31 May 2024: Clinical Research
Differential Inflammatory Responses in Adult and Pediatric COVID-19 Patients: Implications for Long-Term Consequences and Anti-Inflammatory Treatment
Kacper ToczyłowskiDOI: 10.12659/MSM.944052
Med Sci Monit 2024; 30:e944052
Abstract
BACKGROUND: COVID-19 manifests with varying degrees of severity across different age groups; adults typically experience more severe symptoms than children. Matrix metalloproteinases (MMPs), known for their role in tissue remodeling and immune responses, may contribute to the pathophysiological disparities observed between these groups. We sought to delineate differences in serum MMP profiles between adult and pediatric COVID-19 patients, assess the influence of anti-inflammatory treatment on MMP levels, and examine potential implications for long-term consequences.
MATERIAL AND METHODS: Serum samples from adult and pediatric COVID-19 patients, alongside controls, were analyzed for MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, MMP-13, EMMPRIN, TNF-α, TIMP-1, TIMP-2, TIMP-3, and TIMP-4. A subset of adult patients received treatment with glucocorticoids, tocilizumab, and convalescent plasma, and MMP levels were compared with those of untreated patients.
RESULTS: Elevated levels of MMP-1, MMP-7, TIMP-1, and TIMP-2 were observed in adult and pediatric patients. Adult patients displayed higher concentrations of MMP-3, MMP-8, MMP-9, TNF-α, and TIMP-4 than children. Post-treatment reduction in MMP-1, MMP-8, MMP-9 levels was observed, with median decreases from 21% to 70%. MMP-3 and MMP-7 remained largely unchanged, and MMP-2 concentrations increased after treatment. Notably, anti-inflammatory treatment correlated with reduced post-treatment MMP levels, suggesting potential therapeutic benefit.
CONCLUSIONS: Distinctive inflammatory responses in COVID-19 were evident between adults and children. While certain MMPs exhibited post-treatment reduction, the persistence of elevated levels raises concerns about potential long-term consequences, including lung fibrosis. Our findings emphasize the need for personalized treatment strategies and further investigation into the dynamics of MMP regulation in COVID-19.
Keywords: Anti-Inflammatory Agents, COVID-19, Tocilizumab, Matrix Metalloproteinases, Tissue Inhibitor of Metalloproteinases, COVID-19 Serotherapy
Introduction
Matrix metalloproteinases (MMPs) are a family of enzymes that play a pivotal role in tissue remodeling and homeostasis within the extracellular matrix of various tissues and organs. These enzymes are zinc-dependent endopeptidases, and their primary function is to regulate the turnover and degradation of extracellular matrix components, such as collagen, elastin, and proteoglycans. MMPs are crucial for various physiological processes, including embryogenesis, wound healing, tissue repair, and immune response modulation [1]. However, dysregulated MMP activity has been implicated in various pathological conditions, such as cancer metastasis, arthritis, and cardiovascular diseases, due to their ability to degrade extracellular matrix structures and facilitate tissue damage [2–4].
MMPs have emerged as significant players in the context of COVID-19 pathology. Studies have shown that patients with COVID-19, particularly those with severe disease, exhibit elevated levels of certain MMPs in their serum [5]. MMPs are thought to contribute to the pathophysiology of COVID-19 by promoting inflammation and tissue damage. Specifically, they can disrupt the integrity of the endothelial barrier, facilitating the leakage of immune cells and pro-inflammatory molecules into tissues. This endothelial dysfunction is associated with the development of acute respiratory distress syndrome, a severe complication of COVID-19 [6]. Additionally, MMPs can be involved in the remodeling of lung tissue, which could contribute to long-term pulmonary complications in survivors of severe COVID-19 [7]. A study comparing serum MMP-3 and MMP-9 levels in patients with COVID-19 at hospital admission and 1 week later suggests a role for MMP-3 in early lung inflammation stages [8]. Proteomic analysis of lung biopsies from patients with COVID-19 revealed a notable increase in MMP-1, MMP-2, MMP-7, MMP-8, and MMP-14 levels, compared with non-COVID-19 counterparts [9]. Moreover, higher concentrations of MMP-2 and MMP-8 in tracheal aspirate fluid, along with increased active MMP-2 levels, were observed in non-survivors of COVID-19. These findings suggest a strong association between MMPs, particularly the MMP-2/MMP-8 axis, and the severity of lung involvement in COVID-19. Imbalance in neutrophil-derived proteases and their inhibitors, tissue inhibitor of matrix metalloproteinase 2 (TIMP-2) and secretory leukocyte protease inhibitor, contributes to acute lung injury. Animal studies have shown that blocking TIMP-2 and secretory leukocyte protease inhibitor exacerbates lung injury, highlighting the potential therapeutic value of modulating MMP activity in acute lung injury [10].
While COVID-19 affects both adults and children, there are notable differences in the pathophysiology of the disease between these age groups. Generally, children tend to experience milder symptoms and have a lower risk of severe illness than do adults [11]. One key difference is the expression of ACE2 receptors, the cellular entry points for the SARS-CoV-2 virus. Children appear to have fewer ACE2 receptors in their respiratory tract, which may partially explain their reduced susceptibility to severe respiratory symptoms [12]. Additionally, children often exhibit a more robust innate immune response, which can help control viral replication early in the infection [13]. Elevated MMP levels were observed in children with multisystem inflammatory syndrome (MIS-C) requiring intensive care, compared with those not needing it, and in children with severe/moderate COVID-19, compared with those with mild cases [14]. Presently, direct comparisons of MMP concentrations between children and adults with COVID-19 are lacking. Understanding the role of MMPs in COVID-19 may offer valuable insights into disease mechanisms and potential therapeutic strategies aimed at modulating MMP activity to mitigate tissue damage and inflammation. With an understanding of the importance of MMPs in the pathologies of lung and other conditions, we aimed at comparing serum levels of various MMPs in adult and pediatric patients hospitalized with COVID-19.
Material and Methods
ETHICS STATEMENT:
This study was conducted according to the declaration of Helsinki and approved by the Ethics Committee of Medical University of Białystok (approval nos. APK.002.259.2020 and APK.002.428.2022). A written informed consent was obtained from all studied patients or patient parents/caregivers.
STUDY DESIGN AND PATIENT SELECTION:
We included patients who were hospitalized with COVID-19 between March 2020 and April 2021. Adult patients (n=22) were recruited at the Department of Infectious Diseases and Neuroinfections, while children (n=13) were recruited at the Department of Pediatric Infectious Diseases. COVID-19 diagnosis was confirmed using nasopharyngeal swabs collected from all study participants. Reverse transcription polymerase chain reaction (RT-PCR) assays were performed on the collected swab specimens following established protocols recommended by health authorities. Patients who tested positive for SARS-CoV-2 by RT-PCR were categorized as COVID-19 cases. Serum samples were collected from adult participants at 2 distinct time points. The first set of samples (T1) was obtained from all participants upon admission to the hospital and then stored at −80°C for subsequent analysis. For adult patients, a second set of serum samples was collected (T2) after the resolution of COVID-19 symptoms but before their discharge. We excluded patients who did not provide informed written consent for participation in the study. Additionally, individuals for whom serum samples were not collected or were deemed insufficient for analysis were excluded from the study cohort. Patients with pre-existing conditions or comorbidities known to significantly influence MMP levels, such as autoimmune diseases, chronic inflammatory conditions, or recent surgery, were also excluded to minimize confounding variables. Furthermore, individuals with a history of receiving MMP-modulating therapies or immunosuppressive medications were excluded to prevent interference with the interpretation of MMP levels. The control group consisted of 25 healthy individuals, including 13 children and 12 adults, who exhibited no signs of acute infection. Adult serum samples were sourced from voluntary blood donors at the Regional Centre for Transfusion Medicine in Białystok. Healthy children were recruited from the outpatient clinic at the University Children’s Hospital in Białystok, specifically among children seeking medical consultation for reasons unrelated to acute infections.
LABORATORY ANALYSIS:
Luminex assays (R&D Systems, Minneapolis, MN, USA) for the quantitative detection of human MMPs (MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, MMP-13), their inducers: extracellular MMP inducer (EMMPRIN), and tumor necrosis factor alpha (TNF-α), and 4 tissue inhibitors of MMPs (TIMP-1, TIMP-2, TIMP-3, TIMP-4) were performed according to the manufacturer’s protocol on a Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA). This multiplex immunoassay system allows for the simultaneous measurement of multiple analytes in a single serum sample. In this study, we used 4 different Luminex kits. Serum levels of TIMPs were measured with the 4-Plex Luminex assay (catalog number LKT003). Subsequently, 3 distinct custom assays (1-Plex, cat. no. LXSAHM-01, 2-Plex cat. no. LXSAHM-02, and 8-Plex, cat. no. LXSAHM-08) were used to measure other MMPs and their inducers. This approach was necessitated by technical constraints; MMPs cannot be multiplexed with TIMPs, and certain MMPs cannot be multiplexed together due to assay limitations. Furthermore, variations in expected serum levels dictated the division of assays based on the recommended serum dilution, ensuring optimal detection sensitivity and accuracy. The sensitivity of assays was as follows: MMP-1 2.7 pg/mL, MMP-2 108 pg/mL, MMP-3 5.3 pg/mL, MMP-7 23.2 pg/mL, MMP-8 34.2 pg/mL, MMP-9 13.6 pg/mL, MMP-10 1.2 pg/mL, MMP-12 7.1 pg/mL, MMP-13 19.0 pg/mL, IL-6 1.7 pg/mL, TNF 1.2 pg/mL, TIMP-1 1.54 pg/mL, TIMP-2 14.7 pg/mL, TIMP-3 86 pg/mL, and TIMP-4 1.29 pg/mL. Prior to analysis, serum samples were processed and stored according to standardized protocols to ensure sample integrity. Quality control measures were implemented to ensure the accuracy and reproducibility of the assay results, including the use of standard reference materials and calibration curves. The assay methodology and procedures were performed in accordance with manufacturer guidelines and established laboratory protocols.
STATISTICAL ANALYSIS:
Statistical comparisons among the 3 study groups, namely adults with COVID-19, children with COVID-19, and control participants, were conducted using GraphPad Prism version 10.0.0 for Windows (GraphPad Software, Boston, MA, USA,
Results
DATA OF HOSPITALIZATION:
A total of 35 patients (22 adults and 13 children) were recruited from March 2020 to April 2021. Characteristics of the studied groups and patient details are presented in Table 1. Notably, a number of adult patients had chronic comorbidities, mainly hypertension and dyslipidemia. There were no comorbidities in the pediatric cohort (Table 1).
SERUM LEVELS OF MMPS IN ADULT AND PEDIATRIC PATIENTS:
When compared with the control group, both adult and pediatric patients had elevated serum concentrations of MMP-1, MMP-7, TIMP-1, and TIMP-2. Elevated levels of MMP-3, MMP-8, MMP-9, and TIMP-4 were found in the adult COVID-19 patients, but not in infected children. No differences in serum levels of MMP-2, MMP-10, MMP-12, MMP-13, and TIMP-3 between the groups were found.
Compared with children, adult patients showed elevated serum concentrations of MMP-3 (median, 7.4 ng/mL; IQR, 4.7–11.2 vs median, 2.0; IQR, 1.7–2.4; P=0.0003), MMP-8 (median, 4.4 ng/mL; IQR, 2.8–8.4; vs median 0.1; IQR, 0.1–0.9; P<0.0001), MMP-9 (median, 148.9 ng/mL; IQR, 114.8–176.7 vs median, 56.6; IQR, 54.4–66.7; P=0.0016), TNF-α (median, 5.5 pg/mL; IQR, 3.1–9.1 vs median, 0.6; IQR, 0.6–1.3; P=0.0062), and TIMP-4 (median, 0.9 ng/mL; IQR, 0.8–1.3 vs median, 0.3; IQR, 0.07–0.4; P<0.0001), but lower concentrations of EMMPRIN (median, 2.8 ng/mL; IQR, 2.4–3.0 vs median, 4.1; IQR, 3.5–4.5; P=0.0002) (Figure 1).
SERUM PROFILE OF MMPS ON ADMISSION AND AFTER TREATMENT:
Of the 8 proteins that were elevated on T1, compared with controls, only 5 showed a significant reduction at T2 (Table 2). A decrease at T2 was observed for MMP-1 (median decrease 21%), MMP-8 (70%), MMP-9 (48%), TIMP-1 (27%), and TIMP-4 (26%) only. There was no significant decrease in the serum concentrations of MMP-3 and MMP-7 between T1 and T2. Interestingly, concentrations of MMP-2 and TIMP-2 increased after treatment by the median 35% and 13%, respectively, when compared with the baseline (Figure 2). Although not significantly elevated when compared with the control group, concentrations of MMP-13, TNF-α, and TIMP-3 were calculated to be lower at T2 compared with T1, possibly due to single outliers.
EFFECT OF ANTI-INFLAMMATORY TREATMENT ON THE LEVELS OF MMPS:
Treatment was administered according to the current recommendations of scientific societies at the time of the study. Anti-inflammatory treatment and convalescent plasma was administered to a subset of 16 adult patients, with treatment modalities varying among individuals. Specifically, 3 patients received glucocorticoids only, 3 were treated with convalescent plasma only, 3 were treated with a combination of glucocorticoids and tocilizumab, 4 received convalescent plasma in conjunction with glucocorticoids, and 3 underwent a combined treatment of convalescent plasma, glucocorticoids, and tocilizumab. The statistical analysis was constrained by the limited number of enrolled patients, preventing the derivation of statistically significant results. Nevertheless, our observations indicated that the application of anti-inflammatory treatment was associated with a notable reduction in the levels of specific MMPs at T2 (Figure 3). Concentrations of MMP-2 decreased in 25% of treated patients versus 0% of untreated patients. For MMP-3, a decrease was observed in 81% of treated patients, as opposed to 16% untreated patients. Similarly, a more common decrease in treated patients was noted for MMP-8 (96% vs 67%) and MMP-9 (100% vs 50%).
EXPLORING INTERCONNECTIONS BETWEEN LABORATORY ABNORMALITIES AND MMPS/TIMPS IN COVID-19 PATIENTS:
To unveil intricate associations between commonly observed laboratory abnormalities in patients with COVID-19 and the MMP/TIMP levels, we calculated the Spearman r correlation matrix (Figure 4). Notably, our investigation evaluated connections between well-established markers of inflammation, such as C-reactive protein (CRP), neutrophil and lymphocyte count, and alanine aminotransferase (ALT), white blood cell count (WBC), and D-dimers. MMP-1 exhibited positive correlations with TNF-alpha and CRP, while MMP-2 correlated with CRP and neutrophil count. MMP-3 and MMP-7 demonstrated associations with TNF-alpha, CRP, and neutrophil count. Additionally, MMP-9 displayed a notable correlation with ALT, and MMP-10 exhibited connections with CRP and D-dimers. Interestingly, MMP-12 exhibited negative correlations with CRP, WBC, and neutrophil count. Furthermore, significant associations were identified between various MMPs and TIMPs, such as TIMP-1 with MMP-1 and MMP-8, TIMP-2 with MMP-2, and TIMP-3 with MMP-1, MMP-2, and MMP-7. The correlation matrix also unveiled interactions among different MMPs, albeit with lower correlation strengths, underscoring the complexity of the interplay within the MMP system.
Discussion
DIFFERENCES IN MMP RESPONSES BETWEEN ADULTS AND CHILDREN:
In adult patients, we observed a substantial increase in the serum concentrations of MMP-1, MMP-3, MMP-7, MMP-8, and MMP-9, accompanied by elevated levels of TIMP-1, TIMP-2, and TIMP-4. During the convalescent stage, MMP-3 and MMP-7 remained largely unchanged, and MMP-2 concentrations increased. Conversely, the infection had no discernible impact on the concentrations of MMP-10, MMP-12, MMP-13, and TIMP-3. In the pediatric group, we identified a distinct pattern characterized by a lower number of upregulated proteins. Specifically, in children, we found no increase in concentrations of MMP-3, MMP-8, MMP-9, and TIMP-4, as observed in adults. These findings shed light on the divergent MMP responses in adult and pediatric patients with COVID-19, providing a nuanced understanding of the systemic implications of the infection. The rationale behind this investigation stemmed from the critical need to decipher the inflammatory cascades underlying COVID-19 and explore potential variations that might contribute to the observed differences in disease severity and outcomes between adults and children.
In our study, a noteworthy observation emerged, as we found significantly higher serum concentrations of MMP-3, MMP-8, and MMP-9 in adults with COVID-19 than in pediatric patients with the same condition. To the best of our knowledge, this is the first study to directly compare MMP profiles between children and adults with COVID-19. This observation gains further significance when considering existing data on severe manifestations of COVID-19 in children, specifically those with MIS-C. Previous research has indicated elevated baseline levels of MMPs, including MMP-3, MMP-8, and MMP-9, in children with MIS-C requiring intensive care, compared with those with less severe presentations [14,15]. We hypothesize that a number of MMPs were not upregulated in our pediatric cohort, because of a milder course of infection, compared with the adult group. Our findings align with a broader understanding of age-related differences in COVID-19 severity, encompassing variations in innate and adaptive immune responses, endothelial function, and clotting mechanisms [16,17]. Children, benefiting from a more robust and rapid innate immune response, might efficiently control the virus, while adults can experience dysregulated responses, leading to heightened inflammation and tissue damage [11].
ROLE OF SPECIFIC MMPS IN COVID-19:
Existing literature consistently suggests that MMPs play a foundational role in the severity of SARS-CoV-2 infection, contributing to complications such as acute lung injury/acute respiratory distress syndrome [5]. The role of MMPs in COVID-19 stems from the pathogenesis resulting in the release of chemokines and pro-inflammatory markers, which cause pulmonary edema. Therefore, MMPs could be used to identify the severity of the COVID-19 stage to assess the true damage to the lungs [8]. This is highlighted by increased MMP-3 levels as the WHO COVID-19 severity stages progress [8], or by high MMP-7 and MMP-9 levels in patients with acute respiratory distress syndrome during COVID-19 [18]. Moreover, it is evident that MMPs play diverse and distinct functions, determined by their substrate specificity, expression, activation levels, and localization [5,19,20]. For instance, MMP-1, recognized for its pivotal role in vascular remodeling and its association with idiopathic pulmonary fibrosis [21], was elevated in patients hospitalized with COVID-19, particularly in severe cases [22]. The hyperactivation of MMP-1/PAR1 signaling, leading to compromised vascular endothelial cell function and excessive inflammatory cell recruitment, emphasizes its potential as a peripheral blood biomarker [23]. MMP-2 is a crucial member of the MMP family, primarily responsible for the degradation of type IV collagen, a major component of the extracellular matrix. In our study, a notable increase in MMP-2 levels was observed after recovery, indicating its potential involvement in the pathophysiology of COVID-19. The hypothesis posits that the reduction in MMP-2 levels may result from systemic changes induced by SARS-CoV-2, linked to cytokine storms and ACE2 imbalance [24]. MMP-2 deficiency is recognized to predispose to inflammation, with implications for cardiovascular health [25]. Elevated MMP-2 levels can indicate heightened RAS activation, playing a critical role in hypertension and COVID-19 pathophysiology. Additionally, it is suggested that patients with pre-existing hypertension may experience more severe vascular lesions due to higher levels of AngII, which induces MMP-2 via the AT-1 receptor. A proteomic analysis of lung tissue from patients with COVID-19 revealed a positive correlation between MMP-8 expression and MMP-2 levels, indicating a potential link to the shedding of immunosuppressive mediators, such as sHLA-G and sTREM-1. The concurrent upregulation of the MMP-2/MMP-8 axis, along with neutrophil infiltration and the generation of reactive oxygen species, leading to increased lipid peroxidation, may contribute to severe lung tissue damage in COVID-19 [9].
MMP-9, a gelatinase associated with alveolar capillary destruction and lung injury, was markedly increased in plasma levels in patients with COVID-19, compared with in healthy controls in this study. The inflammatory response mediated by neutrophils and the activation of growth factors and TGF-β1 through MMP-9 further underscore its role in COVID-19 pathogenesis [8,18,24]. Notably, our findings align with literature indicating the potential involvement of MMP-9 in COVID-19 [8,26].
MMP-3, also known as stromelysin-1, is recognized for its broad substrate specificity, targeting various components of the extracellular matrix [19]. In the context of COVID-19, our study revealed sustained elevation in MMP-3 levels. This observation aligns with previous findings linking increased MMP-3 expression to inflammatory responses and tissue damage [8]. The persistence of elevated MMP-3 levels suggests its potential involvement in the ongoing inflammatory processes associated with inflammatory diseases [27,28]. Similarly, MMP-7, also known as matrilysin, has been implicated in various pathological conditions, particularly in lung diseases [29,30]. In our study, MMP-7 levels remained consistently elevated, indicating its sustained activity during COVID-19 infection. MMP-7 is known for its role in degrading various extracellular matrix components, including fibronectin and proteoglycans [19]. The prolonged elevation of MMP-7 suggests its potential contribution to tissue remodeling and repair processes in response to the viral insult. Previous research has associated MMP-7 with impaired lung function tests 6 months after COVID-19 [21] and idiopathic lung fibrosis [29,30], highlighting its significance in the context of respiratory diseases.
POTENTIAL THERAPEUTIC IMPLICATIONS OF MMPS:
The potential association between elevated MMP levels and disease severity, as observed in both adults and children, underscores the intricate interplay between MMPs and COVID-19 pathophysiology, offering insights that could contribute to future therapeutic strategies. Our investigation revealed a notable downregulation of MMPs in patients with COVID-19 who received anti-inflammatory treatment, including glucocorticoids, tocilizumab, and convalescent plasma. This finding aligns with the broader understanding of the role of these therapeutic interventions in modulating the inflammatory response associated with severe COVID-19 [31,32]. Glucocorticoids, known for their potent anti-inflammatory effects, are reported to suppress the expression and activity of various MMPs [33–35], potentially contributing to the observed reduction in MMP levels. Tocilizumab, an interleukin-6 receptor antagonist, targets a key mediator of the inflammatory cascade, thus influencing downstream processes, including MMP regulation [36,37]. The use of convalescent plasma, containing neutralizing antibodies and anti-inflammatory factors, can also contribute to dampening the inflammatory milieu [38] and subsequently downregulating MMPs, as it was shown previously in studies linking the use of intravenous immunoglobulins and altered levels of MMPs [39,40]. This observed downregulation aligns with the broader goal of anti-inflammatory treatment in mitigating the exaggerated immune response associated with severe COVID-19, suggesting a potential avenue for therapeutic intervention. However, further research is warranted to elucidate the specific mechanisms through which these treatments modulate MMP activity and the implications of MMP downregulation for the overall disease course and long-term outcomes in patients with COVID-19 undergoing anti-inflammatory therapies.
This concept underscores the potential for both specific and nonspecific inhibition of MMPs as viable therapeutic strategies. Effectively managing the clinical outcome of COVID-19 infection requires precise and timely identification of the risk of mortality. Therefore, gaining a comprehensive understanding of MMP activity at specific time points or in correlation with the severity of the disease becomes crucial. Such knowledge offers significant implications for deciphering the mechanisms underpinning disease progression, empowering clinicians to initiate timely and targeted treatment interventions for improved patient outcomes.
LIMITATIONS:
This study is not without limitations. The relatively small sample size and the study’s observational nature can affect the generalizability of the findings. Additionally, while efforts were made to control for confounding factors, the heterogeneity in treatment regimens and the absence of longitudinal follow-up to assess long-term outcomes limit the ability to draw definitive conclusions regarding the therapeutic implications of MMP modulation. Future research with larger, diverse cohorts and controlled interventional designs will be essential to validate these preliminary findings and elucidate the mechanisms underlying the observed effects. Potential confounding factors, such as differences in baseline health status, the presence of comorbidities, and variations in treatment protocols, may have influenced the observed MMP profiles. Although we attempted to mitigate these effects through careful patient selection and statistical analysis, the potential for residual confounding remains. Acknowledging these limitations is crucial for the accurate interpretation of the results and the design of future studies.
Conclusions
In conclusion, our study highlights the distinctive inflammatory responses to COVID-19 in adults and children, emphasizing the complexity of the disease’s pathophysiology across age groups. We observed a post-treatment reduction in MMP-1, MMP-8, MMP-9 levels, with median decreases ranging from 21% to 70% across these markers in patients receiving glucocorticoids, tocilizumab, or convalescent plasma. Notably, the observed post-treatment reduction in specific MMPs suggests the potential efficacy of anti-inflammatory interventions in modulating the COVID-19-related inflammatory cascade. However, the persistence of elevated MMP-2, MMP-3, and MMP-7, even after treatment, raises concern about potential long-term consequences, including fibrosis and chronic respiratory dysfunction. This underscores the need for further research to delineate the intricate dynamics of MMP regulation and its implications for the clinical course and outcomes of patients with COVID-19.
Figures
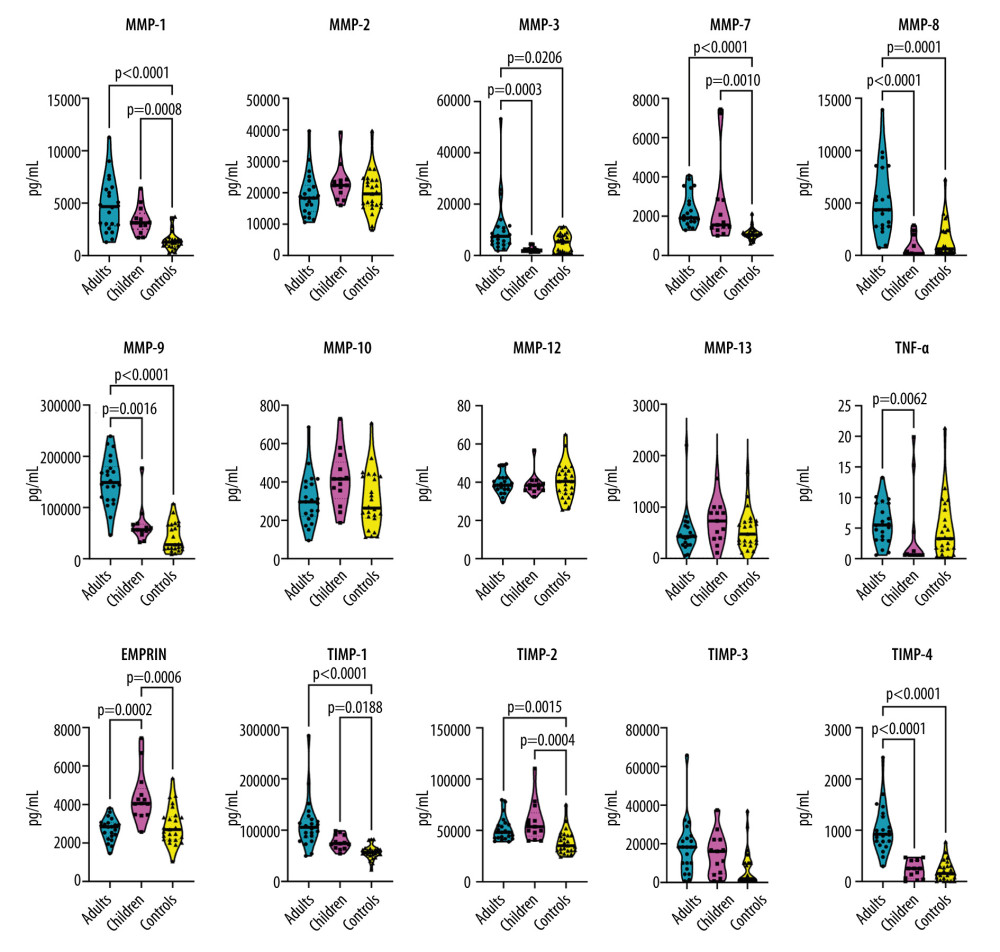 Figure 1. Violin plots illustrating the concentration of serum levels of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) in 3 study groups: (1) adults with COVID-19, (2) children with COVID-19, and (3) healthy control participants. Each panel represents 1 protein, and the violin plots depict the distribution of data within each group. Brackets and corresponding P values indicate significant differences between study groups. Thick horizontal lines drawn inside the plots represent median values.
Figure 1. Violin plots illustrating the concentration of serum levels of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) in 3 study groups: (1) adults with COVID-19, (2) children with COVID-19, and (3) healthy control participants. Each panel represents 1 protein, and the violin plots depict the distribution of data within each group. Brackets and corresponding P values indicate significant differences between study groups. Thick horizontal lines drawn inside the plots represent median values. 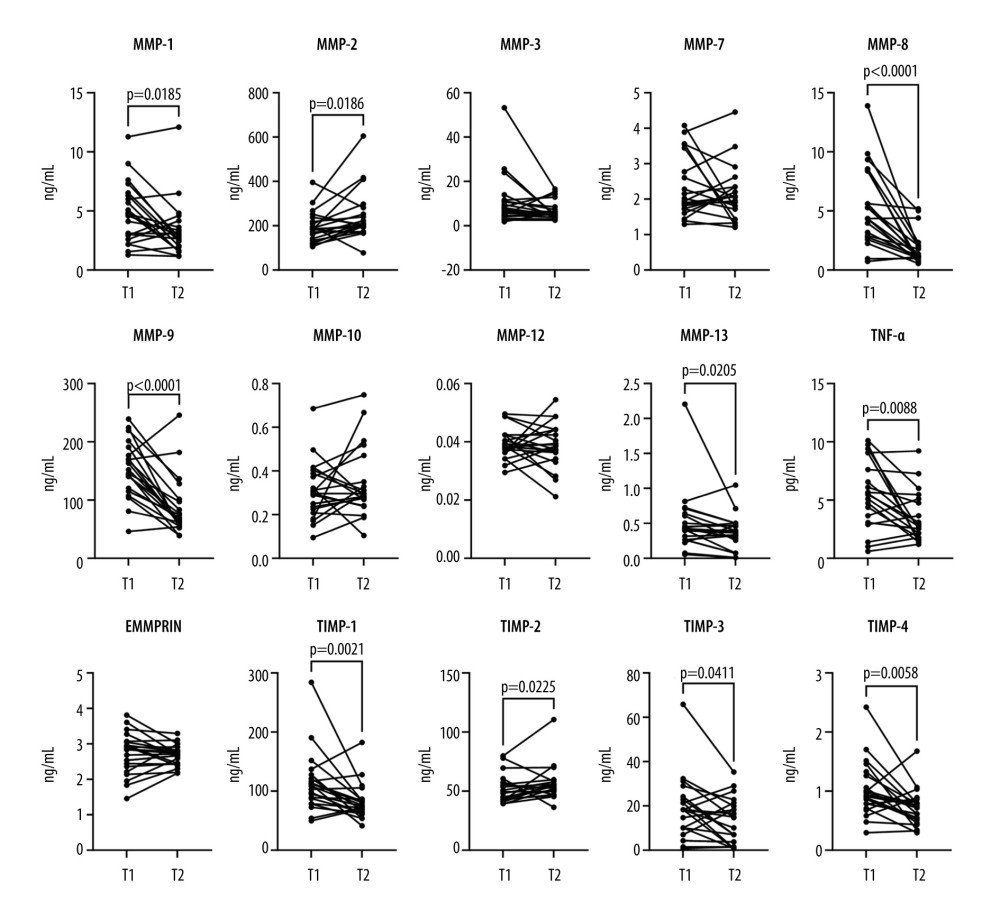 Figure 2. Before-after plots of serum matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) concentrations in adult COVID-19 patients. Within each subplot, lines connect paired data points from the same patient, comparing protein concentrations at 2 time points: T1 (upon admission) and T2 (after symptom resolution and before discharge). Statistical significance assessed using the Wilcoxon matched-pairs signed-rank test is denoted by brackets above the graphs, with corresponding P values.
Figure 2. Before-after plots of serum matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) concentrations in adult COVID-19 patients. Within each subplot, lines connect paired data points from the same patient, comparing protein concentrations at 2 time points: T1 (upon admission) and T2 (after symptom resolution and before discharge). Statistical significance assessed using the Wilcoxon matched-pairs signed-rank test is denoted by brackets above the graphs, with corresponding P values. 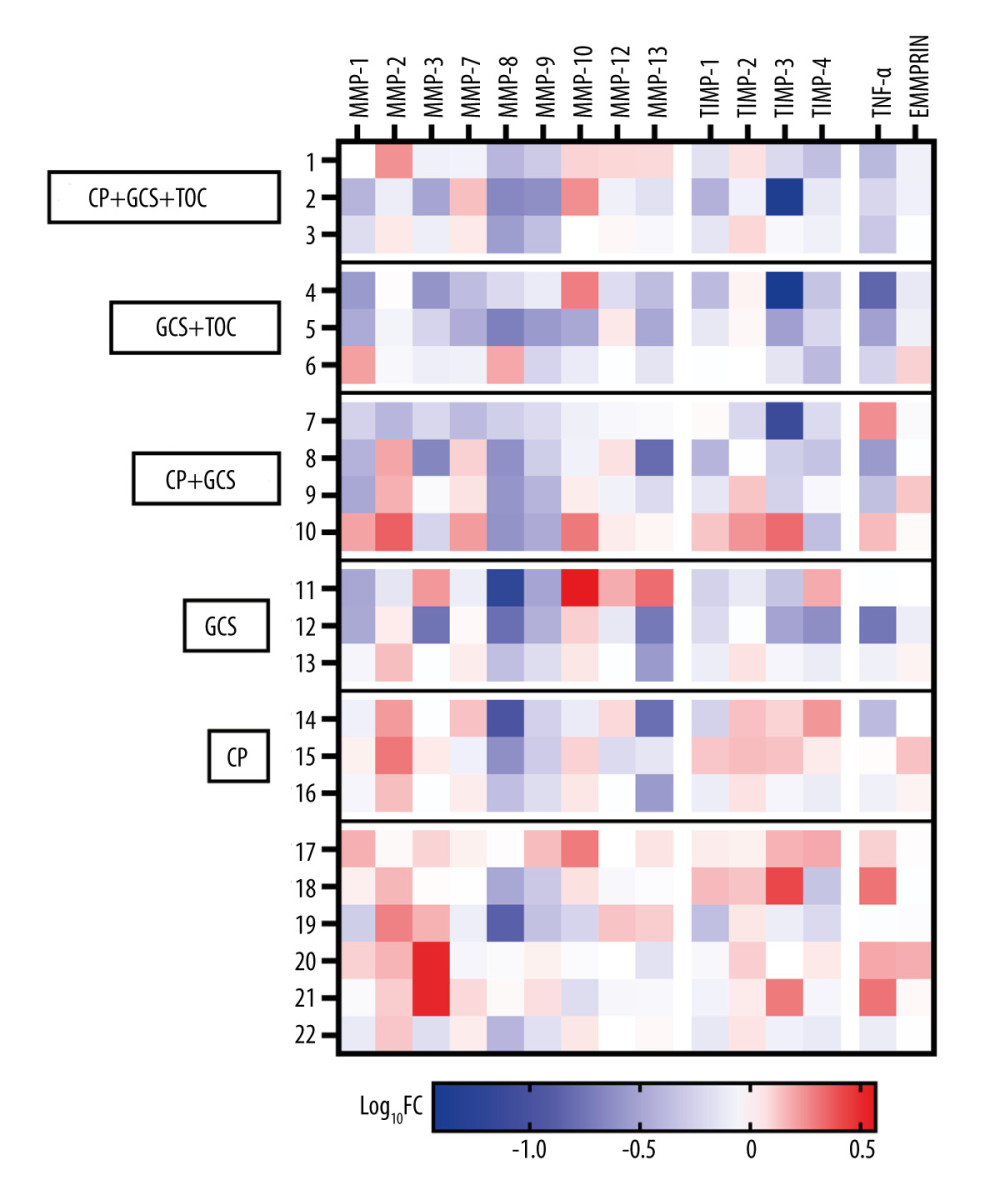 Figure 3. Heatmap of log10 Fold-Change (Log10FC) in serum protein concentrations comparing T2 to T1 in adult COVID-19 patients, divided by the treatment used. The Log10FC shows the change in serum protein concentrations between the 2 time points, T2 (after symptom resolution and before discharge) and T1 (upon admission), for all 22 recruited adult COVID-19 patients. The proteins analyzed include matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), tumor necrosis factor-alpha (TNFα), and extracellular matrix metalloproteinase inducer (EMMPRIN). Each row represents an individual patient, while each column corresponds to a specific protein. The color-coding scheme is designed such that an increase in protein concentration is represented in red, while a decrease is depicted in blue. The legend below the heatmap provides a reference for the Log10FC scale. CP – convalescent plasma; GCS – glucocorticoids; TOC – tocilizumab
Figure 3. Heatmap of log10 Fold-Change (Log10FC) in serum protein concentrations comparing T2 to T1 in adult COVID-19 patients, divided by the treatment used. The Log10FC shows the change in serum protein concentrations between the 2 time points, T2 (after symptom resolution and before discharge) and T1 (upon admission), for all 22 recruited adult COVID-19 patients. The proteins analyzed include matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), tumor necrosis factor-alpha (TNFα), and extracellular matrix metalloproteinase inducer (EMMPRIN). Each row represents an individual patient, while each column corresponds to a specific protein. The color-coding scheme is designed such that an increase in protein concentration is represented in red, while a decrease is depicted in blue. The legend below the heatmap provides a reference for the Log10FC scale. CP – convalescent plasma; GCS – glucocorticoids; TOC – tocilizumab 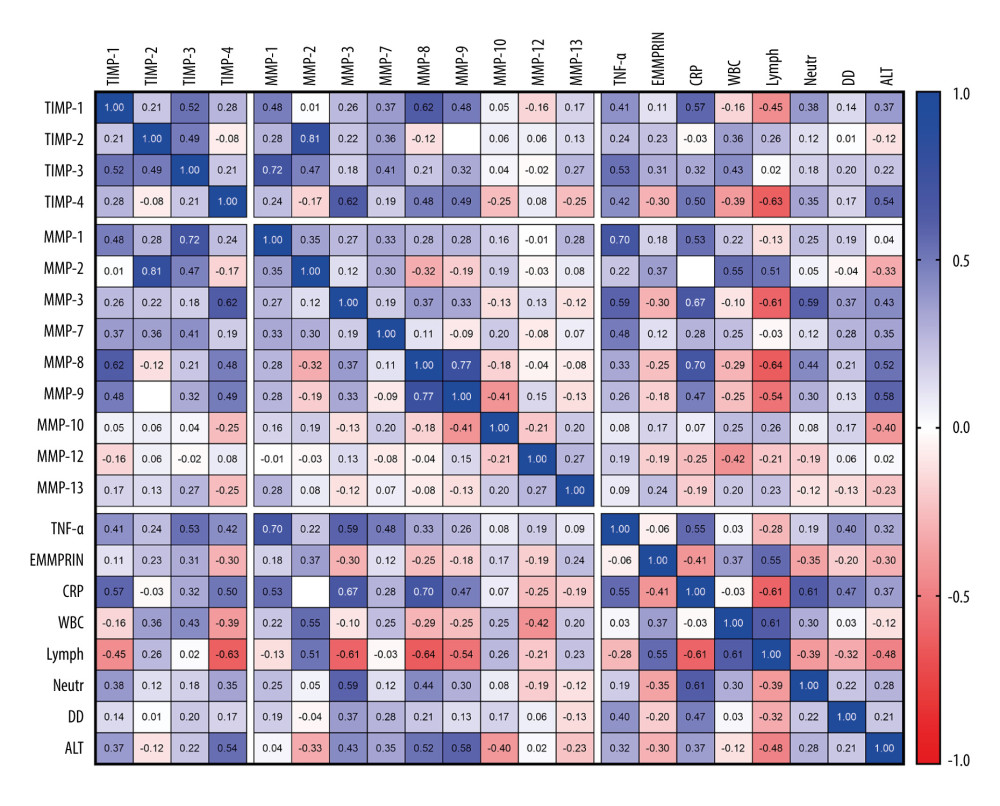 Figure 4. Correlation matrix of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), tumor necrosis factor-alpha (TNFα), and other laboratory results, including C-reactive protein (CRP), white blood cell count (WBC), lymphocytes (Lymph), neutrophils (Neutr), D-dimers, and alanine aminotransferase (ALT) in adult and pediatric COVID-19 patients. Statistical analysis conducted using Spearman’s correlation coefficient. Color-coded representation with positive correlations in blue and negative correlations in red. R values are displayed within cells, and missing values indicate correlations lower than 0.01.
Figure 4. Correlation matrix of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), tumor necrosis factor-alpha (TNFα), and other laboratory results, including C-reactive protein (CRP), white blood cell count (WBC), lymphocytes (Lymph), neutrophils (Neutr), D-dimers, and alanine aminotransferase (ALT) in adult and pediatric COVID-19 patients. Statistical analysis conducted using Spearman’s correlation coefficient. Color-coded representation with positive correlations in blue and negative correlations in red. R values are displayed within cells, and missing values indicate correlations lower than 0.01. References
1. Page-McCaw A, Ewald AJ, Werb Z, Matrix metalloproteinases and the regulation of tissue remodelling: Nat Rev Mol Cell Biol, 2007; 8(3); 221-33
2. Kessenbrock K, Plaks V, Werb Z, Matrix metalloproteinases: Regulators of the tumor microenvironment: Cell, 2010; 141(1); 52-67
3. Burrage PS, Mix KS, Brinckerhoff CE, Matrix metalloproteinases: Role in arthritis: Front Biosci J Virtual Libr, 2006; 11; 529-43
4. Liu P, Sun M, Sader S, Matrix metalloproteinases in cardiovascular disease: Can J Cardiol, 2006; 22(Suppl B); 25B-30B
5. Khalid U, Dimov D, Vlaykova T, Matrix metalloproteinases in COVID-19: Underlying significance: Biotechnol Equip, 2023; 37(1); 295-301
6. Davey A, McAuley DF, O’Kane CM, Matrix metalloproteinases in acute lung injury: Mediators of injury and drivers of repair: Eur Respir J, 2011; 38(4); 959-70
7. Elkington PTG, Friedland JS, Matrix metalloproteinases in destructive pulmonary pathology: Thorax, 2006; 61(3); 259-66
8. Gelzo M, Cacciapuoti S, Pinchera B, Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients: Sci Rep, 2022; 12(1); 1212
9. da Silva-Neto PV, do Valle VB, Fuzo CA, Matrix metalloproteinases on severe COVID-19 lung disease pathogenesis: cooperative actions of MMP-8/MMP-2 axis on immune response through HLA-G shedding and oxidative stress: Biomolecules, 2022; 12(5); 604
10. Gipson TS, Bless NM, Shanley TP, Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury: J Immunol Baltim Md 1950, 1999; 162(6); 3653-62
11. Zimmermann P, Curtis N, Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections: Arch Dis Child, 2021; 106(5); 429-39
12. Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD: Mol Ther Methods Clin Dev, 2020; 18; 1-6
13. Loske J, Röhmel J, Lukassen S, Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children: Nat Biotechnol, 2022; 40(3); 319-24
14. Pavan Kumar N, Venkataraman A, Varadarjan P, Role of matrix metalloproteinases in multi-system inflammatory syndrome and acute COVID-19 in children: Front Med, 2022; 9; 1050804
15. Sacco K, Castagnoli R, Vakkilainen S, Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19: Nat Med, 2022; 28(5); 1050-62
16. Consiglio CR, Cotugno N, Sardh F, The immunology of multisystem inflammatory syndrome in children with COVID-19: Cell, 2020; 183(4); 968-981e7
17. Constantin T, Pék T, Horváth Z, Garan D, Szabó AJ, Multisystem inflammatory syndrome in children (MIS-C): Implications for long COVID: Inflammopharmacology, 2023; 31(5); 2221-36
18. Nasr El-Din A, Ata KAE-S, Abdel-Gawad AR, Fahmy NF, Impact of high serum levels of MMP-7, MMP-9, TGF-β and PDGF macrophage activation markers on severity of COVID-19 in obese-diabetic patients: Infect Drug Resist, 2021; 14; 4015-25
19. Cui N, Hu M, Khalil RA, Chapter one – biochemical and biological attributes of matrix metalloproteinases: Progress in molecular biology and translational science. Vol 147. Matrix metalloproteinases and tissue remodeling in health and disease: Cardiovascular remodeling, 2017; 1-73, Academic Press
20. Nissinen L, Kähäri V-M, Matrix metalloproteinases in inflammation: Biochim Biophys Acta, 2014; 1840(8); 2571-80
21. Safont B, Tarraso J, Rodriguez-Borja E, Lung function, radiological findings and biomarkers of fibrogenesis in a cohort of COVID-19 patients six months after hospital discharge: Arch Bronconeumol, 2022; 58(2); 142-49
22. Syed F, Li W, Relich RF, Excessive matrix metalloproteinase-1 and hyperactivation of endothelial cells occurred in COVID-19 patients and were associated with the severity of COVID-19: J Infect Dis, 2021; 224(1); 60-69
23. Mazor R, Alsaigh T, Shaked H, Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells: J Biol Chem, 2013; 288(1); 598-607
24. D’Avila-Mesquita C, Couto AES, Campos LCB, MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients: Biomed Pharmacother, 2021; 142; 112067
25. Hardy E, Hardy-Sosa A, Fernandez-Patron C, MMP-2: Is too low as bad as too high in the cardiovascular system?: Am J Physiol Heart Circ Physiol, 2018; 315(5); H1332-40
26. Ding L, Guo H, Zhang C, Elevated matrix metalloproteinase-9 expression is associated with COVID-19 severity: A meta-analysis: Exp Ther Med, 2023; 26(6); 545
27. Ma J-D, Zhou J-J, Zheng D-H, Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis: Mediators Inflamm, 2014; 2014; e179284
28. Wan J, Zhang G, Li X, Matrix metalloproteinase 3: A promoting and destabilizing factor in the pathogenesis of disease and cell differentiation: Front Physiol, 2021; 12; 663978
29. Morais A, Beltrão M, Sokhatska O, Serum metalloproteinases 1 and 7 in the diagnosis of idiopathic pulmonary fibrosis and other interstitial pneumonias: Respir Med, 2015; 109(8); 1063-68
30. Khan F, Stewart I, Saini G, A systematic review and individual participant data meta-analysis of MMP-7 and outcomes in idiopathic pulmonary fibrosis: Eur Respir J, 2021; 58
31. Malgie J, Schoones JW, Pijls BG, Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: A rapid systematic review and meta-analysis of observational studies: Clin Infect Dis, 2021; 72(11); e742-49
32. Xu X, Han M, Li T, Effective treatment of severe COVID-19 patients with tocilizumab: Proc Natl Acad Sci USA, 2020; 117(20); 10970-75
33. El-Shabrawi Y, Eckhardt M, Berghold A, Synthesis pattern of matrix metalloproteinases (MMPs) and inhibitors (TIMPs) in human explant organ cultures after treatment with latanoprost and dexamethasone: Eye, 2000; 14(3); 375-83
34. Mäkitalo L, Rintamäki H, Tervahartiala T, Serum MMPs 7–9 and their inhibitors during glucocorticoid and anti-TNF-α therapy in pediatric inflammatory bowel disease: Scand J Gastroenterol, 2012; 47(7); 785-94
35. Eberhardt W, Schulze M, Engels C, Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: Involvement of nuclear factor-kappaB and Ets transcription factors: Mol Endocrinol Baltim Md, 2002; 16(8); 1752-66
36. Mano Y, Shibata K, Sumigama S, Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9 secretions from human amnion cells in preterm premature rupture of membranes: Gynecol Obstet Invest, 2009; 68(3); 145-53
37. Yeo J, Baek HJ, Song YW, Lee EY, Evaluation of serum matrix metalloproteinase-3 as an objective indicator for the disease activity in rheumatoid arthritis patients treated with methotrexate versus tocilizumab: 24-week results from a prospective randomized controlled study: J Rheum Dis, 2022; 29(2); 89-97
38. Franchini M, Glingani C, Liumbruno GM, Potential mechanisms of action of convalescent plasma in COVID-19: Diagn Berl Ger, 2021; 8(4); 413-20
39. Shapiro S, Shoenfeld Y, Gilburd B, Intravenous gamma globulin inhibits the production of matrix metalloproteinase-9 in macrophages: Cancer, 2002; 95(9); 2032-37
40. Hurnaus S, Mueller-Felber W, Pongratz D, Schoser BGH, Serum levels of matrix metalloproteinases-2 and -9 and their tissue inhibitors in inflammatory neuromuscular disorders: Eur Neurol, 2006; 55(4); 204-8
Figures
 Figure 1. Violin plots illustrating the concentration of serum levels of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) in 3 study groups: (1) adults with COVID-19, (2) children with COVID-19, and (3) healthy control participants. Each panel represents 1 protein, and the violin plots depict the distribution of data within each group. Brackets and corresponding P values indicate significant differences between study groups. Thick horizontal lines drawn inside the plots represent median values.
Figure 1. Violin plots illustrating the concentration of serum levels of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) in 3 study groups: (1) adults with COVID-19, (2) children with COVID-19, and (3) healthy control participants. Each panel represents 1 protein, and the violin plots depict the distribution of data within each group. Brackets and corresponding P values indicate significant differences between study groups. Thick horizontal lines drawn inside the plots represent median values. Figure 2. Before-after plots of serum matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) concentrations in adult COVID-19 patients. Within each subplot, lines connect paired data points from the same patient, comparing protein concentrations at 2 time points: T1 (upon admission) and T2 (after symptom resolution and before discharge). Statistical significance assessed using the Wilcoxon matched-pairs signed-rank test is denoted by brackets above the graphs, with corresponding P values.
Figure 2. Before-after plots of serum matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), and tumor necrosis factor-alpha (TNF-α) concentrations in adult COVID-19 patients. Within each subplot, lines connect paired data points from the same patient, comparing protein concentrations at 2 time points: T1 (upon admission) and T2 (after symptom resolution and before discharge). Statistical significance assessed using the Wilcoxon matched-pairs signed-rank test is denoted by brackets above the graphs, with corresponding P values. Figure 3. Heatmap of log10 Fold-Change (Log10FC) in serum protein concentrations comparing T2 to T1 in adult COVID-19 patients, divided by the treatment used. The Log10FC shows the change in serum protein concentrations between the 2 time points, T2 (after symptom resolution and before discharge) and T1 (upon admission), for all 22 recruited adult COVID-19 patients. The proteins analyzed include matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), tumor necrosis factor-alpha (TNFα), and extracellular matrix metalloproteinase inducer (EMMPRIN). Each row represents an individual patient, while each column corresponds to a specific protein. The color-coding scheme is designed such that an increase in protein concentration is represented in red, while a decrease is depicted in blue. The legend below the heatmap provides a reference for the Log10FC scale. CP – convalescent plasma; GCS – glucocorticoids; TOC – tocilizumab
Figure 3. Heatmap of log10 Fold-Change (Log10FC) in serum protein concentrations comparing T2 to T1 in adult COVID-19 patients, divided by the treatment used. The Log10FC shows the change in serum protein concentrations between the 2 time points, T2 (after symptom resolution and before discharge) and T1 (upon admission), for all 22 recruited adult COVID-19 patients. The proteins analyzed include matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), tumor necrosis factor-alpha (TNFα), and extracellular matrix metalloproteinase inducer (EMMPRIN). Each row represents an individual patient, while each column corresponds to a specific protein. The color-coding scheme is designed such that an increase in protein concentration is represented in red, while a decrease is depicted in blue. The legend below the heatmap provides a reference for the Log10FC scale. CP – convalescent plasma; GCS – glucocorticoids; TOC – tocilizumab Figure 4. Correlation matrix of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), tumor necrosis factor-alpha (TNFα), and other laboratory results, including C-reactive protein (CRP), white blood cell count (WBC), lymphocytes (Lymph), neutrophils (Neutr), D-dimers, and alanine aminotransferase (ALT) in adult and pediatric COVID-19 patients. Statistical analysis conducted using Spearman’s correlation coefficient. Color-coded representation with positive correlations in blue and negative correlations in red. R values are displayed within cells, and missing values indicate correlations lower than 0.01.
Figure 4. Correlation matrix of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), extracellular matrix metalloproteinase inducer (EMMPRIN), tumor necrosis factor-alpha (TNFα), and other laboratory results, including C-reactive protein (CRP), white blood cell count (WBC), lymphocytes (Lymph), neutrophils (Neutr), D-dimers, and alanine aminotransferase (ALT) in adult and pediatric COVID-19 patients. Statistical analysis conducted using Spearman’s correlation coefficient. Color-coded representation with positive correlations in blue and negative correlations in red. R values are displayed within cells, and missing values indicate correlations lower than 0.01. Tables
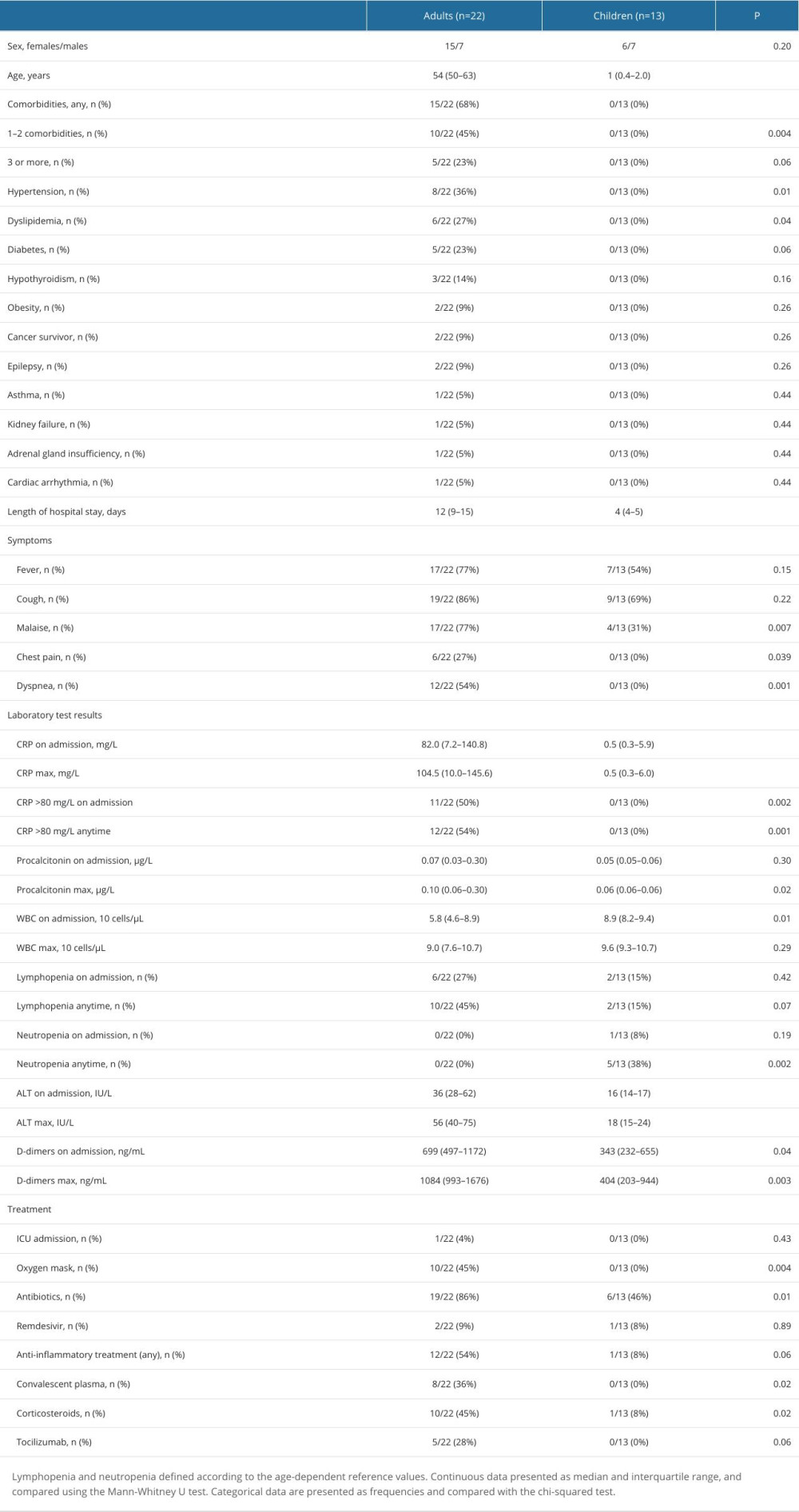 Table 1. Characteristics of the study groups.
Table 1. Characteristics of the study groups.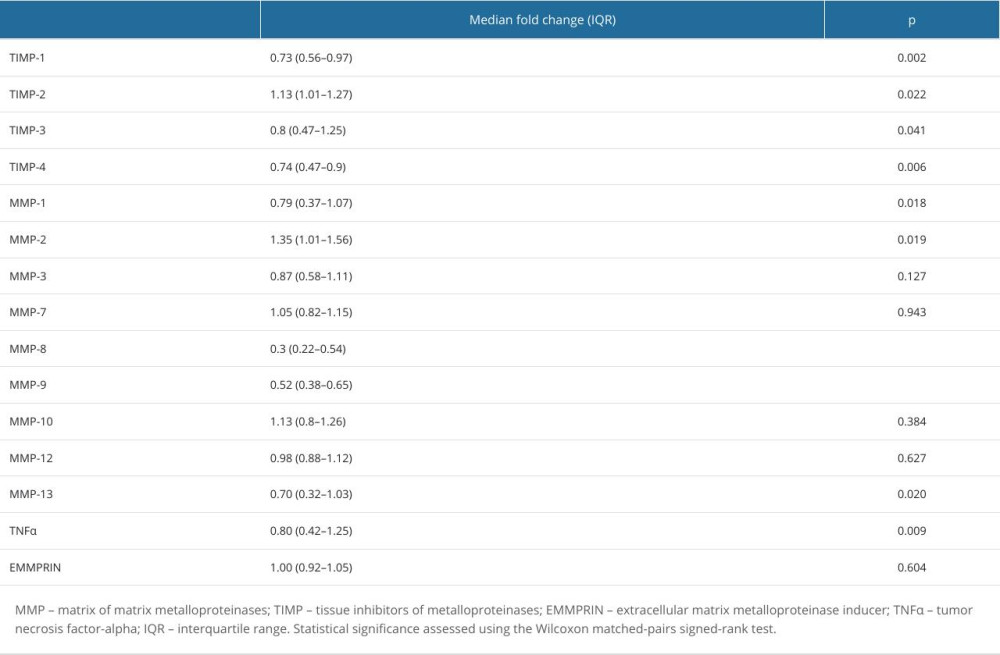 Table 2. Change in the serum concentrations of measured proteins in adult COVID-19 patients concentrations between 2 time points: T2 (after symptom resolution and before discharge) and T1 (upon admission).
Table 2. Change in the serum concentrations of measured proteins in adult COVID-19 patients concentrations between 2 time points: T2 (after symptom resolution and before discharge) and T1 (upon admission). Table 1. Characteristics of the study groups.
Table 1. Characteristics of the study groups. Table 2. Change in the serum concentrations of measured proteins in adult COVID-19 patients concentrations between 2 time points: T2 (after symptom resolution and before discharge) and T1 (upon admission).
Table 2. Change in the serum concentrations of measured proteins in adult COVID-19 patients concentrations between 2 time points: T2 (after symptom resolution and before discharge) and T1 (upon admission). In Press
Clinical Research
Comparative Evaluation of Digital Cephalometric Tracing Applications on Mobile Devices and Manual TracingMed Sci Monit In Press; DOI: 10.12659/MSM.944628
Clinical Research
Impact of COVID-19-Induced Academic Stress on Insomnia and Suicidal Ideation among Taiwanese Health Trainee...Med Sci Monit In Press; DOI: 10.12659/MSM.944932
Review article
Impact of Workplace Bullying on Nursing Care Quality: A Comprehensive ReviewMed Sci Monit In Press; DOI: 10.12659/MSM.944815
Clinical Research
Anterior Plate-Supported Cannulated Screw Surgery for Ankle Arthrodesis: Clinical and Radiologic Results in...Med Sci Monit In Press; DOI: 10.12659/MSM.944452
Most Viewed Current Articles
17 Jan 2024 : Review article 1,882,405
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research 1,538,203
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research 690,402
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial 50,329
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








