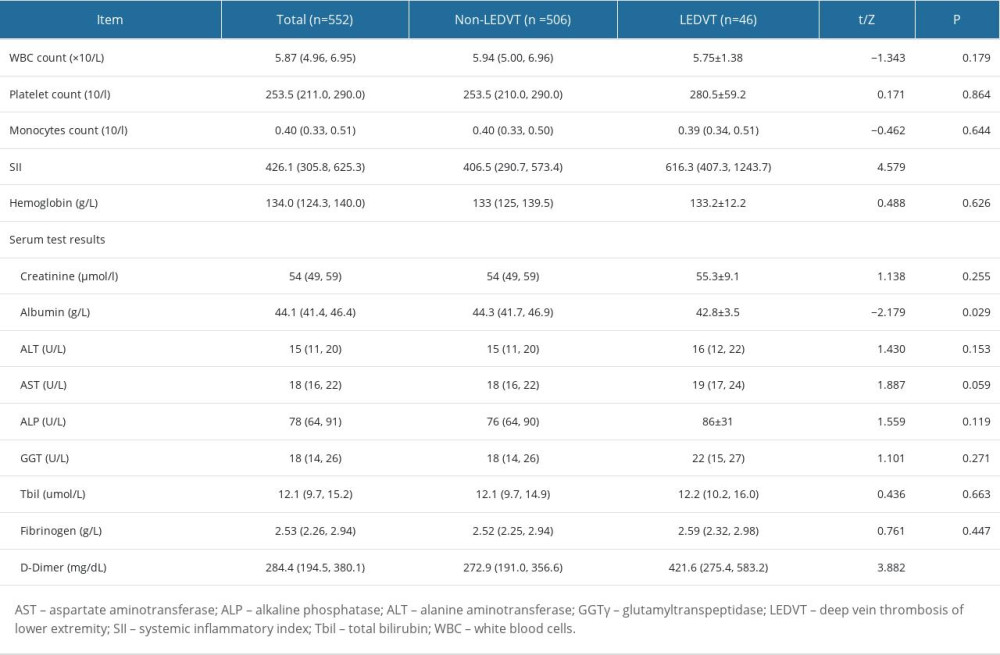 Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups.
Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups.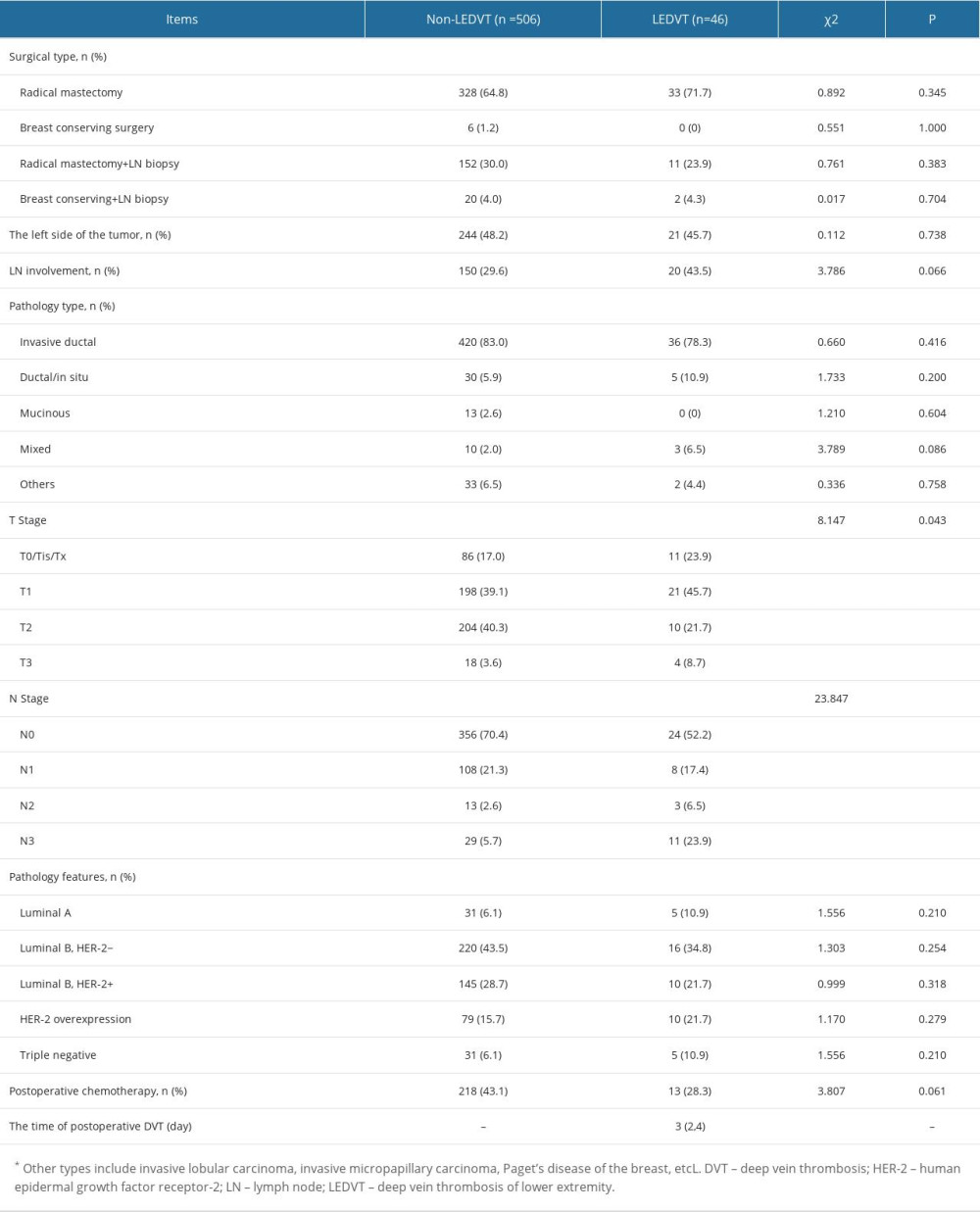 Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT.
Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT.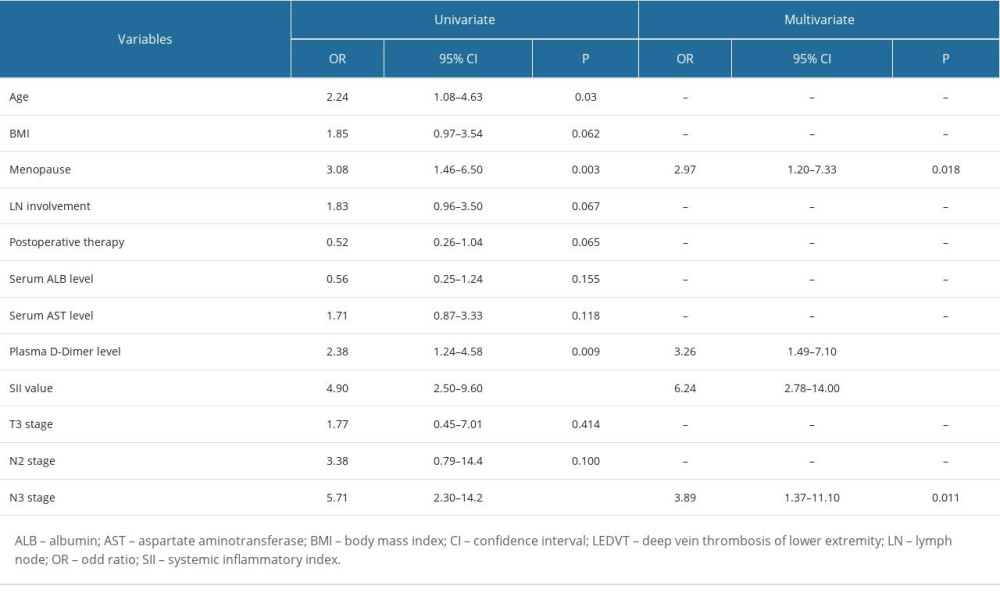 Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves.
Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves.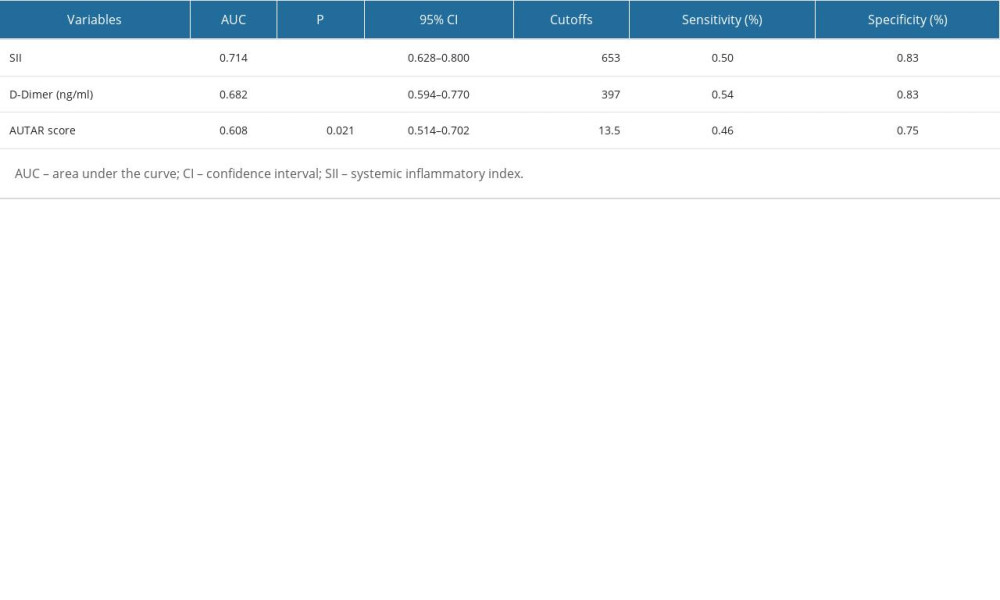
References
1. Mahajan A, Brunson A, White R, Wun T, The epidemiology of cancer-associated venous thromboembolism: An update: Semin Thromb Hemost, 2019; 45(4); 321-25
2. Trott T, Bowman J, Diagnosis and management of pulmonary embolism: Emerg Med Clin North Am, 2022; 40(3); 565-81
3. Falanga A, Ay C, Di Nisio MESMO Guidelines Committee, Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline: Ann Oncol, 2023; 34(5); 452-67
4. Giaquinto AN, Sung H, Miller KD, Breast Cancer Statistics, 2022: Cancer J Clin, 2022; 72(6); 524-41
5. Key NS, Khorana AA, Kuderer NM, Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline update: J Clin Oncol, 2020; 38(5); 496-520
6. Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023: Cancer J Clin, 2023; 73(1); 17-48
7. Autar R, Nursing assessment of clients at risk of deep vein thrombosis (DVT): The Autar DVT scale: J Adv Nurs, 1996; 23(4); 763-70
8. Edwards MA, Brennan E, Rutt AL, Venous thromboembolism prophylaxis in otolaryngologic patients using caprini assessment: Laryngoscope, 2023 Available from: https://doi.org/10.1002/lary.31041
9. Ha H, Ko YH, Kim K, Application of the Khorana score for cancer-associated thrombosis prediction in patients of East Asian ethnicity undergoing ambulatory chemotherapy: Thromb J, 2023; 21(1); 63
10. Jonczyk MM, Fisher CS, Babbitt R, Surgical predictive model for breast cancer patients assessing acute postoperative complications: The breast cancer surgery risk calculator: Ann Surg Oncol, 2021; 28(9); 5121-31
11. Harbsmeier AN, Altintas I, Iversen K, Biomarkers and the post-thrombotic syndrome: A systematic review of biomarkers associated with the occurrence of the post-thrombotic syndrome after lower extremity deep venous thrombosis: Phlebology, 2023; 38(9); 577-98
12. Bodnar P, Klishch I, Bodnar Y, The role of markers of systemic inflammatory response in pathogenesis of thrombotic complications in malignancy: Georgian Med News, 2022(332); 121-24
13. Navarrete S, Solar C, Tapia R, Pathophysiology of deep vein thrombosis: Clin Exp Med, 2023; 23(3); 645-54
14. Hu B, Yang XR, Xu Y, Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma: Clin Cancer Res, 2014; 20(23); 6212-22
15. Hu X, Shao YX, Yang ZQ, Preoperative systemic immune-inflammation index predicts prognosis of patients with non-metastatic renal cell carcinoma: A propensity score-matched analysis: Cancer Cell Int, 2020; 20; 222
16. Sun Y, Li W, Li AJ, Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients: Cancer Manag Res, 2019; 11; 3153-62
17. Yu J, Li D, Lei D, Tumor-specific D-dimer concentration ranges and influencing factors: A cross-sectional study: PLoS One, 2016; 11(11); e0165390
18. Zhang L, Fang Y, Xing J, The efficacy of the systemic immune-inflammation index and prognosis nutritional index for the diagnosis of venous thromboembolism in gastrointestinal cancers: J Inflamm Res, 2022; 15; 4649-61
19. Zhang L, Liu X, Yang R, The diagnostic value of the systemic immune-inflammation index for venous thromboembolism in lung cancer patients: A retrospective study: Mediators Inflamm, 2022; 2022; 9215311
20. Ji Y, Wang H, Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: A meta-analysis: World J Surg Oncol, 2020; 18(1); 197
21. Ramcharitar RK, Man L, Khaja MS, A Review of the past, present and future of cancer-associated thrombosis management: Heart Int, 2022; 16(2); 117-23
22. Khan UT, Walker AJ, Baig S, Venous thromboembolism and mortality in breast cancer: Cohort study with systematic review and meta-analysis: BMC Cancer, 2017; 17(1); 747
23. Park JH, Ahn SE, Kwon LM, The risk of venous thromboembolism in korean patients with breast cancer: A single-center experience: Cancers (Basel), 2023; 15(12); 3124
24. Zheng E, Tang Y, Yang MCurrent status of prevention and nursing on venous thromboembolism among perioperative patients with lung cancer: Zhongguo Fei Ai Za Zhi, 2017; 20(10); 661-66 [in Chinese]
25. Brinkmann V, Neutrophil extracellular traps in the second decade: J Innate Immun, 2018; 10(5–6); 414-21
26. Hassanian SM, Avan A, Ardeshirylajimi A, Inorganic polyphosphate: A key modulator of inflammation: J Thromb Haemost, 2017; 15(2); 213-18
27. Kiris T, Çelik A, Variş E, Association of lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention: Angiology, 2017; 68(8); 707-15
28. Guo TM, Cheng B, Ke L, Prognostic value of neutrophil to lymphocyte ratio for in-hospital mortality in elderly patients with acute myocardial infarction: Curr Med Sci, 2018; 38(2); 354-59
29. Huang J, Zhang Q, Wang R, Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention: Med Sci Monit, 2019; 25; 9690-701
30. Chen XJ, Wang YJ, Yu JJ, Effect observation on application of Autar scale in prevention of postoperative lower extremity of deep venous thrombosis in breast cancer patients: Chin Nurs Res, 2016; 7(30); 2414-15
31. Mangold A, Alias S, Scherz T, Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size: Circ Res, 2015; 116(7); 1182-92 Erratum in: Circ Res. 2021;128(2):e26
32. Sharma DJ, Nath HJ, Batta A, Goala AK, Neutrophil-to-lymphocyte ratio (NLR) useful as a cost-effective preliminary prognostic marker in st-elevation myocardial infarction (STEMI): an observational study from a tertiary care hospital in Northeast India: Cureus, 2023; 15(3); e36885
33. Sezer M, Okcular I, Goren T, Association of haematological indices with the degree of microvascular injury in patients with acute anterior wall myocardial infarction treated with primary percutaneous coronary intervention: Heart, 2007; 93(3); 313-18
34. Couturaud F, Mahé I, Schmidt J, Adult breast, lung, pancreatic, upper and lower gastrointestinal cancer patients with hospitalized venous thromboembolism in the national French hospital discharge database: BMC Cancer, 2023; 23(1); 531
35. Peng M, Yang S, Li G, Solid tumor complicated with venous thromboembolism: A 10-year retrospective cross-sectional study: Clin Appl Thromb Hemost, 2021; 27; 1076029620975484
Tables
 Table 1. Univariate analysis of general clinical data between the LEDVT and non-LEDVT groups.
Table 1. Univariate analysis of general clinical data between the LEDVT and non-LEDVT groups. Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups.
Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups. Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups.
Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups. Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT.
Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT. Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves.
Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves. Table 1. Univariate analysis of general clinical data between the LEDVT and non-LEDVT groups.
Table 1. Univariate analysis of general clinical data between the LEDVT and non-LEDVT groups. Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups.
Table 2. Univariate analysis of peripheral blood test results between the LEDVT and non-LEDVT groups. Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups.
Table 3. Univariate analysis of surgical data between the LEDVT and non-LEDVT groups. Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT.
Table 4. Univariate and multivariate logistic regression of predictors of postoperative LEDVT. Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves.
Table 5. Assessment of the predictive ability of the independent risk factors for LEDVT by receiver operating characteristic curves. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952