09 January 2024: Clinical Research
Impact of Respiratory Viruses and SARS-CoV-2 on Febrile Seizures in Saudi Children: Insights into Etiologies, Gender, and Familial Associations
Saleh Fahad AlFulayyihDOI: 10.12659/MSM.942478
Med Sci Monit 2024; 30:e942478
Abstract
BACKGROUND: Childhood febrile seizures occur between 5 months and 6 years of age in children without a previous history of seizure and are associated with high temperature in the absence of intracranial infection. This retrospective study identified 71 children aged 6 months to 5 years with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia and aimed to identify an association between common respiratory virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
MATERIAL AND METHODS: Pediatric nasopharyngeal specimens were tested using a multiplex PCR respiratory panel detecting human coronaviruses (NL63, 229E, OC43, HKU1), influenza A/B, human adenovirus, parainfluenza viruses 1-4, respiratory syncytial virus, human metapneumovirus, rhinovirus/enterovirus, Middle East respiratory syndrome coronavirus, and, as of September 2021, SARS-CoV-2, confirmed using the Cepheid Xpert Xpress SARS-CoV2 RT-PCR kit.
RESULTS: In a cohort of 71 pediatric patients (median age, 19 months; 54.9% female), dominant pathogens included human rhinovirus/enterovirus (23.9%), influenza A/B (26.8%), and SARS-CoV-2 (14.1%). Concurrent infections were noted in 28.2%. Simple seizures occurred in 69%, and complex seizures in 31%. Females exhibited an 8.18-fold increased risk for complex seizures. Each additional fever day reduced complex seizure risk by 36%. Familial seizure history increased risk 8.76-fold. Human rhinovirus/enterovirus or parainfluenza infections inversely affected complex seizure likelihood compared with adenovirus.
CONCLUSIONS: In Saudi children with febrile seizures, distinct viral etiologies, sex, and familial links play pivotal roles. Given regional viral variations, region-tailored diagnostic and therapeutic strategies are paramount. A multicenter prospective cohort study is essential for comprehensive understanding.
Keywords: Febrile Seizures Associated with Afebrile Seizures, Multiplex Polymerase Chain Reaction, Respiratory Tract Infections, SARS-CoV-2, Saudi Arabia
Background
Febrile seizures, the most common seizure phenotype in children, affect roughly 2% to 5% of the global pediatric population [1,2]. The prevalence in Saudi Arabia exceeds global averages, at 8% [3], with seizures often induced by rapid febrile episodes during diseases such as respiratory infections. Respiratory viruses, the primary cause of febrile infections in pediatric cohorts, have been consistently linked to the development of febrile seizures [4,5]. In pediatric etiologies, influenza, respiratory syncytial virus (RSV), adenovirus, and human rhinovirus appear as major agents [6]. Multiplex polymerase chain reaction (PCR) testing and other advances in molecular diagnostics now allow for the simultaneous detection of a wide range of respiratory viruses, improving our understanding of the viral determinants of febrile seizures [7,8].
This association has been extensively researched, yet results vary depending on region. In Japan, influenza has a clear link to febrile seizures [9], but in the United States, the link is stronger with human rhinovirus [10]. Similarly, adenoviruses dominate in Turkey [11], rhinoviruses in Australia (with a 21.7% incidence) [12], and influenza in both Canada [13] and the Netherlands – the latter emphasizing the role of influenza A [14]. However, from a Korean perspective, influenza and enterovirus are both important factors [15]. According to Tso et al, 0.5% of pediatric patients with COVID-19 experienced febrile seizures, with 9% requiring critical care [16]. This contrasts with Hong Kong’s higher rates, particularly among unvaccinated children during the Omicron surge [17], and the 10.7% prevalence reported by Pascarella [18]. Another study found that 12.8% of those with the Omicron variant of COVID-19 experienced febrile seizures [19]. However, as Kurd et al reported [20], febrile seizures associated with SARS-CoV-2 are less prevalent than those associated with other respiratory viruses. The links between SARS-CoV-2 and febrile seizures in children require more investigation. Localized viral epidemiology, underlying genetic predispositions, and unique environmental vectors can all contribute to such geographical variations.
The literature on this topic in Saudi Arabia is still in its infancy. A Riyadh-based study identified RSV as the most common virus in pediatric febrile seizure settings [21], whereas a Jeddah-based study identified adenovirus [22]. The absence of multiplex PCR in this research is a major constraint, resulting in limited sample dimensions. The increased occurrence of febrile seizures in Saudi Arabia, set against the backdrop of respiratory infections, emphasizes the importance of thorough examinations. In the present study, we used a multiplex PCR respiratory panel, which promises a broad detection range of respiratory viruses while also identifying the viral factors triggering febrile seizures in Saudi pediatric demographics. Therefore, this retrospective study identified 71 children aged between 6 months and 5 years with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia and aimed to identify an association between common respiratory virus and SARS-CoV-2 infection.
Material and Methods
ETHICAL CONSIDERATIONS:
The King Abdullah International Medical Research Center’s (KAIMRC) Institutional Review Board approved the study protocol (reference: RD20/004/D). The investigation strictly followed relevant ethical requirements. Notably, KAIMRC waived the necessity for informed consent, which is generally required for human research, for this study (reference: RD22D/001/01). All data were handled anonymously to protect participant confidentiality and privacy.
STUDY DESIGN AND SETTING:
We performed an observational, retrospective cohort analysis by carefully reviewing electronic medical records from January 2017 to December 2021. The study was carried out in Dammam, Saudi Arabia, at the Imam Abdulrahman bin Faisal Hospital.
PARTICIPANTS:
The study included infants and children aged 6 months to 5 years who were admitted to the hospital with a confirmed diagnosis of febrile seizures. Patients who tested positive for common respiratory viruses by nasopharyngeal aspirate by use of the respiratory viral panel multiplex nucleic acid assay PCR or had a positive PCR for SARS-CoV-2 were included in the study.
EXCLUSION CRITERIA:
Children with a documented history of afebrile seizures or epilepsy were excluded from the trial. Additionally, those with obvious neurological abnormalities, such as cerebral palsy, neurodegenerative disorders, or developmental delays, were omitted. Any patient exhibiting signs suggestive of central nervous system infections was also barred from participating. This strict method removed potential confounding factors from our analysis. These stringent criteria were developed to ensure that the study sample was ideally targeted to investigate the research question, with the least amount of potential confounding variables. While the inclusion criteria sought to represent the larger community of Saudi Arabian infants and children experiencing febrile seizures, the exclusion criteria sought to exclude instances with probable other etiologies for seizures.
MULTIPLEX NUCLEIC ACID ASSAY FOR RESPIRATORY VIRAL PANEL:
The BioFire respiratory panel 2.1 multiplex PCR technology was used in our study to detect a range of respiratory pathogens. The technique begins with the collection of a nasopharyngeal swab or aspirate, which is then transmitted to the laboratory under controlled conditions to maintain sample integrity by viral transport medium [23]. In this method, specific segments of the pathogen’s DNA/RNA, termed targeted genes, are amplified for detection. For instance, human coronavirus NL63 and 229E are detected using the nucleocapsid (N) gene; influenza A targets the matrix (M) gene, while influenza B targets the non-structural (NS) gene; human adenovirus targets the hexon gene; parainfluenza viruses 1–4 target the hemagglutinin-neuraminidase (HN) gene; both human coronavirus OC43 and RSV are identified through the N gene; human metapneumovirus targets the matrix (M) gene; coronavirus HKU1 targets the N gene; human rhinovirus/enterovirus is detected through the 5′ non-coding region (5′NCR); Middle East respiratory syndrome coronavirus utilizes the upstream of the E protein (UpE) and ORF1b genes; and SARS-CoV-2 often targets the ORF1ab, N, and spike (S) genes. In the PCR process of the BioFire respiratory panel 2.1, these targeted genes, represented by specific primers, are mixed in a single reaction, and their amplification signifies the presence of the respective pathogen in the sample [24]. It allows for the rapid detection of the causative virus [25].
SARS-COV-2 DETECTION:
A reverse transcription-PCR kit type Xpert Xpress SARS-CoV2 test by manufacturer Cepheid, licensed by the Saudi Food and Drug Authority in October 2020, confirmed the presence of SARS-CoV-2. Moreover, SARS-CoV-2 was included in our institute’s PCR multiplex respiratory panels in September 2021.
COVARIATES:
Age, sex, duration of fever prior to febrile seizure, temperature at presentation, maximum temperature, type of seizure (simple or complex), family history of febrile seizure, length of hospital stay, lumbar puncture, and nasopharyngeal aspirate were all investigated as covariates.
Febrile seizure is defined as a convulsion episode that occurs in children aged 6 months to 5 years, in association with a fever of 38°C (100.4°F), with no prior history of afebrile seizure, evidence of central nervous system infection (meningitis, encephalitis, and brain abscess), metabolic disturbance, traumatic cause, or history of afebrile seizure or underlying neurological disorders. The American Academy of Pediatrics’ diagnostic criteria were used in this study [26,27].
Simple febrile seizure is defined as a primary generalized convulsion that lasts less than 15 min and does not reoccur within 24 h [26,28,29].
A complex febrile seizure is defined as a focused or prolonged convulsion lasting more than 15 min and/or more than 1 convulsion in 24 h [30,31].
STATISTICAL ANALYSIS:
We first conducted data processing. This rigorous phase detected and rectified missing values, along with correcting outliers and discrepancies. In this phase, we used the ‘tidyverse’ package in R, which integrates the functionalities of the ‘dplyr’ and ‘tidyr’ packages (packages that are known for their superior data manipulation). We then generated descriptive statistics, including the median, interquartile range (IQR), and proportions. Baseline attributes were created using the ‘TableOne’ package in R (this package was created to reference the ubiquitous “Table 1” in research narratives).
Next, we conducted inferential analysis using the t test, chi-square test, and regression analyses, as appropriate. The base of the analysis was conducted using the ‘stats’ package in R, supplemented by the ‘DescTools’ package for descriptive statistics, and the ‘Finalfit’ package to create tables for binary logistic regression outcomes. In addition, to aid in the visual display and interpretation of findings, we used the ‘ggplot2’ package in R.
The results are presented as odds ratios and 95% confidence intervals (CIs). Statistical significance is indicated by a
The entirety of our analyses was conducted using R on the Windows platform, version 4.2.2, dated 2022–10–31 (ucrt).
Results
DEMOGRAPHIC AND CLINICAL PROFILE:
In this retrospective cohort study, the characteristics of 71 pediatric patients, aged between 6 months and 5 years, diagnosed with febrile seizures from 2017 to 2021 at a single medical center in Saudi Arabia are presented. The median age of the children was 19 months (IQR, 12 to 36 months). The cohort had a slightly higher proportion of female (54.9%) than male (45.1%) patients. Clinically, the median duration of fever prior to presentation was 2 days, with temperatures on admission having a median of 37.9°C. The peak temperatures these children experienced were notably high, with a median of 39.1°C. A significant proportion of the cohort, 35.2%, had a positive family history of seizures. The average length of hospital stay was 3 days. A lumbar puncture was performed in 23.9% of the children, suggesting the need for cerebrospinal fluid analysis in these cases. Most of the seizures were classified as simple (69.0%), with the remaining classified as complex seizures (31.0%). Baseline characteristics of the study population are shown in Table 1.
VIRAL ETIOLOGIES AND SARS-COV-2 PREVALENCE:
In the context of the viral etiologies identified among the pediatric cohort diagnosed with febrile seizures, several respiratory viruses were found. Most prominently, influenza A or B were identified in 26.8% of the children, making it the most prevalent pathogen in this cohort. Closely following this, human rhinovirus/enterovirus was detected in 23.9% of cases. Other viruses, including adenovirus, parainfluenza, and RSV, constituted a smaller fraction, with 18.3%, 8.5%, and 8.5%, respectively. The category labeled “others” included 14.1% of cases, suggesting the presence of a diverse array of other viral agents not distinctly categorized (Figure 1, Table 1).
The emergence and global spread of SARS-CoV-2 has been a focal point of many recent clinical investigations. In our cohort, the prevalence of SARS-CoV-2 was 14.1%. This indicates a substantial proportion of children diagnosed with febrile seizures were concurrently infected with this novel coronavirus. Notably, the presence of SARS-CoV-2 was almost equivalent to some of the more traditionally recognized respiratory viruses in the context of febrile seizures. This analysis provides a detailed examination of the viral etiologies and the prevalence of SARS-CoV-2 in the studied cohort (Table 1).
INFERENTIAL ANALYSIS OF COMPARATIVE CHARACTERISTICS STRATIFIED BY SEIZURE TYPE:
Table 2 shows a comprehensive comparison of demographic, clinical, and virological attributes among pediatric patients diagnosed with simple and complex febrile seizures. The following observations and implications were noted.
DEMOGRAPHICS AND CLINICAL PARAMETERS:
Age: The median age for children with simple seizures was 19.0 months, while it was slightly elevated, at 20.5 months, for those with complex seizures. However, the IQR indicates a wider age distribution in the complex group. The difference between these groups was not statistically significant (
Sex: There was a sex disparity, especially in the complex seizure group, in which female patients constituted 68.2%, compared with 49.0% in the simple group. The difference did not reach statistical significance (
Clinical Metrics: The median duration of fever was higher for the simple seizure group (3.00 days) than the complex group (1.50 days), but the difference was not significant (
VIROLOGICAL CHARACTERISTICS:
Virus type: The distribution of viral etiologies varied between the groups. Adenovirus was notably more prevalent in the complex seizure group (36.4%) than in the simple group (10.2%). However, the overall distribution of viruses did not reach statistical significance (
SARS-CoV-2 prevalence: The presence of SARS-CoV-2 was higher in the simple seizure group (18.4%) than in the complex group (4.5%), although this difference was not statistically significant (
CLINICAL INTERVENTIONS AND OUTCOMES:
Length of hospital stay: Both groups had a median hospital stay of 3 days, but with differing IQRs. The statistical significance (
Lumbar puncture: A significantly higher proportion of children with complex seizures (45.5%) underwent lumbar puncture than did those with simple seizures (14.3%;
Factors influencing seizure types: We conducted logistic regression analysis, as shown in Figure 2, to identify factors concomitant with seizure classifications, principally distinguishing between simple and complex typologies. The presented odds ratios (ORs) characterize the propensity for a complex seizure manifestation relative to a simple seizure, with respect to incremental alterations in the predictor variables, while keeping all other parameters unchanged. Pertinent variables, including sex, fever duration, familial seizure antecedents, certain viral species, namely human rhinovirus/enterovirus and parainfluenza, and the administration of lumbar puncture, were found to have significant correlations with seizure phenotypes. Intriguingly, female patients manifested an 8.18-fold increased likelihood for complex seizures in comparison with male patients (P=0.035). Each successive day of fever was inversely associated with complex seizure predisposition, with a decrease of 36% in odds (OR=0.64, P=0.031). Patients with a hereditary inclination for seizures had an 8.76-fold augmented probability for complex seizures (P=0.021). In comparison, patients with human rhinovirus/enterovirus or parainfluenza had a fewer complex seizures, particularly when compared with patients with adenovirus (OR=0.01, P=0.006 and OR=0.04, P=0.047, respectively). Furthermore, patients with complex seizures underwent up to 17.91 times more lumbar puncture than did patients with simple seizures (P=0.033). Parameters including age, temperature on hospital admission, maximum temperature, duration of hospitalization, co-infection, and the presence of the SARS-CoV-2 virus were not statistically significantly associated with seizure phenotypes. Cumulatively, these findings underscore the importance of sex, fever duration, familial seizure histories, viral classifications, and lumbar puncture as pivotal considerations in the holistic management and therapy for pediatric patients with seizures.
Discussion
VIROLOGICAL ASSOCIATIONS WITH FEBRILE SEIZURES:
The association between various viral agents and the incidence of febrile seizures (febrile seizure) has been a topic of ongoing interest in the pediatric research community. In the present study, influenza A or B emerged as the predominant strain associated with febrile seizure, being detected in 26.8% of cases. This is consistent with multiple studies that have underscored the significance of influenza A or B in febrile seizure episodes. Stokes et al found that the causative agent in febrile seizure in 40% of cases was influenza A [44]. Schanzer et al reported a similar association, indicating that influenza A accounted for 35% of febrile seizure-related hospital admissions [13]. Furthermore, Chiu et al observed a higher incidence of febrile seizure in pediatric patients with influenza A (19.5%) than in those with adenovirus or parainfluenza infections [45]. Tang et al also reported a considerably higher occurrence of influenza A infection (19.6%) in patients with febrile seizure, especially among older children [38].
In our findings, the next significant virological entity was human rhinovirus/enterovirus, which accounted for 23.9% of cases. This corroborates the results of Francis et al, in which rhinoviruses and enteroviruses were identified as the most common agents, making up 22% and 20% of their cohort, respectively [12]. In contrast, Pokorn et al found influenza, RSV, and parainfluenza as the predominant viruses linked to febrile seizure [34]. Adenoviruses were the causative agent in 18.3% of our cases. In other studies, such as the one conducted by Cadet et al, adenovirus was identified as a prevalent viral entity in children with febrile seizure [16]. Naric et al also found adenovirus was the most frequently identified virus in their cohort [33].
NOVEL SARS-COV-2 PREVALENCE IN PEDIATRIC FEBRILE SEIZURES:
Our present study on the prevalence of SARS-CoV-2 in pediatric patients diagnosed with febrile seizures provided notable insights. Specifically, SARS-CoV-2 was detected in 14.1% of patients; however, most (85.9%) were found to not have the virus. Furthermore, our data did not show a statistically significant relationship between the presence of SARS-CoV-2 and the specific type of seizure. Comparative findings from other studies present an intriguing landscape. For instance, Cadet et al identified febrile seizures attributed to SARS-CoV-2 infection in 0.5% of their sample, a relatively low prevalence compared with our observation [16]. Their findings also indicated that most of the febrile seizure cases related to SARS-CoV-2 did not coexist with other infections. Conversely, Pascarella et al found a higher incidence of febrile seizures in children with a SARS-CoV-2 infection, at 18.4%, slightly surpassing our own results of 14.1% [18]. Park et al cited an interesting finding, showing that despite shifts in the patterns of respiratory viral infections, the clinical features and outcomes of febrile seizures remained consistent before and throughout the COVID-19 pandemic [46]. Moreover, Dimopoulou et al identified 44.8% of patients having febrile seizures during the SARS-CoV-2 Omicron wave [47]. This study highlighted that while SARS-CoV-2 can be a triggering factor, other pre-existing conditions or viruses play a synergistic role in the development and presentation of the febrile seizure and raises pertinent questions about the diverse clinical presentations across pediatric populations and the possible geographical or cohort-specific variations that exist.
Furthermore, our observation that co-infections were present in 28.2% of febrile seizure cases underscores the complex interplay of viral agents in this clinical scenario. A similar observation was made by Carman et al, who noted the co-detection of multiple viruses in 58.3% of children with febrile seizures [11]. Co-infections were also identified in 32% of patients in the study by Rudolph et al [35]. These findings, in conjunction with ours, accentuate the multifaceted nature of virological associations with febrile seizure and the need for comprehensive virological evaluations in affected children. Indeed, our results contribute to the expanding body of evidence that characterizes the diverse virological agents implicated in febrile seizures. Although influenza A and B continue to be predominant contributors, the new involvement of SARS-CoV-2, alongside the intricate interplay arising from co-infections, necessitates more comprehensive investigation and scrutiny in future research.
RISK FACTORS ASSOCIATED WITH COMPLEX SEIZURES IN PEDIATRIC PATIENTS:
Sex variations in the susceptibility and presentation of complex febrile seizures have received significant attention in the pediatric neurology research paradigm. The elucidation of patient sex as a potential modulating factor can offer critical insights into the pathophysiological processes underlying febrile seizure manifestation and prognosis. Our retrospective study presents compelling evidence regarding sex disparities in the occurrence of complex febrile seizures. Interestingly, female patients were more vulnerable, demonstrating an 8.18-fold increased propensity for complex seizures. However, the sex-related landscape of febrile seizure risk is not unidimensional. Contrary to our findings, Esmaili Gourabi reported no discernible sex-based disparities in the occurrence of either simple or complex seizures [39]. Similarly, another facet of our study’s observations indicated a lack of significant differences in patient sex in the incidence rates of both simple and complex seizure types. Furthermore, Rudolph et al found that male patients faced an increased risk of complex seizure, specifically 3.3% greater, of undergoing complex seizures than did their female counterparts [35]. This discrepancy underscores the intricate and perhaps multifactorial nature of sex as a determinant in febrile seizure susceptibility and manifestation. Intriguingly, while sex differences in the occurrence of febrile seizures are evident, the risk factors for recurrent seizures notably include male sex and age under 1 year [48]. Moreover, Chiu et al emphasized that the elevated seizure incidence linked with influenza A was independent of variations in age, sex, or familial seizure histories [45]. The differential sex-based response was further highlighted in cases of febrile status epilepticus, with female patients having significantly better outcomes than male patients [49]. While our study, in agreement with certain studies in the literature, underscores the increased susceptibility of females to complex febrile seizures, other studies reflect a multifaceted and occasionally contradictory picture. The observed variations might be influenced by the underlying viral etiologies, individual neurodevelopmental trajectories, genetic predispositions, or other yet unidentified factors. A consolidated, multicenter study with larger cohorts might be essential to unravel the intricate interplay of patient sex in febrile seizure onset, progression, and outcomes.
The temporal relationship between the onset of fever and the emergence of complex febrile seizures remains a subject of considerable debate in pediatric neurology. The exact duration of fever preceding seizure manifestation is thought to influence the presentation and recurrence of these seizure episodes, although research outcomes differ on the specifics. Our retrospective analysis, involving a cohort of 71 children, revealed an intriguing inverse association between fever duration and complex seizures. Namely, a shorter fever duration prior to first seizure appeared to be predictive of complex seizure presentations. This observation greatly differs from the findings of Tang et al, which showed extended febrile periods were positively correlated with recurrent and complex seizure events [38]. The inconsistency in these findings underscores the complexity of the pathophysiological dynamics behind febrile seizures and might be indicative of variations in the study populations or potentially region-specific therapeutic interventions.
Corroborating our study’s observations, Deborah G. Hirtz emphasized that a brief fever duration prior to the initial seizure serves as a risk marker for the recurrence of the complex seizure phenotype [50]. Similarly, John M. Pellock found that the likelihood of a recurrence or the manifestation of a complex febrile seizure escalates when the body temperature remains below 40.0°C during the inaugural convulsive event and when the interval between the onset of the febrile episode and the first convulsion is less than an hour [51]. In agreement with these findings, Sajun Chung found a heightened recurrence risk is associated with a shorter duration of clinically discernible fever [52].
The observed inverse association in our study between the presence of human rhinovirus/enterovirus or parainfluenza and the risk of complex seizures is compelling, particularly when compared with the elevated risk noted in patients with adenovirus (OR=0.01, P=0.006 and OR=0.04, P=0.047, respectively). Notably, these findings differ from those presented by Chung et al, who identified no significant variance in complex febrile seizure risk among patients harboring 5 prevalent viruses [53]. Cha et al postulated that such discrepancies could arise from unique population dynamics or other unaccounted-for variables. Further complicating the issue, RSV-associated febrile seizures had an incidence of 5.3%, with 13.6% being complex, figures that are notably lower than those for influenza and adenovirus [54]. This observation agrees with our data, underscoring the propensity of adenovirus to predispose individuals to complex seizures. In contrast, Pokorn et al found no marked distinction in viral loads among children with either simple or complex febrile seizures [55]. Similarly, Suga et al did not identify significant variations in odds ratios for complex seizures across various viruses, placing their results in opposition to our present findings. The underlying pathophysiological pathways responsible for these discrepancies remain unknown, highlighting the multifaceted nature of viral-neurological interactions. Notably, infections associated with HHV-6 have been linked with elevated rates of complex seizures, recurrences, and febrile status epilepticus [56]. Although HHV-6 was not included in our investigation, its established link with increased seizure risk highlights the importance of continually refining our understanding of the neurological effects of diverse viral pathogens.
CLINICAL IMPLICATIONS:
In this Saudi Arabian retrospective study, a high prevalence of febrile seizures was seen in female children, particularly those with a family history of seizures. The presence of influenza A or B, human rhinovirus/enterovirus, and SARS-CoV-2 highlights the need of performing comprehensive viral tests in febrile children, especially during the pandemic. While adenovirus was found to be related to complicated seizures, most seizures were simple. Because prominent viral etiologies differ among Saudi regions, with RSV in Riyadh, adenovirus in Jeddah, and human rhinovirus/enterovirus and parainfluenza in the Eastern Province, our study highlights the need for region-specific diagnostic and therapeutic techniques. Human rhinovirus/enterovirus and parainfluenza infections appear to be more benign than adenovirus infections, particularly in the Eastern Province. The absence of multiplex PCR testing in earlier investigations emphasizes the requirement of these diagnostic and therapeutic techniques. The effects of patient sex and SARS-CoV-2 prevalence should be confirmed in larger cohorts. Overall, this study provides important insights into individualized pediatric febrile seizure management, highlighting characteristics such as sex, family history, and particular viral correlations.
STUDY STRENGTHS AND LIMITATIONS:
This study had several strengths. First, the study stands out for its timely relevance, particularly considering the SARS-CoV-2 crisis, as well as an extensive literature review highlighting the regional epidemiological peculiarities of febrile seizures. Second, the use of sophisticated molecular diagnostics, particularly multiplex PCR, fills diagnostic gaps in previous Saudi-centric research, providing precision. Third, by deliberately focusing on Saudi Arabia’s pediatric population aged 6 months to 5 years, this study uncovered regionally relevant insights regarding the epidemiology of febrile seizures. Fourth, the study’s comprehensiveness and specificity are supported by the 5-year retrospective cohort design, which was strengthened by thorough inclusion and exclusion criteria. Fifth, the study’s attention to ethical standards and emphasis on data confidentiality demonstrate its dedication to research integrity. Sixth, the findings have been compared with those of recognized studies, such as from Keum et al and others. Seventh, the detailed observation on consanguineous marriages in Saudi Arabia provides critical cultural insights that could influence regional medical thinking. Finally, key interrelationships of variables were identified in our statistical analysis, which included advanced regression analyses, strengthening this study’s standing in pediatric virological research.
This study also had several limitations. First, the conclusions are based on a small sample of 71 children from a single location, which may be not be representative. Second, the retrospective approach based on historical data could have created biases due to insufficient or inconsistently preserved records. Third, the changing viral landscape, particularly with the introduction of SARS-CoV-2, may not have been adequately recorded during a 5-year period spanning pre-pandemic to pandemic periods. Fourth, while genetic predispositions and environmental vectors are mentioned, they are not addressed specifically. Fifth, the limitations of the PCR assays, along with potential biases introduced by the waiver of informed permission and classification as “others” for viral etiologies, confuse the findings. Sixth, there is a lack of long-term follow-up data, which hinders understanding of post-seizure outcomes. Seventh, the study’s tiny female majority may have skewed findings, especially because several studies have demonstrated male dominance in febrile seizures. The reported greater risk for complex seizures in female patients differs from other studies, implying that there can be geographical or sample-specific variances that are not generally relevant. Finally, the study does not definitively resolve the function of SARS-CoV-2 in febrile seizures.
Future research should use a prospective cohort study approach to obtain more solid and complete results. A multi-center study, perhaps in partnership with institutions in adjacent countries, would yield a more diverse and representative dataset. Accepting advances in PCR technology and adhering to strict sample collection and transportation guidelines can help to reduce diagnostic errors. A larger sample size would provide more statistical power, and a more precise classification of viral etiologies would show a clearer picture of viral effect. In future research, using long-term follow-up data could provide insight on febrile seizure recurrence and long-term neurological consequences.
Conclusions
In our retrospective study of Saudi children, we observed distinct correlations between febrile seizures and various viral etiologies, emphasizing the role of sex and familial history. The prominence of different viruses, such as RSV in Riyadh and adenovirus in Jeddah, underscores the necessity for region-specific diagnostic and therapeutic approaches. Notably, the benign nature of human rhinovirus/enterovirus and parainfluenza compared with adenovirus, particularly in the Eastern Province, demands tailored clinical responses. The study prepares the way for individualized pediatric febrile seizure management. For a comprehensive understanding and to confirm these findings, a multicenter prospective cohort study is urgently needed.
Figures
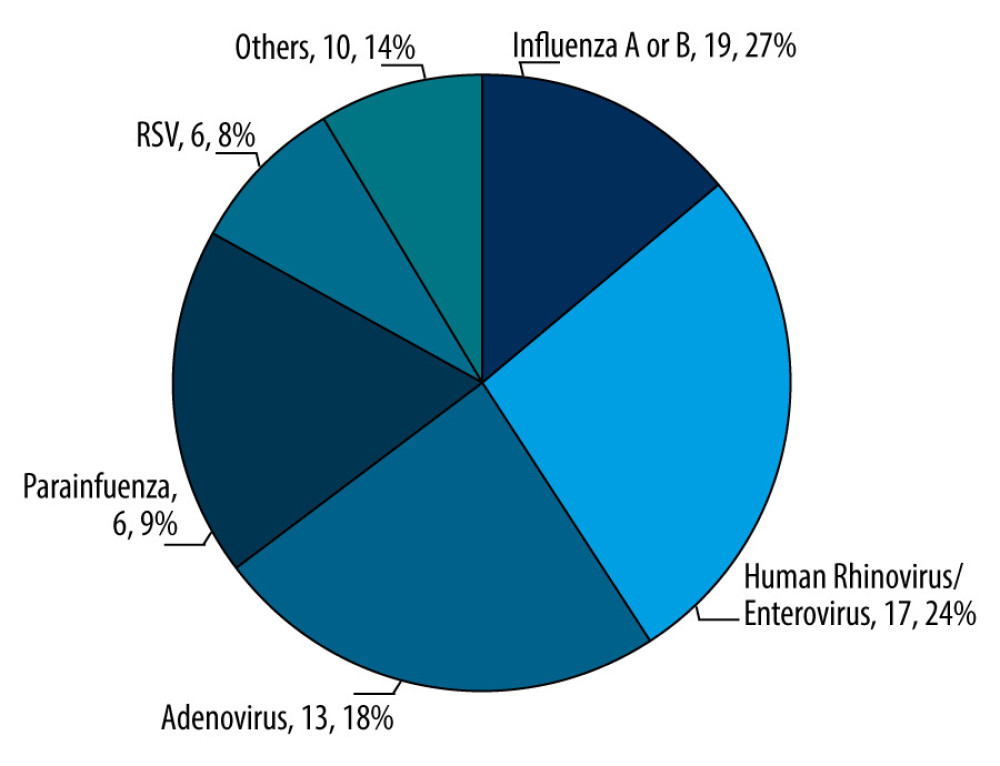 Figure 1. Proportional representation of viral etiologies in the pediatric cohort (N=71). The chart shows the distribution of viral agents identified within the cohort of 71 pediatric patients. Values of each pie segment are given as numerical count and percentage of the total. (This chart was generated using Microsoft Excel 2021. Data points were inputted into an Excel spreadsheet).
Figure 1. Proportional representation of viral etiologies in the pediatric cohort (N=71). The chart shows the distribution of viral agents identified within the cohort of 71 pediatric patients. Values of each pie segment are given as numerical count and percentage of the total. (This chart was generated using Microsoft Excel 2021. Data points were inputted into an Excel spreadsheet). 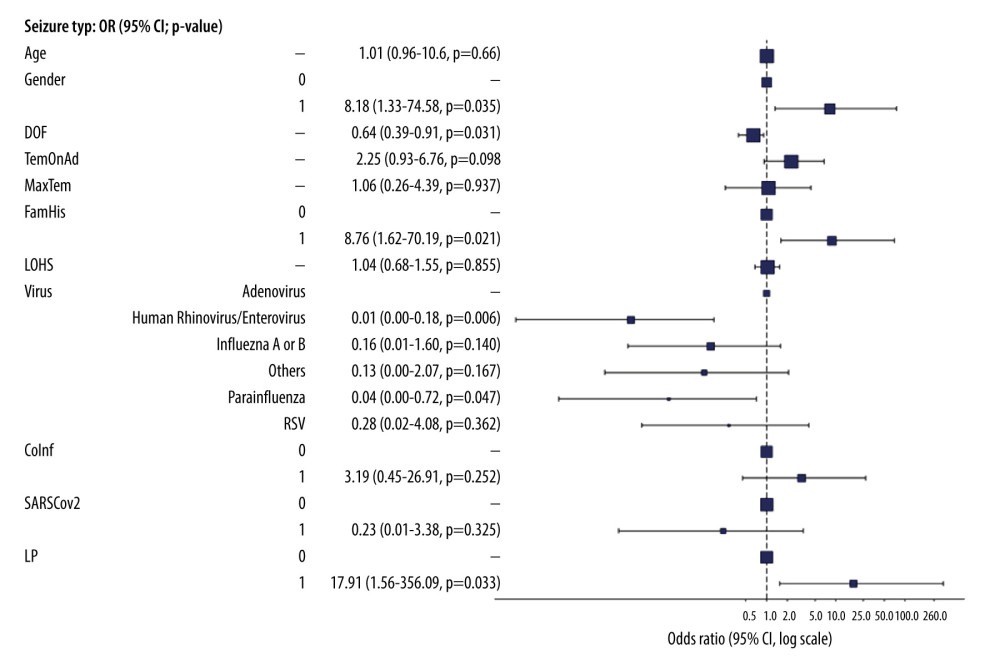 Figure 2. Multivariate logistic regression analysis of febrile seizure types in pediatric cohort aged 6 months to 5 years. Key findings include the 8.18-fold risk of female sex for complex seizures (P=0.035); 36% reduced odds with each duration of fever (DOF) day (OR=0.64, P=0.031); 8.76-fold risk with family history (FamHis) (P=0.021); Reduced risk with human rhinovirus/enterovirus or parainfluenza vs adenovirus (OR=0.01, P=0.006; OR=0.04, P=0.047); 17.91-fold risk lumbar puncture for complex seizure (P=0.033). Factors like age, temperature on admission (TemonAd), maximum temperature (MaxTem), length of hospital stay (LOHS), co-infection (Coinf), and SARS-CoV-2 were non-significant. P<0.05 is statistically significant. (This figure created by ‘Finalfit’ R package, version 1.0.6, was published on January 14, 2023. The primary authors are Ewen Harrison, Tom Drake, and Riinu Ots).
Figure 2. Multivariate logistic regression analysis of febrile seizure types in pediatric cohort aged 6 months to 5 years. Key findings include the 8.18-fold risk of female sex for complex seizures (P=0.035); 36% reduced odds with each duration of fever (DOF) day (OR=0.64, P=0.031); 8.76-fold risk with family history (FamHis) (P=0.021); Reduced risk with human rhinovirus/enterovirus or parainfluenza vs adenovirus (OR=0.01, P=0.006; OR=0.04, P=0.047); 17.91-fold risk lumbar puncture for complex seizure (P=0.033). Factors like age, temperature on admission (TemonAd), maximum temperature (MaxTem), length of hospital stay (LOHS), co-infection (Coinf), and SARS-CoV-2 were non-significant. P<0.05 is statistically significant. (This figure created by ‘Finalfit’ R package, version 1.0.6, was published on January 14, 2023. The primary authors are Ewen Harrison, Tom Drake, and Riinu Ots). Tables
Table 1. Baseline characteristics of study participants (N=71).Demographic, clinical, and virological characteristics of 71 children aged 6 months to 5 years who were diagnosed with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia.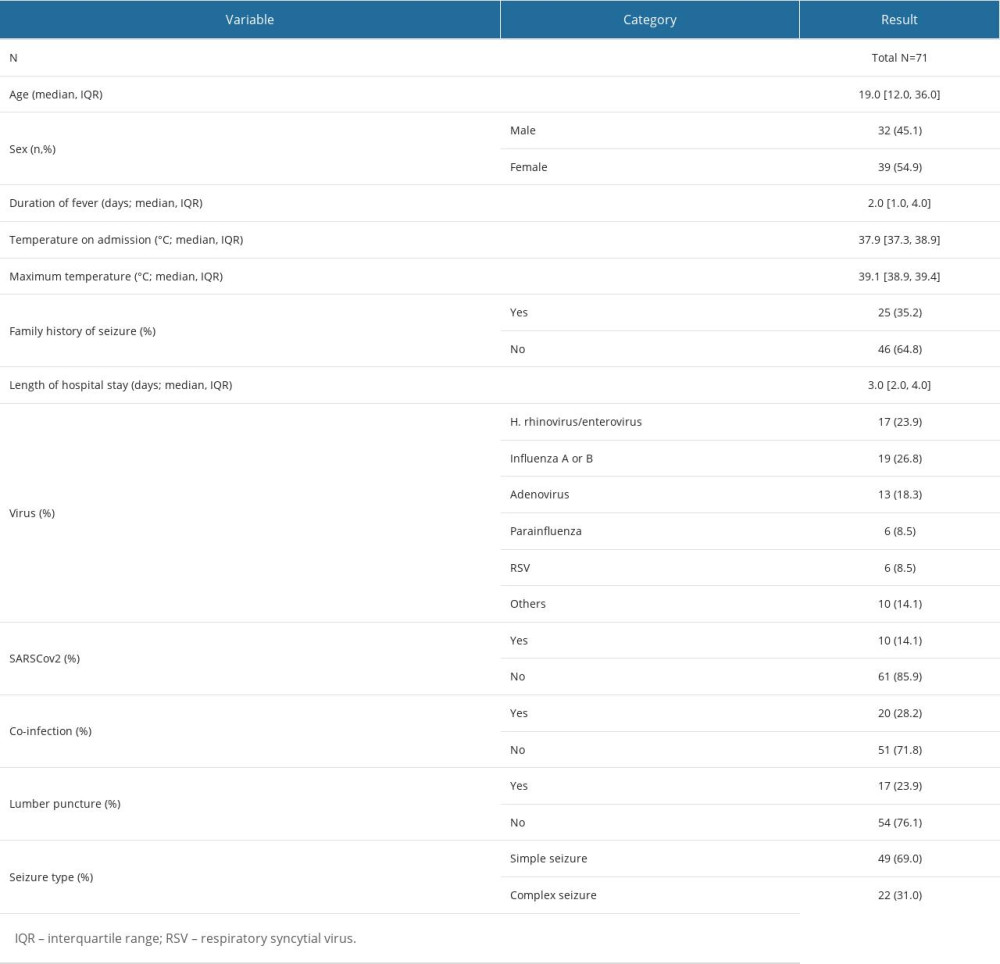 Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex.
Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex.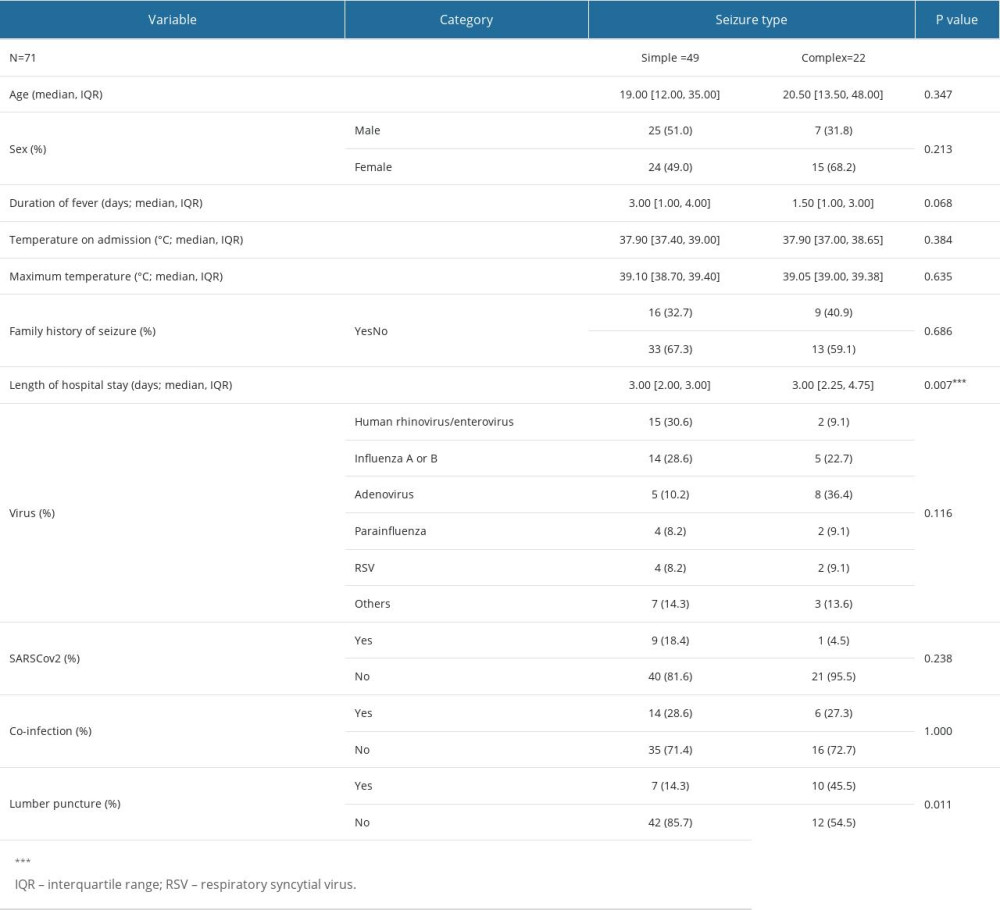
References
1. Pavlidou E, Hagel C, Panteliadis C, Febrile seizures: Recent developments and unanswered questions: Child’s Nerv Syst, 2013; 29; 2011-17
2. Patterson JL, Carapetian SA, Hageman JR, Kelley KR, Febrile seizures: Pediatr Ann, 2013; 42(12); 249-54
3. Choi YJ, Jung JY, Kim JH, Febrile seizures: Are they truly benign? Longitudinal analysis of risk factors and future risk of afebrile epileptic seizure based on the national sample cohort in South Korea, 2002–2013: Seizure, 2019; 64; 77-83
4. Millichap JG, Millichap JJ, Role of viral infections in the etiology of febrile seizures: Pediatr Neurol, 2006; 35; 165-72
5. Chung B, Wong V, Relationship between five common viruses and febrile seizure in children: Arch Dis Child, 2007; 92(7); 589-93
6. Jansen RR, Wieringa J, Koekkoek SM, Frequent detection of respiratory viruses without symptoms: Toward defining clinically relevant cutoff values: J Clin Microbiol, 2011; 49(7); 2631-36
7. Mahony JB, Detection of respiratory viruses by molecular methods: Clin Microbiol Rev, 2008; 21; 716-47
8. Jartti T, Söderlund-Venermo M, Hedman K, New molecular virus detection methods and their clinical value in lower respiratory tract infections in children: Paediatr Respir Rev, 2013; 14; 38-45
9. Sugata K, Taniguchi K, Yui A, Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis: Pediatrics, 2008; 122(2); 392-97
10. Krief WI, Levine DA, Platt SL, Influenza virus infection and the risk of serious bacterial infections in young febrile infants: Pediatrics, 2009; 124(1); 30-39
11. Carman KB, Calik M, Karal Y, Viral etiological causes of febrile seizures for respiratory pathogens (EFES Study): Hum Vaccin Immunother, 2019; 15(2); 496-502
12. Francis JR, Richmond P, Robins C, An observational study of febrile seizures: The importance of viral infection and immunization: BMC Pediatr, 2016; 16(1); 202
13. Schanzer DL, Langley JM, Tam TWS, Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children: Pediatr Infect Dis J, 2006; 25(9); 795-800
14. Han DH, Kim SY, Lee NM, Seasonal distribution of febrile seizure and the relationship with respiratory and enteric viruses in Korean children based on nationwide registry data: Seizure, 2019; 73; 9-13
15. Van Zeijl JH, Mullaart RA, Borm GF, Galama JMD, Recurrence of febrile seizures in the respiratory season is associated with influenza A: J Pediatr, 2004; 145(6); 800-5
16. Cadet K, Boegner J, Ceneviva GD, Evaluation of febrile seizure diagnoses associated with COVID-19: J Child Neurol, 2022; 37(5); 410-15
17. Tso WWY, Kwan MYW, Kwok JSY, Clinical characteristics of unvaccinated or incompletely vaccinated children with neurological manifestations due to SARS-CoV-2 Omicron infection: J Med Virol, 2023; 95(7); e28895
18. Pascarella A, Maglione M, Lenta S, Seizures in children with SARS-CoV-2 infection: Epidemiological, clinical and neurophysiological characterization: Children (Basel), 2022; 9(12); 1923
19. Kim JM, Park EG, Lee JY, Characteristics of febrile seizures with SARS-CoV-2 infection in the Omicron era: Transl Pediatr, 2023; 12(5); 807-15
20. Kurd M, Hashavya S, Benenson S, Gilboa T, Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children: Seizure, 2021; 92; 89-93
21. Alhumaidy M, Al-Mulhim Y, Alabdullah W, Prevalence and risk factors of febrile convulsions among infants and children in Saudi Arabia: Int J Med Dev Countries, 2020; 1520-26
22. Ahmed AJA, Alenezi AT, Alanazi AMS, Febrile convulsions in children, Arar, Northern Saudi Arabia: Egypt J Hosp Med, 2018; 71(3); 2738-41
23. Pierce VM, Hodinka RL, Comparison of the GenMark diagnostics eSensor respiratory viral panel to real-time PCR for detection of respiratory viruses in children: J Clin Microbiol, 2012; 50(11); 3458-65
24. Precit MR, He K, Mongkolrattanothai K, Impact of FilmArray™ Respiratory Panel testing on the clinical management of pediatric bone marrow transplant patients: Eur J Clin Microbiol Infect Dis, 2022; 41(3); 395-405
25. Ginocchio CC, McAdam AJ, Current best practices for respiratory virus testing: J Clin Microbiol, 2011; 49(9 Suppl); S44-48
26. Duffner PK, Berman PH, Baumann RJ, Clinical practice guideline – neurodiagnostic evaluation of the child with a simple febrile seizure: Pediatrics, 2011; 127; 389-94
27. Dougherty D, Duffner PK, Baumann RJ, Febrile seizures: Clinical practice guideline for the long-term management of the child with simple febrile seizures: Pediatrics, 2008; 121(6); 1281-86
28. Gerber MA, Berliner BC, The child with a ’simple’ febrile seizure: Appropriate diagnostic evaluation: Am J Dis Child, 1981; 135(5); 431-33
29. Freeman JM, Febrile seizures: A consensus of their significance, evaluation, and treatment: Pediatrics, 1980; 66(6); 1009
30. Patel AD, Vidaurre J, Complex febrile seizures: A practical guide to evaluation and treatment: J Child Neurol, 2013; 28; 759-64
31. Berg AT, Shinnar S, Complex febrile seizures: Epilepsia, 1996; 37(2); 126-33
32. Keum HR, Lee SJ, Kim JM, Seasonal trend of viral prevalence and incidence of febrile convulsion: A Korea Public Health Data Analysis: Children, 2023; 10(3); 529
33. Naric J, Rissland J, Simon A, Role of multiplex PCR analysis in children with febrile seizures: Wiener Medizinische Wochenschrift, 2017; 167(11–12); 246-50
34. Pokorn M, Jevšnik M, Petrovec M, Respiratory and enteric virus detection in children: A prospective study comparing children with febrile seizures and healthy controls: J Child Neurol, 2017; 32(1); 84-93
35. Rudolph H, Gress K, Weiss C, General characteristics of children with single-and co-infections and febrile seizures with a main focus on respiratory pathogens: Preliminary results: Pathogens, 2021; 10(8); 1061
36. Hautala M, Arvila J, Pokka T, Respiratory viruses and febrile response in children with febrile seizures: A cohort study and embedded case-control study: Seizure, 2021; 84; 69-77
37. Shabbir Hussain S, Hashmi MUDS, Febrile seizures: Demographic, clinical and etiological profile of children admitted with febrile seizures in a tertiary care hospital: J Pak Med Assoc, 2015; 65(9); 1008-10
38. Tang J, Yan W, Li Y, Relationship between common viral upper respiratory tract infections and febrile seizures in children from Suzhou, China: J Child Neurol, 2014; 29(10); 1327-32
39. Esmaili Gourabi H, Bidabadi E, Cheraghalipour F, Febrile seizure: Demographic features and causative factors: Iran J Child Neurol, 2012; 6(4); 33-37
40. Van Esch A, Steyerberg EW, Berger MY, Family history and recurrence of febrile seizures: Arch Dis Child, 1994; 70(5); 395-99
41. Camfield P, Camfield C, Febrile seizures and genetic epilepsy with febrile seizures plus (GEFS+): Epileptic Disord, 2015; 17(2); 124-33
42. Nakayama J, Arinami T, Molecular genetics of febrile seizures: Epilepsy Res, 2006; 70; 190-98
43. Kira R, Ishizaki Y, Torisu H, Genetic susceptibility to febrile seizures: Case-control association studies: Brain Dev, 2010; 32; 57-63
44. Stokes MJ, Downham MAPS, Webb JKG, Viruses and febrile convulsions: Arch Dis Child, 1977; 52(2); 129-33
45. Chiu SS, Tse CY, Lau YL, Peiris M, Influenza A infection is an important cause of febrile seizures: Pediatrics, 2001; 108(4); E63
46. Park EG, Kim JM, Suh W, Impact of COVID-19 on the incidence of respiratory viral infections and clinical characteristics of associated febrile seizures: Transl Pediatr, 2023; 12(4); 528-37
47. Dimopoulou D, Dasoula F, Liaska M, Rise of neurologic manifestations during SARS-CoV-2 Omicron wave in children with COVID-19: Pediatr Infect Dis J, 2023; 42(4); e128-e29
48. Ojha AR, Shakya KN, Aryal UR, Recurrence risk of febrile seizures in children: J Nepal Paediatr Soc, 2012; 32(1); 33-36
49. Kloc ML, Marchand DH, Holmes GL, Cognitive impairment following experimental febrile seizures is determined by sex and seizure duration: Epilepsy Behav, 2022; 126; 108430
50. Hirtz DG, Febrile seizures: Pediatr Rev, 1997; 18(1); 5-9
51. Pellock JM, Shields WD, Recent research on febrile seizures: A review: J Neurol Neurophysiol, 2013; 4(165); 19519
52. Chung S, Febrile seizures: Korean J Pediatr, 2014; 57(9); 384-95
53. Chung B, Wong V, Relationship between five common viruses and febrile seizure in children: Arch Dis Child, 2007; 92(7); 589-93
54. Cha T, Choi YJ, Oh JW, Respiratory syncytial virus-associated seizures in Korean children, 2011–2016: Korean J Pediatr, 2019; 62(4); 131-37
55. Pokorn M, Jevšnik M, Petrovec M, Respiratory and enteric virus detection in children: A prospective study comparing children with febrile seizures and healthy controls: J Child Neurol, 2017; 32(1); 84-93
56. Suga S, Suzuki K, Ihira M, Clinical characteristics of febrile convulsions during primary HHV-6 infection: Arch Dis Child, 2000; 82(1); 62-66
Figures
 Figure 1. Proportional representation of viral etiologies in the pediatric cohort (N=71). The chart shows the distribution of viral agents identified within the cohort of 71 pediatric patients. Values of each pie segment are given as numerical count and percentage of the total. (This chart was generated using Microsoft Excel 2021. Data points were inputted into an Excel spreadsheet).
Figure 1. Proportional representation of viral etiologies in the pediatric cohort (N=71). The chart shows the distribution of viral agents identified within the cohort of 71 pediatric patients. Values of each pie segment are given as numerical count and percentage of the total. (This chart was generated using Microsoft Excel 2021. Data points were inputted into an Excel spreadsheet). Figure 2. Multivariate logistic regression analysis of febrile seizure types in pediatric cohort aged 6 months to 5 years. Key findings include the 8.18-fold risk of female sex for complex seizures (P=0.035); 36% reduced odds with each duration of fever (DOF) day (OR=0.64, P=0.031); 8.76-fold risk with family history (FamHis) (P=0.021); Reduced risk with human rhinovirus/enterovirus or parainfluenza vs adenovirus (OR=0.01, P=0.006; OR=0.04, P=0.047); 17.91-fold risk lumbar puncture for complex seizure (P=0.033). Factors like age, temperature on admission (TemonAd), maximum temperature (MaxTem), length of hospital stay (LOHS), co-infection (Coinf), and SARS-CoV-2 were non-significant. P<0.05 is statistically significant. (This figure created by ‘Finalfit’ R package, version 1.0.6, was published on January 14, 2023. The primary authors are Ewen Harrison, Tom Drake, and Riinu Ots).
Figure 2. Multivariate logistic regression analysis of febrile seizure types in pediatric cohort aged 6 months to 5 years. Key findings include the 8.18-fold risk of female sex for complex seizures (P=0.035); 36% reduced odds with each duration of fever (DOF) day (OR=0.64, P=0.031); 8.76-fold risk with family history (FamHis) (P=0.021); Reduced risk with human rhinovirus/enterovirus or parainfluenza vs adenovirus (OR=0.01, P=0.006; OR=0.04, P=0.047); 17.91-fold risk lumbar puncture for complex seizure (P=0.033). Factors like age, temperature on admission (TemonAd), maximum temperature (MaxTem), length of hospital stay (LOHS), co-infection (Coinf), and SARS-CoV-2 were non-significant. P<0.05 is statistically significant. (This figure created by ‘Finalfit’ R package, version 1.0.6, was published on January 14, 2023. The primary authors are Ewen Harrison, Tom Drake, and Riinu Ots). Tables
 Table 1. Baseline characteristics of study participants (N=71).Demographic, clinical, and virological characteristics of 71 children aged 6 months to 5 years who were diagnosed with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia.
Table 1. Baseline characteristics of study participants (N=71).Demographic, clinical, and virological characteristics of 71 children aged 6 months to 5 years who were diagnosed with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia. Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex.
Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex. Table 1. Baseline characteristics of study participants (N=71).Demographic, clinical, and virological characteristics of 71 children aged 6 months to 5 years who were diagnosed with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia.
Table 1. Baseline characteristics of study participants (N=71).Demographic, clinical, and virological characteristics of 71 children aged 6 months to 5 years who were diagnosed with febrile seizures between 2017 and 2021 at a single center in Saudi Arabia. Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex.
Table 2. Comparative clinical and virological characteristics stratified by seizure type.Clinical and virological attributes of 71 pediatric patients, aged 6 months to 5 years, diagnosed with febrile seizures between 2017 and 2021 at a single medical center in Saudi Arabia, further stratified by the type of seizure: simple versus complex. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








