21 February 2024: Clinical Research
Prognostic Assessment of Colorectal Cancer Patients after Laparoscopic Surgery: A Comprehensive Evaluation of the Glasgow Prognostic Score and Fibrinogen-to-Prealbumin Ratio
Bing Liu1ABCDE, Jiangfeng Qian1BE, Yuan Zhou1CD, Ning Chen1EF, Haiwen Zhuang1EF, Jian Wang1EF, Xiaoyu Zhang1AG*, Haijian Zhao1DGDOI: 10.12659/MSM.942658
Med Sci Monit 2024; 30:e942658
Abstract
BACKGROUND: Previous studies have shown that systemic inflammation and suboptimal nutritional status are associated with poor cancer prognosis. This study aims to investigate the prognostic value of preoperative Glasgow prognostic score (GPS) and fibrinogen-to-prealbumin ratio (FPR) in patients with CRC (colorectal cancer) after laparoscopic surgery.
MATERIAL AND METHODS: In this study, the clinical data of 112 patients with CRC who underwent laparoscopic surgery were retrospectively analyzed, and the 3-year and 5-year survival rates of these patients were evaluated. In addition, the prognostic role of preoperative FPR and GPS in CRC patients was assessed using X-tile software, Kaplan-Meier analysis, and Cox regression analysis. Receiver operating characteristic (ROC) curves were generated to assess the predictive value of FPR, GPS, and FPR-GPS for the survival of these patients.
RESULTS: The results revealed a significant negative correlation between high FPR, elevated GPS, and overall survival (OS) in patients with CRC. Univariate and multivariate Cox regression analyses identified GPS (HR=3.207, 95% CI: 1.746~6.126), FPR (HR=2.669, 95% CI: 1.052~6.772), and lymph node metastasis (HR=2.222, 95% CI: 1.199~4.115) as independent prognostic indicators for overall survival. The ROC analysis demonstrated that the prediction based on FPR and GPS outperformed a single indicator in accurately predicting the prognosis of CRC patients.
CONCLUSIONS: Combining the preoperative FPR with the GPS contributes to accurate prognosis assessment for CRC patients after laparoscopic surgery. Patients exhibiting high FPR and GPS values are associated with a worse prognosis.
Keywords: Adenocarcinoma, Colorectal Neoplasms, Prognosis
Background
Colorectal cancer (CRC) is one of the most prevalent malignant neoplasms affecting the gastrointestinal tract. As per the Global Cancer Observatory (GCO) compiled by the International Agency for Research on Cancer (IARC) in 2020, CRC ranks third in terms of the incidence worldwide and stands as the second leading cause of cancer-related mortality [1]. Currently, surgical intervention remains one of the most efficacious approaches for managing colorectal cancer, with laparoscopic surgery emerging as the standard technique owing to its reduced postoperative complications and comparable long-term prognosis to open surgery. Currently, less invasive and more cost-effective screening methods, such as tumor markers (CEA and CA199), fecal immunohistochemistry examination, and colonoscopy examination, along with timely surgical resection, have significantly enhanced the survival rate of CRC patients. However, their long-term clinical outcomes remain suboptimal. Several studies have reported a 5-year survival rate for CRC ranging from 57.0% to 77.0% [2]. This range indicates that there are differences in the clinical treatment effects, and patients may obtain different prognostic outcomes. Currently, there are many indicators to assess the prognosis of CRC patients, such as TNM staging, Dukes’ staging, and degree of cell differentiation [3,4]. However, patients with the same tumor grading during the same period may still have different prognoses. The predictive accuracy of these biomarkers for CRC patients is not always reliable. It has been previously demonstrated that evaluation based on preoperative nutritional status and systemic inflammatory markers can partially predict postoperative complications, tumor recurrence, metastasis, and overall survival (OS) outcomes [5,6]. Currently, there is a growing trend of incorporating comprehensive prognostic biomarkers that can integrate inflammation and nutrition, such as GPS and FPR, into studies aiming to predict the prognosis of diverse tumors [7–10]. However, there is a paucity of research on the combined utilization of GPS and FPR for prognostic sensitivity prediction in patients with CRC. Therefore, this study was conducted to investigate the prognostic significance of preoperative fibrinogen-to-prealbumin ratio (FPR), Glasgow prognostic score (GPS), C-reactive protein (CRP), fibrinogen (Fib), albumin (Alb), and prealbumin (pre-Alb) in CRC patients after laparoscopic surgery. These scientific efforts are expected to provide novel approaches for predicting early clinical prognosis and improving outcomes in patients with CRC.
Material and Methods
PATIENTS:
This study aimed to collect comprehensive clinical data, including hematological indicators and relevant clinical information, from patients with colorectal cancer who underwent laparoscopic surgery at the Affiliated Huai’an Hospital of Xuzhou Medical University between January 2016 and August 2018. Inclusion criteria were: laparoscopic surgery for colorectal cancer, achieving R0 resection; confirmation of colorectal cancer through pathological examination; absence of preoperative radiotherapy or chemotherapy treatment; and availability of complete clinical data. Exclusion criteria were: patients with recurrent tumors or concurrent malignancies; patients with hepatic, renal, or cardiac dysfunction; patients with blood system disorders; patients who have undergone palliative surgery; patients who have previously received adjuvant therapy; and patients with incomplete clinical data. The study was approved by the Ethics Committee of Huai’an Hospital Affiliated to Xuzhou Medical University.
METHOD:
Serum levels of fibrinogen, albumin, prealbumin, and C-reactive protein were assessed in patients during the week preceding surgery. Hospital information systems were utilized for the collection of clinical data, encompassing admission time, duration of hospitalization, sex, age, body mass index (BMI), preoperative comorbidities (diabetes, hypertension and cardiovascular diseases), postoperative chemotherapy administration, tumor location, surgical type, depth of infiltration, perineural invasion presence, vascular invasion occurrence, pathological type identification, histological grade determination, and American Society of Anesthesiologists (ASA) classification. The AJCC 8th edition was utilized for the classification of colorectal cancer staging based on TNM staging criteria.
The prognostic research indicators were as follows: FPR, which represents the ratio of fibrinogen to prealbumin; GPS score of 0 is assigned when albumin levels are normal (>35 g/L) and CRP levels are below 10mg/L; GPS score of 1 is assigned when either albumin (≤35 g/L) or CRP (≥10 mg/L) deviates from the normal range; the total GPS score is obtained by summing these 2 scores. After discharge, follow-up will be conducted through methods such as telephone consultations and reviewing hospital or outpatient medical records. Post-surgery check-ups will be scheduled every 3 months for a total of 2 years, followed by once every 6 months for a total of 5 years. After the completion of the fifth year, annual check-ups will be conducted [11]. The overall survival (OS) is defined as the duration from diagnosis until death or the termination of follow-up. The follow-up period concludes in August 2023.
STATISTICAL ANALYSIS:
SPSS 26.0 (SPSS, Inc., Chicago, IL, USA) was utilized to analyze data. The optimal threshold for FPR was determined using X-tile 3.6.1 (Yale University, New Haven, CT, USA). The Shapiro-Wilk test was employed to assess the normality of continuous variable distributions. In the case of normally distributed data, the
Results
CHARACTERISTICS OF PATIENTS AT BASELINE:
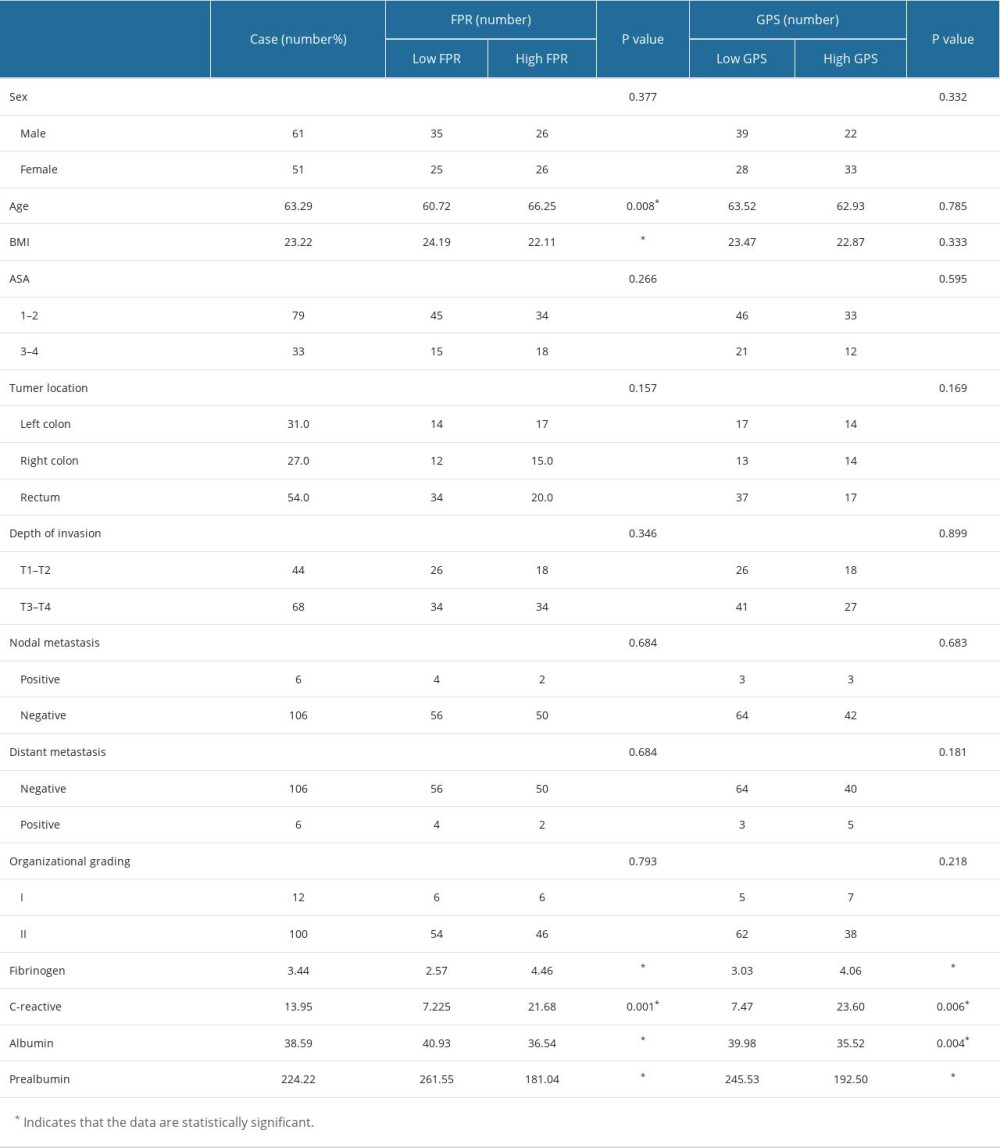
A total of 112 eligible patients were enrolled in the study, with a median age of 63.5 years. Among them, 16 patients (14.2%) were younger than 50 years old, while the remaining 96 patients (85.8%) fell within the age range of 50 to 80. After surgery, adjuvant chemotherapy was administered to a total of 75 patients (66.9%). In this study, the 3-year and 5-year survival rates were 74.1% and 58%, respectively. Patients were included based on their FPR and GPS scores: there were 67 patients with a score of 0, 36 patients with a score of 1, and 9 patients with a score of 2. The group with a score of 0 was categorized as the normal group (low GPS), while the group encompassing scores ranging from 1 to 2 comprised abnormal cases (high GPS). The median of FPR was 0.137. The optimal cutoff value for FPR was determined to be 0.139 using the X-tile software. Based on this threshold, patients were categorized into a high FPR group (52 patients) and a low FPR group (60 patients). Table 1 shows the correlation between FPR, GPS, and clinical pathological factors. The fibrinogen-to-prealbumin ratio (FPR) had a significant positive correlation with body mass index (BMI) (P<0.001) and fibrinogen levels (P≤0.001). Additionally, it demonstrates a significant negative correlation with age (P=0.008), albumin levels (P<0.001), prealbumin levels (P<0.001), and C-reactive protein levels (CRP) (P=0.001). GPS was significantly correlated with fibrinogen (P<0.001), CRP (P=0.006), albumin (P=0.004), and prealbumin (P<0.001). There was no correlation between GPS grouping and sex, age, tumor location, depth of infiltration, or tumor grade. GPS was significantly correlated with fibrinogen (P<0.001), CRP (P=0.006), albumin (P=0.004), and prealbumin (P<0.001).
PROGNOSTIC FACTORS:
The univariate analysis results showed that BMI, lymph node metastasis, albumin, prealbumin, fibrinogen, and GPS can predict the survival rate of CRC patients (Figure 1A). Additionally, a Cox proportional hazard model was constructed for multivariate analyses. The results revealed that lymph node metastasis (P=0.017), GPS (P<0.001), and FPR (P<0.001) were independent prognostic factors for CRC patients (Figure 1B). Meanwhile, this experiment demonstrated the superior discriminatory ability of GPS for CRC (HR=3.270), followed by FPR (HR=2.669) and lymph node metastasis (HR=2.222).
OVERALL SURVIVAL:
The median overall survival of all enrolled patients in the study was 63 months, with a follow-up period extending to 84 months. To date, a total of 47 deaths have been recorded, with 42 deaths attributed to tumors and complications arising from advanced-stage tumors, while the cause of death for the remaining 5 individuals remains undetermined. The 5-year overall survival (OS) rate of these patients was 36.5% in the high FPR group, whereas it reached 76.7% in the low FPR group (P<0.001) (Figure 2A). The 5-year OS rate was 24.4% in the high GPS group and 75.6% in the low GPS group, demonstrating a significant difference (P<0.001) (Figure 2B). The lymph node metastasis group had a 5-year OS rate of 33.3%, whereas the non-lymph node metastasis group showed a higher rate of 59.4% (P=0.005) (Figure 2C). The 5-year OS rate was 13.8% in the high FPR and high GPS group (29 cases), 53.8% in the low FPR and high GPS group (13 cases), 71.4% in the high FPR and low GPS group (21 cases), and 79.6% in the low FPR and low GPS group (49 cases) (P=0.001) (Figure 2D). According to these results, patients in the high FPR and high GPS group had the highest likelihood of an unfavorable prognosis, followed by those in the high FPR and low GPS group, then the low FPR and high GPS group, and finally the low FPR and low GPS group.
ROC CURVE FOR OVERALL SURVIVAL PREDICTION:
FPR and GPS were further analyzed through binary logistic regression analyses to obtain a new composite predictor. Then, the ROC analysis was performed on the new composite predictor and individual predictors. The results indicated that the AUC of 3-year OS prediction using FPR-GPS as a combined predictor was 0.868 (95% CI: 0.796~0.940, P<0.001), which was significantly higher than that using FPR alone (AUC=0.823, 95% CI: 0.724~0.923, P<0.0001) and GPS alone (AUC=0.768, 95% CI: 0.665~0.870, P<0.001) (Figure 3A). Meanwhile, the composite predictor (FPR-GPS) demonstrated higher accuracy in predicting the 5-year OS rate, with an AUC of 0.816 (95% CI: 0.732~0.900, P<0.001). In comparison, using GPS alone resulted in an AUC of 0.785 (95% CI: 0.695~0.875, P<0.0001), and using FPR alone resulted in an AUC of 0.761 (95% CI: 0.668~0.853, P<0.001). Both individual predictors were inferior to FPR-GPS in terms of predictive performance (Figure 3B). The above results show that the new composite predictor (FPR-GPS) was more accurate in predicting the OS of CRC patients compared with FPR or GPS alone.
Discussion
In recent years, there has been increasing attention on the relationship between inflammation and malignant tumors [12–14]. On the one hand, long-term chronic inflammation stimulation can cause uncontrolled cell proliferation and lead to cell growth and tumor formation [15]. On the other hand, there is a mutual induction effect between inflammation and malignant tumors [16], where tumor growth may result in visceral tissue inflammation and elevated CRP levels. The association between the inflammatory response and poor prognosis in cancer patients has been demonstrated by multiple studies, and inflammation markers that reflect the status of the body’s inflammatory response are widely employed for predicting tumor prognosis. The nutritional status is another important factor that affects the prognosis of cancer patients. As the nutritional status of patients gradually declines, the immune response to tumors will be suppressed, leading to tumor spread and disease progression. Various prognostic indicators based on overall inflammation and nutritional status have demonstrated favorable performance in predicting clinical outcomes for patients with colorectal cancer (CRC). To the best of our knowledge, this is the first study to assess the utility of FPR in combination with GPS in predicting the prognosis of CRC patients [17,18].
In this study, the correlation of clinical and pathological factors with survival rates of CRC patients after surgical resection was examined, and we also assessed the prognostic value of the inflammation-based markers (FPR and GPS). The correlation analysis results revealed that high FPR was significantly associated with low BMI, high CPR, and low albumin levels. This indicated that elevated FPR may reflect poor physical and nutritional status of CRC patients. Additionally, inflammatory factors can induce malnutrition in patients, which was consistent with previous research findings [19,20]. Meanwhile, a close correlation was observed between elevated GPS and decreased prealbumin levels of fibrinogen. The survival analysis results indicated that high FPR, high GPS, and lymph node metastasis were independent prognostic factors affecting the OS of CRC patients, and these factors were associated with the poor prognosis of these patients. In addition, by pairing high FPR with low FPR and high GPS with low GPS, these patients were divided into 4 groups: the high FPR and high GPS group, the high FPR and low GPS group, the low FPR and high GPS group, and the low FPR and low GPS group. The Kaplan-Meier method was used to plot survival curves and calculate the 5-year OS rates of patients in these groups. The study results showed that the 5-year OS rate reached the minimum when both FPR and GPS increased simultaneously; whereas the 5-year OS rate was highest when both FPR and GPS decreased simultaneously. Additionally, it was found that the 5-year OS rate in the high FPR and low GPS group was significantly higher than that in the low FPR and high GPS group, indicating a higher risk of long-term adverse prognosis associated with high GPS compared with high FPR. This result was further confirmed by the K-M curve (Figure 2D) and ROC curve for the 5-year OS (Figure 3B).
This was the first scientific attempt to incorporate FPR and GPS into binary logistic regression analyses in CRC-related research, resulting in a new composite predictor – FPR-GPS. Through the ROC analysis of the prognosis of CRC patients using FPR, GPS, and FPR-GPS as predictors, the results demonstrated that the composite predictor had higher sensitivity in predicting the prognosis of CRC patients after surgical treatment compared with FPR or GPS alone.
In this study, fibrinogen, proalbumin, albumin, and CRP did not demonstrate independent prognostic significance in CRC. Owing to its ability to balance the effects of inflammation and nutrition, FPR can provide a more comprehensive reflection of the biological status of patients. High FPR levels indicated the up-regulation of fibrinogen and down-regulation of prealbumin, which suggested the poor nutritional status and inflammatory burden related to cancer [19]. Zhang et al [20] investigated the relationship between preoperative FPR and prognosis in patients with gastric cancer (GC). They found that elevated FPR was significantly associated with increased tumor diameter, deeper infiltration depth, and lymph node metastasis. In addition, it was revealed that GC patients with a high FPR had advanced tumor staging, lower degree of differentiation, and shorter survival time. However, these results were inconsistent with the findings of the present study, possibly due to differences in the types of diseases. Sun et al [6] investigated the correlation between FPR and the development as well as prognosis of CRC at stages II-III, finding that FPR was a more precise predictor of prognosis during the assessment of tumor burdens and progression in these patients. In addition to individual inflammatory biomarkers, various emerging prognostic scores, such as the Glasgow prognostic score (GPS) and modified Glasgow prognostic score (mGPS), also exhibited robust performance in evaluating the progression and survival rates of CRC. Kenta Kasahara et al [18] conducted a retrospective study and revealed that GPS can serve as a prognostic indicator for predicting the outcome of patients with resectable CRC at an advanced stage, demonstrating higher practicality in the prognostic model compared to mGPS. The meta-analysis conducted by Hae Dong Woo et al [21] demonstrated a significant association between preoperative CRP levels and the survival rate of CRC patients after surgery.
Nevertheless, there are some potential limitations in this study. Firstly, this was a retrospective study based on a single-center analysis, and there is a lack of a validation group for further investigation. Moreover, the focus of this study was on the 5-year survival outcomes of CRC patients after surgery, and the accurate and sufficient data on progression-free survival were not considered. Thus, the overall survival situation of these patients cannot be fully depicted. Therefore, it is imperative to conduct more extensive multicenter clinical trials and perform stratified studies based on a larger sample size to validate these findings.
Conclusions
The findings of this study suggest that preoperative FPR, GPS, and lymph node metastasis are independent prognostic factors for patients with CRC. In comparison to high FPR levels, elevated GPS values are associated with a higher risk of long-term adverse prognoses. Moreover, the combined utilization of FPR and GPS demonstrates enhanced sensitivity in predicting both short-term and long-term survival outcomes compared with the application of a single predictor.
Figures
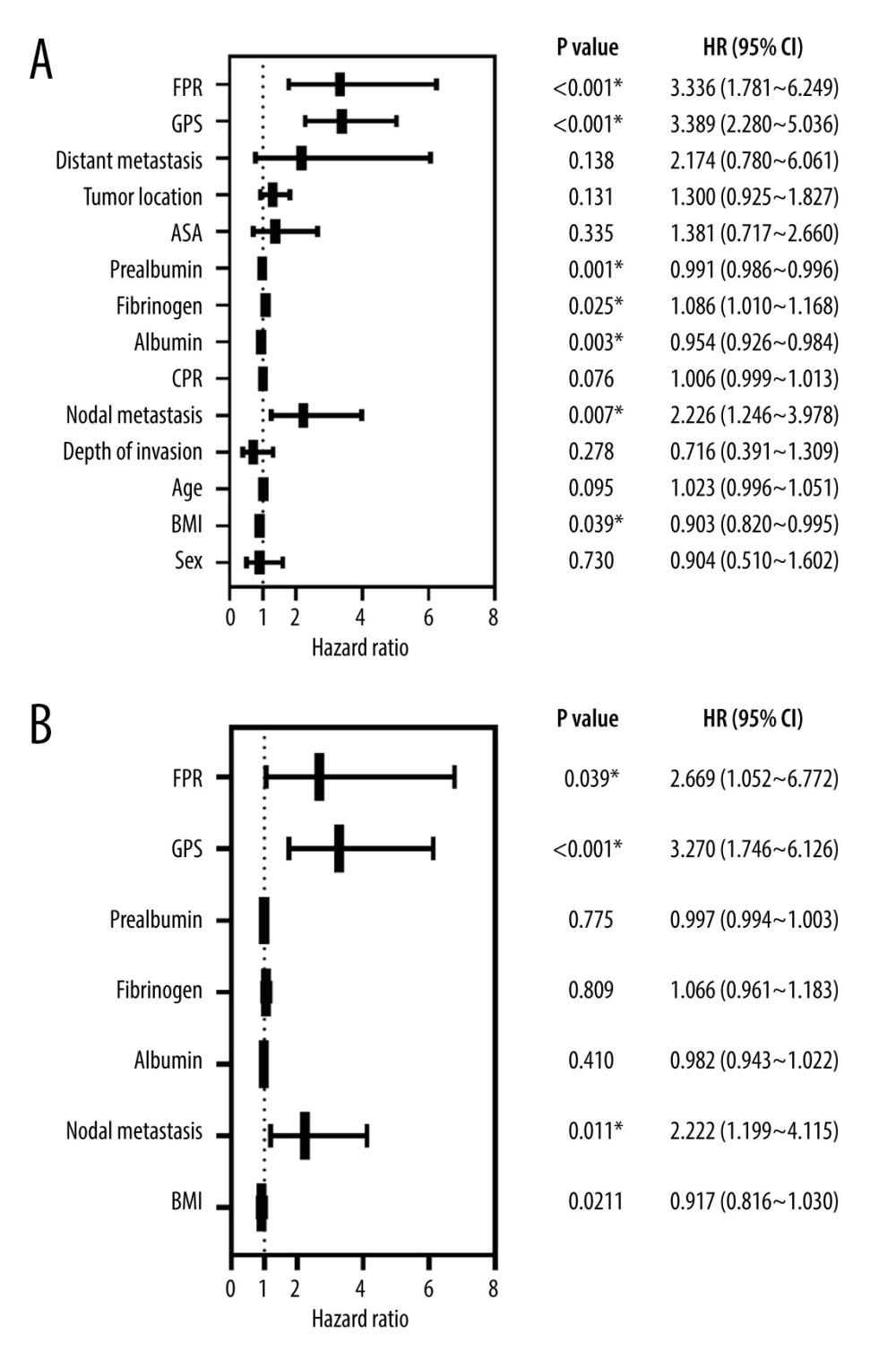 Figure 1. Forest plots of univariate (A) and multivariate (B) analyses associated with prognostic factors in patients. * Statistical results of Cox regression analysis. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 1. Forest plots of univariate (A) and multivariate (B) analyses associated with prognostic factors in patients. * Statistical results of Cox regression analysis. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. 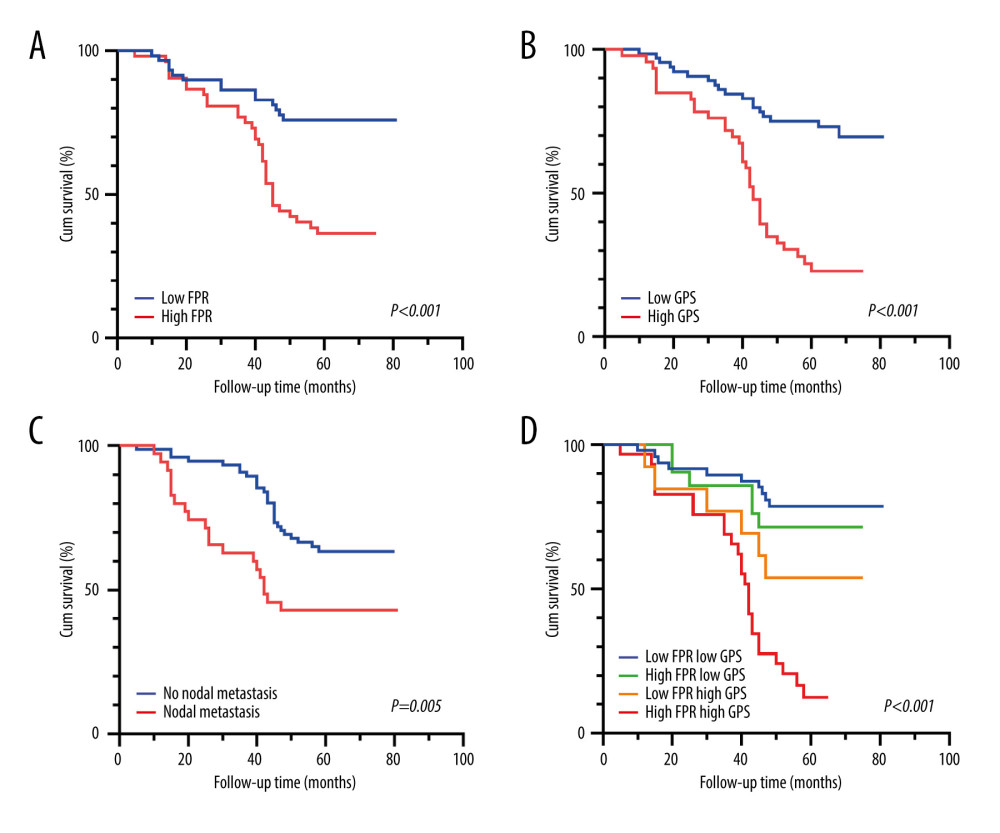 Figure 2. (A–D) The overall survival was stratified based on FPR, GPS, lymph node metastasis, and the combined effect of FPR and GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 2. (A–D) The overall survival was stratified based on FPR, GPS, lymph node metastasis, and the combined effect of FPR and GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. 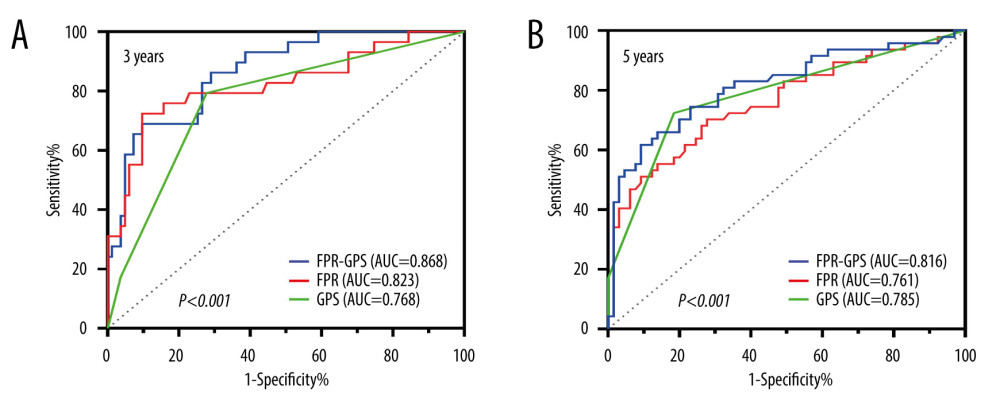 Figure 3. The ROC curves are grouped by FPR, GPS, and FPR-GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 3. The ROC curves are grouped by FPR, GPS, and FPR-GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. References
1. Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71; 209-49
2. Wang R, Lian J, Wang X, Survival rate of colorectal cancer in China: A systematic review and meta-analysis: Front Oncol, 2023; 13; 1033154
3. Hou S, Jing J, Wang Y, Evaluation of clinical diagnostic and prognostic value of preoperative serum carcinoembryonic antigen, CA19-9, and CA24-2 for colorectal cancer: Altern Ther Health M, 2023; 29; 192-97
4. Zhang Q, Li B, Zhang S, Prognostic impact of tumor size on patients with metastatic colorectal cancer: A large SEER-based retrospective cohort study: Updates surg, 2023; 75; 1135-47
5. Balahura LR, Selaru A, Dinescu S, Inflammation and Inflammasomes: Pros and cons in tumorigenesis: J Immunol Res, 2020; 2020; 2549763
6. Sun F, Peng HX, Gao QF, Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II–III colorectal cancer patients: Cancer Manag Res, 2018; 10; 2151-61
7. Li C, Fan Z, Guo W, Fibrinogen-to-prealbumin ratio: A new prognostic marker of resectable pancreatic cancer: Front Oncol, 2023; 13; 1149942
8. Li S, Zhang X, Lou C, Preoperative peripheral blood inflammatory markers especially the fibrinogen-to-lymphocyte ratio and novel FLR-N score predict the prognosis of patients with early-stage resectable extrahepatic cholangiocarcinoma: Front Oncol, 2022; 12; 1003845
9. Saal J, Bald T, Eckstein M, Integrating on-treatment modified Glasgow Prognostic Score and imaging to predict response and outcomes in metastatic renal cell carcinoma: JAMA Oncol, 2023; 9; 1048-55
10. Sun DW, An L, Lv GY, Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: An updated meta-analysis: World J Surg Oncol, 2020; 18; 9
11. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2023 edition): Zhonghua Wai Ke Za Zhi, 2023; 61; 617-44 [in Chinese]
12. Feng Q, Chang W, Mao Y, Tumor-associated macrophages as prognostic and predictive biomarkers for postoperative adjuvant chemotherapy in patients with stage II colon cancer: Clin Cancer res, 2019; 25; 3896-907
13. Okugawa Y, Toiyama Y, Yamamoto A, Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer: Ann Surg, 2020; 272; 342-51
14. Sjoquist KM, Renfro LA, Simes RJ, Personalizing survival predictions in advanced colorectal cancer: The ARCAD nomogram project: J Natl Cancer Inst, 2018; 110; 638-48
15. Roxburgh CS, McMillan DC, Role of systemic inflammatory response in predicting survival in patients with primary operable cancer: Future Oncol, 2010; 6; 149-63
16. Moore MM, Chua W, Charles KA, Inflammation and cancer: Causes and consequences: Clin Pharmacol Ther, 2010; 87; 504-8
17. Chen C, Liu Y, Han P, Research progress of preoperative FPR, FAR or AFR in patients with colorectal cancer: Cancer Manag Res, 2021; 13; 1791-801
18. Kasahara K, Enomoto M, Udo R, Prognostic value of preoperative high-sensitivity modified Glasgow prognostic score in advanced colon cancer: A retrospective observational study: BMC Cancer, 2022; 22; 20
19. Hailun X, Huang S, Yuan G, Prognostic significance of preoperative fibrinogen-to-prealbumin ratio in patients with stage I-III colorectal cancer undergoing surgical resection: A retrospective cohort study: Biomed Res Int, 2021; 2021; 3905353
20. Zhang F, Wang Y, Sun P, Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma: J Cancer Res Clin, 2017; 143; 2413-24
21. Woo HD, Kim K, Kim J, Association between preoperative C-reactive protein level and colorectal cancer survival: A meta-analysis: Cancer Cause Control, 2015; 26; 1661-70
Figures
 Figure 1. Forest plots of univariate (A) and multivariate (B) analyses associated with prognostic factors in patients. * Statistical results of Cox regression analysis. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 1. Forest plots of univariate (A) and multivariate (B) analyses associated with prognostic factors in patients. * Statistical results of Cox regression analysis. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. Figure 2. (A–D) The overall survival was stratified based on FPR, GPS, lymph node metastasis, and the combined effect of FPR and GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 2. (A–D) The overall survival was stratified based on FPR, GPS, lymph node metastasis, and the combined effect of FPR and GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. Figure 3. The ROC curves are grouped by FPR, GPS, and FPR-GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph.
Figure 3. The ROC curves are grouped by FPR, GPS, and FPR-GPS. We used GraphPad Prism 9 (Dotmatics, San Diego, USA) to generate this graph. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952