04 August 2020: Database Analysis
Sirtuin 3 mRNA Expression is Downregulated in the Brain Tissues of Alzheimer’s Disease Patients: A Bioinformatic and Data Mining Approach
Shuang Song12ABCDEF, Bin Li12A, Zhen Jia12C, Li Guo12AG*DOI: 10.12659/MSM.923547
Med Sci Monit 2020; 26:e923547
Abstract
BACKGROUND: Emerging experimental evidence has shown that sirtuin 3 (SIRT3), which is a class III histone deacetylase, participates in the pathological process of Alzheimer’s disease (AD). However, data mining of current gene expression databases, such as Gene Expression Omnibus (GEO), has not been previously performed to determine whether SIRT3 expression is upregulated or downregulated in the brain tissues of AD patients.
MATERIAL AND METHODS: Eight RNA expression chip datasets of AD brains in the GEO database were selected, and GEO2R analysis was conducted to identify the differentially expressed genes (DEGs) between the AD and control groups. Furthermore, the SIRT3 mRNA levels between the AD and control groups and their relationships with the DEGs and diagnosis of AD were evaluated. Finally, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of both the AD-related DEGs and the SIRT3-related DEGs were conducted.
RESULTS: The SIRT3 mRNA levels were downregulated in 7 of 8 databases and were related to the diagnosis of AD in 7 databases, with an area under the curve (AUC) of the receiver operating characteristic curve (ROC curve) greater than 50%. Additionally, GO and KEGG analyses showed that SIRT3 downregulation could affect neuroactive ligand-receptor interactions, the MAPK signaling pathway, long-term potentiation, the calcium signaling pathway and axon guidance in AD patients.
CONCLUSIONS: SIRT3 mRNA is downregulated in the brain tissues of AD patients, promoting the progression of AD.
Keywords: Alzheimer Disease, Computational Biology, Sirtuin 3, Aged, 80 and over, Area Under Curve, Brain, data mining, Databases, Genetic, Disease Progression, Down-Regulation, Gene Expression Profiling, gene ontology, RNA, Messenger, ROC Curve, young adult
Background
Alzheimer’s disease (AD) is one of most common neurodegenerative diseases, but the pathogenesis has not been fully elucidated. Globally, it is estimated that approximately 24 million individuals, most of whom are elderly people, are suffering from this disease, but some individuals show early onset disease [1]. Patients usually present with memory loss and sometimes display other atypical changes, such as behavioral and psychological symptoms and even motor dysfunction [2]. In addition, AD can occur with other neurodegenerative diseases, such as Parkinson’s disease, suggesting possible common etiologies and pathogenic mechanisms. Given the variance of the clinical manifestations of AD, its pathogenesis is suggested to be complex and multifactorial. The major pathological changes of this disease are aberrant Aβ protein accumulation and toxicity from hyperphosphorylated tau, leading to senile plaques and neurofibrillary tangles. However, the causes and changes in the downstream pathways of these pathological events remain unclear.
The sirtuin family is a 7-member protein family that mediates deacetylation [3]. Thus, these enzymes can post-translationally interact with a large group of proteins carrying lysine residues, including transcription factors [4], histones and enzymes [5]. Sirtuins are class III histone deacetylases (HDACs) because of a unique property – the requirement for nicotinamide adenine dinucleotide (NAD+) as a cofactor – which allows these proteins to sense and orchestrate the metabolic homeostasis of cells to fight against age-related diseases. Sirtuins participate in many aging-related cellular processes, such as the tricarboxylic acid cycle, elimination of reactive oxygen species (ROS), and mitophagy. These proteins show different localizations in cells. Sirtuin 3 (SIRT3) is mainly expressed in mitochondria, which have high levels of NAD+ and its substrates; thus, this enzyme can sense changes in cellular metabolic status and regulate various metabolic processes.
We selected SIRT3 because recent experimental evidence has shown that low SIRT3 protein expression is observed in the brain tissues of AD patients and correlates with the pathological and clinical severity of this disease. In brain samples from AD patients for autopsy and the 3xTG mouse model of AD (Psen1, APPSwe, and TauP301L mutations), SIRT3 protein expression was downregulated [6]. Moreover, the SIRT3 protein levels in the brain tissues of AD patients were negatively correlated with the levels of tau tangles and amyloid plaques and positively correlated with cognitive function scores, including the 60-item Boston Naming Test and the Mattis dementia rating scale [7,8]. An
Theoretically, SIRT3 mRNA levels may not show the same trend as that of its translated product. SIRT3 mRNA levels were also reported to be downregulated in the brain tissue cortex of AD patients [15], but some researchers reported opposite findings [16]. To date, data mining of RNA microarray results from current databases to determine whether SIRT3 is downregulated in brain tissues has not been performed. Using public AD-related RNA microarray datasets, one can easily analyze changes in mRNAs of interest from a large sample to obtain more convincing results than those from a single experiment.
In this study, 8 datasets including RNA expression data from the brain tissues of AD patients were selected and analyzed using bioinformatic methods to determine whether the SIRT3 mRNA levels were downregulated in these samples. Moreover, we investigated whether this change was unique for AD or was found in other neurodegenerative diseases and discussed whether this change contributes to the diagnosis of AD.
Material and Methods
DATABASE SELECTION:
Eight datasets of mRNA expression data from microarray analyses of AD brain tissues were selected from the NCBI Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds) because they had a relatively large sample number. The GEO accession numbers were GSE33000 [17], GSE15222 [18], GSE48350 [19], GSE122063, GSE132903 [20], GSE44770 [21], GSE44771 [21], and GSE44768 [21]. The demographic features of these datasets are summarized. Among them, GSE33000 includes brains samples from Huntington’s disease (HD) patients, and GSE122063 includes brain samples from VaD patients. In addition, 2 databases of blood samples from AD patients (GSE63061 [22] and GSE63060 [22]), 2 of brain tissue samples from PD patients (GSE7621 [23] and GSE20291 [24]) and 1 of blood samples from HD patients (GSE1751 [25]) were also selected to determine whether the change in SIRT3 mRNA is specific to AD or is found in many neurodegenerative diseases. All these open access datasets and related research were approved by the local ethics committee, and our further data mining of these datasets complied with the Declaration of Helsinki.
GEO2R ANALYSIS, RECEIVER OPERATING CHARACTERISTIC (ROC) CURVE ANALYSIS AND MULTIVARIATE LOGISTIC REGRESSION:
GEO2R, which is an online tool offered by the GEO database website aiming to identify differentially expressed genes (DEGs) between selected groups, was used to analyze these datasets. The
DEG SELECTION AND GO AND KYOTO ENCYCLOPEDIA OF GENES AND GENOMES (KEGG) ANALYSES:
DEGs were selected from the GEO2R analysis results according to the following criteria: 1) |log2FC| ≥0.5, 2) adjusted
SOFTWARE AND STATISTICAL METHODS:
RStudio 3.6.1, SPSS 25.0 and Excel 2010 were used for statistical analysis. GEO data were downloaded into local RStudio using the GEOquery package, and statistical processing was conducted using the dplyr package. The Kruskal-Wallis test and Wilcoxon rank sum test/Mann-Whitney test were performed using the stats package and ggpubr package. Spearman’s rank correlation coefficients were calculated by the Hmisc package. Logistic regression was performed using SPSS 25.0. The pROC package was used to generate ROC curves and related figures. Other plots were generated using ggplot2, ggpubr, UpSetR, ggplotify, ggsignif, and the cowplot package in RStudio.
DEGs were identified by the GEO2R webtool, which can conduct
Results
SIRT3 MRNA WAS DOWNREGULATED IN THE BRAIN TISSUES OF AD PATIENTS:
The demographic features of the 8 datasets in which the AD patients were all pathologically diagnosed with AD and the samples were all from brain tissues are summarized in Table 1. According to the GEO2R analysis results, SIRT3 was downregulated in 7 of them, and the upregulation or downregulation of sirtuin mRNA is summarized in Table 2. In addition to SIRT3, SIRT5 was downregulated in the majority of the datasets. In contrast, SIRT1, SIRT2, and SIRT7 were upregulated in the majority of the datasets. However, the fold changes of these sirtuin-related RNA expression levels between the case and control groups were relatively small, while the |log2FC| of only 4 of all 82 sirtuin-related RNA samples in these datasets was larger than 0.5 (Figure 1).
SIRT3 MRNA DOWNREGULATION WAS A UNIQUE TREND IN THE BRAIN TISSUES OF AD PATIENTS:
When conducting GEO2R analysis in GSE63060, GSE63061 (AD blood samples), GSE20291 and GSE7621 (PD brain tissues), as well as GSE1751 (HD blood samples), we found that the SIRT2, SIRT6,and SIRT7 transcriptional levels were upregulated in the blood samples from AD patients, while SIRT1 and SIRT5 were differentially expressed in the blood samples from the HD patients. In PD brain tissues, no significant changes in sirtuin-related RNA were detected. However, this result in the PD group might be biased due to the small sample number of each group. The GEO2R results of other neurodegenerative diseases and AD blood samples are shown in Table 3.
In dataset GSE33000, there were 2 probes related to SIRT5, named SIRT5.1 (probe ID 10023826451, GenBank accession number NM_012241) and SIRT5.2 (probe ID 10025904845, GenBank accession number NM_031244). The multigroup Kruskal-Wallis test showed that the RNA levels of the sirtuins, except for SIRT6, were significantly different among the 3 groups, and the SIRT1-SIRT4 as well as the SIRT5.2 RNA levels were significantly different between the HD and AD groups, and the P-values (Bonferroni method and adjusted Wilcoxon rank sum test/Mann-Whitney test) were 3.90E-06, 1.50E-05, 5.60E-07, 1.00E-04, and 0.029. The multigroup comparison results are shown in Figure 2.
In dataset GSE122063, there were 2 probes related to SIRT2: SIRT2.1 (probe ID 2946, GenBank accession number NM_001193286) and SIRT2.2 (probe ID 41723, GenBank accession number NM_012237). There were 2 probes related to SIRT3: SIRT3.1 (probe ID 27463, GenBank accession number AK074992) and SIRT3.2 (probe ID 50835, GenBank accession number NM_012239). There were 4 probes related to SIRT5: SIRT5.1 (probe ID 27999, GenBank accession number NM_031244), SIRT5.2 (probe ID 40657, GenBank accession number NM_012241), SIRT5.3 (probe ID 44391, GenBank accession number NM_012241), and SIRT5.4 (probe ID 56262, GenBank accession number NM_031244). There were 2 probes related to SIRT7: SIRT7.1 (probe ID 23571, GenBank accession number NM_016538) and SIRT7.2 (probe ID 27708, GenBank accession number NM_016538). The multigroup Kruskal-Wallis test showed that the RNA levels of the sirtuins, except for SIRT4, were significantly different among the VaD, AD, and control groups. Further post hoc comparison between the AD and VaD groups showed that SIRT1, SIRT3.2, and SIRT5.3 were significantly differentially expressed, with adjusted P-values of 0.042, 0.0068, and 0.019, respectively. The multigroup comparison results are shown in Figure 3.
Combining these findings, we found that the SIRT3 mRNA (NM_012239) expression levels in the AD group were significantly different from those of the healthy control group and the other neurodegenerative disease groups in the brain tissues (PD, HD, and VaD). Moreover, SIRT3 mRNA downregulation was not detected in the blood samples from the AD and HD groups in our analysis.
SIRT3 MRNA DOWNREGULATION WAS RELATED TO THE DIAGNOSIS AND PROMOTED THE PROGRESSION OF AD:
To evaluate the diagnostic value of SIRT3 mRNA expression in AD, we generated ROC curves for 8 AD brain tissue datasets. In 7 of the datasets, the 95% CI of the AUC was greater than 50%, which suggested that SIRT3 mRNA expression has diagnostic value in AD. For these 7 datasets, further logistic regression for diagnosis including 3 variables showed that all 95% CIs of the OR for SIRT3 mRNA expression were less than 1, indicating that high SIRT3 expression is a protective factor against AD. The ROC curve and the ORs for logistic regression are shown in Figure 4.
GO AND KEGG ANALYSES OF THE AD-RELATED DEGS AND THE SIRT3 DOWNREGULATION-RELATED DEGS:
Finally, 1610 AD-related DEGs were selected and are shown in Figure 5A, and 507 SIRT3-related DEGs were selected and are shown in Figure 5B. GO and KEGG analyses of these DEGs showed that several biological processes, cellular components, molecular functions and pathways were enriched in the brain tissues of AD patients (Figures 6, 7), and several were correlated with SIRT3 downregulation.
Discussion
AD is the most common cause of dementia, which results in a heavy burden on both individuals and societies, leading to substantial public health expenses. In the USA, more than 4.7 million individuals greater than 65 years of age suffered from AD in 2010, and the total number of AD patients will reach 13.8 million in 2050 [26]. Furthermore, the number of deaths among elderly AD patients is estimated to reach 1.6 million [27] in 2050, comprising 43% of the deaths among the elderly population. Elucidation of the associated changes in the molecular pathways in AD will help reveal the pathogenesis and identify potential targets to develop effective drugs for treatment of AD.
Sirtuins, including SIRT1–SIRT7, are an enzyme family characterized by their deacetylation activity. They are NAD+-dependent enzymes; widespread in the cytoplasm, nucleus, and mitochondria; and participate in many biological processes, especially energy production and biogenesis [3,28]. These enzymes can affect cell signal transduction through deacetylation of not only histones but also many extranuclear proteins. Some evidence has shown that sirtuins play an important role in neurodegenerative disease. A case-control study of 2 Caucasian cohorts found that the single nucleotide polymorphism (SNP) SIRT2 rs10410544 (T allele) was linked to the diagnosis of AD (95% CI for the OR, 1.02–1.43,
Previous findings suggested that SIRT3 may play an important role in AD, and AD and SIRT3 interactions have been identified during the disease process. On the one hand, SIRT3 protein upregulation seems to be a protective factor in AD, and SIRT3 protein or mRNA upregulation was observed when many kinds of neuroprotective drugs were administered to animal and cell models of AD [12–14,32]. SIRT3 protein downregulation was found in the brain tissues of AD patients [7,9]. On the other hand, some evidence has shown that AD may affect the expression of SIRT3. The oxidative stress associated with AD can activate poly (ADP-ribose) polymerase 1 (PARP-1), which can decrease NAD+ and promote ROS generation, leading to upregulation of SIRT3 expression [3]. Aβ can inhibit the activity of SIRT3 [9]. The detailed interaction between AD and SIRT3 has not been fully elucidated.
The detailed protective mechanism of SIRT3 in AD is still unclear, but to date, some experimental evidence has been published. First, Aβ can disrupt the metabolism and function of the mitochondria, leading to an insufficient energy supply [9] and brain aging. However, SIRT3 can post-translationally deacetylate many key enzymes in the Krebs cycle and fat metabolism, including complex I subunit NDUFA9 [33], lactate dehydrogenase A (LDHA) [34] and MnSOD [35], to improve mitochondrial metabolism and the ATP supply and ameliorate hypometabolism in the brain tissues of AD individuals. Second, honokiol, a SIRT3 activator, can reduce the Aβ level, which may be related to the activation of SIRT3-PGC-1a, and then downregulate the activity of β-site-APP cleaving enzyme (BACE) [32]. Third, due to the sufficient ATP supply and deacetylation of key proteins such as PGC-1a and MnSOD, the oxidative stress level is controlled. Fourth, p53 can downregulate the transcription of ND2 and ND4 in mitochondrial DNA, increase the production of ROS and damage brain tissue. Utilizing the GST technique, researchers found that SIRT3 can directly interact with p53, deacetylate it at the K320 site, and then downregulate ROS production [15]. Fifth, SIRT3 can enhance the activity of OGG1, which can repair DNA damaged by ROS [36]. Sixth, SIRT3 can interact with FOXO3a, a transcription factor related to longevity, and then affect mitochondrial homeostasis [37].
Because of the unstable and changeable character of mRNA, its detection through quantitative real-time PCR has a relatively large experimental error. Due to regulatory mechanisms such as microRNAs, lncRNAs and circRNAs, SIRT3 transcription may be upregulated, downregulated or unchanged in AD and is not necessarily consistent with the SIRT3 protein levels. Contradictory findings about the mRNA changes in AD patients and animal brain tissues have been reported [15,16,38]. In transgenic PDAPP mice, the SIRT3 mRNA levels were spatially upregulated and correlated with Aβ protein accumulation, and the same result was found in autopsies of human AD brains [16]. However, another report showed that SIRT3 mRNA is downregulated in cortex samples from AD individuals [15]. A meta-analysis using data mining methods in large samples will likely identify actual changes in SIRT3 mRNA in AD. In our research, we used a public microarray database to validate the SIRT3 mRNA expression and found that the SIRT3 mRNA level was downregulated in the brain tissues of AD patients, which was consistent with the change in SIRT3 protein.
Further analysis showed that SIRT3 mRNA downregulation was significantly different in AD compared to HD and VaD, indicating that SIRT3 mRNA downregulation may play a unique role in AD. We did not detect any sirtuin RNA changes in PD brain tissue using GEO2R, perhaps because of the small sample size of the selected datasets. Some evidence has shown that PD can affect sirtuins. Compared with Aβ, which can damage the function of SIRT3, extracellular alpha-synuclein (eASN), which is an important component of the Lewy bodies in PD, can activate the transcription of SIRT1 and SIRT3 [39].
ROC curve results and ORs generated from logistic regression analyses further indicated that SIRT3 mRNA downregulation contributes to the final diagnosis of AD because the 95% CI of the AUC was greater than 50% in 7 of 8 analyzed datasets. Multifactor regression demonstrated that SIRT3 mRNA upregulation is a protective factor against AD. However, it is impossible to diagnose AD through brain biopsy, and a single RNA measurement is not sufficient to diagnose such a complicated disease. Furthermore, we investigated the possible changes in SIRT3 mRNA levels in the blood samples from AD and HD patients. We did not detect any changes possibly because mRNA is unstable and can be degraded by RNases in peripheral blood serum. The ROC analysis indicated the importance of SIRT3 mRNA changes in the pathogenesis of AD, which should be investigated in detail in the future. Our multifactor regression analysis suggests that SIRT3 upregulation may be an effective way to inhibit the progression of AD, and further KEGG and GO analyses identified promising pathways and genes to investigate in the future, such as cell-cell signaling, transmission of nerve impulse and synaptic transmission (the first 3 had the most significant adjusted P values). According to our analysis, neuroactive ligand-receptor interactions, the MAPK signaling pathway, long-term potentiation, the calcium signaling pathway and axon guidance are involved in SIRT3 mRNA downregulation in AD.
A change in a single mRNA cannot explain the disease pathogenesis of AD, which is a limitation of our research. Further SIRT3 mRNA-microRNA-circRNA regulatory networks and SIRT3 gene methylation should be assessed in the future. A profile of blood microRNAs and circRNAs, which are more stable in blood samples, can be identified by machinery learning, such as the kNN technique, to promote early diagnosis of AD.
Conclusions
SIRT3 mRNA levels are downregulated in the brain tissues of AD patients, which is unique in AD compared to other neurodegenerative diseases, such as PD, HD, and VaD. SIRT3 mRNA downregulation in brain tissues can help diagnose AD with good sensitivity and specificity, and SIRT3 mRNA upregulation is a protective factor against AD. However, SIRT3 mRNA levels do not change in the blood samples of AD patients. Many biological processes, such as cell-cell signaling, transmission of nerve impulse and synaptic transmission, are related to SIRT3 mRNA downregulation.
Figures
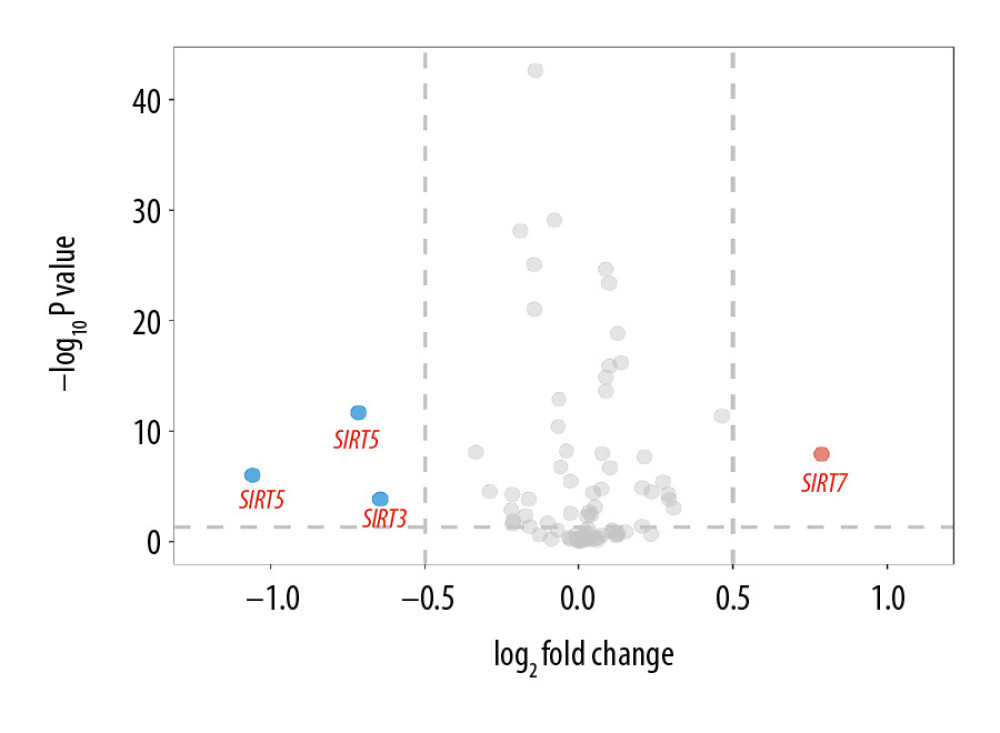 Figure 1. Volcano plot of sirtuin-related RNA changes in 8 datasets between the Alzheimer’s disease and control groups.
Figure 1. Volcano plot of sirtuin-related RNA changes in 8 datasets between the Alzheimer’s disease and control groups. 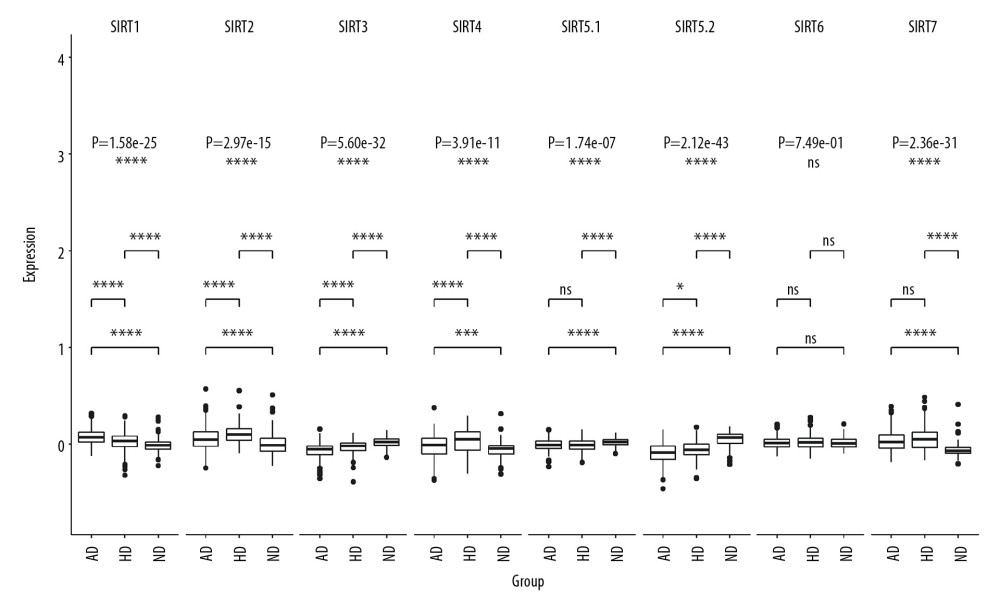 Figure 2. Boxplot of multigroup comparisons of sirtuin-related RNA levels among the Huntington’s disease, Alzheimer’s disease (AD) and control groups in dataset GSE33000. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P-values among the Huntington’s disease, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. **** P<0.0001, *** P<0.001, ** P<0.01, * P<0.05 and ns P≥0.05. ND indicates “no dementia” as the control group.
Figure 2. Boxplot of multigroup comparisons of sirtuin-related RNA levels among the Huntington’s disease, Alzheimer’s disease (AD) and control groups in dataset GSE33000. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P-values among the Huntington’s disease, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. **** P<0.0001, *** P<0.001, ** P<0.01, * P<0.05 and ns P≥0.05. ND indicates “no dementia” as the control group. 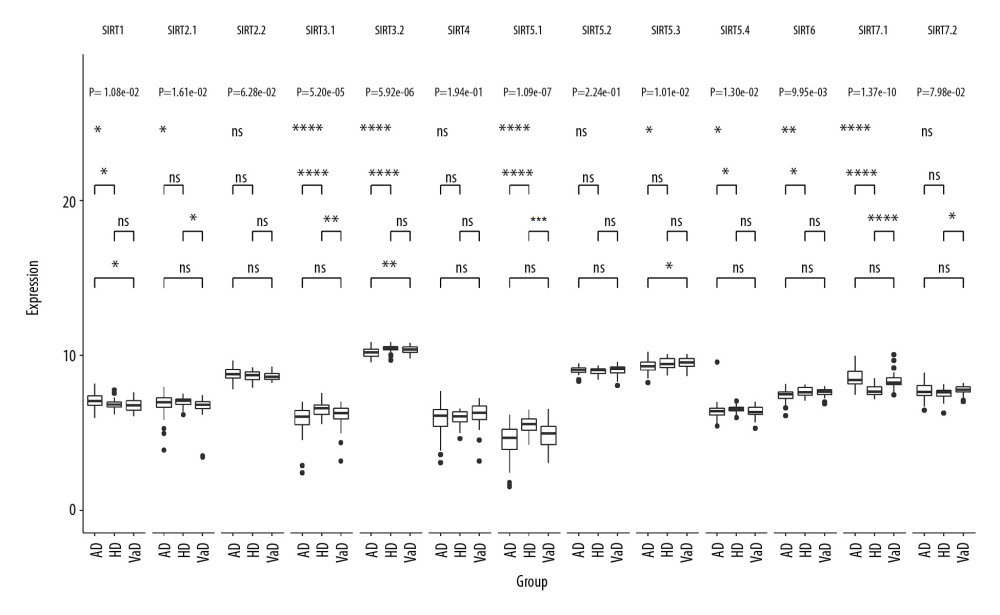 Figure 3. Boxplot of multigroup comparisons of the sirtuin-related RNA expression levels among the VaD, Alzheimer’s disease (AD) and control groups in dataset GSE122063. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P values among the VaD, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. Represents P<0.0001, represents P<0.001, ** P<0.01, ** P<0.05, and ns P≥0.05. ND indicates “no dementia” as the control group.
Figure 3. Boxplot of multigroup comparisons of the sirtuin-related RNA expression levels among the VaD, Alzheimer’s disease (AD) and control groups in dataset GSE122063. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P values among the VaD, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. Represents P<0.0001, represents P<0.001, ** P<0.01, ** P<0.05, and ns P≥0.05. ND indicates “no dementia” as the control group. 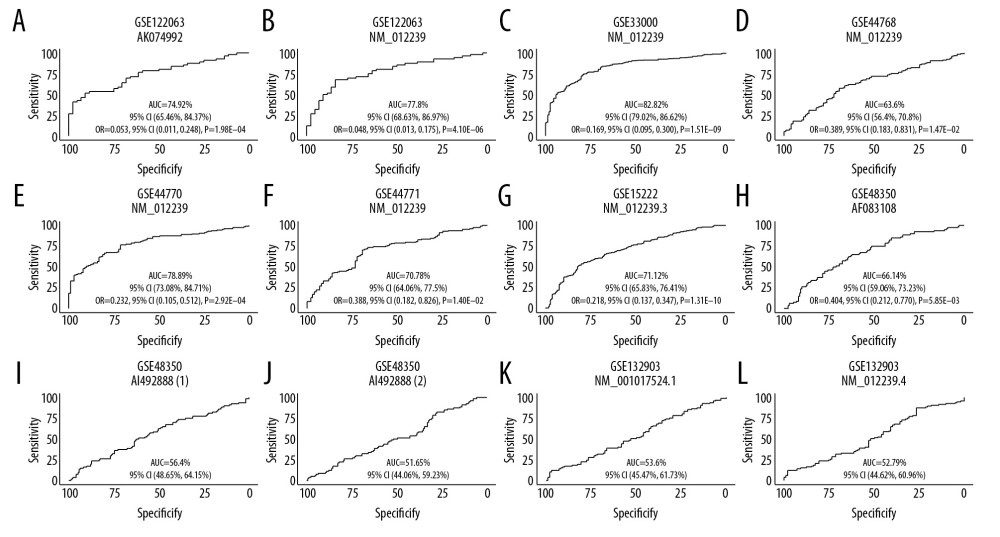 Figure 4. (A–L) ROC curve of the SIRT3 RNA levels for diagnosis of Alzheimer’s disease in 8 datasets. The gene symbol and GenBank accession ID on top of each ROC curve provide the subgroup information. The AUC, 95% CI of the AUC, OR, 95% CI of the OR and Wald test P value of SIRT3 RNA expression are shown under the curve. If the 95% CI of the AUC included 50%, further logistic regression would not proceed. ROC – receiver operating characteristic curve; AUC – area under the curve; CI – confidence interval; OR – odds ratio.
Figure 4. (A–L) ROC curve of the SIRT3 RNA levels for diagnosis of Alzheimer’s disease in 8 datasets. The gene symbol and GenBank accession ID on top of each ROC curve provide the subgroup information. The AUC, 95% CI of the AUC, OR, 95% CI of the OR and Wald test P value of SIRT3 RNA expression are shown under the curve. If the 95% CI of the AUC included 50%, further logistic regression would not proceed. ROC – receiver operating characteristic curve; AUC – area under the curve; CI – confidence interval; OR – odds ratio. 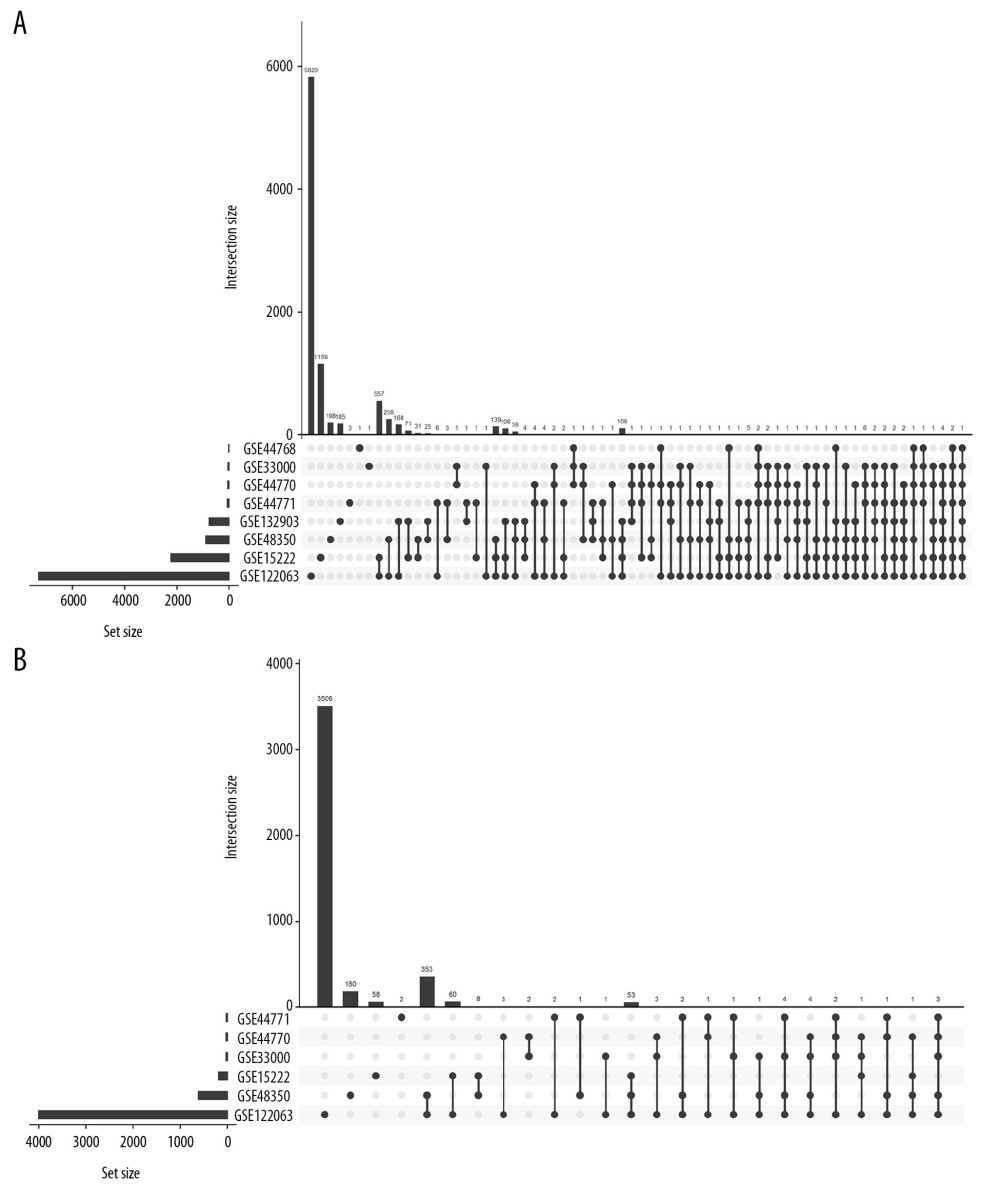 Figure 5. Histogram of the differentially expressed genes (DEGs) and the SIRT3-related DEGs in 8 datasets. (A) Histogram of the Alzheimer’s disease (AD)-related DEGs in 8 datasets. The bars parallel to the X axis represent gene counts in each dataset, and the bars parallel to the Y axis represent the intersection gene counts of selected datasets. The dots below the X axis indicate which datasets make up the intersection. (B) Histogram of the SIRT3-related DEGs in 6 datasets, as there was no SIRT3-related DEG in GSE44768 and SIRT3 did not have a significant difference between the AD and control groups in GSE132903.
Figure 5. Histogram of the differentially expressed genes (DEGs) and the SIRT3-related DEGs in 8 datasets. (A) Histogram of the Alzheimer’s disease (AD)-related DEGs in 8 datasets. The bars parallel to the X axis represent gene counts in each dataset, and the bars parallel to the Y axis represent the intersection gene counts of selected datasets. The dots below the X axis indicate which datasets make up the intersection. (B) Histogram of the SIRT3-related DEGs in 6 datasets, as there was no SIRT3-related DEG in GSE44768 and SIRT3 did not have a significant difference between the AD and control groups in GSE132903. 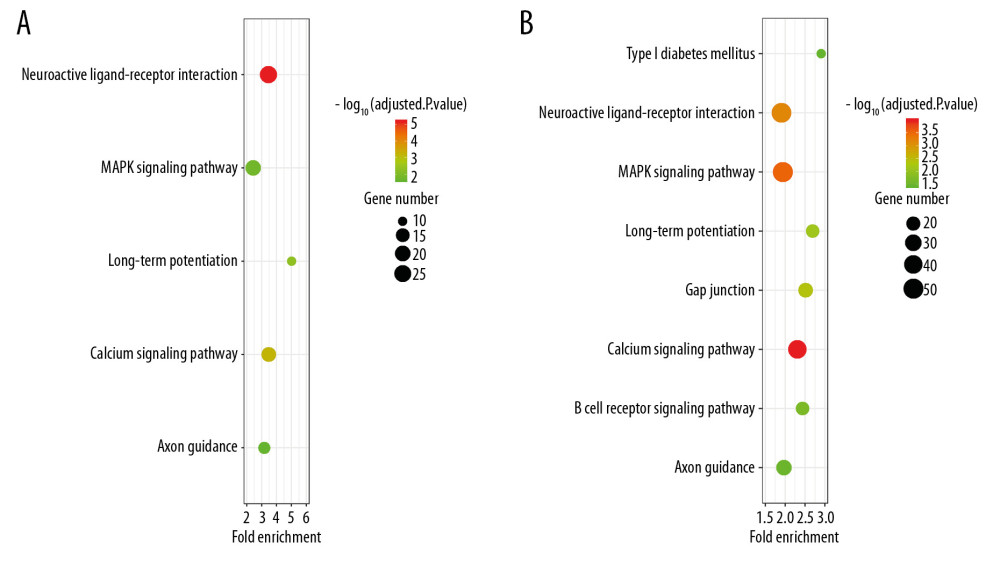 Figure 6. Bubble plot for the enriched Kyoto Encyclopedia of Genes and Genomes pathways of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A) The enriched pathways of the SIRT3-related DEGs. (B) The enriched pathways of the AD-related DEGs. The size of the bubble represents the gene number enriched in that pathway, and the color of the bubble represents the adjusted P-value of the pathway. The X axis represents the fold change of the pathway.
Figure 6. Bubble plot for the enriched Kyoto Encyclopedia of Genes and Genomes pathways of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A) The enriched pathways of the SIRT3-related DEGs. (B) The enriched pathways of the AD-related DEGs. The size of the bubble represents the gene number enriched in that pathway, and the color of the bubble represents the adjusted P-value of the pathway. The X axis represents the fold change of the pathway. 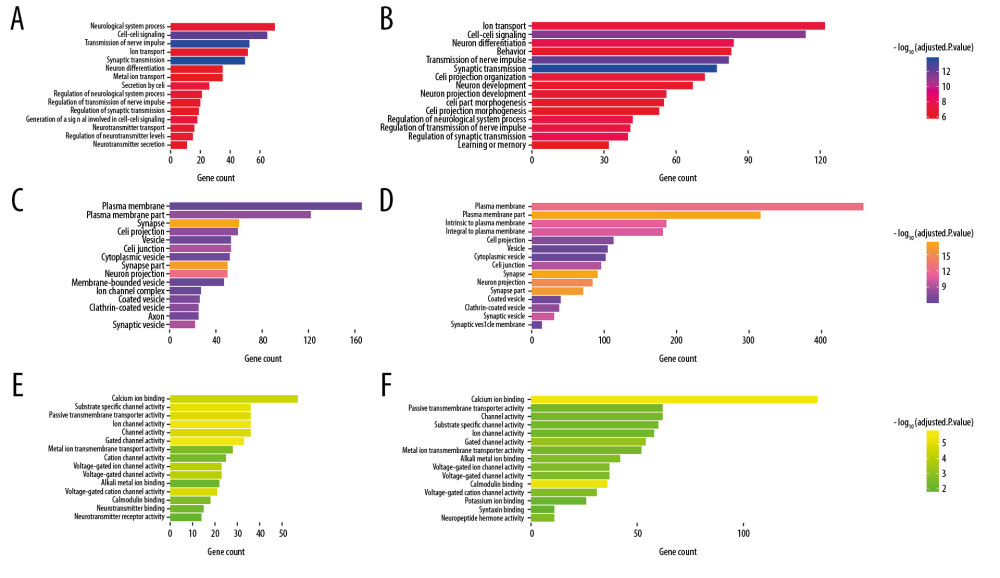 Figure 7. Box plot for enriched Gene Ontology biological process, cellular component, and molecular function categories of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A, B) Top 15 enriched biological processes of the SIRT3-related DEGs and the AD-related DEGs, respectively, ranked by adjusted P-value. (C, D) Top 15 enriched cellular components of the SIRT3- and AD-related DEGs, respectively. (E, F) Top 15 enriched molecular functions of the SIRT3- and AD-related DEGs, respectively. The color of the box represents the adjusted P-value of the entities, and the X axis represents the gene number involved in the entity.
Figure 7. Box plot for enriched Gene Ontology biological process, cellular component, and molecular function categories of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A, B) Top 15 enriched biological processes of the SIRT3-related DEGs and the AD-related DEGs, respectively, ranked by adjusted P-value. (C, D) Top 15 enriched cellular components of the SIRT3- and AD-related DEGs, respectively. (E, F) Top 15 enriched molecular functions of the SIRT3- and AD-related DEGs, respectively. The color of the box represents the adjusted P-value of the entities, and the X axis represents the gene number involved in the entity. Tables
Table 1. Demographic features of 8 RNA expression datasets from microarray analyses of Alzheimer’s disease brain tissues included in the study.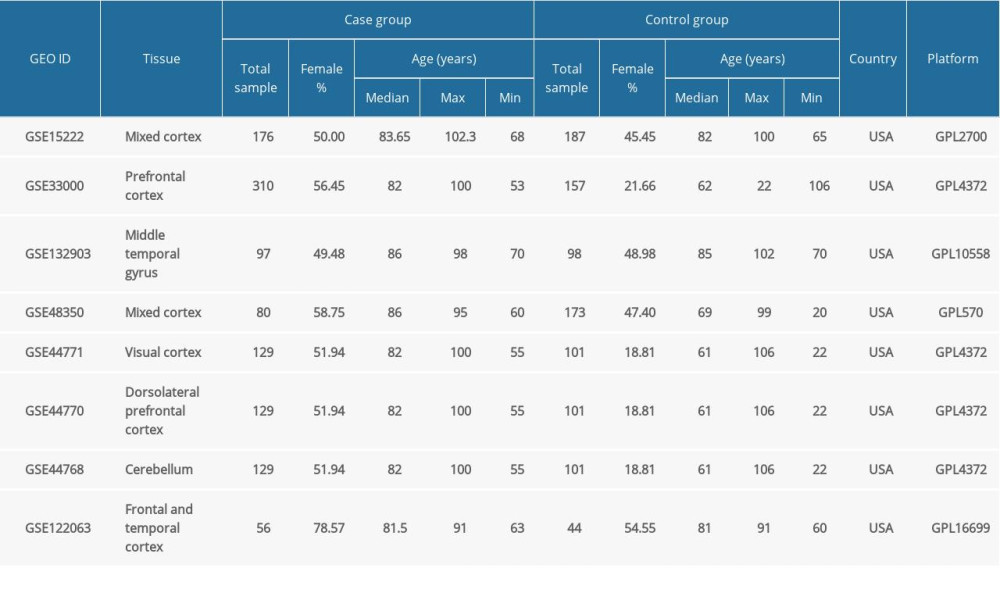 Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets.
Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets.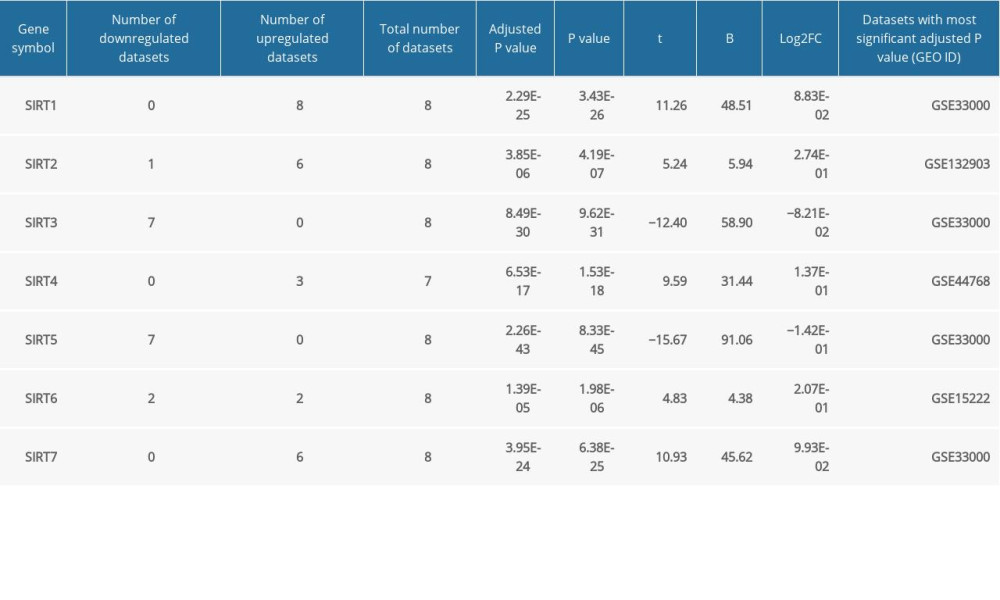 Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients.
Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients.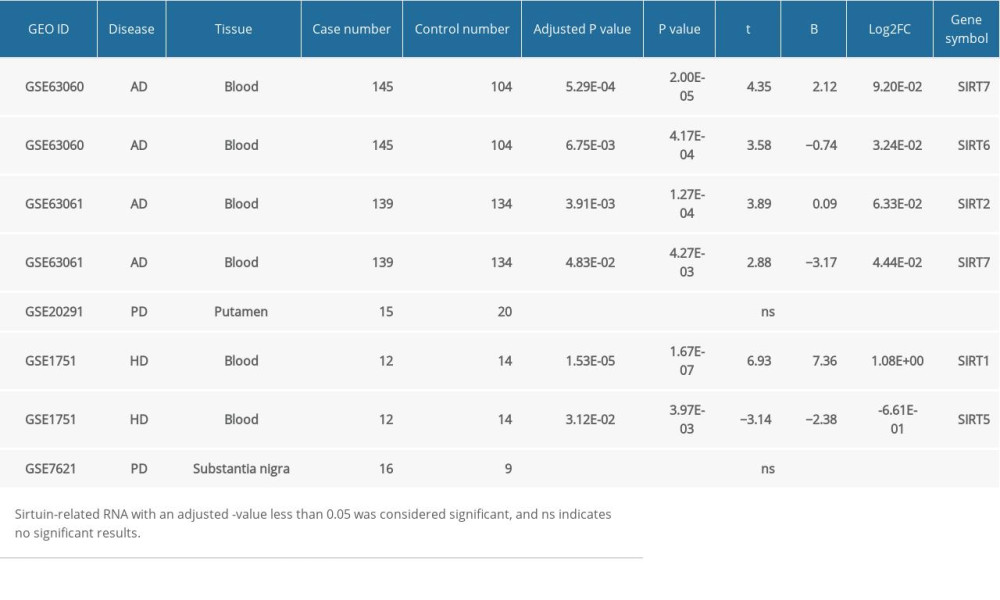
References
1. Erkkinen MG, Kim M-O, Geschwind MD, Clinical neurology and epidemiology of the major neurodegenerative diseases: Cold Spring Harb Perspect Biol, 2018; 10(4); a033118
2. Vöglein J, Paumier K, Jucker M, Clinical, pathophysiological and genetic features of motor symptoms in autosomal dominant Alzheimer’s disease: Brain, 2019; 142(5); 1429-40
3. Ansari A, Rahman MS, Saha SK, Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease: Aging Cell, 2017; 16(1); 4-16
4. Jęśko H, Strosznajder RP, Sirtuins and their interactions with transcription factors and poly(ADP-ribose) polymerases: Folia Neuropathol, 2016; 54(3); 212-33
5. van de Ven RAH, Santos D, Haigis MC, Mitochondrial sirtuins and molecular mechanisms of aging: Trends Mol Med, 2017; 23(4); 320-31
6. Han P, Tang Z, Yin J, Pituitary adenylate cyclase-activating polypeptide protects against beta-amyloid toxicity: Neurobiol Aging, 2014; 35(9); 2064-71
7. Li S, Yin J, Nielsen M, Sirtuin 3 mediates tau deacetylation: J Alzheimers Dis, 2019; 69(2); 355-62
8. Yin J, Han P, Song M, Amyloid-beta increases tau by mediating sirtuin 3 in Alzheimer’s disease: Mol Neurobiol, 2018; 55(11); 8592-601
9. Yin J, Li S, Nielsen M, Sirtuin 3 attenuates amyloid-beta induced neuronal hypometabolism: Aging, 2018; 10(10); 2874-83
10. Liu Y, Cheng A, Li YJ, SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice: Nat Commun, 2019; 10(1); 1886
11. Yin J, Nielsen M, Li S, Shi J, Ketones improves apolipoprotein E4-related memory deficiency via sirtuin 3: Aging, 2019; 11(13); 4579-86
12. Ruankham W, Suwanjang W, Wongchitrat P, Sesamin and sesamol attenuate H2O2 -induced oxidative stress on human neuronal cells via the SIRT1, SIRT3-FOXO3a signaling pathway: Nutr Neurosci, 2019 [Online ahead of print]
13. Cosin-Tomas M, Senserrich J, Arumi-Planas M, Role of resveratrol and selenium on oxidative stress and expression of antioxidant and anti-aging genes in immortalized lymphocytes from Alzheimer’s disease patients: Nutrients, 2019; 11(8); 1764
14. Li H, Jia J, Wang W, Honokiol alleviates cognitive deficits of Alzheimer’s disease (PS1V97L) transgenic mice by activating mitochondrial SIRT3: J Alzheimers Dis, 2018; 64(1); 291-302
15. Lee J, Kim Y, Liu T, SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease: Aging Cell, 2018; 17(1); e12679
16. Weir HJ, Murray TK, Kehoe PG, CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer’s disease: PLoS One, 2012; 7(11); e48225
17. Narayanan M, Huynh JL, Wang K, Common dysregulation network in the human prefrontal cortex underlies two neurodegenerative diseases: Mol Syst Biol, 2014; 10; 743
18. Webster JA, Gibbs JR, Clarke J, Genetic control of human brain transcript expression in Alzheimer disease: Am J Hum Genet, 2009; 84(4); 445-58
19. Blair LJ, Nordhues BA, Hill SE, Accelerated neurodegeneration through chaperone-mediated oligomerization of tau: J Clin Invest, 2013; 123(10); 4158-69
20. Piras IS, Krate J, Delvaux E, Transcriptome changes in the Alzheimer’s disease middle temporal gyrus: importance of RNA metabolism and mitochondria-associated membrane genes: J Alzheimers Dis, 2019; 70(3); 691-713
21. Zhang B, Gaiteri C, Bodea L-G, Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease: Cell, 2013; 153(3); 707-20
22. Sood S, Gallagher IJ, Lunnon K, A novel multi-tissue RNA diagnostic of healthy ageing relates to cognitive health status: Genome Biol, 2015; 16; 185
23. Lesnick TG, Papapetropoulos S, Mash DC, A genomic pathway approach to a complex disease: axon guidance and Parkinson disease: PLoS Genet, 2007; 3(6); e98
24. Zhang Y, James M, Middleton FA, Davis RL, Transcriptional analysis of multiple brain regions in Parkinson’s disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms: Am J Med Genet B Neuropsychiatr Genet, 2005; 137B(1); 5-16
25. Borovecki F, Lovrecic L, Zhou J, Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease: Proc Natl Acad Sci USA, 2005; 102(31); 11023-28
26. Hebert LE, Weuve J, Scherr PA, Evans DA, Alzheimer disease in the United States (2010–2050) estimated using the 2010 census: Neurology, 2013; 80(19); 1778-83
27. Weuve J, Hebert LE, Scherr PA, Evans DA, Deaths in the United States among persons with Alzheimer’s disease (2010–2050): Alzheimers Dement, 2014; 10(2); e40-46
28. Albani D, Polito L, Forloni G, Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: Experimental and genetic evidence: J Alzheimers Dis, 2010; 19(1); 11-26
29. Polito L, Kehoe PG, Davin A, The SIRT2 polymorphism rs10410544 and risk of Alzheimer’s disease in two Caucasian case-control cohorts: Alzheimers Dement, 2013; 9(4); 392-99
30. Braidy N, Poljak A, Grant R, Differential expression of sirtuins in the aging rat brain: Front Cell Neurosci, 2015; 9; 167
31. Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG, Distinct patterns of sirtuin expression during progression of Alzheimer’s disease: Neuromolecular Med, 2014; 16(2); 405-14
32. Ramesh S, Govindarajulu M, Lynd T, SIRT3 activator Honokiol attenuates beta-Amyloid by modulating amyloidogenic pathway: PLoS One, 2018; 13(1); e0190350
33. Ahn B-H, Kim H-S, Song S, A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis: Proc Natl Acad Sci USA, 2008; 105(38); 14447-52
34. Cui Y, Qin L, Wu J, SIRT3 enhances glycolysis and proliferation in SIRT3-expressing gastric cancer cells: PLoS One, 2015; 10(6); e0129834
35. Zou X, Santa-Maria CA, O’Brien J, Manganese superoxide dismutase acetylation and dysregulation, due to loss of SIRT3 activity, promote a luminal B-Like breast carcinogenic-permissive phenotype: Antioxid Redox Signal, 2016; 25(6); 326-36
36. Cheng Y, Ren X, Gowda AS, Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress: Cell Death Dis, 2013; 4; e731
37. Jacobs KM, Pennington JD, Bisht KS, SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression: Int J Biol Sci, 2008; 4(5); 291-99
38. Cieslik M, Czapski GA, Strosznajder JB, The molecular mechanism of amyloid beta42 peptide toxicity: The role of sphingosine kinase-1 and mitochondrial sirtuins: PLoS One, 2015; 10(9); e0137193
39. Motyl J, Wencel PL, Cieslik M, Alpha-synuclein alters differently gene expression of Sirts, PARPs and other stress response proteins: implications for neurodegenerative disorders: Mol Neurobiol, 2018; 55(1); 727-40
Figures
 Figure 1. Volcano plot of sirtuin-related RNA changes in 8 datasets between the Alzheimer’s disease and control groups.
Figure 1. Volcano plot of sirtuin-related RNA changes in 8 datasets between the Alzheimer’s disease and control groups. Figure 2. Boxplot of multigroup comparisons of sirtuin-related RNA levels among the Huntington’s disease, Alzheimer’s disease (AD) and control groups in dataset GSE33000. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P-values among the Huntington’s disease, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. **** P<0.0001, *** P<0.001, ** P<0.01, * P<0.05 and ns P≥0.05. ND indicates “no dementia” as the control group.
Figure 2. Boxplot of multigroup comparisons of sirtuin-related RNA levels among the Huntington’s disease, Alzheimer’s disease (AD) and control groups in dataset GSE33000. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P-values among the Huntington’s disease, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. **** P<0.0001, *** P<0.001, ** P<0.01, * P<0.05 and ns P≥0.05. ND indicates “no dementia” as the control group. Figure 3. Boxplot of multigroup comparisons of the sirtuin-related RNA expression levels among the VaD, Alzheimer’s disease (AD) and control groups in dataset GSE122063. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P values among the VaD, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. Represents P<0.0001, represents P<0.001, ** P<0.01, ** P<0.05, and ns P≥0.05. ND indicates “no dementia” as the control group.
Figure 3. Boxplot of multigroup comparisons of the sirtuin-related RNA expression levels among the VaD, Alzheimer’s disease (AD) and control groups in dataset GSE122063. At the top of the figure, sirtuin gene symbols indicate subgroup information; below, Kruskal-Wallis P values among the VaD, AD, and control groups are shown. At the bottom of the figure, boxes representing the sirtuin-related RNA expression values from the microarray are shown, and the post hoc subgroup comparison results are shown between the RNA expression boxes and Kruskal-Wallis P-values. Represents P<0.0001, represents P<0.001, ** P<0.01, ** P<0.05, and ns P≥0.05. ND indicates “no dementia” as the control group. Figure 4. (A–L) ROC curve of the SIRT3 RNA levels for diagnosis of Alzheimer’s disease in 8 datasets. The gene symbol and GenBank accession ID on top of each ROC curve provide the subgroup information. The AUC, 95% CI of the AUC, OR, 95% CI of the OR and Wald test P value of SIRT3 RNA expression are shown under the curve. If the 95% CI of the AUC included 50%, further logistic regression would not proceed. ROC – receiver operating characteristic curve; AUC – area under the curve; CI – confidence interval; OR – odds ratio.
Figure 4. (A–L) ROC curve of the SIRT3 RNA levels for diagnosis of Alzheimer’s disease in 8 datasets. The gene symbol and GenBank accession ID on top of each ROC curve provide the subgroup information. The AUC, 95% CI of the AUC, OR, 95% CI of the OR and Wald test P value of SIRT3 RNA expression are shown under the curve. If the 95% CI of the AUC included 50%, further logistic regression would not proceed. ROC – receiver operating characteristic curve; AUC – area under the curve; CI – confidence interval; OR – odds ratio. Figure 5. Histogram of the differentially expressed genes (DEGs) and the SIRT3-related DEGs in 8 datasets. (A) Histogram of the Alzheimer’s disease (AD)-related DEGs in 8 datasets. The bars parallel to the X axis represent gene counts in each dataset, and the bars parallel to the Y axis represent the intersection gene counts of selected datasets. The dots below the X axis indicate which datasets make up the intersection. (B) Histogram of the SIRT3-related DEGs in 6 datasets, as there was no SIRT3-related DEG in GSE44768 and SIRT3 did not have a significant difference between the AD and control groups in GSE132903.
Figure 5. Histogram of the differentially expressed genes (DEGs) and the SIRT3-related DEGs in 8 datasets. (A) Histogram of the Alzheimer’s disease (AD)-related DEGs in 8 datasets. The bars parallel to the X axis represent gene counts in each dataset, and the bars parallel to the Y axis represent the intersection gene counts of selected datasets. The dots below the X axis indicate which datasets make up the intersection. (B) Histogram of the SIRT3-related DEGs in 6 datasets, as there was no SIRT3-related DEG in GSE44768 and SIRT3 did not have a significant difference between the AD and control groups in GSE132903. Figure 6. Bubble plot for the enriched Kyoto Encyclopedia of Genes and Genomes pathways of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A) The enriched pathways of the SIRT3-related DEGs. (B) The enriched pathways of the AD-related DEGs. The size of the bubble represents the gene number enriched in that pathway, and the color of the bubble represents the adjusted P-value of the pathway. The X axis represents the fold change of the pathway.
Figure 6. Bubble plot for the enriched Kyoto Encyclopedia of Genes and Genomes pathways of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A) The enriched pathways of the SIRT3-related DEGs. (B) The enriched pathways of the AD-related DEGs. The size of the bubble represents the gene number enriched in that pathway, and the color of the bubble represents the adjusted P-value of the pathway. The X axis represents the fold change of the pathway. Figure 7. Box plot for enriched Gene Ontology biological process, cellular component, and molecular function categories of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A, B) Top 15 enriched biological processes of the SIRT3-related DEGs and the AD-related DEGs, respectively, ranked by adjusted P-value. (C, D) Top 15 enriched cellular components of the SIRT3- and AD-related DEGs, respectively. (E, F) Top 15 enriched molecular functions of the SIRT3- and AD-related DEGs, respectively. The color of the box represents the adjusted P-value of the entities, and the X axis represents the gene number involved in the entity.
Figure 7. Box plot for enriched Gene Ontology biological process, cellular component, and molecular function categories of the Alzheimer’s disease (AD)-related differentially expressed genes (DEGs) and the SIRT3-related DEGs. (A, B) Top 15 enriched biological processes of the SIRT3-related DEGs and the AD-related DEGs, respectively, ranked by adjusted P-value. (C, D) Top 15 enriched cellular components of the SIRT3- and AD-related DEGs, respectively. (E, F) Top 15 enriched molecular functions of the SIRT3- and AD-related DEGs, respectively. The color of the box represents the adjusted P-value of the entities, and the X axis represents the gene number involved in the entity. Tables
 Table 1. Demographic features of 8 RNA expression datasets from microarray analyses of Alzheimer’s disease brain tissues included in the study.
Table 1. Demographic features of 8 RNA expression datasets from microarray analyses of Alzheimer’s disease brain tissues included in the study. Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets.
Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets. Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients.
Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients. Table 1. Demographic features of 8 RNA expression datasets from microarray analyses of Alzheimer’s disease brain tissues included in the study.
Table 1. Demographic features of 8 RNA expression datasets from microarray analyses of Alzheimer’s disease brain tissues included in the study. Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets.
Table 2. Summary of the GEO2R analysis of the sirtuin mRNA levels between the Alzheimer’s disease and control groups in 8 datasets. Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients.
Table 3. GEO2R analysis of microarray datasets of samples from other neurodegenerative diseases and blood samples from Alzheimer’s disease patients. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








