23 January 2024: Review Articles
Decoding the Neurological Sequelae of General Anesthesia: A Review
Paweł RadkowskiDOI: 10.12659/MSM.942740
Med Sci Monit 2024; 30:e942740
Abstract
ABSTRACT: General anesthesia is an integral part of modern surgical practice, but it is associated with a number of complications, including neurological ones. This article provides a thorough analysis of these complications, taking into account the most common ones like drug complications, through delirium, postoperative cognitive impairment (POCD), to the rarest ones like perioperative stroke (POS), spinal cord ischemia (SCI), and postoperative visual loss (POVL). Its main goal is to familiarize healthcare professionals, especially those involved in anesthesiology, with the intricacies of neurological complications. Given their specificity and frequency of occurrence, it is well known that the diagnosis and management of these complications can sometimes cause problems for physicians without advanced neurological knowledge. Also, in addition to complex diagnostics, the pathomechanism of non-pharmacological complications is often not fully understood due to their multifactoriality and sometimes paucity of research. For this reason, an increasing amount of work is being done in the medical community to better understand this group of conditions, enabling faster diagnosis and more effective treatment, as well as perioperative prevention. This paper aims to increase awareness and vigilance among physicians across the spectrum of surgical patient care, from premedication to postoperative follow-up. Drawing on the authors' experience and the extensive medical literature, this paper includes 39 selected articles from 1994 to 2023, seeking the latest insights in the constantly evolving field of neurology and anesthesiology. This article aims to review the neurological complications of general anesthesia.
Keywords: Anesthesia, General, cognitive dysfunction, Critical Care Outcomes, Neurology
Background
General anesthesia is a widely used type of anesthesia, used in almost every surgical field. In addition to the most common respiratory complications associated with intubation, maintenance of the patient’s artificial airway, and mechanical ventilation, as well as the wide-ranging activities of the surgical team, various neurological complications are encountered after general anesthesia in the practice of anesthesiology. The most common are drug complications, related to inadequate dosage, residual effects, or drug interactions. Less commonly, delirium and postoperative cognitive dysfunction (POCD) are found in clinical practice. In contrast, the occurrence of neurological complications with potentially serious consequences for the patient, such as perioperative stroke (POS), spinal cord ischemia (SCI), or postoperative vision loss (POVL), are very rare. Diagnosing or differentiating individual complications can sometimes be problematic, especially for inexperienced clinicians, and often requires further diagnosis and assistance from a neurologist.
The purpose of this article is to introduce the most important complications after general anesthesia, including pathophysiology and preventive and therapeutic options. It is intended to familiarize and better understand the group of neurological complications, especially to anesthesia personnel, since this type of complication, due to its often ambiguous clinical picture and sometimes very rare incidence, may create a problem even for experienced health care professionals. We aim to raise awareness and arouse alertness among physicians at every stage of surgical patient management, from premedication and ending with postoperative care.
The work is based on the experience and knowledge of the authors and also on the available medical literature. The selection of 39 articles published from 1994 to 2023 was based on a review of the titles and abstracts of the available literature independently by 2 researchers. Because the field of neurology is constantly evolving and leaves many questions unanswered, the purpose was to find the most up-to-date information possible. In addition, we tried to include the latest research in animal models to present the latest findings in the pathophysiology of selected conditions and potential future therapeutic options.
Delayed Awakening After General Anesthesia
Delayed recovery from general anesthesia, by definition, is the failure to regain consciousness within 30–60 min after anesthesia. This condition, which is still one of the biggest challenges for the anesthetist, is mainly caused by pharmacological agents used in the perioperative period, patient pathologies, or accompanying intraoperative complications. The clinical picture mainly includes the altered mental state of the patient and the accompanying respiratory complications [1,3].
Pharmacological Agents
The most common pharmacological cause of prolonged awakening is the pre-pacification of the anesthetic. Residual effects of other drugs used, overly abundant premedication, or drug interactions, especially those unrelated to the operation itself, that prolong the effects of anesthetic drugs are also significant.
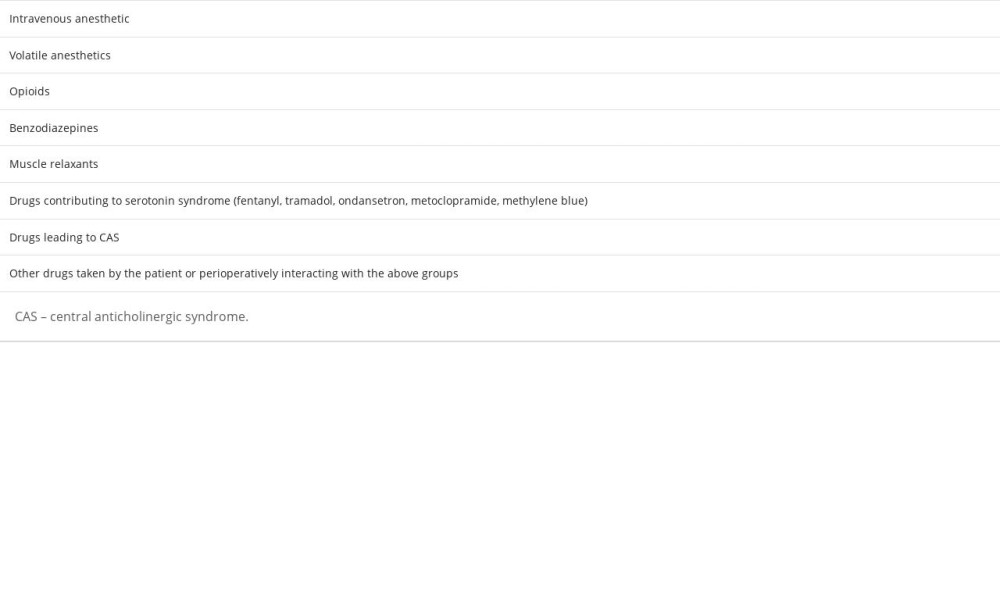
The continuous infusion of intravenous anesthetics to maintain anesthesia, their half-life, and interactions with other drugs are the key mechanisms causing prolonged waking in this group of drugs, while the use of their induction doses does not seem to cause this complication. The adverse effects of volatile anesthetics occur in cases of agents with a higher blood: gas partition coefficient (such as isoflurane), used in obese patients, during longer surgeries (prolonged use), and also take longer to eliminate from the system in hypoventilating patients. Opioids extend recovery by a dual mechanism: directly causing sedation through modulation of opioid receptors, as well as inducing respiratory depression, causing hypercapnia, or slower diffusion of inhaled anesthetics. Benzodiazepines, especially when used in premedication, exacerbate the depressant effects of anesthetics on the CNS (central nervous system) and, especially when combined with high doses of opioids, exacerbate hypoventilation, leading to hypocapnia and coma. Residual action, overdosing or impaired elimination of muscle relaxants results in paralysis, which can be very difficult to differentiate from a prolonged waking state. In addition, drugs like succinylcholine and mivacurium may show prolonged effects due to pseudocholinesterase (PChE) deficiency, caused by selected pathologies, drugs, or genetically [3]. Also, incompletely reversed respiratory muscle relaxation results in impaired lung ventilation. Neuromuscular block is prolonged in patients in hypothermia, with acid-base and electrolyte imbalances, and is caused by many pharmacological interactions, such as aminoglycoside antibiotics, which prolong the action of myorelaxants.
Perioperative serotonin syndrome may also contribute to prolonged recovery. We can distinguish a group of drugs used perioperatively (such as fentanyl, tramadol, ondansetron, metoclopramide, and methylene blue) which, in patients taking drugs with serotonergic effects – drugs from the group of monoamine oxidase inhibitors (MAOIs) or selective serotonin reuptake inhibitors (SSRIs) – can lead to the above syndrome. Also, it is worth remembering the central anticholinergic syndrome (CAS) and the drugs that cause it, which are described in the next section of this article [1,2]. Table 1 summarizes the drugs most frequently causing delayed recovery.
Non-Pharmacological Agents
Non-pharmacological risk factors for prolonged awakening include numerous patient-related risk factors along with comorbidities and other disorders of body function, as well as adverse factors stemming from the surgery itself.
Geriatric patients are often burdened with multimorbidity, are more sensitive to perioperative medications used, and are often characterized by deterioration of CNS function. Pediatric patients also belong to the risk group due to susceptibility to hypothermia resulting in slower drug metabolism or an incompletely mature CNS. There is increasing progress in identifying genetic factors responsible for, among other things, malfunctioning metabolic pathways resulting in prolonged drug effects. Both overweight (larger volume of distribution, forcing higher drug doses) and underweight remain important. Also, it takes significantly longer for men to awake [1,2]. A significant number of comorbidities and metabolic disorders have been described, and the most important ones are listed in Table 2. In addition, patients undergoing prolonged neurosurgery or cardiac surgery (especially when extracorporeal circulation is used) are at increased risk of hypotension and are at higher risk of this complication.
Anticholinergic Syndrome
Anticholinergic syndrome is a collection of symptoms caused by the blocking of muscarinic (M) receptors by drugs that have antimuscarinic effects. In anesthesiology, such effects are mainly manifested by atropine, opioids, benzodiazepines, propofol, ketamine, etomidate, nitrous oxide, and other volatile anesthetics, as well as antipsychotic phenothiazines or butrylphenones. The central receptors are M1 subtype receptors – located mainly in the CNS – are involved in cognitive functions such as perception or attention (anticholinergic delirium is caused by blockade of only this subtype).
Peripheral subtypes, as part of the autonomic nervous system (ANS), include M2, M3, or M4 receptors found in organs such as the heart, lungs, and salivary glands. The clinical picture of anticholinergic syndrome is characterized by a heterogeneity of symptoms, due to which subtypes of muscarinic receptors blockade will be most effective. Based on the predominant symptoms, we can distinguish central and peripheral forms of anticholinergic syndrome. The central (CAS) form is most often manifested by agitation, which can evolve into hyperactive delirium. Hypoactive or mixed forms are much less common, and causes of delirium other than cholinergic blockade should be considered. Other symptoms of the central form include visual and auditory hallucinations, or speech disorders. In contrast, the predominant symptoms of the peripheral form include dilated pupils causing blurred vision and photophobia, hyperthermia, dry skin, mucous membranes, difficulty swallowing, decreased gastrointestinal motility, or urinary retention. There may be a decrease in blood pressure (due to peripheral vasodilatation) or an increase (as a response to agitation) and also sinus tachycardia. Diagnosis of CAS is straightforward when peripheral symptoms are present along with it. However, when, especially in the postoperative setting, peripheral symptoms are negligible or absent and central symptoms are atypical, often co-occurring with sedation or coma, diagnosis becomes much more difficult. The incidence of CAS after general anesthesia is estimated at 8–12% and is known to occur more frequently than after regional anesthesia.
Diagnosing CAS, we should first rule out the usual causes of analogous neurological symptoms, which most often include hypoxemia, hypercapnia, hypoglycemia, or prolonged (rather than toxic in the form of excessive anticholinergic effects) effects of drugs like opioids, muscle relaxants, or sedatives. For the treatment of symptoms of anticholinergic syndrome, physostigmine, which is an acetylcholinesterase inhibitor, is most effective. The dose of physostigmine used to treat postoperative CAS is 1 mg i.v. (it can be divided into doses of 0.5 mg) and the maximum dose in this case is 2 mg i.v. [4].
Postoperative Delirium
Delirium, otherwise known as acute brain syndrome or confusion, is an unstable neurological condition reflected by cognitive dysfunction, characterized primarily by disorganized thinking and lack of attention. The onset is acute (it develops over a period ranging from hours to days) and the above symptoms are usually reversible. Three subtypes can be distinguished: hyperactive (hypervigilance, agitation, aggression); hypoactive (lethargy, decreased psychomotor performance); and mixed, with symptoms common to both of the above. The hypoactive and mixed subtypes are the most common, but can often remain undiagnosed without use of screening tests [5–7]. Delirium is probably the most important postoperative complication, affecting up to 70% of elderly patients (over 60 years old) undergoing major surgery [6,8]. On the other hand, a more recent study showed that of 462 patients operated on for hip fracture, only 74 (16.02%) manifested symptoms of postoperative delirium (however, this result may have been influenced by the following factors: varying sample sizes across studies, different types of delirium, or differences in diagnostic criteria); is often invisible, and is missed by health care professionals in up to 75% of cases [7,9].
Often, postoperative delirium is a marker of brain vulnerability, suggesting the possibility of previously undiagnosed neurological diseases such as depression or early dementia [10], but the pathogenesis of delirium is still unclear and involves many pathophysiological processes taking place. Among the most important appear to be disturbed nerve cell metabolism (abnormal glucose metabolism – putting forward diabetes as a leading cause, imbalance of neurotransmitters, or ongoing inflammation in nerve tissue), endothelial dysfunction, impaired oxygen metabolism, or malnutrition leading to hypoalbuminemia and reduced availability of neutral amino acids.
Despite the complex and not entirely clear pathomechanism of postoperative delirium, there are a number of known risk factors that can cause it. Important known risk factors are pain and discomfort (associated with immobilization, inserted drains, and catheters), infection and inflammation, dehydration, water-electrolyte and acid-base imbalance, blood loss and transfusion, temperature changes, hypoxemia, endocrine disorders, the occurrence of seizures and social isolation or daylight deficiency accompanying the patient’s hospitalization [11,12]. In addition, recent publications have attributed a significant role to patients’ advanced age, low body mass index (BMI (≤19.35 kg/m2), hypoalbuminemia, or abnormal glycemia. The risk of delirium is increased by hyperglycemia >9.5 mmol/L; ≥8.05 mmol/L according to Chu et al [9], but to prevent equally adverse hypoglycemia, more liberal glycemic control should be used in the preoperative period to achieve the desired euglycemic state) [6,9]. The validity of the above factors is supported by the previously mentioned pathophysiological processes leading to postoperative delirium.
Also, drugs used in the perioperative period can trigger more frequent manifestation of delirium in patients at risk. These include opioids, benzodiazepines, volatile anesthetics [6], propofol, antihistamines, glucocorticosteroids, and atropine. In such a situation, it may be necessary to reduce the target dose of the drug [13].
Due to the widespread occurrence of postoperative delirium, great clinical value is attributed to attempts to prevent it, or, if that is not possible, to reduce its complications.
One way to prevent this is to use intravenous anesthesia with simultaneous monitoring by electroencephalography (EEG). This allows strict control of the doses of administered anesthetics in patients prone to developing postoperative delirium, which was also recently confirmed by a retrospective study where 4648 adult patients undergoing various surgical procedures were analyzed [14]. On this basis, it can be expected that the use of regional anesthesia instead of general anesthesia (in patients for whom such a change can be made) would reduce the incidence of postoperative mayhem. However, the research conclusions are not optimistic. Based on small studies where patients were randomly assigned to regional (with moderate sedation) and general anesthesia, Mason et al [15] conducted a meta-analysis. Its results did not reveal a higher incidence of delirium after general than regional anesthesia. Also, a more recent study that analyzed high-risk patients undergoing hip fracture surgery found no difference in the incidence of delirium after each of the 2 anesthesia methods. Another study only found that patients who received deeper anesthesia were more likely to exhibit postoperative delirium [6].
Another method of preventing postoperative delirium is the pharmacological agents used in the therapeutic process. Neuroleptics such as haloperidol, olanzapine, quetiapine, or risperidone may be helpful. Also, subanesthetic doses of ketamine, or the use of dexmedetomidine [9] as a drug for postoperative sedation of patients, may have a beneficial effect on reducing delirium episodes. In high-risk patients, it is advisable to use non-sedative analgesics and, although the advantage over general anesthesia was not established in the study, to use regional anesthesia if possible.
Postoperative Cognitive Dysfunction (POCD)
Postoperative cognitive dysfunction is a complex of neurological disorders, usually of mild severity, mainly impairing memory and concentration; elderly patients are at highest risk. According to a multicenter study of patients over 60 years of age, the incidence of POCD was 25% at 1 week after surgery and 10% after 3 months. Persistent POCD is estimated to occur in 1% of patients. The exact cause of the development of POCD is still unknown, but its cause could be a combination of sources like surgery, anesthesia, or the patient’s co-existing diseases (a significant role is attributed to vascular pathology leading to various types of ischemic strokes in the brain) [16]. The results of the study did not show that the use of general or regional anesthesia affects the incidence of this complication [17]. It is now known that hypoxemia and inadequate cerebral perfusion are not the leading pathophysiological mechanisms leading to POCD [18]. Based on animal models, there is a theory that an important component of POCD pathophysiology is the inflammatory response to anesthesia and surgery [19,20]. Recent studies show that prolonged or repeated general anesthesia can lead to cognitive impairment due to the neurotoxic properties of inhaled sevoflurane and intravenous ketamine. These anesthetics disrupt iron metabolism, leading to ferropoptosis of neurons [21].
Perioperative Stroke (POS) After General Anesthesia
Perioperative stroke is a stroke (of ischemic or hemorrhagic etiology, where 95% of cases are due to ischemia) occurring during or up to 30 days after surgery [22]. Surgeries other than cardiology, vascular, or neurology cause far fewer strokes than the 3 mentioned above. Non-cardiac, vascular, and neurological surgeries result in a much lower incidence of stroke than the 3 mentioned above. According to recent literature, the incidence of POS in patients undergoing non-cardiac and non-neurosurgical surgery ranges from 0.1% to 0.8%. In contrast, the averaged incidence after cardiac surgery is 4.6%, but there are large discrepancies depending on the type of surgery [23]. In reality, these results appear to be much higher. Patients may experience clinically silent strokes, with no apparent neurological dysfunction, and stroke symptoms often appear late and are thus diagnosed and classified differently from POS by other clinical centers. In addition, patients may be exposed to anesthetics or sedatives during the development of stroke symptoms [22,24]. The Neuro Vision Pilot Study suggests that the incidence of clinically unmanifested silent strokes confirmed by neuroimaging – MRI (magnetic resonance imaging) and CT (computed tomography) – after non-cardiac surgery is up to 10% [25]. The mortality rate can be up to 8 times higher than for patients suffering non-operative strokes and can reach 26%.
The risk factors for POS generally overlap with those causing non-operative strokes. Also, the pathophysiological background is complex and for each type of surgery, a specific model of the pathophysiological mechanism leading to CNS stroke plays a key role. For example, in cardiac surgery patients we can distinguish between 2 types of stroke: early (intraoperative) and late (postoperative). They differ in their pathophysiological mechanisms. In early stroke, atherosclerotic plaque embolization and cerebral hypoperfusion seem to play a key role; therefore, it is important to maintain MAP more than 64 mmHg during surgery. Late stroke, on the other hand, results from cardiac rhythm abnormalities such as atrial fibrillation, hypercoagulability, or intracranial atherosclerosis [23]. Recent studies in animal models have also shown that increased neuroinflammatory responses induced by both trauma itself and surgical interference can influence the pathogenesis of POS [22].
Since intravenous thrombolysis is contraindicated after the procedure, the therapeutic options remain intravenous thrombolytic administration and mechanical thrombectomy, which is currently the most important therapeutic tool [23,24]. Stroke prevention plays a very important role, which includes other methods in addition to maintaining optimal blood pressure during the procedure, or individually selected anticoagulant treatment for a particular condition predisposing to excessive clotting, like neuromonitoring during surgery. These include electroencephalography (EEG), somatosensory evoked potentials (SSEP), transcranial Doppler study (TCD) [24], and near-infrared spectroscopy (NIRS) [26,27].
Due to the discovery of neuroinflammation as a potential pathophysiological pathway leading to perioperative stroke, modulation of inflammation plays an important role in prevention and treatment. In addition to some inhaled and intravenous anesthetics commonly used in anesthesiology, there is research into new therapies that have neuroprotective effects by combating neuroinflammation. These include using gases (such as hydrogen, argon, or xenon), pharmaceuticals (eg, nerinetide to counteract oxidative stress), immune or stem cell therapies (Treg and mesenchymal lymphocytes or neural stem cells, respectively) and the use of preconditioning or follow-up procedures [22]. The hope in therapy is also to use the possibilities of nanotechnology, which makes it possible to use nanoparticles as nanocarriers to precisely deliver drugs with revascularization, anti-inflammatory, neuroregenerative, or anti-reactive oxygen species effects to brain tissue [28].
Spinal Cord Ischemia (SCI)
Spinal cord ischemia, as a serious postoperative complication, can arise at any stage of the operation and is most often associated with surgical treatment of aneurysms of the thoracoabdominal segment of the aorta. Ischemia can lead to incomplete spinal cord injury, and causes certain neurological manifestations; paraplegia and paraparesis are mentioned most often. The clinical presentation resembles a “man in a barrel,” and it is reported that weakness more often involves the upper extremities [29,30]. On the other hand, Xue et al [31], studying SCI after thoracic endovascular aortic surgery (TEVAR), define this complication as the onset of transient or permanent paraplegia or paraparesis occurring after the above surgeries, manifested by losses in motor or sensory functions of the lower extremities, or urinary or bowel incontinence. They found that 1.69% of the 650 patients selected for the study developed SCI after TEVAR, more of whom underwent surgery under general anesthesia (45.5% vs 17.7%,
The spinal cord is supplied by segmental arteries, which are branches of the aorta, vertebral, intercostal, lumbar, and sacral arteries. The decrease in core perfusion includes a group of risk factors specific to vascular surgery such as extensive endograft coverage of the aorta, previous or current surgery on the abdominal or thoracic aorta [31], or the use of an aortic clamp for longer than 60 min. We can surmise that there are also risk factors that are not specific only to vascular surgery. A very important one is perioperative hypotension (mean arterial pressure (MAP) <70 mmHg) and any disorder that can lead to hemodynamic instability. Therefore, counteracting hypotension is one of the preventive strategies for SCI. A low hemoglobin level is among these factors and it was found that a high normal baseline level is a protective factor and, if necessary, attempts should be made to normalize it. Other proven preventive interventions include controlling CSF (cerebrospinal fluid) pressure and, if it is elevated, dropping fluid level by lumbar puncture, or using intraoperative hypothermia [31].
The literature also considers sudden intubation or prolonged neck flexion during surgery may be associated with ischemic pathology [29]. Also at risk for SCI are geriatric patients with frequent spondyloid lesions. For this reason, it is important to carefully evaluate patients’ cervical spine for stenosis or the aforementioned spondylosis and to avoid straightening the neck during intubation [30]. Recent studies in a mouse model with transient spinal ischemia found that intranasal administration of polysulfides protected against delayed paraplegia. In the future, this may prove to be an invaluable therapeutic method for treating patients with SCI [33].
Postoperative Visual Loss (POVL)
Although up to 4% of patients reported blurred vision lasting at least 3 days, most of these were caused by mechanical abrasions of the cornea and can be easily prevented by carefully taping the eye during general anesthesia. Postoperative vision loss is a much rarer but serious complication that occurs after non-ophthalmic surgeries [34,35]. The most common causes of this complication are ischemic neuropathy of the optic nerve and obstruction of the retinal arteries, while less common causes include cortical blindness, acute glaucoma, or hemorrhage in the choroid or vitreous body. The incidence of POVL ranges from 1 in 125 000 to 1 in 60 000 anesthesia procedures counted in the general surgical population in the USA. It was also report that ischemic neuropathy of the optic nerve is the most common complication of cardiac surgery (0.086%) and spinal surgery (0.03%) [34]. Another source (The 2017 Florida State Inpatient Database) [35] reports that out of 630 439 patients undergoing surgery, POVL developed in 76 patients, of which 46 cases were due to retinal vascular obstruction, 24 were due to ischemic neuropathy of the optic nerve, and the remaining 6 were sudden loss of vision. Retinal artery obstruction is more common in elderly patients and in patients with hypotension. In 75% of cases, the embolic material is of atherosclerotic origin. Treatment methods include the use of hypo-osmotic vitreous volume-lowering agents and anti-edema drugs, as well as the use of vasodilators, alteplase, or breaking up the embolus with a YAG (yttrium aluminum garnet) laser.
Ischemic neuropathy of the optic nerve is caused by impaired flow in the posterior short ciliary arteries and involves partial or complete infarction of the intraocular portion of the II nerve. The cause of perfusion abnormalities is most often a prolonged drop in blood pressure, intraoperative hypovolemia, and thrombotic or embolic material. Among the group of patients particularly vulnerable to ischemic neuropathy are those suffering from diabetes, hypertension, vascular atherosclerosis, or atrial fibrillation. Carey et al also report that ischemic nerve neuropathy is also associated with an increase in intraocular pressure (IOP), which is influenced by the patient’s abdominal position (prone position) during surgery. In a randomized trial [36], the authors report that reversal of the Trendelenburg position on the abdomen significantly reduces IOP, which may have a role in the prevention of POLV of ischemic neuropathy etiology. Also, the use of brimonidine (an α2 agonist) has been proven to outweigh the use of colloids or crystalloids in reducing ICP (intracranial pressure) during surgery performed in the abdominal position, but the researchers attributed a more important role to maintaining adequate blood pressure [37].
Much rarer cortical blindness occurs as a result of global or focal (in the occipital lobes) hypoperfusion of the cerebral cortex, caused in addition to hypoxemia, vasoconstriction, or cerebral vascular thrombosis and intracranial pressure increase or hemorrhage.
Conclusions
The most common cause of neurological complications in anesthesiology is drug effects, and this cause in the case of complications in waking a patient should be excluded at the beginning. The next in terms of prevalence are postoperative delirium or postoperative cognitive dysfunction (POCD), whose not rare overlapping clinical picture means that they can often be difficult to differentiate. On the other hand, focal neurological disorders are extremely rare in clinical practice, which include perioperative stroke (POS), spinal cord ischemia (SCI), and postoperative visual loss (POVL).
The spectrum of neurological complications after general anesthesia is certainly much broader than the pathologies described above. Examples include seizures after surgery, but there is still a paucity of literature on this problem. Neurosurgery is known to be a risk factor [38], but it is believed that the main problem is poor control of epileptic seizures before surgery rather than the type of anesthesia, and most of the anesthetics used have anticonvulsant effects. Because of the lack of data and the uncertain role of general anesthesia, it is difficult to determine the incidence – one study puts the incidence of epilepsy within 1 year after general anesthesia at 0.41/1000 patients [39].
Future Directions
To reduce the incidence of neurological complications after general anesthesia, it must be managed carefully. The best solution is to stick to the “5N” rule, that is, to keep the surgical patient normotensive, normovolemic, normocapnic, normoglycemic, and normothermic. In the case of prolonged disorders of consciousness, an overdose of anesthetic and muscle relaxant drugs should be ruled out first, while a further step is to consider a CT scan of the head to detect possible organic CNS lesions. Also, one should be aware that the incidence rates of individual complications given above are subject to underestimation due to the complex clinical picture of disease entities that are difficult to differentiate clearly, especially by inexperienced doctors. Symptoms can be subtle and under-expressed, and often masked by other symptoms. Anesthesiologists should be aware of the patient’s baseline neurological status before proceeding with general anesthesia, the patient’s modifiable and non-modifiable risk factors, and the risk of particular types of surgery on particular neurological disorders. This knowledge will allow both to appropriately modify the patient’s perioperative management and to be prepared for the occurrence of specific neurological complications, which will allow for easier diagnosis and faster internal treatment.
References
1. Misal US, Joshi SA, Shaikh MM, Delayed recovery from anesthesia: A postgraduate educational review: Anesth Essays Res, 2016; 10(2); 164-72
2. Thomas E, Martin F, Pollard B, Delayed recovery of consciousness after general anaesthesia: BJA Educ, 2020; 20(5); 173-79
3. Ellis TA, Edberg JL, Kumar N, Applefield DJ, Delayed emergence from anesthesia: A simulation case for anesthesia learners: MedEdPORTAL, 2017; 13; 10628
4. Dawson AH, Buckley NA, Pharmacological management of anticholinergic delirium – theory, evidence and practice: Br J Clin Pharmacol, 2016; 81(3); 516-24
5. Meagher DJ, Moran M, Raju B, Motor symptoms in 100 patients with delirium versus control subjects: Comparison of subtyping methods: Psychosomatics, 2008; 49(4); 300-8
6. Lee YL, Wong J, Ng SY, Delirium in patients following general anaesthesia: Ann Acad Med Singap, 2022; 51(2); 71-73
7. Kotfis K, Ely EW, Shehabi Y, Intensive care unit delirium – a decade of learning: Lancet Respir Med, 2023; 11(7); 584-86
8. Mashour GA, Woodrum DT, Avidan MS, Neurological complications of surgery and anaesthesia: Br J Anaesth, 2015; 114(2); 194-203
9. Chu Z, Wu Y, Dai X, The risk factors of postoperative delirium in general anesthesia patients with hip fracture: Attention needed: Medicine (Baltimore), 2021; 100(22); e26156
10. Davis DH, Muniz Terrera G, Keage H, Delirium is a strong risk factor for dementia in the oldest-old: A population-based cohort study: Brain, 2012; 135(Pt 9); 2809-16
11. Stefano GB, Bilfinger TV, Fricchione GL, The immune-neuro-link and the macrophage: Postcardiotomy delirium, HIV-associated dementia and psychiatry: Prog Neurobiol, 1994; 42(4); 475-88
12. Lynch EP, Lazor MA, Gellis JE, The impact of postoperative pain on the development of postoperative delirium: Anesth Analg, 1998; 86(4); 781-85
13. Clegg A, Young JB, Which medications to avoid in people at risk of delirium: A systematic review: Age Ageing, 2011; 40(1); 23-29
14. Sumner M, Deng C, Evered L, Processed electroencephalography-guided general anaesthesia to reduce postoperative delirium: A systematic review and meta-analysis: Br J Anaesth, 2023; 130(2); e243-e53
15. Mason SE, Noel-Storr A, Ritchie CW, The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: A systematic review with meta-analysis: J Alzheimers Dis, 2010; 22(Suppl 3); 67-79
16. Evered LA, Silbert BS, Postoperative cognitive dysfunction and noncardiac surgery: Anesth Analg, 2018; 127(2); 496-505
17. Bhushan S, Huang X, Duan Y, Xiao Z, The impact of regional versus general anesthesia on postoperative neurocognitive outcomes in elderly patients undergoing hip fracture surgery: A systematic review and meta-analysis: Int J Surg, 2022; 105; 106854
18. Moller JT, Cluitmans P, Rasmussen LS, Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction: Lancet, 1998; 351(9106); 857-61 Erratum in: Lancet 1998;351(9117):1742
19. Terrando N, Monaco C, Ma D, Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline: Proc Natl Acad Sci USA, 2010; 107(47); 20518-22
20. Cibelli M, Fidalgo AR, Terrando N, Role of interleukin-1beta in postoperative cognitive dysfunction: Ann Neurol, 2010; 68(3); 360-68
21. Wu J, Yang JJ, Cao Y, Iron overload contributes to general anaesthesia-induced neurotoxicity and cognitive deficits: J Neuroinflammation, 2020; 17(1); 110
22. Jin X, Li P, Michalski D, Perioperative stroke: A perspective on challenges and opportunities for experimental treatment and diagnostic strategies: CNS Neurosci Ther, 2022; 28(4); 497-509
23. Leary MC, Varade P, Perioperative stroke: Curr Neurol Neurosci Rep, 2020; 20(5); 12
24. Jacas A, Magaldi M, Fàbregas N, Valero R, Postoperative stroke: The picture is out of focus: Eur J Anaesthesiol, 2018; 35(11); 896-98
25. Mrkobrada M, Hill M, Chan M, The neurovision pilot study: Noncardiac surgery carries a significant risk of acute covert stroke: Stroke, 2013; 44; ATMP9
26. Murkin JM, Adams SJ, Novick RJ, Monitoring brain oxygen saturation during coronary bypass surgery: A randomized, prospective study: Anesth Analg, 2007; 104(1); 51-58
27. Ono M, Joshi B, Brady K, Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke: Br J Anaesth, 2012; 109(3); 391-98
28. An J, Zhao L, Duan R, Potential nanotherapeutic strategies for perioperative stroke: CNS Neurosci Ther, 2022; 28(4); 510-20
29. https://doi.org/10.1055/s-0042-1744397
30. Buchowski JM, Kebaish KM, Suk KS, Kostuik JP, Central cord syndrome after total hip arthroplasty: A patient report: Spine (Phila Pa 1976), 2005; 30(4); E103-5 Erratum in: Spine. 2005 Apr 1;30(7):845
31. Xue L, Luo S, Ding H, Risk of spinal cord ischemia after thoracic endovascular aortic repair: J Thorac Dis, 2018; 10(11); 6088-96
32. Martin DJ, Martin TD, Hess PJ, Spinal cord ischemia after TEVAR in patients with abdominal aortic aneurysms: J Vasc Surg, 2009; 49(2); 302-6 discussion 306–7
33. Kanemaru E, Miyazaki Y, Marutani E, Intranasal administration of polysulfide prevents neurodegeneration in spinal cord and rescues mice from delayed paraplegia after spinal cord ischemia: Redox Biol, 2023; 60; 102620
34. Raphael J, Moss HE, Roth S, Perioperative visual loss in cardiac surgery: J Cardiothorac Vasc Anesth, 2019; 33(5); 1420-29
35. Lin JC, French DD, Margo CE, Greenberg PB, Epidemiology of postoperative visual loss for non-ocular surgery in a cohort of inpatients: Eye (Lond), 2022; 36(6); 1323-25
36. Carey TW, Shaw KA, Weber ML, DeVine JG, Effect of the degree of reverse Trendelenburg position on intraocular pressure during prone spine surgery: A randomized controlled trial: Spine J, 2014; 14(9); 2118-26
37. Farag E, Sessler DI, Kovaci B, Effects of crystalloid versus colloid and the α-2 agonist brimonidine versus placebo on intraocular pressure during prone spine surgery: A factorial randomized trial: Anesthesiology, 2012; 116(4); 807-15
38. Elbashary AS, Alshwairikh KA, Almaanie R, Seizure following general anesthesia for cystoscopy and urethral dilatation: A case report: Saudi J Anaesth, 2022; 16(2); 246-48
39. Chang HC, Liao CC, Chang CC, Risk of epilepsy in surgical patients undergoing general or neuraxial anaesthesia: Anaesthesia, 2018; 73(3); 323-31
In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952