10 February 2024: Database Analysis
Association Between Albumin-Corrected Anion Gap and In-Hospital Mortality and Sepsis-Associated Acute Kidney Injury
Haixu Guo12BCDEF, Jie Wang13A*DOI: 10.12659/MSM.943012
Med Sci Monit 2024; 30:e943012
Abstract
BACKGROUND: This study aimed to investigate the association between albumin-corrected anion gap (ACAG) and in-hospital mortality in sepsis-associated acute kidney injury (S-AKI).
MATERIAL AND METHODS: We conducted this retrospective study based on data from the Medical Information Mart for Intensive Care IV database, and assessed the prognostic capabilities of ACAG in comparison with albumin (ALB) and anion gap (AG) to predict in-hospital mortality of patients with S-AKI. Binomial logistic regression analysis was performed to identify whether ACAG was an independent risk factor for in-hospital mortality for the patients, and receiver operating characteristic (ROC) curves were plotted to clarify its efficacy in predicting in-hospital mortality. We also performed a decision curve analysis (DCA) to determine whether there were net clinical benefits for patients when ACAG was used to predict in-hospital mortality.
RESULTS: Binary logistic regression analysis showed that ACAG was an independent risk factor for in-hospital mortality in patients with S-AKI, with an area under the ROC (AUC) curve of 0.675 (moderate predictive value) for the prediction of in-hospital mortality, higher than that of ALB or AG alone, with the highest Youden’s index (0.2675). The DCA substantiated the superiority of ACAG in net clinical benefits at various threshold probability, enhancing its clinical applicability.
CONCLUSIONS: The research emphasizes the potential of ACAG as a valuable predictive tool for in-hospital mortality in S-AKI patients, which is better than albumin and AG, encouraging its consideration in clinical practice.
Keywords: Acute Kidney Injury, Mortality, Acid-Base Equilibrium
Background
Sepsis, caused by a maladaptive host response to infection, precipitates acute organ dysfunction, prominently impacting the kidneys [1], with acute kidney injury (AKI) manifesting in 40–50% of septic patient, which has a very high mortality rate of up to 60% [2]. Sepsis-associated acute kidney injury (S-AKI) is typically defined as AKI concomitant with sepsis, devoid of other significant causative factors, or as the co-existence of both Kidney Disease: Improving Global Outcomes (KDIGO) criteria and Sepsis-3 criteria [3]. Due to its intricate pathophysiology, the therapeutic arsenal for S-AKI remains limited and lacks consensus [4]. Current interventions – fluid resuscitation, vasopressor medications, and renal replacement therapy (RRT) – are commonly performed but are not very effective in reducing patient mortality [3]. Its global incidence and fatality rates continue to rise, making it a critical public health issue and potentially increasing the risk of mortality in the Intensive Care Unit (ICU) setting. Despite its significance, the effective prediction of mortality rates in S-AKI patients presents substantial challenges. The complexity lies in identifying precise indicators that can accurately predict mortality [5–7].
The anion gap (AG) serves as a crucial measurement in assessing acid-base balance, maintaining its function within a standard range under normal circumstances, and thus providing valuable insight into the acid-base status [8]. In instances of severe sepsis, however, the scenario becomes complicated due to the onset of hyperlactatemia, which triggers a high anion gap metabolic acidosis [9]. Elevated anion gap has been observed in patients with acute kidney injury [10]. Notably, when AG reaches or exceeds 14 mmol/L, there is a heightened risk of short-term mortality during ICU stay for patients with S-AKI [11]. Furthermore, serum albumin (ALB), endowed with a critical role as a negatively charged protein, contributes significantly to the plasma’s buffering capacity by binding to cations such as sodium. This binding propensity, in turn, impacts the calculated anion gap. In patients with AKI, diminished serum albumin levels are frequently observed [12]. It is noteworthy, however, that hypoalbuminemia can lead to a reduction in the anion gap, adding another layer of complexity to the assessment [9]. Hence, to facilitate a more precise evaluation of acid-base balance, particularly when confronted with conditions of hypoalbuminemia, the concept of serum ACAG has been introduced [13].
Despite these advancements, the mechanism underlying ACAG’s influence on S-AKI patients remains largely uncharted. In the present study, we hypothesized that there is an association between ACAG and ICU mortality rates within this patient population. To test this hypothesis, we used the MIMIC database as our investigative tool, seeking to increase understanding of sepsis-associated AKI and to provide insights potentially helpful in clinical practice. Confirmation of this hypothesis would further underscore the importance of routinely considering ACAG in clinical assessments of S-AKI patients.
Material and Methods
DATABASE:
The MIMIC-IV (Medical Information Mart for Intensive Care IV, version 2.2) database, is a contemporary, freely available repository of critical care information. It offers data that previously received institutional review board consent. Capturing records from the period 2008–2019, this database aggregates a wealth of clinical data from patients treated at the Beth Israel Deaconess Medical Center [14–16]. After successfully navigating our online training modules and passing the Protecting Human Research Participants assessment, we were granted permission to extract data from the MIMIC-IV databases. Our research gained ethical clearance from a department linked to the Massachusetts Institute of Technology (Approval Code 56616826). All the information about patients in the database is rendered anonymous, thus eliminating the requirement for explicit informed consent from individual patients. We structured and presented our research in alignment with the guidelines from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement, ensuring that every step met the ethical benchmarks established in the Declaration of Helsinki.
STUDY POPULATION:
The inclusion criteria were as follows: (1) adults aged 18 years and older; (2) with acute kidney injury (AKI) secondary to sepsis. The diagnostic criteria for sepsis followed Sepsis-3 – for patients with infections or suspected infections in the ICU, the diagnosis of sepsis was made when the SOFA score was ≥2 [17]. Exclusion criteria encompassed the following scenarios: (1) instances where the duration of AKI was less than 48 h; (2) when the patient’s survival period was less than 7 days; (3) patients with stage 5 chronic kidney disease (CKD) or end-stage kidney disease, defined as an estimated glomerular filtration rate of less than 15 ml/min/1.73 m2; and (4) cases with missing key data, such as demographic details or variables needed to calculate traditional severity scores. These criteria were meticulously designed to ensure a robust and relevant study.
DATA EXTRACTION:
Information was sourced from the MIMIC-IV database (v2.2). We selected critical data points which comprised demographic details like age, sex, ethnicity, weight, height, and length of hospital/ICU stay, along with coexisting comorbidities (hypertension, diabetes, chronic pulmonary disease, and myocardial infarction), laboratory tests (hemoglobin, platelets, white blood cell, pH, glucose, calcium, blood urea nitrogen, creatinine, sodium, potassium, chloride, bicarbonate, albumin, and anion gap), vital signs (mean blood pressure, respiratory rate, and heart rate), urine output, Sequential Organ Failure Assessment (SOFA) score, and therapeutic interventions like mechanical ventilation and renal replacement therapy. For metrics that were recorded several times within the first day of admission, we determined an average to maintain data uniformity. The AG was calculated using the formula:
AG (mmol/l)=(sodium+potassium)-(chloride+bicarbonate).
Subsequently, the ACAG was determined using the formula:
ACAG (mmol/l)=[4.4-{albumin(g/dl)}]×2.5+AG [13].
STATISTICAL ANALYSIS:
To assess the distribution of continuous variables, we employed the Kolmogorov-Smirnov test. Variables conforming to a normal distribution are presented as mean±SD and were compared using the independent sample
We investigated the association between ACAG and in-hospital mortality rates in ICU-admitted patients afflicted with S-AKI. Binomial logistic regression analysis was performed to identify whether ACAG was an independent risk factor for in-hospital mortality for the patients (variables with P values less than 0.1 in the univariable regression analysis were included in the multivariable regression analysis), and receiver operating characteristic (ROC) curves were plotted to clarify its efficacy in predicting in-hospital mortality. The area under the ROC (AUC) curves were compared using the Z-test. We also performed a decision curve analysis (DCA) to clarify whether there were net clinical benefits for patients when ACAG was used to predict in-hospital mortality [18].
Our statistical evaluations were carried out utilizing a combination of IBM SPSS Statistics (v27.0), MedCalc Statistical Software (v19.6.1), and R software (v4.0.3, CRAN). We adopted the approach by Delong et al for the Z-test, facilitated through the MedCalc platform [19]. The DCA was conducted using the R environment, leveraging the “rmda” package. Throughout our analyses, we deemed a P value below 0.05 as indicative of statistical significance.
Results
BASELINE CHARACTERISTICS:
The foundation of our research is built on the MIMIC-IV dataset, which includes an extensive set of 73 181 ICU admissions. From this considerable data pool, we meticulously selected a group of 7750 subjects, separated into 1909 individuals who unfortunately died in the ICU and 5591 who made a successful recovery. Through rigorous analysis, we noted a considerable decrease in both hospital and ICU duration in the death group compared to the survival group (P<0.001). An array of health indicators showed significantly higher values in the death group, including age, SOFA score, levels of blood urea nitrogen, white blood cell count, urea, creatinine, potassium, and AG. Additionally, we noticed a higher frequency of interventions (mechanical ventilation and renal replacement therapy) in the death group. In contrast, the survival group we identified higher levels of hemoglobin, platelets, chloride, bicarbonate, and ALB, as well as increased urine output and a higher pH value, each demonstrating statistical significance (P<0.001). Despite these differences, the 2 groups showed consistency in sex distribution, ethnicity, sodium levels, and the occurrence of chronic pulmonary disease. Table 1 shows the fundamental attributes of the research cohort.
ACAG IS ASSOCIATED WITH S-AKI IN-HOSPITAL MORTALITY:
We discerned marked multicollinearity between AG and ACAG, prompting us to omit AG from subsequent binomial logistic regression evaluations. As ACAG was the subject of the study, the variables we included in the regression analyses were predominantly electrolytes, and due to the multivariable regression variable limitations (which would result in an unstable model if there were too many variables), we included as many variables as possible that were related to the death event. The outcomes of this analysis are detailed in Table 2. ACAG was identified as an independent risk factor for in-hospital mortality in the patients after adjusting for serial confounders (OR=1.129, 95% CI 1.109–1.150, P<0.001).
Through ROC curve evaluations, we gauged the prognostic efficacy of ACAG, AG, and ALB in predicting in-hospital mortality for ICU-admitted S-AKI patients (Table 3). The AUC metrics for ALB, AG, and ACAG were 0.648, 0.598, and 0.675, respectively, as illustrated in Figure 1. In comparing these AUCs, distinct disparities emerged between ALB versus AG (Z=4.449, P=0.0001), ALB versus ACAG (Z=8.702, P<0.0001), and AG versus ACAG (Z=8.171, P<0.0001). Of these metrics, ACAG exhibited the highest sensitivity (56.00%) and the highest Youden’s index (0.2675). In fact, it is difficult to reconcile sensitivity and specificity, as setting high specificity usually leads to a decrease in sensitivity. Therefore, we need a composite indicator (Youden ‘s index) to judge the predictive efficacy of an indicator in a comprehensive way. In contrast, ALB displayed the highest specificity, registering at 72.24%.
COMPARISON OF DECISION CURVES:
In the DCA, ACAG, denoted by the solid red line, consistently surpassed AG, represented by the solid blue line. Moreover, ACAG consistently outperformed ALB, indicated by the solid green line. The descending order of net benefits was observed as ACAG, AG, and ALB, suggesting ACAG is the optimal predictor among the 3 prognostic indicators (Figure 2).
Discussion
Our study draws attention to the importance of the ACAG as a predictor of mortality among patients with S-AKI. Importantly, when ACAG levels surpass the threshold of 20.25 mmol/L, they serve as a potent predictor for in-hospital mortality among intensive care patients with S-AKI. This metric offers healthcare providers a concrete, actionable criterion for patient surveillance, potentially guiding therapeutic interventions and adjustments in treatment strategy. While ALB demonstrated a slightly higher specificity, ACAG consistently showed a higher Youden’s index and sensitivity, thus underscoring the potential clinical utility of ACAG. Relying solely on a single indicator to predict mortality in S-AKI is inappropriate because the predictive value of a single indicator is inevitably not high enough. We demonstrated in this study that ACAG has a better predictive value than AG in the prognostic prediction of S-AKI, which implies that ACAG may be an excellent alternative variable in constructing a scoring system. Meanwhile, the advantage of ACAG over AG may not be an isolated case, but may also be a pattern in other diseases (some studies have already confirmed this, including sepsis [20], acute pancreatitis [21], and acute myocardial infarction [22]), and our study provides more evidence for subsequent studies. Furthermore, this advantage of ACAG over AG has some value in the clinic, and perhaps, in the future, ACAG could be a direct substitute for AG in certain diseases.
The DCA in our study offers a nuanced approach to evaluating the ACAG as a prognostic factor for S-AKI. By quantifying the net benefit, a delicate balance between true positives and false positives, DCA provides a robust method to assess the overall advantages and risks of ACAG’s clinical application [18]. The versatility of DCA lies in its threshold flexibility, allowing comparative assessments across a spectrum of values, and imparting relevant insights across varied clinical contexts and risk profiles. This results in an intelligible interpretation of the predictive capability of ACAG, facilitating a parallel evaluation against other predictive parameters. Further, DCA’s ability to offer quantifiable net benefits at divergent thresholds underpins a tailored decision-making process. It empowers clinicians and patients to arrive at optimal choices aligned with individual circumstances and preferences. Complementing the receiver operating characteristic (ROC) curves, DCA adds valuable dimensions concerning decision thresholds, thereby elevating its practical relevance in clinical practice. The integrated use of DCA and ROC curves in our analysis culminates in a comprehensive appraisal of ACAG’s predictive efficacy for S-AKI mortality. In summary, the incorporation of DCA in our research not only substantiates the significance of ACAG in predicting S-AKI mortality but also highlights the multidimensional strengths of DCA as an evaluative instrument. The synergistic application of DCA with ACAG furnishes refined insights into S-AKI management, contributing to the broader conversation surrounding personalized healthcare strategies.
The relationship between the AG and S-AKI is well established, as AG serves as a crucial clinical indicator for assessing the acid-base balance within the body [23]. In patients with sepsis, underlying mechanisms such as insufficient tissue perfusion, inflammation, and immunosuppression can alter the metabolic profile, causing AG elevation [24]. This rise in AG has been observed to correlate with higher in-hospital mortality rates [25]. Hypoalbuminemia is a common complication in sepsis and has been correlated with mortality rates in ICU patients with sepsis [26,27], and ALB infusion can improve the short-term mortality rates in patients with AKI and septic shock [28]. Specific research demonstrated that with a decrease of 10 mg/L in serum ALB, there is a corresponding drop of 2.5 mmol/L in AG. This relationship is attributable to the charged nature of ALB, leading to circumstances where AG might present as falsely “normal” [29]. Such an understanding emphasizes the need for careful consideration of ALB levels when evaluating AG as a clinical metric, especially in the context of sepsis-related complications. Enter the ACAG, which enhances AG by incorporating the ALB level. ACAG presents a more nuanced perspective on AG, refining our understanding of a patient’s acid-base status. By reflecting the complex interplay between nutritional condition, immune function, inflammation, and tissue injury, ACAG stands out as a more comprehensive tool for evaluating patients with S-AKI. ACAG’s potential superiority over AG and ALB levels as a prognostic factor stems from its multidimensional approach. It not only corrects the AG’s potential discrepancies due to hypoalbuminemia but also provides insights into the overall health status of the patient, thereby yielding a more accurate prediction of mortality risk. While AG remains an invaluable metric for assessing acid-base balance, its prognostic efficacy may be limited by variations in ALB levels. ACAG, by integrating ALB, offers a more holistic patient assessment, underscoring its potential to predict patient outcomes more precisely in the context of S-AKI. This insight into ACAG’s potential advantage calls for more extensive research to validate its application in clinical settings and emphasizes its potential role as a more effective prognostic tool in managing S-AKI [30,31].
Our study introduces a nuanced perspective to the existing body of knowledge by carefully evaluating the potential of ACAG in predicting outcomes for patients with S-AKI. While recognizing the complexity of the condition, we found that the relatively simple and cost-effective measurement of ACAG may have merit, especially in resource-constrained settings. We also noted the ongoing relevance of both AG and ALB in the context of S-AKI, reaffirming their importance in prognostic models. Our findings suggest that ACAG’s predictive efficacy needs further exploration, rather than regarding it as an absolute superior metric. The strength of ACAG, as observed in our study, could potentially contribute to earlier identification of patients at increased risk of mortality, facilitating timely and resource-efficient interventions. However, the integration of ACAG into routine practice for S-AKI must be approached with caution and further substantiated through ongoing research and clinical trials. In this way, our research offers a step toward understanding, rather than a definitive conclusion, highlighting a path that could eventually lead to improved patient care.
While our study provides valuable insights, it is important to recognize its inherent limitations. First, the retrospective nature of the model could introduce potential biases. Second, as the data were sourced from an American population, questions arise regarding the applicability of our findings to other ethnic or regional groups. Moreover, we were not able to identify a causal relationship between ACAG and in-hospital mortality – did elevated ACAG lead to increased mortality, or did the deterioration of patients predisposed to death for a variety of reasons ultimately lead to elevated ACAG? Lastly, the absence of dynamic tracking of ACAG might result in an under- or overestimation of its prognostic significance. However, the robustness of our large sample size lends credence to our conclusions and strengthens our study. Despite these limitations, the scope of our study underscores the reliability of our findings and encourages further exploration in this field. Our research opens the door to understanding the central role ACAG may play in the management of S-AKI, providing a foundation for future work. Nevertheless, we strongly advocate for additional prospective studies and research across diverse populations to firmly establish ACAG’s role as a consistent prognostic tool in the context of S-AKI.
Conclusions
We observed that elevated ACAG levels were correlated with higher risk of mortality for S-AKI patients during their in-hospital period. Furthermore, ACAG appears to be a more valuable predictor in this context than ALB and AG, enhancing its potential clinical utility.
Figures
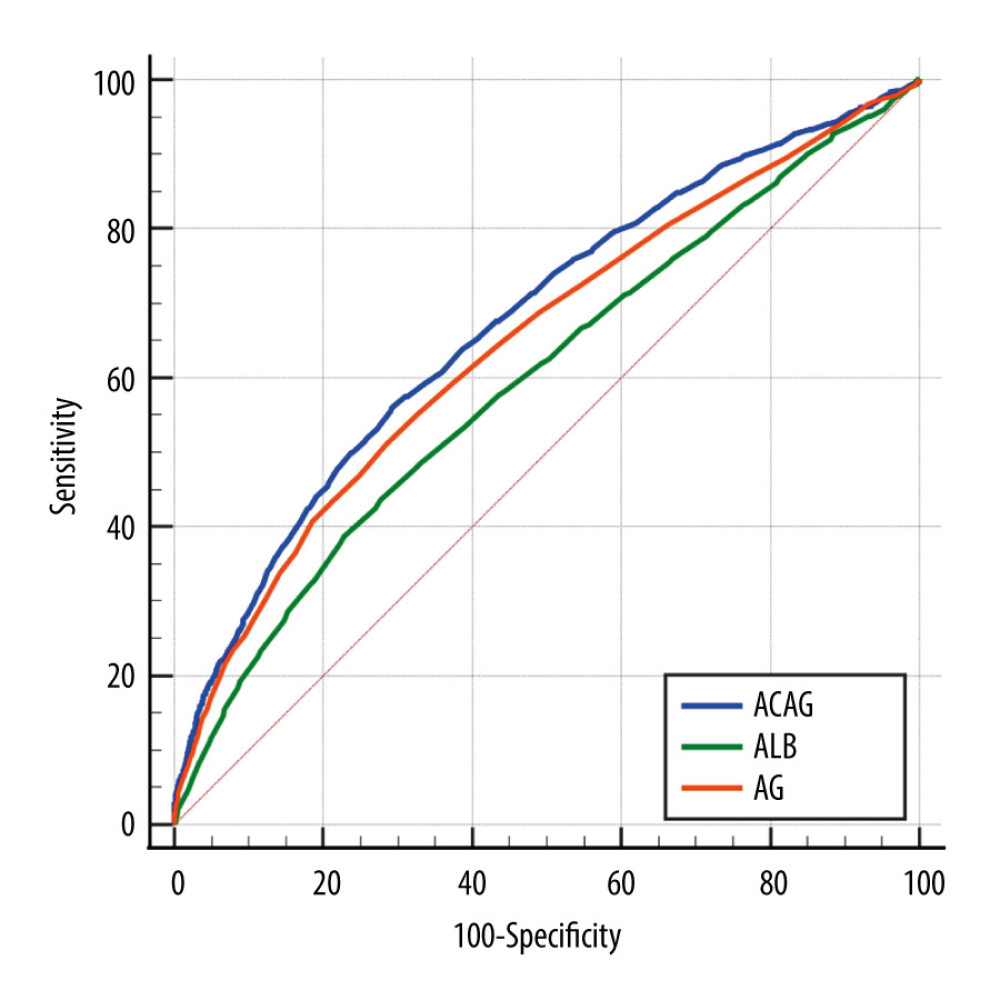 Figure 1. ROC curves of ACAG, AG, and ALB.The AUC for ALB, AG, and ACAG were 0.648, 0.598, and 0.675, respectively. ACAG – albumin-corrected anion gap.
Figure 1. ROC curves of ACAG, AG, and ALB.The AUC for ALB, AG, and ACAG were 0.648, 0.598, and 0.675, respectively. ACAG – albumin-corrected anion gap. 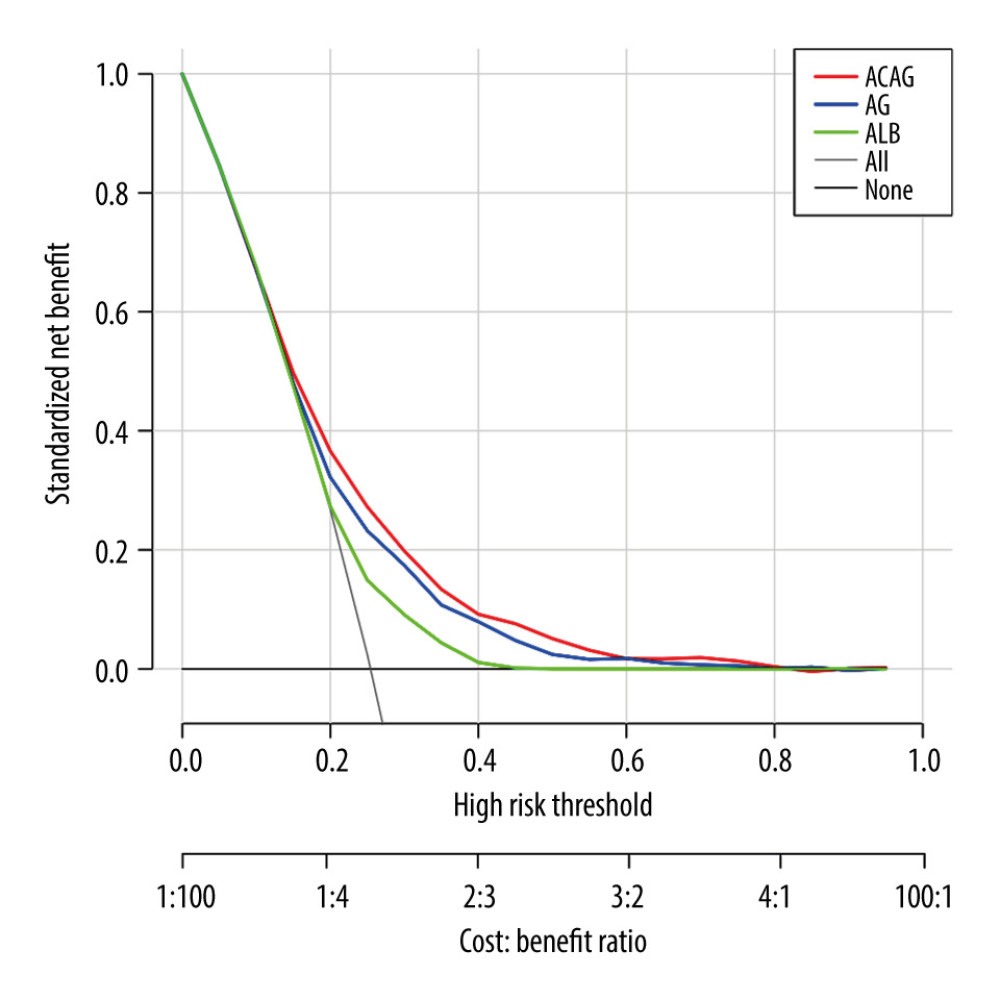 Figure 2. ROC curves of ACAG, AG, and ALB.Compared with AG and ALB, ACAG had the highest net clinical benefit at almost all threshold probabilities. ACAG – albumin-corrected anion gap.
Figure 2. ROC curves of ACAG, AG, and ALB.Compared with AG and ALB, ACAG had the highest net clinical benefit at almost all threshold probabilities. ACAG – albumin-corrected anion gap. References
1. Cecconi M, Evans L, Levy M, Rhodes A, Sepsis and septic shock: Lancet (London, England), 2018; 392(10141); 75-87
2. Zhao Y, Feng X, Li B, Dexmedetomidine protects against lipopolysaccharide-induced acute kidney injury by enhancing autophagy through inhibition of the PI3K/AKT/mTOR pathway: Front Pharmacol, 2020; 11; 128
3. Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA, Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment: Kidney Int, 2019; 96(5); 1083-99
4. Sung PH, Chiang HJ, Wallace CG, Exendin-4-assisted adipose derived mesenchymal stem cell therapy protects renal function against co-existing acute kidney ischemia-reperfusion injury and severe sepsis syndrome in rat: Am J Transl Res, 2017; 9(7); 3167-83
5. Lorente-Sorolla C, Garcia-Gomez A, Català-Moll F, Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis: Genome Med, 2019; 11(1); 66
6. Jia Y, Li Z, Feng Y, Methane-rich saline ameliorates sepsis-induced acute kidney injury through anti-inflammation, antioxidative, and antiapoptosis effects by regulating endoplasmic reticulum stress: Oxid Med Cell Longev, 2018; 2018; 4756846
7. Li P, Bledsoe G, Yang ZR, Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis: Immunology, 2014; 142(2); 216-26
8. Pratumvinit B, Lam L, Kongruttanachok N, Anion gap reference intervals show instrument dependence and weak correlation with albumin levels: Clin Chim Acta, 2020; 500; 172-79
9. Booth AL, Abels E, McCaffrey P, Development of a prognostic model for mortality in COVID-19 infection using machine learning: Mod Pathol, 2021; 34(3); 522-31
10. Alhamad T, Blandon J, Meza AT, Acute kidney injury with oxalate deposition in a patient with a high anion gap metabolic acidosis and a normal osmolal gap: J Nephropathol, 2013; 2(2); 139-43
11. Jiang L, Wang Z, Wang L, Predictive value of the serum anion gap for 28-day in-hospital all-cause mortality in sepsis patients with acute kidney injury: A retrospective analysis of the MIMIC-IV database: Ann Transl Med, 2022; 10(24); 1373
12. Pereira M, Rodrigues N, Godinho I, Acute kidney injury in patients with severe sepsis or septic shock: A comparison between the ‘Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease’ (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications: Clin Kidney J, 2017; 10(3); 332-40
13. Song R, Cao S, Prediabetes directly deteriorates into diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome triggered by acute pancreatitis: A case report illustrating a “chicken and egg” paradigm in ketosis-prone diabetes: Diabetes Ther, 2018; 9(3); 1377-83
14. Goldberger AL, Amaral LA, Glass L, PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals: Circulation, 2000; 101(23); E215-20
15. Lu X, Wang X, Gao Y, Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: A cohort study from MIMIC: Am J Emerg Med, 2021; 42; 121-26
16. Yang R, Huang T, Shen L, The use of antibiotics for ventilator-associated pneumonia in the MIMIC-IV fatabase: Front Pharmacol, 2022; 13; 869499
17. Singer M, Deutschman CS, Seymour CW, The third international consensus definitions for sepsis and septic shock (Sepsis-3): JAMA, 2016; 315(8); 801-10
18. Vickers AJ, Elkin EB, Decision curve analysis: A novel method for evaluating prediction models: Med Decis Making, 2006; 26(6); 565-74
19. DeLong ER, DeLong DM, Clarke-Pearson DL, Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach: Biometrics, 1988; 44(3); 837-45
20. Hu T, Zhang Z, Jiang Y, Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: A retrospective propensity score matching analysis: Clinica Chim Aacta, 2021; 521; 272-77
21. Li P, Shi L, Yan X, Albumin corrected anion gap and the risk of in-hospital mortality in patients with acute pancreatitis: A retrospective cohort study: J Inflamm Res, 2023; 16; 2415-22
22. Jian L, Zhang Z, Zhou Q, Association between albumin corrected anion gap and 30-day all-cause mortality of critically ill patients with acute myocardial infarction: a retrospective analysis based on the MIMIC-IV database: BMC Cardiovasc Disord, 2023; 23(1); 211
23. Xu S, Gao Y, Zhang Q, SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model: Oxid Med Cell Longev, 2016; 2016; 7296092
24. Abramowitz MK, Hostetter TH, Melamed ML, The serum anion gap is altered in early kidney disease and associates with mortality: Kidney Int, 2012; 82(6); 701-9
25. Fenves AZ, Emmett M, Approach to patients with high anion gap metabolic acidosis: Core Curriculum 2021: Am J Kidney Dis, 2021; 78(4); 590-600
26. Gatta A, Verardo A, Bolognesi M, Hypoalbuminemia: Intern Emerg Med, 2012; 7(Suppl 3); S193-99
27. Hu J, Lv C, Hu X, Liu J, Effect of hypoproteinemia on the mortality of sepsis patients in the ICU: A retrospective cohort study: Sci Rep, 2021; 11(1); 24379
28. Nanji AA, Campbell DJ, Pudek MR, Decreased anion gap associated with hypoalbuminemia and polyclonal gammopathy: JAMA, 1981; 246(8); 859-60
29. Lee SH, Park S, Lee JW, The anion gap is a predictive clinical marker for death in patients with acute pesticide intoxication: J Korean Med Sci, 2016; 31(7); 1150-59
30. Zhao B, Li Y, Lang X, Increased serum albumin corrected anion gap levels are associated with increased incidence of new-onset HF and poor prognosis in patients with acute myocardial infarction: Clin Chim Acta, 2023; 544; 117354
31. Zhong L, Xie B, Ji XW, Yang XH, The association between albumin corrected anion gap and ICU mortality in acute kidney injury patients requiring continuous renal replacement therapy: Intern Emerg Med, 2022; 17(8); 2315-22
Figures
 Figure 1. ROC curves of ACAG, AG, and ALB.The AUC for ALB, AG, and ACAG were 0.648, 0.598, and 0.675, respectively. ACAG – albumin-corrected anion gap.
Figure 1. ROC curves of ACAG, AG, and ALB.The AUC for ALB, AG, and ACAG were 0.648, 0.598, and 0.675, respectively. ACAG – albumin-corrected anion gap. Figure 2. ROC curves of ACAG, AG, and ALB.Compared with AG and ALB, ACAG had the highest net clinical benefit at almost all threshold probabilities. ACAG – albumin-corrected anion gap.
Figure 2. ROC curves of ACAG, AG, and ALB.Compared with AG and ALB, ACAG had the highest net clinical benefit at almost all threshold probabilities. ACAG – albumin-corrected anion gap. Tables
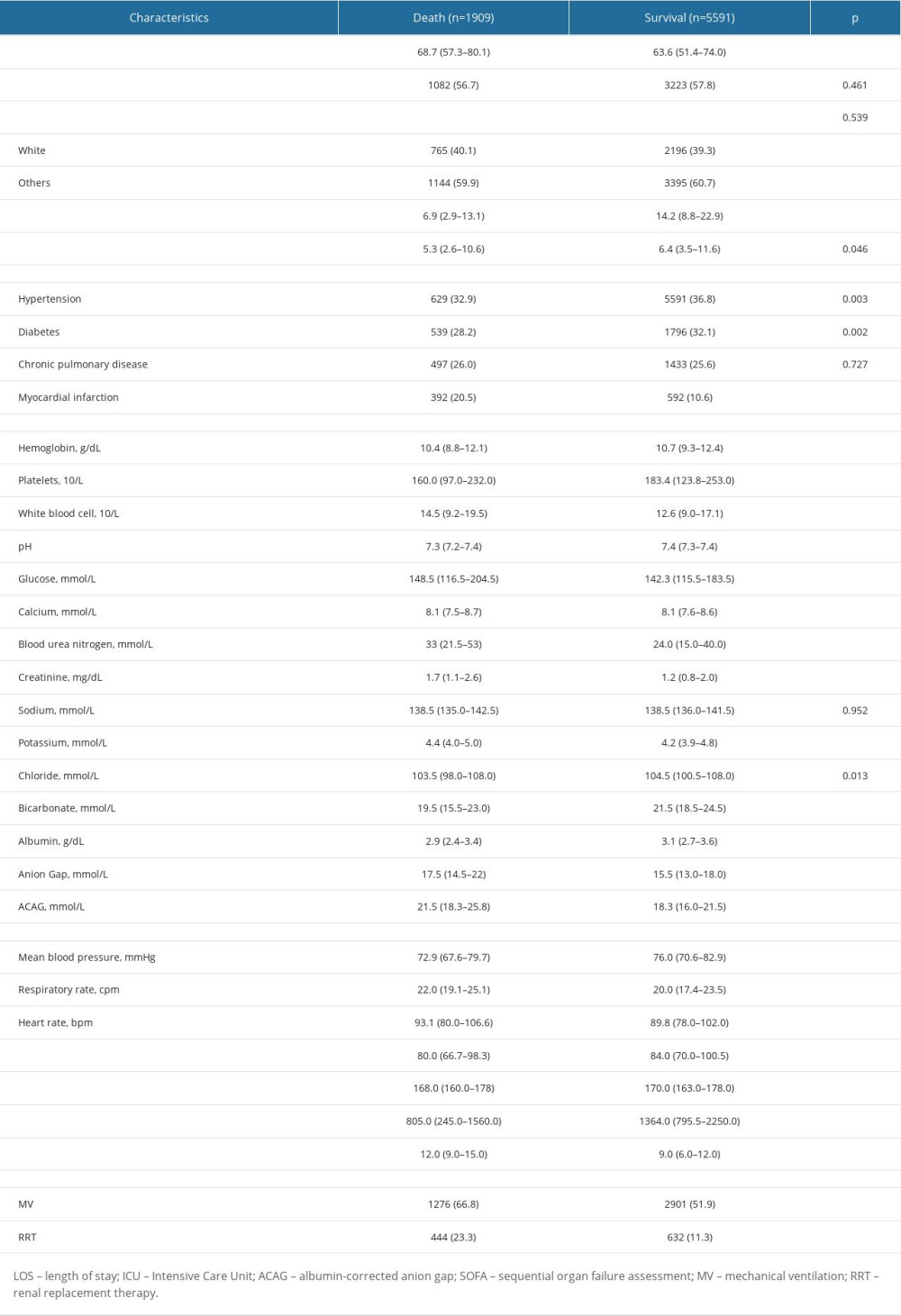 Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population.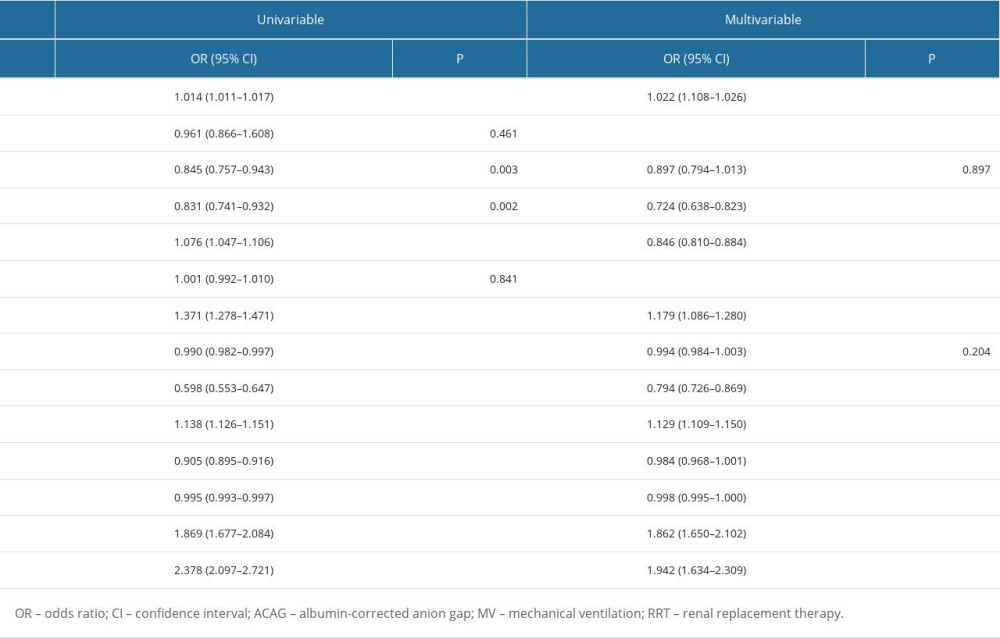 Table 2. Binomial Logistic regression analysis of ACAG for in-hospital mortality among intensive care patients with S-AKI.
Table 2. Binomial Logistic regression analysis of ACAG for in-hospital mortality among intensive care patients with S-AKI.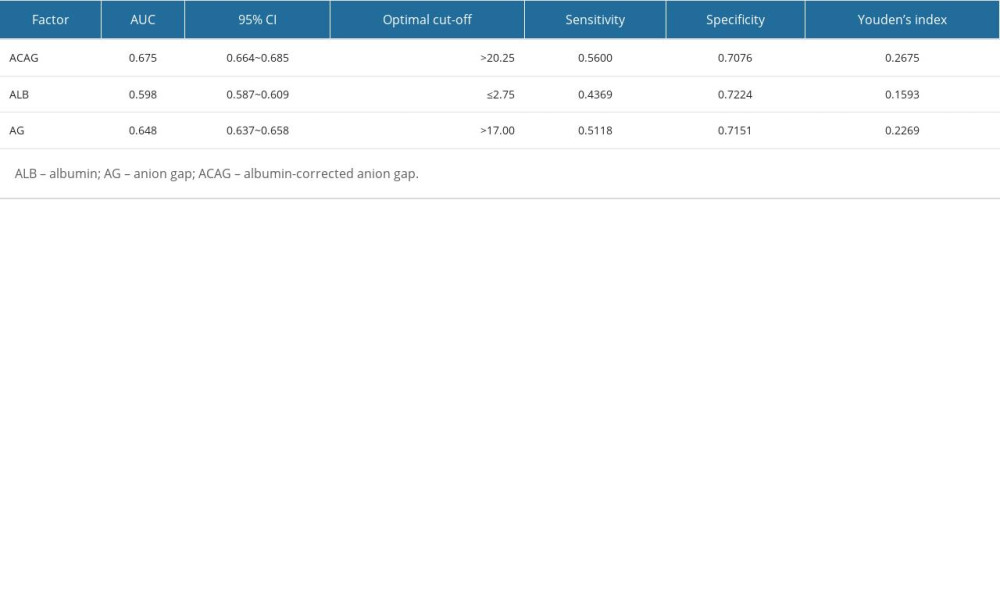 Table 3. Comparison of ROC curves.
Table 3. Comparison of ROC curves. Table 1. Baseline characteristics of the study population.
Table 1. Baseline characteristics of the study population. Table 2. Binomial Logistic regression analysis of ACAG for in-hospital mortality among intensive care patients with S-AKI.
Table 2. Binomial Logistic regression analysis of ACAG for in-hospital mortality among intensive care patients with S-AKI. Table 3. Comparison of ROC curves.
Table 3. Comparison of ROC curves. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








