23 March 2024: Clinical Research
Etiology, Clinical Presentation, and Outcomes of Bacterial Meningitis in Adult Patients: A Retrospective Study in Lithuania (2018–2021)
Greta Rynkevič1BCDEF*, Emilijus Žilinskas1BCDEF, Dovilė Streckytė1ADEF, Daiva Radzišauskienė2AE, Rūta Mameniškienė3ADEDOI: 10.12659/MSM.942904
Med Sci Monit 2024; 30:e942904
Abstract
BACKGROUND: Bacterial meningitis is a serious and life-threatening condition that requires prompt diagnosis and treatment. This retrospective study aimed to identify causes, presentation, and predictive factors for outcomes of community-acquired bacterial meningitis in 86 adults in Vilnius, Lithuania between 2018 and 2021.
MATERIAL AND METHODS: We performed a retrospective study of demographic, clinical, and laboratory records of 86 adult patients admitted to Vilnius University Hospital Santaros Clinics with a diagnosis of acute bacterial meningitis during the period of 2018-2021.
RESULTS: Of 86 patients, 54 (62.79%) were men. The median (Md) age of patients was 58 (range, 18-83) years and the median duration of hospitalization was 20 (range, 3-92) days. Patients were first hospitalized in the Intensive Care Unit (ICU) in 59.3% of cases. The most prevalent concerns were headache (66.28%), febrile temperature (56.98%), general fatigue (53.49%), and confusion/sleepiness (52.33%). Of 57 (66.28%) etiologically confirmed cases, the most prevalent agent was Listeria monocytogenes (29.82%), followed by Streptococcus pneumoniae (28.07%) and Neisseria meningitidis (28.07%). Patients with meningitis caused by L. monocytogenes were the oldest (P=0.003) and had the longest hospitalization (P<0.001). Fatigue was the prominent symptom in patients with meningococcal meningitis (81.2%, P=0.010). Twelve patients (13.95%) have died. Advanced age and low (<100 cells per μL) white blood cell (WBC) count in cerebrospinal fluid (CSF) were associated with lethal outcome, whereas headache was associated with favorable outcome.
CONCLUSIONS: Clinical characteristics of community-acquired acute bacterial meningitis differ based on etiological factors. Patient age, CSF WBC count, and headache may be significant predictive factors for outcomes of bacterial meningitis.
Keywords: Listeria monocytogenes, bacterial meningitis, Neisseria meningitidis, Streptococcus pneumoniae
Introduction
Acute bacterial meningitis remains a devastating infectious disease with substantial mortality rates globally [1]. According to the World Health Organization, around 1 in 6 people who get bacterial meningitis die and 1 in 5 have severe complications [2]. For example, bacterial meningitis was responsible for approximately 500 deaths annually between 2003 and 2007 in the United States [3]. Developing countries face an even higher burden of disease [4].
Despite progress in the prevention of community-acquired bacterial meningitis by implementing intensive vaccination programs against the 3 most common causative pathogens –
Studies also indicate various clinical and laboratory variables that may determine the outcome of patients with acute bacterial meningitis [6,13–16]. For example, 1 study highlighted that factors indicative of systemic compromise, a low level of consciousness, and infection with
According to the data from the National Information System on Communicable Diseases and Infectious Agents, the incidence of bacterial meningitis in Lithuania in 2019 was 0.9/100 000 [17]. From 2010 to 2019, new yearly cases of bacterial meningitis in Lithuania ranged from 26 to 78 [17], and almost all these patients were hospitalized [17]. Similar prevalence rates have been estimated in other European countries, such as The Netherlands and Finland [18,19]. A few studies of causes, socio-demographic factors, clinical presentation, and outcomes of infectious diseases have been conducted in Lithuania [15,20]. For example, Matulyte et al investigated data of patients with a diagnosis of bacterial meningitis treated between 2009 and 2016 [15]. However, since then, no known studies have been conducted to investigate community-acquired bacterial meningitis cases in Lithuania.
As current data on causes, diagnostics, treatment, and prediction of outcomes of bacterial meningitis are heterogeneous, and morbidity and mortality rates remain high, this retrospective study aimed to identify the causes, presentation, and outcomes of community-acquired bacterial meningitis in 86 adults in Vilnius, Lithuania between 2018 and 2021.
Material and Methods
ETHICS STATEMENT:
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Regional Biomedical Research Ethics Committee of Vilnius (no. 158200-18-984-490). All medical records analyzed in the study were completely de-personalized; thus, an informed consent statement was not required.
STUDY SETTINGS:
A retrospective study was performed by analyzing data extracted from the Vilnius University Hospital Santaros Clinics database. The current population of Lithuania is around 2.62 million people and Vilnius is the capital and the largest city in Lithuania. Vilnius University Hospital Santaros Clinics is the main reference center for adult neuroinfectious diseases in Vilnius.
STUDY SUBJECTS:
A retrospective analysis was performed of adult patients (18 years or older) diagnosed with acute bacterial meningitis from January 1, 2018, through December 31, 2021, at the Infectious Diseases Center or Center for Neurology of Vilnius University Hospital Santaros Clinics. Inclusion criteria were: patients with a diagnosis of bacterial meningitis (ICD-10: A32.1, A39.0, G00.0-3, G00.8-9, G03.8, G04.2). Exclusion criteria were: 1) patients with meningitis caused by
DATA COLLECTION:
Demographic factors (age, sex), clinical characteristics (symptoms, onset of symptoms, chronic diseases, meningeal signs during first neurological examination after admission, site, and duration of hospitalization), laboratory results of CSF sampling (white blood cell count,% of granulocytes, protein concentration), and a specific aspect of treatment – administration of glucocorticosteroids – were analyzed.
To assess details of differences in demographic, clinical and laboratory variables based on etiological agents, rare cases (
STATISTICAL ANALYSIS:
Statistical analysis of the data was conducted in Microsoft Excel v16 and IBM SPSS v26. Categorical variables were expressed as counts and percentages, and frequency distributions were assessed with Fisher’s exact test. Continuous variables were expressed as the median and minimal–maximal values. Nonparametric tests (Mann-Whitney U or Kruskal-Wallis) were used to identify differences between groups in continuous variables. Unfavorable outcome was defined as death. Univariate analysis was used to explore unadjusted associations between variables and outcome.
Results
GENERAL CHARACTERISTICS:
We extracted data on 114 patients diagnosed with bacterial meningitis. Of them, 86 (75.44%) were included in further analysis. Fifty-four (62.79%) patients were men. The median age of patients was 58 years (range, 18–83). Median time from onset of symptoms to hospitalization was 2 days (range, 1–14). The most common clinical features at presentation were headache (66.28%), fever (56.98%), fatigue (53.49%), confusion/sleepiness (52.33%), and other symptoms (Figure 1). Meningeal signs (neck stiffness and/or positive Kernig’s sign) were identified in 73.8% of all cases.
Causative agents of meningitis were identified in 57 (66.28%) cases. Of confirmed cases, meningitis was most often caused by Listeria monocytogenes (17, 19.8%), followed by Streptococcus pneumoniae (16, 18.6%), Neisseria meningitidis (16, 18.6%), and other pathogens (Table 1).
Median time of hospitalization was 20 (range, 3–92) days. Fifty-one (59.3%) patients were first hospitalized in the ICU. During hospitalization, 64 patients (74.4%) were treated in the ICU. The median duration of hospitalization in the ICU was 4 (range, 1–37) days. The median number of white blood cells (cytosis) in CSF samples was 1181 (range, 25–32 426) cells per μl. The median percentage of polymorphonuclear cells in CSF samples was 88%. The median neutrophil-to-lymphocyte ratio was 6.82, and the median concentration of protein in CSF samples was 2.97 (range, 0.29–27.88). Treatment with glucocorticoids was administered to 54 patients (62.79%).
ETIOLOGICAL FACTOR-BASED COMPARISON:
Patients with meningitis caused by
Differences in demographic, clinical, laboratory, and hospitalization-related characteristics based on all etiological factors of meningitis and unknown origin of meningitis are summarized in Tables 2–4.
OUTCOMES:
Twelve (13.95%) patients died. Based on univariate regression analysis, older patients (65 years or more) were more likely to die from bacterial meningitis in our sample (OR 6.65 [1.65–26.88]). Further, <100 CSF WBC count was associated with higher risk of death (OR 5.17 [1.42–18.76]). In contrast, the presence of headache was related with a favorable outcome (OR 0.12 [0.03–0.50]). Factors and their impact on the outcome are presented in Table 5 and Figure 2. However, multivariate analysis did not reveal factors that may be significant in predicting unfavorable outcome (Table 6).
Discussion
Our study provides additional data regarding causes, presentation, and outcomes of community-acquired bacterial meningitis in adults. Unexpectedly,
Further, our study revealed that the most common clinical manifestations of bacterial meningitis in adults were headache, fever (≥38.5°C), fatigue, and confusion/sleepiness. These findings are quite similar compared to those found in the earlier Lithuanian study, which showed that the most common symptoms of bacterial meningitis were headache, fever (≥38°C), and nausea [15]. However, headache was reported in 84.3% of patients, more frequently than in our study (66.28%). In addition, we compared our findings to a Dutch study in which headache (87%) and fever (77%) occurred more often as well [6]. However, another retrospective study from western Canada noted more commensurate results as 66% of patients had headache and 56% had confusion [24]. On one hand, such differences again shed light on methodological inconsistencies between studies that prevent comparison of the research. On the other hand, the results of the present study highlight that only a complex of characteristic symptoms allows diagnosing bacterial meningitis accurately, as solitary symptoms are nonspecific.
Our study indicates that the highest CSF protein level was in the
Acute changes in the level of consciousness, severe sepsis, or septic shock with multiple-organ failure are the main reasons for admission of patients with meningitis to the ICU [16]. In our study, 74.4% of patients were treated in the ICU, and 59.3% of patients were admitted directly to the ICU from the hospital emergency department. The need for hospitalization in the ICU was more common in the
In this study, the mortality rate was 13.9% and did not differ significantly by etiological factor. In contrast to the latter finding, a previously mentioned Dutch study revealed that patients with
Our study has a few limitations to mention. Firstly, the retrospective nature of the study did not allow us to minutely examine some variables in the diagnosis and management of bacterial meningitis. For example, due to lack of information in the medical records we were not able to measure the exact period from the time of hospitalization to administration of antibiotics. The latter aspect may be of great significance when predicting outcomes of bacterial meningitis cases [29–31]. Moreover, the number of patients in the current sample was relatively small. Further, our study was limited to 1 tertiary care center hospital; thus, the patients may not accurately represent the population of bacterial meningitis cases within Lithuania.
Conclusions
Bacterial meningitis remains a dangerous and fatal condition, whether left untreated or, in some cases, even when treated properly. By analyzing the data of the patients hospitalized with bacterial meningitis, this study established that clinical characteristics and laboratory results differ based on etiological factors of the disease. The study indicates that
Figures
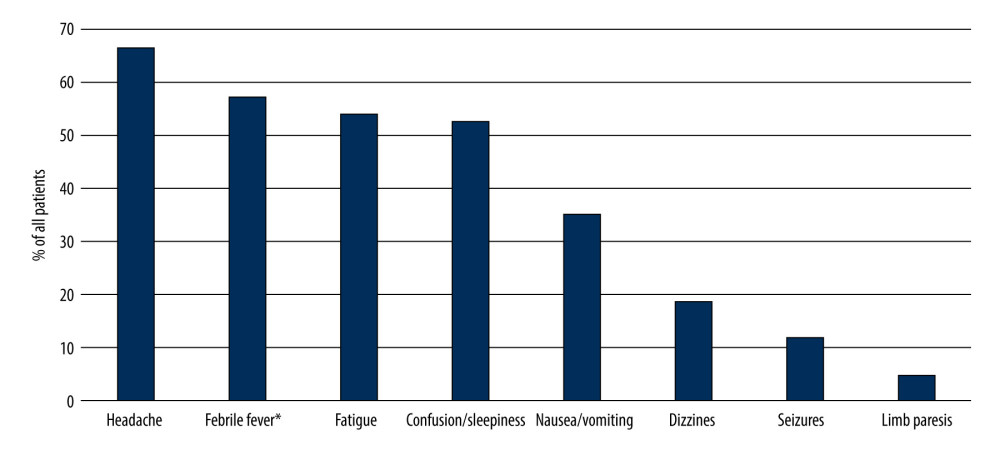 Figure 1. Prevalence of patient concerns before admission to hospital. * >38.5°C. (Microsoft Excel, version 16.74, Microsoft).
Figure 1. Prevalence of patient concerns before admission to hospital. * >38.5°C. (Microsoft Excel, version 16.74, Microsoft). 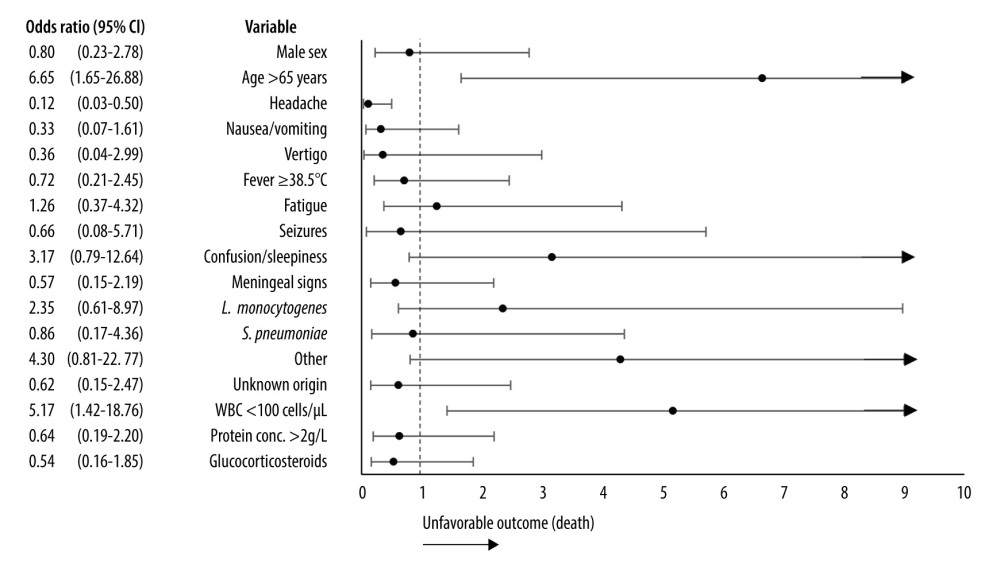 Figure 2. Association between demographic, etiological, clinical factors, laboratory results, treatment, and unfavorable outcome (death). CI – confidence interval, WBC – white blood cells. Certain nominal variables, such as N. meningitidis as an etiological agent, the need for intensive care, and limb paresis, were excluded from the graph as the odds ratio for unfavorable outcome could not be computed. Arrows indicate high values of the upper 95% confidence interval that could not be visualized in the graph. (Microsoft Excel, version 16.74, Microsoft).
Figure 2. Association between demographic, etiological, clinical factors, laboratory results, treatment, and unfavorable outcome (death). CI – confidence interval, WBC – white blood cells. Certain nominal variables, such as N. meningitidis as an etiological agent, the need for intensive care, and limb paresis, were excluded from the graph as the odds ratio for unfavorable outcome could not be computed. Arrows indicate high values of the upper 95% confidence interval that could not be visualized in the graph. (Microsoft Excel, version 16.74, Microsoft). Tables
Table 1. Distribution of etiological factor of bacterial meningitis (n=86).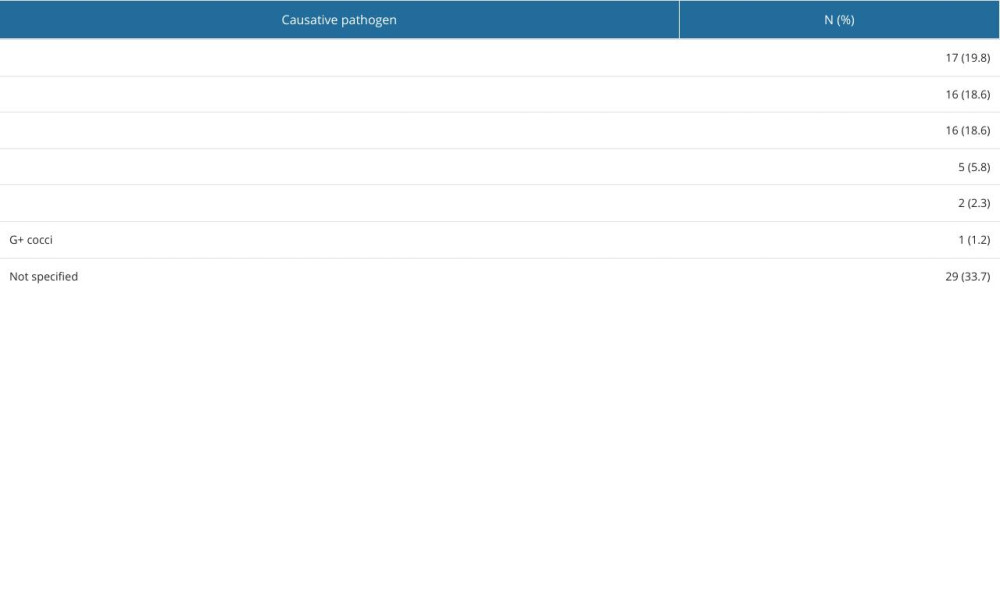 Table 2. Demographic and clinical characteristics of cases based on etiological factor.
Table 2. Demographic and clinical characteristics of cases based on etiological factor.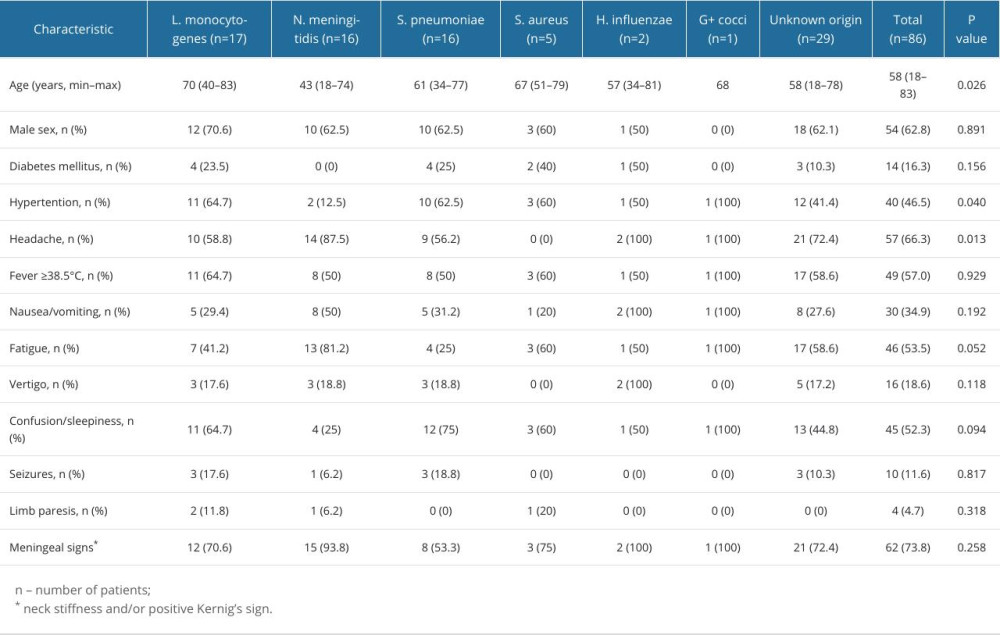 Table 3. Laboratory findings of cases based on etiological factor.
Table 3. Laboratory findings of cases based on etiological factor.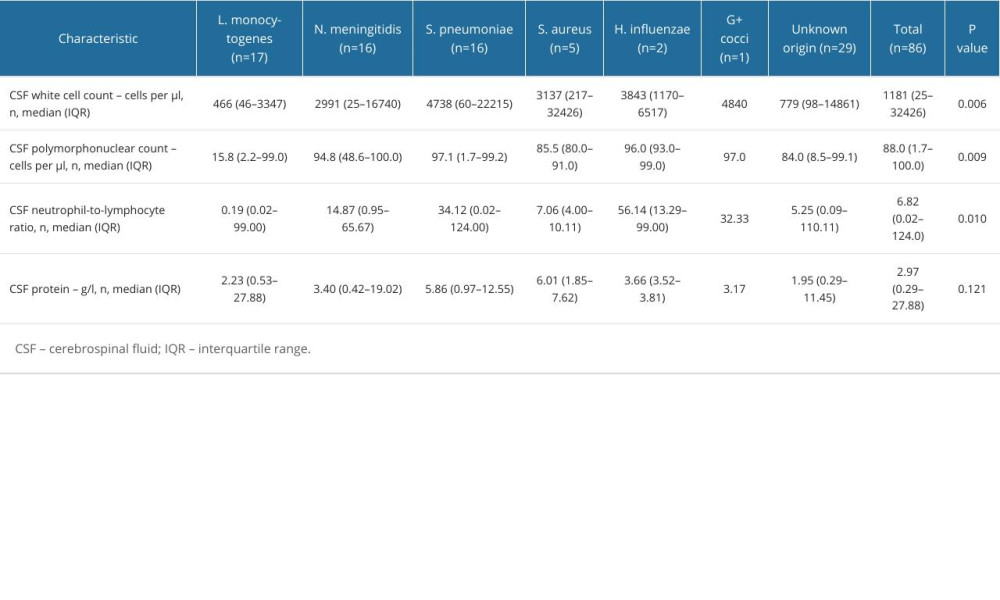 Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient.
Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient.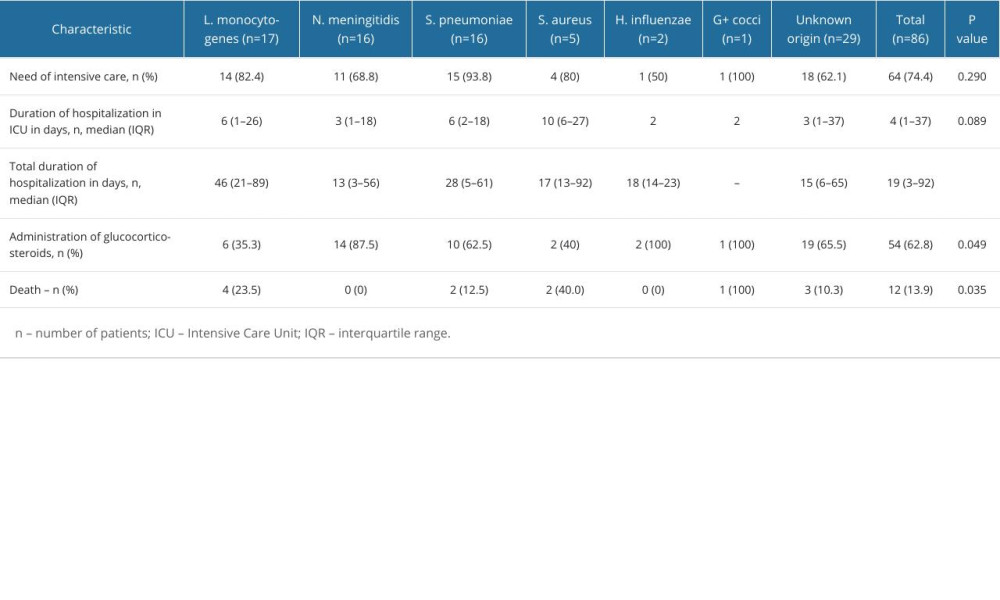 Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis.
Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis.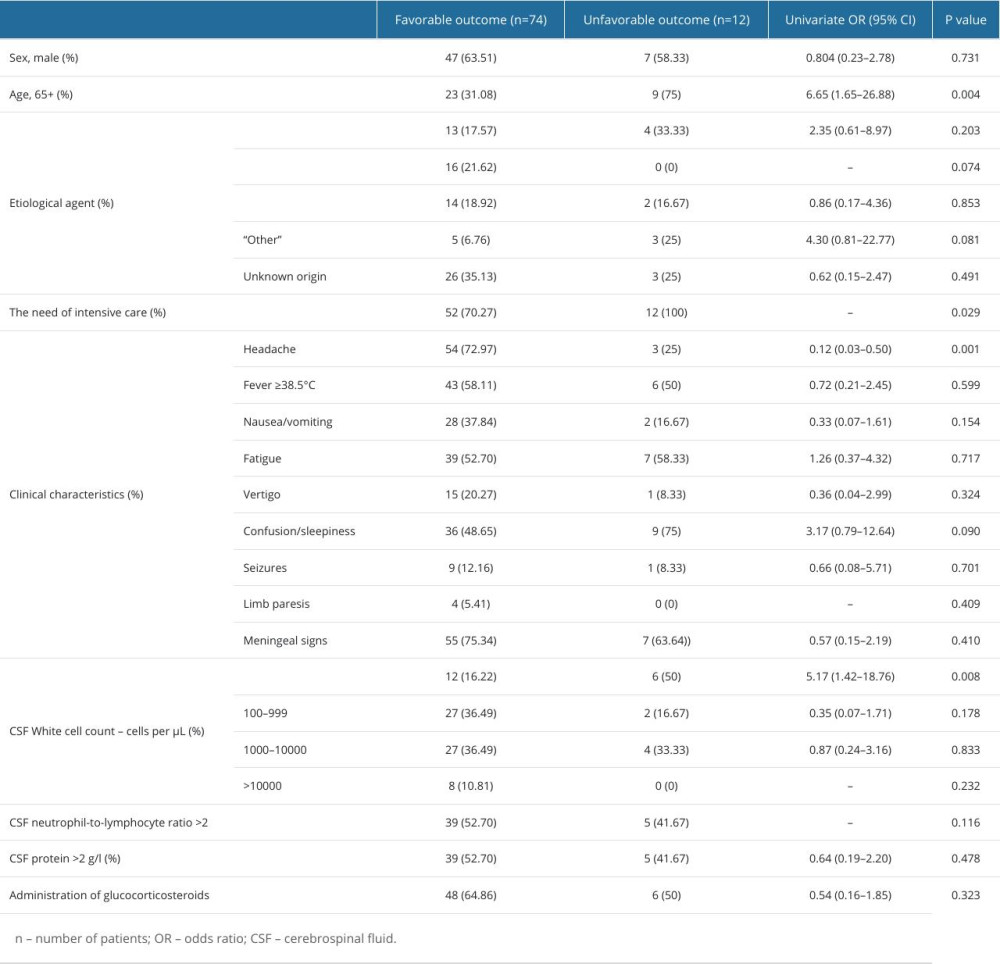 Table 6. Multivariate analysis of factors associated with an unfavorable outcome.
Table 6. Multivariate analysis of factors associated with an unfavorable outcome.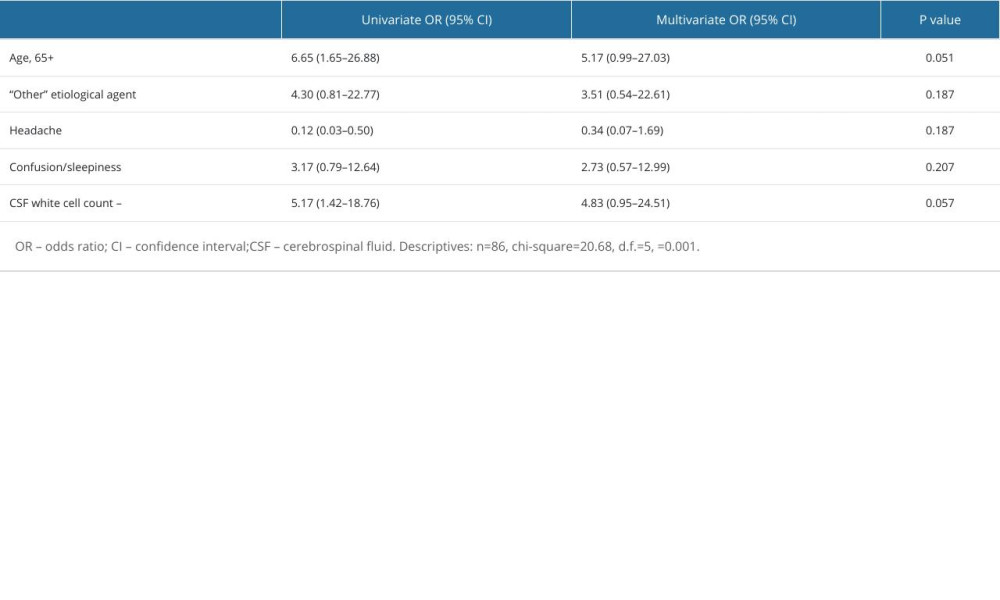
References
1. McGill F, Heyderman RS, Panagiotou S, Acute bacterial meningitis in adults: Lancet, 2016; 388(10063); 3036-47
2. World Health Organisation: Meningitis, 2023 https://www.who.int/news-room/fact-sheets/detail/meningitis
3. Thigpen MC, Whitney CG, Messonnier NE, Bacterial meningitis in the United States, 1998–2007: N Engl J Med, 2011; 364(21); 2016-25
4. Brouwer MC, Tunkel AR, van de Beek D, Epidemiology, Diagnosis, and antimicrobial treatment of acute bacterial meningitis: Clin Microbiol Rev, 2010; 23(3); 467-92
5. van de Beek D, Brouwer MC, Koedel U, Wall EC, Community-acquired bacterial meningitis: Lancet, 2021; 398(10306); 1171-83
6. van de Beek D, de Gans J, Spanjaard L, Clinical features and prognostic factors in adults with bacterial meningitis: N Engl J Med, 2004; 351(18); 1849-59
7. Khatib U, van de Beek D, Lees JA, Brouwer MC, Adults with suspected central nervous system infection: A prospective study of diagnostic accuracy: J Infect, 2017; 74(1); 1-9
8. Costerus JM, Lemmens CMC, van de Beek D, Brouwer MC, Cranial imaging and lumbar puncture in patients with suspected central nervous system infection: Clin Infect Dis, 2020; 70(12); 2469-75
9. Lo SW, Gladstone RA, vanTonder AJ, Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study: Lancet Infect Dis, 2019; 19(7); 759-69
10. Castelblanco RL, Lee M, Hasbun R, Epidemiology of bacterial meningitis in the USA from 1997 to 2010: A population-based observational study: Lancet Infect Dis, 2014; 14(9); 813-19
11. Brouwer MC, McIntyre P, deGans J, Corticosteroids for acute bacterial meningitis: Cochrane Database Syst Rev, 2010(9); CD004405
12. Charlier C, Perrodeau É, Leclercq A, Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study: Lancet InfectDis, 2017; 17(5); 510-19
13. Katchanov J, Heuschmann PU, Endres M, Weber JR, Cerebral infarction in bacterial meningitis: Predictive factors and outcome: J Neurol, 2010; 257(5); 716-20
14. Singhi P, Bansal A, Geeta P, Singhi S, Predictors of long-term neurological outcome in bacterial meningitis: Indian J Pediatr, 2007; 74(4); 369-74
15. Matulyte E, Kiveryte S, Paulauskiene R, Retrospective analysis of the etiology, clinical characteristics and outcomes of community-acquired bacterial meningitis in the University Infectious Diseases Centre in Lithuania: BMC Infect Dis, 2020; 20(1); 733
16. Fernandes D, Gonçalves-Pereira J, Janeiro S, Acute bacterial meningitis in the intensive care unit and risk factors for adverse clinical outcomes: Retrospective study: J Crit Care, 2014; 29(3); 347-50
17. National Public Health Centre under the Ministry of Health: Communicable diseases in Lithuania in 2019, 2020 https://nvsc.lrv.lt/uploads/nvsc/documents/files/Apzvalga_2019_web2.pdf
18. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: A prospective cohort study: Lancet Infect Dis, 2016; 16(3); 339-47
19. Polkowska A, Toropainen M, Ollgren J, Bacterial meningitis in Finland, 1995–2014: A population-based observational study: BMJ Open, 2017; 7(5); e015080
20. Matulyte E, Davidaviciene E, Kancauskiene Z, The socio-demographic, clinical characteristics and outcomes of tuberculosis among HIV infected adults in Lithuania: A thirteen-year analysis: PLoS One, 2023; 18(3); e0282046
21. van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM, Community-acquired bacterial meningitis in adults: N Engl J Med, 2006; 354(1); 44-53
22. Flores-Cordero JM, Amaya-Villar R, Rincón-Ferrari MD, Acute community-acquired bacterial meningitis in adults admitted to the Intensive Care Unit: Clinical manifestations, management and prognostic factors: Intensive Care Med, 2003; 29(11); 1967-73
23. Sigurdardóttir B, Már Björnsso Ó, Jónsdóttir KE, Acute bacterial meningitis in adults: A 20-year overview: Arch Intern Med, 1997; 157(4); 425-30
24. Hussein AS, Shafran SD, Acute bacterial meningitis in adults. A 12-year review: Medicine (Baltimore), 2000; 79(6); 360-68
25. Brouwer MC, Beek D, van de Heckenberg SGB: Clin Infect Dis, 2006; 43(10); 1233-38
26. Koopmans MM, Brouwer MC, Bijlsma MW: Clin Infect Dis, 2013; 57(2); 247-53
27. Auburtin M, Porcher R, Bruneel F, Pneumococcal meningitis in the Intensive Care Unit: Am J Respir Crit Care Med, 2002; 165(5); 713-17
28. Yau B, Hunt NH, Mitchell AJ, Too LK: Int J Mol Sci, 2018; 19(11); 3555
29. Køster-Rasmussen R, Korshin A, Meyer CN, Antibiotic treatment delay and outcome in acute bacterial meningitis: J Infect, 2008; 57(6); 449-54
30. Dzupova O, Rozsypal H, Prochazka B, Benes J, Acute bacterial meningitis in adults: Predictors of outcome: Scand J Infect Dis, 2009; 41(5); 348-54
31. Lepur D, Baršić B, Community-acquired bacterial meningitis in adults: Antibiotic timing in disease course and outcome: Infection, 2007; 35(4); 225-31
Figures
 Figure 1. Prevalence of patient concerns before admission to hospital. * >38.5°C. (Microsoft Excel, version 16.74, Microsoft).
Figure 1. Prevalence of patient concerns before admission to hospital. * >38.5°C. (Microsoft Excel, version 16.74, Microsoft). Figure 2. Association between demographic, etiological, clinical factors, laboratory results, treatment, and unfavorable outcome (death). CI – confidence interval, WBC – white blood cells. Certain nominal variables, such as N. meningitidis as an etiological agent, the need for intensive care, and limb paresis, were excluded from the graph as the odds ratio for unfavorable outcome could not be computed. Arrows indicate high values of the upper 95% confidence interval that could not be visualized in the graph. (Microsoft Excel, version 16.74, Microsoft).
Figure 2. Association between demographic, etiological, clinical factors, laboratory results, treatment, and unfavorable outcome (death). CI – confidence interval, WBC – white blood cells. Certain nominal variables, such as N. meningitidis as an etiological agent, the need for intensive care, and limb paresis, were excluded from the graph as the odds ratio for unfavorable outcome could not be computed. Arrows indicate high values of the upper 95% confidence interval that could not be visualized in the graph. (Microsoft Excel, version 16.74, Microsoft). Tables
 Table 1. Distribution of etiological factor of bacterial meningitis (n=86).
Table 1. Distribution of etiological factor of bacterial meningitis (n=86). Table 2. Demographic and clinical characteristics of cases based on etiological factor.
Table 2. Demographic and clinical characteristics of cases based on etiological factor. Table 3. Laboratory findings of cases based on etiological factor.
Table 3. Laboratory findings of cases based on etiological factor. Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient.
Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient. Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis.
Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis. Table 6. Multivariate analysis of factors associated with an unfavorable outcome.
Table 6. Multivariate analysis of factors associated with an unfavorable outcome. Table 1. Distribution of etiological factor of bacterial meningitis (n=86).
Table 1. Distribution of etiological factor of bacterial meningitis (n=86). Table 2. Demographic and clinical characteristics of cases based on etiological factor.
Table 2. Demographic and clinical characteristics of cases based on etiological factor. Table 3. Laboratory findings of cases based on etiological factor.
Table 3. Laboratory findings of cases based on etiological factor. Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient.
Table 4. Hospitalization characteristics and outcomes of bacterial meningitis patient. Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis.
Table 5. Relationship between demographic, etiological, clinical factors, laboratory results and unfavorable outcome (death) in patients with bacterial meningitis. Table 6. Multivariate analysis of factors associated with an unfavorable outcome.
Table 6. Multivariate analysis of factors associated with an unfavorable outcome. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








