17 March 2024: Lab/In Vitro Research
Analysis of Wet Pack Incidence in Steam Sterilization: A Study in a Chinese Medical Center
Yanhua Chen12BEF, Li Bao3BC, Liangying Yi12AG*, Ruixue Hu12CDDOI: 10.12659/MSM.942601
Med Sci Monit 2024; 30:e942601
Abstract
BACKGROUND: Central sterile supply departments (CSSDs) play a vital role in hospital infection control. We investigate the factors associated with wet pack occurrence after steam sterilization.
MATERIAL AND METHODS: We designed a log sheet to record information concerning sterilized packs. The data included the type of sterilized pack; outside weather (sunny, overcast, or rainy); the item in the sterilized pack; packaging material; whether the item had been packaged in compliance with guidelines; whether the pack had been laid flat, upright, or leaning at an acute angle; which sterilizer was used for sterilization of the pack; whether the pack had been placed on the top or bottom shelf inside the sterilizer chamber; whether the pack had been loaded in compliance with guidelines; the drying time following sterilization; and cooling time after sterilization. The sterilized packs in our study were selected from all of the packs that were sterilized in the CSSD of the authors’ institution during June to December 2021.
RESULTS: Factors associated with wet pack occurrence after steam sterilization include: outside weather on the day of sterilization; the item in the sterilized pack; packaging material; whether the item had been packaged in compliance with guidelines; whether the pack had been placed on the top or bottom shelf; and cooling time after sterilization. Statistically significant differences (P<0.05) in wet pack incidence were identified for all of these factors.
CONCLUSIONS: Various factors are associated with wet pack occurrence after steam sterilization. Recommendations for reducing the risk of wet packs include regular maintenance of the steam pipeline, regular replacement of thermal insulation materials for the steam pipeline, and extension of the drying time.
Keywords: Steam, Sterilization, Surgical Instruments
Introduction
Central sterile supply departments (CSSDs) play a vital role in hospital infection control [1]. Steam sterilization is the preferred method for heat- and moisture-tolerant surgical instruments, with the prevention of the occurrence of wet packs being a key criterion of CSSD quality control. A study conducted in 125 hospitals showed that wet packs occurred in 78% of the surveyed hospitals and the frequency of occurrence of wet packs varied significantly [2]. As noted by Seavey [3], wet packs constitute one of the most complex and time-consuming problems in sterilization. Studies by Sun et al [4] and Zhou [5] have shown that the rate of wet pack occurrence varies significantly in China, ranging from 1.67–19.7%. Basu [6] reported that the incidence rate of wet packs in eastern India is around 1%. Seavey [3] found that the bacterial culture positive rate in the central part of wet packs can be as high as 50%. Due to the wet environment inside and outside the pack, a channel connecting the inside and outside environment enables microorganisms to enter the pack due to the siphon principle, rendering the pack susceptible to secondary contamination, leading to hospital infections. The occurrence of a wet pack should be regarded as a sterilization failure [7] that can delay the regular supply of sterile instruments, affect surgical procedures, and undermine the quality of diagnosis and treatment. Moreover, repeated sterilization of the same instruments may affect the properties of instrument materials [8,9] and constitutes a waste of hospital labor, materials, and financial resources. In recent years, researchers worldwide have been paying attention to wet pack occurrence following pressure steam sterilization. Affected by various factors, wet pack occurrence has not been effectively solved. It is still a thorny issue, causing concern for disinfection and sterilization in hospitals. Wet packs can be caused by several factors, such as steam quality (60%), equipment performance (30%) and loading defects (10%) [10]. The results of Panta et al [11] have shown that approximately 60% of the wet packs caused by equipment factors can be attributed to steam problems. The results of He et al [12] have shown that when steam supplied by the boiler system is too wet or insufficient, the pressure can become unstable and the non-condensable air can result in the occurrence of wet packs. During sterilization, factors such as pressure sensor failure, water drainage filter net clogging, or an incomplete door gasket in the chamber can prevent steam evacuation from the sterilization chamber and lead to steam dampness. All of this can result in wet pack occurrence, accounting for approximately 30% of wet pack incidence [13]. The study of Huang et al [14] shows that if instruments packaged using ordinary cotton wraps are placed at random or stacked up during loading for sterilization, a large amount of condensed water will be generated in the upper packs and drop on the lower packs, resulting in wet pack occurrence. In this study, we investigated the factors associated with wet pack occurrence after steam sterilization and prescribe preventive measures for reducing the risk of wet packs.
Material and Methods
STUDY SETTING:
A total of 4099 sterilized packs were selected by quality controllers from all of the packs that had been sterilized in the CSSD of the authors’ institution during June to December 2021. The packs were sampled using random cluster sampling. Only packs that had been sterilized by steam sterilization were selected. Contaminated packs and packs with damaged packaging were excluded from this study.
SURVEY TOOLS:
After consulting the literature [10–14] and CSSD guidelines [15–18], we listed the possible factors associated with wet pack occurrence using root cause analysis and brainstorming. Then, 5 CSSD experts were invited via email to consult on the factors associated with wet pack occurrence. The experts were working in CSSDs at different hospitals in different provinces, possessed more than 10 years of CSSD work experience, and were members of their respective provincial-level sterile supply committees. Of them, 2 were chief nurses and 3 were co-chief nurses. Factors associated with wet pack occurrence were revised through a consensus of experts. The following factors were identified: type of pack (eg, laparoscopic instrument pack, uterine instrument pack, surgical gown pack) as shown on the label outside the pack; weather conditions on the day that sterilization took place (sunny, overcast, or rainy); the item in the pack; packaging material; whether the item had been packaged in compliance with guidelines; whether the pack had been laid flat, leaning at an acute angle (defined as laid at between a 0° and 90° angle from the shelf), or upright (at a 90° angle from the shelf); the person responsible for sterilization; which sterilizer (of 2 sterilizers at the study hospital) was used for sterilization of packs; whether the pack had been placed on the top or bottom shelf inside the sterilizer chamber; whether the pack had been loaded in compliance with guidelines; the drying time following sterilization; and cooling time after sterilization.
We designed a log sheet for wet pack occurrence following pressure steam sterilization. We recorded information for the following variables: the type of the sterilized pack, as listed on the pack label; weather conditions; the item in the pack (instrument or dressing); packaging material; whether the item had been packaged in compliance with guidelines; whether the pack had been laid flat, leaning at an acute angle (defined as laid at between a 0° and 90° angle from the shelf), or upright (at a 90° angle from the shelf); the person responsible for sterilization; which sterilizer was used; whether the pack had been placed on the top or bottom shelf inside the sterilizer chamber; whether the pack had been loaded in compliance with guidelines; the drying time following sterilization; and cooling time after sterilization. The log sheet was filled out by quick response code scan. The distribution personnel strictly inspected the sterilized packages to identify wet packs and accurately recorded the information concerning wet packs.
CRITERIA FOR DETERMINING A WET PACK:
According to the CSSD directive (Part 2: Standard Operating Procedure of Cleaning, Disinfection, and Sterilization) released by the National Health Commission of the People’s Republic of China [15], a wet pack is characterized by visible moisture or water droplets found in or on the pack after sterilization and cooling. According to the Technical Requirements for Large Steam Sterilizers – Automatic Type released by the Standardization Administration of the People’s Republic of China [16], a pack is considered wet if any of the following conditions are present: its wrapper feels wet; visible moisture or evident water staining is found on the wrapper or on the chemical indicator tape; water droplets, water mist, or water puddles are present inside the pack; or the dressing was moist after sterilization. The Association for the Advancement of Medical Instrumentation [17] and Centers for Disease Control and Prevention [18] of the United States define a wet pack as one in which moisture, water droplets, or water puddles are left in or on the pack following a complete sterilization cycle.
Based on the above-mentioned definitions, a pack was considered wet in our study if any of the following conditions were met: its wrappers felt wet; visible moisture or evident water staining was found in or on the wrapper or on the chemical indicator tape; presence of water droplets, water mist, or water puddles in the pack; or moist dressing after sterilization.
DATA COLLECTION:
The log sheet for wet pack occurrence following pressure steam sterilization was used for collecting the data concerning wet packs. Data entry was double checked by 2 researchers. Training on relevant knowledge, work procedures and evaluation criteria was provided for CSSD staff members who participated in this study to achieve consistency and accuracy in the study and to strictly control any deviation from study protocol.
STATISTICAL ANALYSIS:
IBM SPSS version 25.0 was used for data analysis. Categorical data are described as N. Pearson’s chi-square test was used to identify differences. The odds ratio (OR) and 95% confidence interval (CI) for each variable were analyzed by binary logistic regression to assess which factors were correlated with wet pack occurrence. A Pearson’s chi-square test was used to determine the factors associated with wet pack occurrence. All variables that were significantly correlated with the outcome (identified by
Results
SINGLE-FACTOR ANALYSIS OF FACTORS ASSOCIATED WITH WET PACK OCCURRENCE:
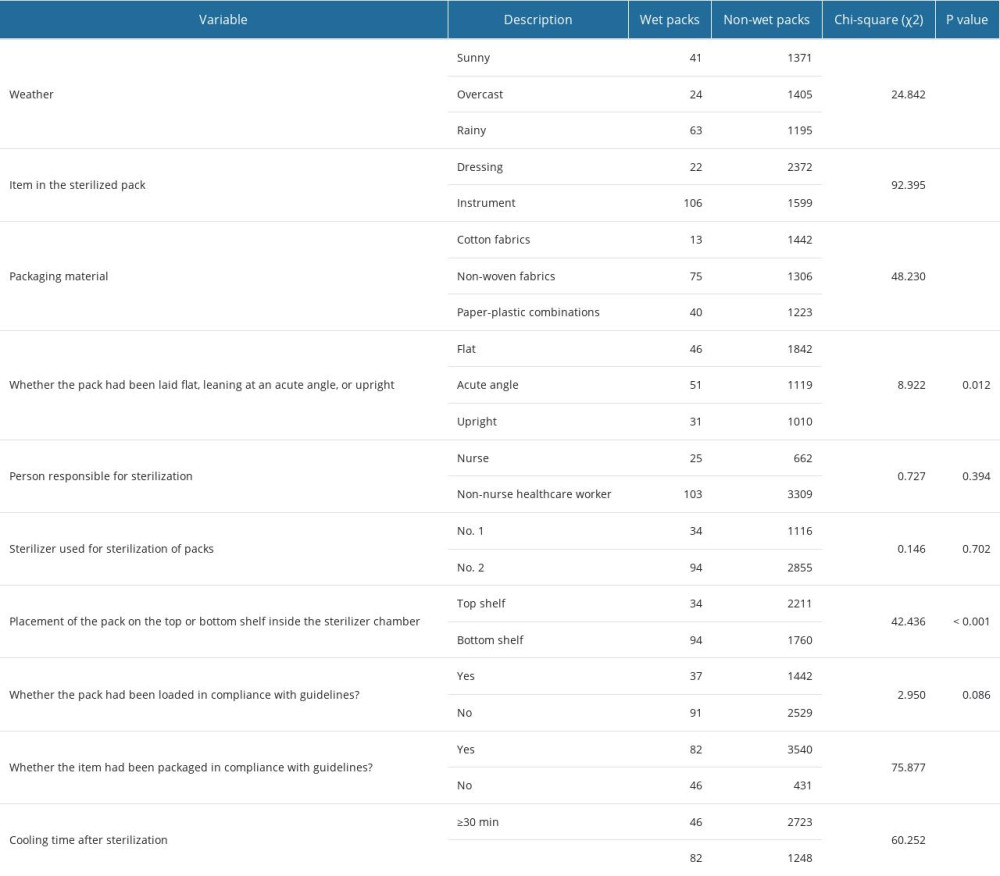
Of the 4099 sterilized packs, 128 (3.12%) were considered wet packs and 3971 (96.88%) were considered non-wet packs. Statistically significant differences (P<0.05) in wet pack presence were associated with the following variables: weather, the item in the sterilized pack, packaging material, whether the item had been packaged in compliance with guidelines, whether the pack had been placed on the top or bottom shelf inside the sterilizer chamber, and cooling time. No statistically significant differences were found for the other variables (P>0.05) (Table 1).
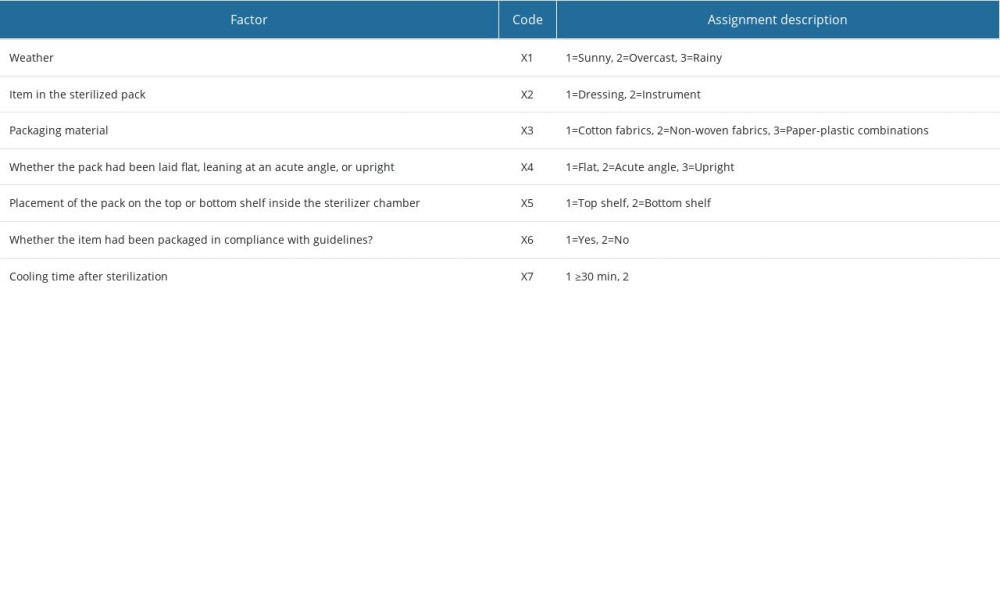
The above statistically significant influencing factors (weather, the item in the sterilized pack, packaging material, whether the item had been packaged in compliance with guidelines, whether the pack had been placed on the top or bottom shelf, and cooling time) were regarded as the independent variables in a single-factor analysis, with wet pack occurrence regarded as the dependent variable. Assignments of the influencing factors are shown in Table 2.
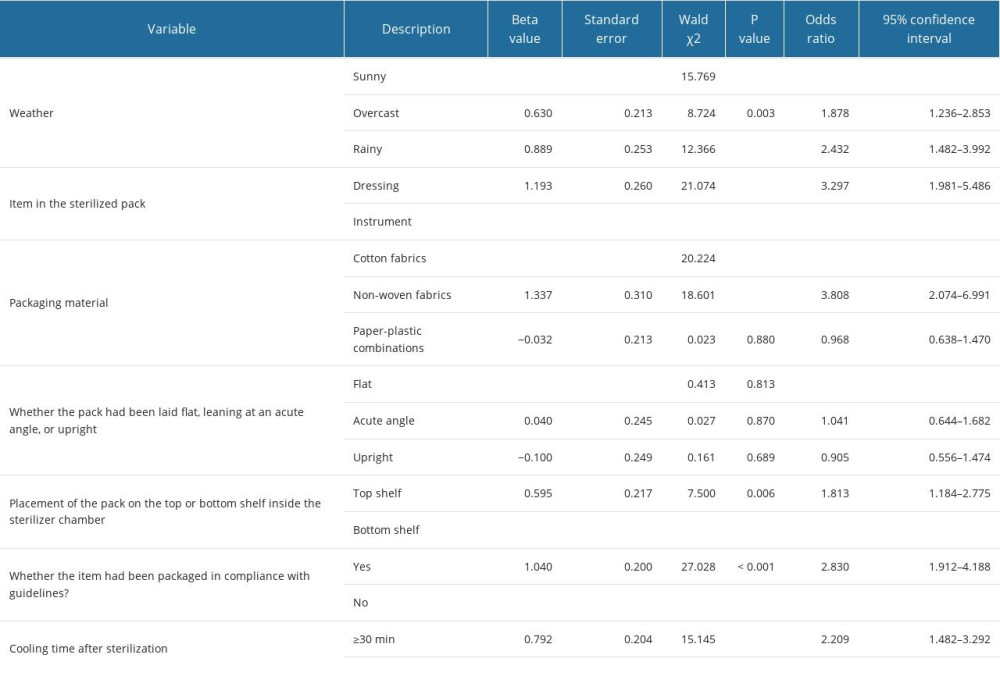
Logistic regression analysis results show that the probability of wet pack occurrence on overcast and rainy days was 1.878 and 2.432 times higher, respectively, than on sunny days; the probability of wet pack occurrence in the instrument packs was 3.297 times higher than in the dressing packs; the probability of wet pack occurrence in the packs packaged using non-woven fabrics was 3.808 times higher than those in cotton wraps; the probability of wet pack occurrence in the packs not packaged in compliance with guidelines was 2.830 times higher than the packs packaged in compliance with guidelines; and the probability of wet pack occurrence when the cooling time of the sterilized pack was less than 30 minutes was 2.209 times higher than when the cooling time was 30 minutes or more (Table 3).
Discussion
LIMITATIONS:
Our study has some limitations. First, we did not investigate the influence of the sterilization cycle protocol parameters on wet pack occurrence. Second, this was a cross-sectional study that analyzed the data concerning wet pack occurrence in a specific time period. Further, there should be more in-depth analysis of the role of sample size in future studies. Multi-center research is required for a more rigorous investigation of the influence of different sterilizers and different parameters on wet pack occurrence.
Conclusions
Our research findings show that the following factors are associated with wet pack occurrence after steam sterilization: outside weather, the item in the sterilized pack, packaging material, whether the item had been packaged in compliance with guidelines, whether the pack had been placed on the top or bottom shelf, and cooling time after sterilization.
Recommendations for reducing the risk of wet packs include: regular maintenance of the steam pipeline, regular replacement of thermal insulation materials for the steam pipeline, extension of the drying time, appropriate packaging of items in compliance with guidelines, purchasing specialized surgical instruments, adding a cooling time alarm system module to the traceability system, and inclusion of a handheld infrared thermometer for accurate measurement of the surface temperature of the packs.
References
1. Liu M, Huang X, Zhang J, Investigation on the current status of central sterile supply department management during the COVID-19 pandemic: Chinese J Disinfect, 2022; 39(5); 363-66
2. van Doornmalen JPCM, Tessarolo F, Lapanaitis N, A survey to quantify wet loads after steam sterilization processes in healthcare facilities: J Hosp Infect, 2019; 103(1); e105-e9
3. Seavey R, Troubleshooting failed sterilization loads: Process failures and wet packs/loads: Am J Infect Control, 2016; 44(5 Suppl); e29-34
4. Sun H, Yang L, Xiang M, Zhao L, Application of FOCUS-PDCA program to reduce the incidence of sterilization wet package of foreign medical devices: Chinese Nurs Res, 2018; 32(14); 2232-37
5. Zhou Q, The effect of processing quality control on the wet package of foreign medical devices: Chinese J Disinfect, 2017; 34(2); 175-76
6. Basu D, Reason behind wet pack after steam sterilization and its consequences: An overview from Central Tterile Supply Department of a cancer center in eastern India: J Infect Public Health, 2017; 10; 235-39
7. Fan X, Ren G, Yang M, Investigation on usage status of pressure steam sterilizers in basic medical institutions: Chinese J Disinfect, 2017; 34(6); 554-56
8. Stošić N, Popović J, Anđelković Apostolović M, Effects of autoclave sterilization on cyclic fatigue resistance in 5 types of rotary endodontic instruments: An in vitro study: Med Sci Monit, 2023; 29; e939694
9. Gupta S, Sayed ME, Gupta B, Comparison of composite resin (duo-shade) shade guide with vita ceramic shades before and after chemical and autoclave sterilization: Med Sci Monit, 2023; 29; e940949
10. Barbosa Rodrigues S, Queiroz de Souza R, Uchikawa Graziano K, Sidnei Erzinger G, Specialists’ opinions regarding factors related to wet loads after steam sterilization: J Hosp Infect, 2022; 120; 117-22
11. Panta G, Richardson AK, Shaw IC, Effectiveness of autoclaving in sterilizing reusable medical devices in healthcare facilities: J Infect Dev Ctries, 2019; 13(10); 858-64
12. He HY, Huang FY, Wu XH, Risk study by fault simulation of entering air at heating-up stage in pulsating vacuum autoclave: Chin J Nosocomiol, 2019; 29(24); 3830-35
13. Xu HF, Cause and control countermeasures of pulsating vacuum sterilization: Med Equip, 2019; 32(3); 78-79
14. Huang Q, Wang XQ, Lin CL, Effect of quality control circle on reduction of wet bag rate of medical instrument packages in sterilization and supply room: Chin J Nosocomiol, 2016; 26(22); 5243-45
15. National Health Commission of the People’s Republic of China: Health Industrial Standard (WS310.2.2016) of the People’s Republic of China – Central sterile supply department (CSSD) – Part 2 : Standard for operating procedure of cleaning, disinfection and sterilization, 2016 http//www.nhc.gov.cn/ewebeditor/uploadfile/2017/01/20170105090606684.pdf
16. Standardization Administration of the People’s Republic of China: Technical requirements for large steam sterilizers-Automatic type, 2008 http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=0A26F133371312C94529CDCB4C723FD0
17. : ANSI/AAMI ST79: 2017 Comprehensive guide to steam sterilization and sterility assurance in health care facilities, 2017, Arlington, VA, Association for the Advancement of surgical instrumentation https://aami.org/standards/featured-standards/ansi-aami-st79
18. Centers for Disease Control and Prevention (CDC): Guidelines for disinfection and sterilization in healthcare facilities, 2019 http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_May_2019.pdf
19. Yu G, Li Y, Cause analysis and coping strategies of pulse pressure steam sterilizers: Med Pharm Yunnan, 2020; 41(6); 615-16
20. Duan M, Wang J, Wang H, Effect of double non-woven packing on wet packing rate in pulsating vacuum sterilizers under different conditions: Chinese J Rural Med Pharm, 2022; 29(2); 17-18
21. He H, Li S, Tang H, Study on the effect of vacuum degree on wet pack rate in the drying stage of pulsating pressure steam sterilization: Chinese J Disinfect, 2020; 37(5); 342-44
22. Fan S, Jiang S, Wei Y, Application of approach to sterilizing and drying orthopedics instrument packs provided by other companies via using air pulser: Guangxi Med J, 2021; 43(9); 1164-65
23. Kang F, The effect of different absorb moisture materials on wet surgical equipment package: Gansu Sci Tech, 2019; 35(14); 123-24
24. Yuan Y, Chao X, Sun X, Influence of packaging materials and methods on wet packs: Chinese J Nosocomiol, 2014; 24(19); 4905-7
25. Xu C, Causes and management countermeasures of wet package in sterilization supply room after steam sterilization: Contemporary Med, 2018; 24(27); 47-49
26. Wei D, Jiang Y, Effect of PDCA in reducing the rate of abortion packet: Modern Nurse, 2017; 1; 154-56
27. Gao L, Zhang X, Sun F, Causes and improvement measures of pressure steam sterilization wet packs for foreign surgical instruments: China Health Industry, 2023; 20(7); 211-213
28. Pang Q, Zhang J, Application of root cause analysis in reducing the wet package rate of pulse vacuum sterilization: Chinese J Med Dev, 2023; 36(7); 44-46
29. Liu C, Zhang L, Control of wet bag and case analysis: China Modern Doctor, 2016; 54(21); 141-44
30. Wang M, Jin C, Zhao X, Cause and improvement measures of wet bag production in autoclaved steam sterilization: Electronic J Pract Clin Nurs Science, 2018; 3(10); 191-95
31. Li X, Di R, Cui Z, Effect of infrared thermometer on evaluating unloading temperature of items in pressure steam sterilizer: Chinese J Disinfect, 2019; 36(9); 659-61
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952