16 June 2020: Clinical Research
Lower Platelet Aggregation Is a Risk Factor for Dual Antiplatelet Therapy-Associated Bleeding: A Preliminary Retrospective Study with Genotype Analysis
Dongdong Yuan1ABCD, Xiangfen Shi2BCD, Liping Guo1BF, Gaobiao Wang1BF, Yujie Zhao1B, Yuling Yang1B, Hanjuan Zhang1B, Qiong Huang1B, Yiqiang Yuan1AE*DOI: 10.12659/MSM.923758
Med Sci Monit 2020; 26:e923758
Abstract
BACKGROUND: The purpose of this study was to investigate factors influencing bleeding in patients with acute coronary syndrome (ACS) who are on aspirin and ticagrelor as dual antiplatelet therapy.
MATERIAL AND METHODS: This retrospective case-control study included 50 patients with ACS (25 with reported bleeding events and 25 without) on aspirin and ticagrelor. Adenosine diphosphate (ADP)- and arachidonic acid (ACA)-induced platelet aggregation rates were measured using light transmission aggregometry. Single-nucleotide polymorphisms (SNPs) in PEAR1, GP1BA, and GSTP1 were genotyped.
RESULTS: ACA-induced platelet aggregation rates were obviously lower in patients with bleeding events than in those without (13.28±8.46% vs. 24.93±9.89%, P<0.001). No significant differences in ADP-induced platelet aggregation rates were observed between the 2 groups (16.17±9.74% vs. 16.88±12.69%, P>0.05). Among those with bleeding events and among controls, 70% and 80% had an ACA-induced platelet aggregation rate of 0–18% and 18–50%, respectively. Mutation rates of rs6065 in GP1BA and rs1695, rs4891, and rs8191439 in GSTP1 also differed significantly between the 2 groups.
CONCLUSIONS: Lower ACA-induced platelet aggregation rates are associated with increased risk of bleeding in patients with ACS who are on aspirin and ticagrelor. An ACA-induced platelet aggregation rate of 18% may be considered the cutoff point for identifying high risk of aspirin-associated bleeding events in patients with ACS. SNP genotyping may also help predict the risk of bleeding in patients with ACS.
Keywords: acute coronary syndrome, Genotype, Hemorrhage, Platelet Aggregation, Adenosine Diphosphate, Arachidonic Acid, Aspirin, Case-Control Studies, Dual Anti-Platelet Therapy, Glutathione S-Transferase pi, percutaneous coronary intervention, Platelet Aggregation Inhibitors, Platelet Glycoprotein GPIb-IX Complex, Polymorphism, Single Nucleotide, Receptors, Cell Surface, ticagrelor
Background
Patients with acute coronary syndrome (ACS) often need percutaneous coronary intervention, such as stenting [1]. However, the procedure may cause injury to the arterial endothelium and platelet activation. Platelet activation and aggregation increase the risk of coronary thrombosis and recurrent ACS. Therefore, antiplatelet therapy is necessary in ACS patients after interventional treatment to reduce the risk of ischemic events without increasing the risk of bleeding. Recent guidelines from the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommend aspirin and ticagrelor as post-procedural dual antiplatelet therapy in patients with ACS [2,3].
Ticagrelor is an oral, direct, reversibly binding, P2Y12 receptor antagonist [4]. The P2Y12 receptor is the predominant receptor involved in the adenosine diphosphate (ADP)-stimulated activation of the glycoprotein IIb/IIIa receptor. Activation of the glycoprotein IIb/IIIa receptor results in enhanced platelet degranulation and thromboxane production, and prolonged platelet aggregation. Aspirin (acetylsalicylic acid) irreversibly inhibits prostaglandin H synthase (cyclooxygenase-1) in platelets, and thereby blocks the formation of thromboxane A2, which has a potent pro-aggregant effect on platelets. However, associated bleeding events can lead to patient distress and premature discontinuation of the recommended therapy.
Platelet function can be evaluated by measuring platelet aggregation, and platelet aggregation rates reflect bleeding risk in patients with ACS who are on ticagrelor. Platelet endothelial aggregation receptor-1 (PEAR1) is crucial in platelet aggregation and function. Further, single-nucleotide polymorphisms (SNPs) in
The purpose of our study was to investigate the potential association between bleeding and platelet aggregation and SNPs in
Material and Methods
PATIENT SELECTION:
Our study was approved by the Ethics Committee of our hospital. This retrospective case-control study included 50 age- and platelet count-matched patients with ACS. The inclusion criteria were: diagnosed with ACS and went through percutaneous coronary intervention; age >18 years; and used aspirin and ticagrelor as post-procedural dual antiplatelet therapy. Post-procedural dual antiplatelet therapy comprised 100 mg aspirin once daily and 90 mg ticagrelor twice daily. Patients were subdivided into the case group (25 patients with reported bleeding events) and the control group (25 patients without reported bleeding events). Bleeding was defined as skin petechiae, nosebleed, hematuria, black or tarry stools, mucosal hematomas, and gum bleeding. Patients with active infections, renal or liver dysfunction, uncontrolled hypertension, history of allergy to the investigated drugs, and history of bleeding were excluded.
PLATELET AGGREGATION ASSAY:
Platelet aggregation rates were measured using light transmission aggregometry [8]. Platelet aggregation was induced with either 10 μmol/L ADP or 0.7 mmol/L arachidonic acid (ACA). Blood was tested within 2 h of collection.
GENOTYPING ASSAY:
Venous blood from each patient was collected, and 2 ml of the sample was used for genotyping. Genomic DNA was isolated for genotyping of SNPs in
STATISTICAL ANALYSIS:
Continuous data are expressed as means and standard deviations. Platelet aggregation rates were expressed as percentages. Comparisons were made using the
Results
There were no obvious differences between the demographic data of the patients in the case and control groups (Table 1). ACA-induced platelet aggregation rates were obviously lower in the case group than in the control group (13.28±8.46%
Discussion
Our study demonstrated that the ACA-induced platelet aggregation rate was significantly lower in patients with reported bleeding events than in those without. However, no significant differences were found in the ADP-induced platelet aggregation rates between the 2 groups. The mutation rates of the
Ticagrelor is a potent platelet inhibitor that binds reversibly to P2Y12, an ADP receptor. ADP-induced platelet aggregation is a good indicator of ticagrelor pharmacodynamics [9], which may explain the lack of a significant difference in the ADP-induced platelet aggregation rate observed between the 2 groups. However, the variation in the pharmacodynamics of aspirin is greater than the variation in that of ticagrelor, which is correlated with aspirin metabolism-associated SNPs such as
We also found that 70% of the patients with reported bleeding had an ACA-induced platelet aggregation rate of 0–18% and that 80% of the non-bleeding patients had an ACA-induced platelet aggregation rate of 18–50%, which is similar to previous findings [13]. We speculate that an ACA-induced platelet aggregation rate of 18% is a satisfactory cutoff for identifying patients with ACS with a high risk of aspirin-associated bleeding. This finding may provide a useful biomarker for assessing the risk of aspirin-associated bleeding in ACS patients.
We found that the
Our study had some limitations. First, platelet function and the SNPs were tested after bleeding events occurred, which may not support causative relationships. Second, our sample size was small, and the study was conducted at a single hospital, which may limit the generalization of our findings.
Conclusions
Low ACA-induced platelet aggregation rates were associated with an increased risk of bleeding in patients with ACS on aspirin and ticagrelor dual antiplatelet therapy. An ACA-induced platelet aggregation rate of 18% might be considered a cutoff for identifying high risk of aspirin-associated bleeding in patients with ACS. Genotyping the
Tables
Table 1. Patients’ demographic data.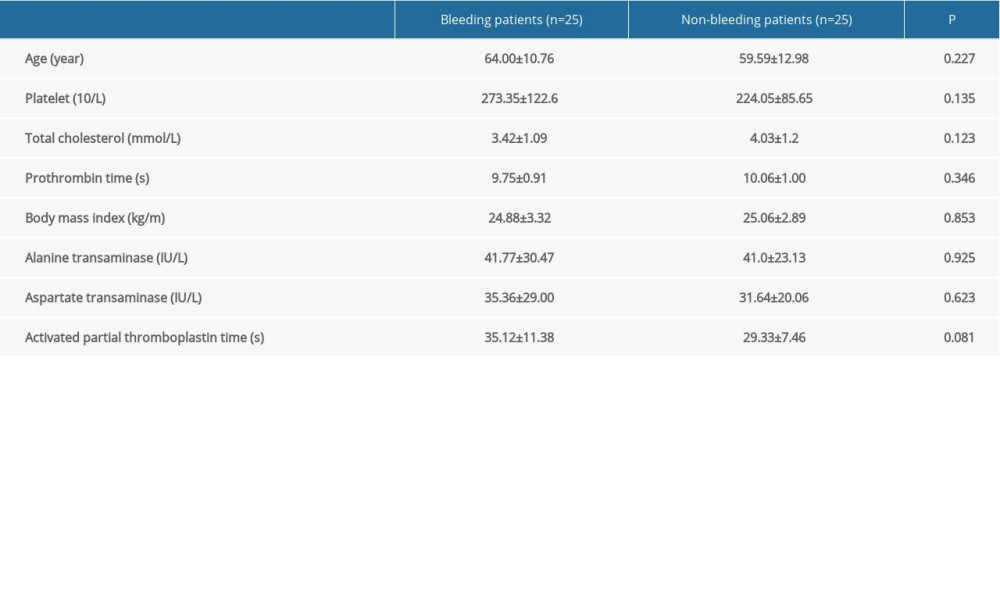 Table 2. Comparison of the platelet aggregation rates of the patients with bleeding events and those without bleeding events.
Table 2. Comparison of the platelet aggregation rates of the patients with bleeding events and those without bleeding events.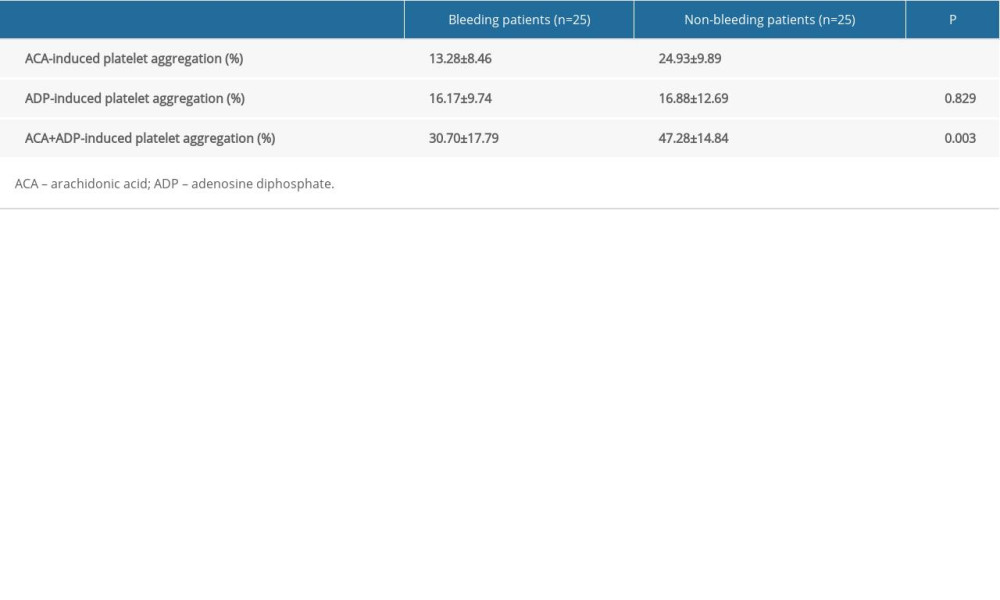 Table 3. Distribution of arachidonic acid-induced platelet aggregation rates in patients with reported bleeding events.
Table 3. Distribution of arachidonic acid-induced platelet aggregation rates in patients with reported bleeding events.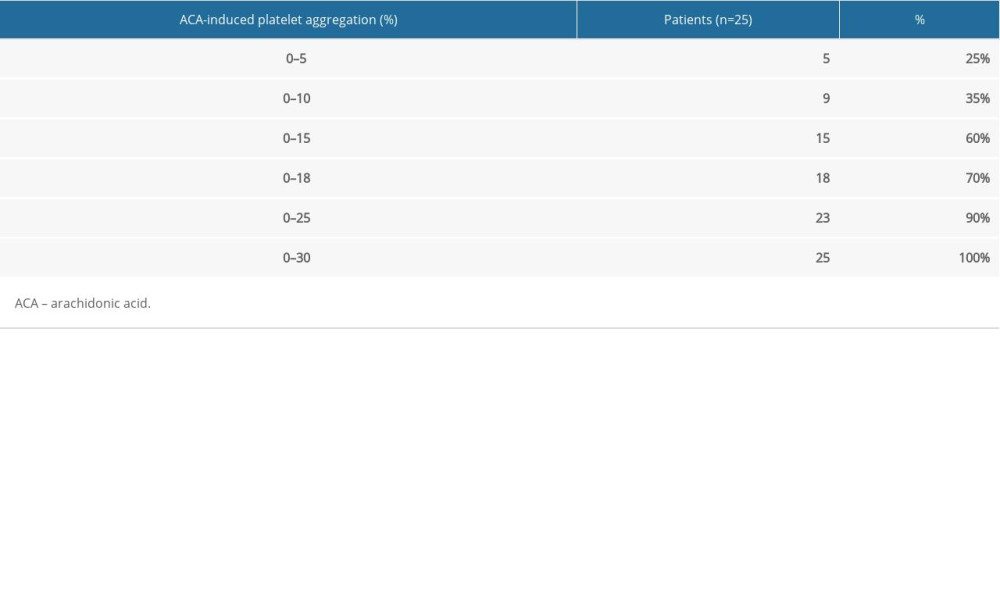 Table 4. Distribution of arachidonic acid-induced platelet aggregation rates in patients without reported bleeding events.
Table 4. Distribution of arachidonic acid-induced platelet aggregation rates in patients without reported bleeding events.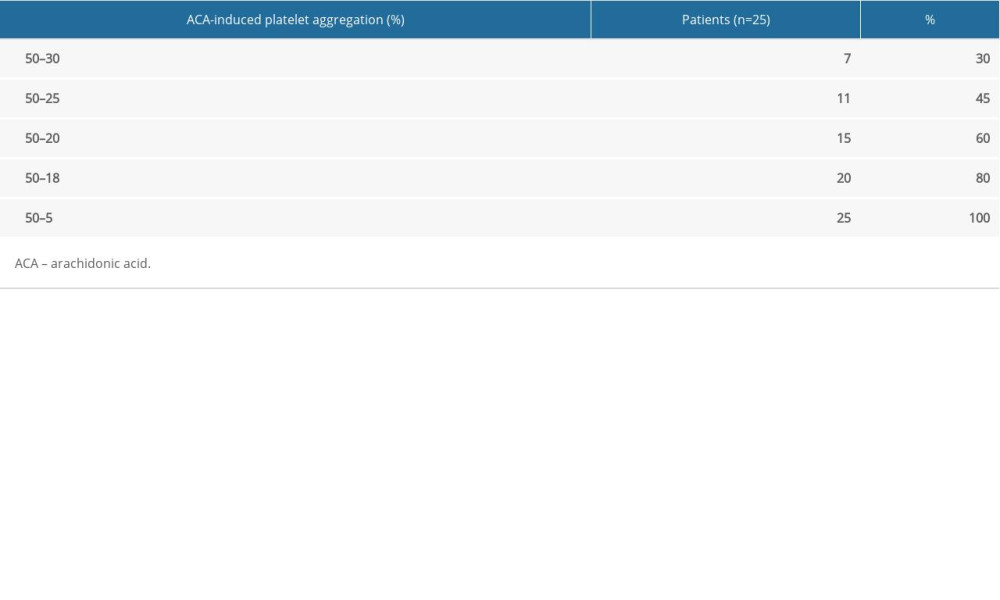 Table 5. Comparison of single-nucleotide polymorphisms between patients with and without reported bleeding events.
Table 5. Comparison of single-nucleotide polymorphisms between patients with and without reported bleeding events.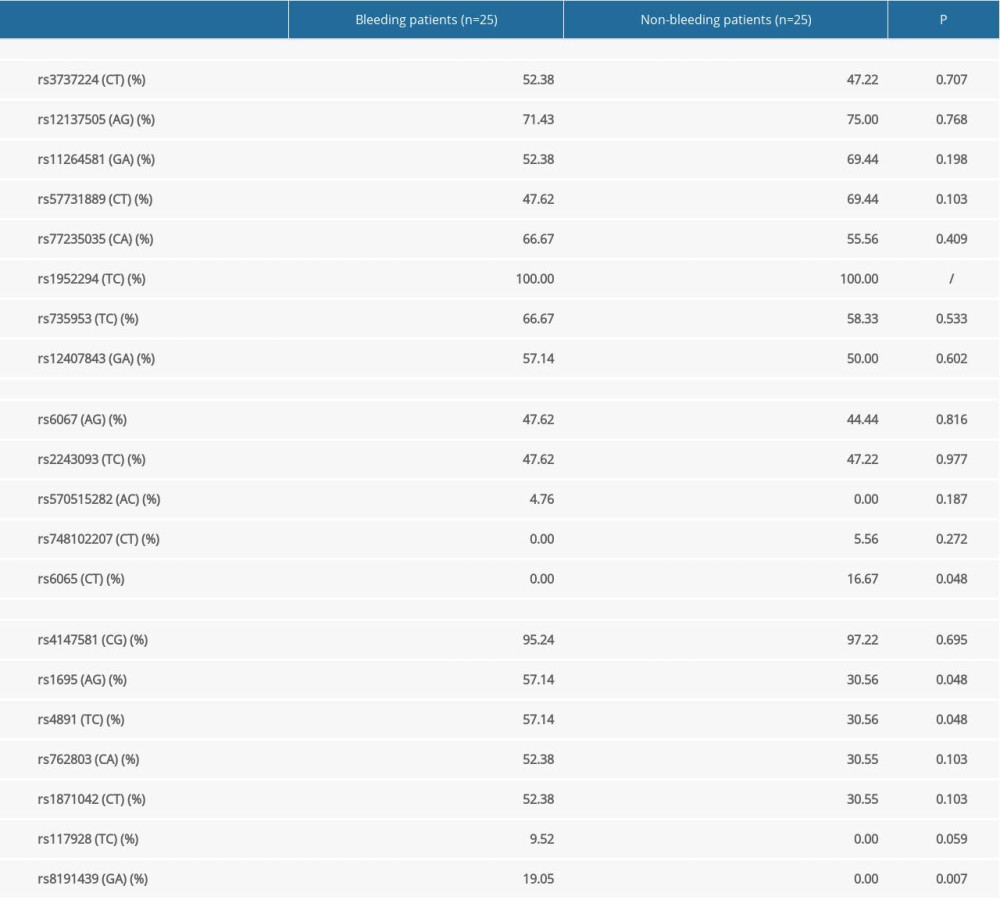
References
1. Mahmud E, Ben-Yehuda O, Percutaneous coronary intervention in acute coronary syndrome: completing the job saves lives: J Am Coll Cardiol, 2018; 72(17); 2000-2
2. Neumann FJ, Sousa-Uva M, Ahlsson A, 2018 ESC/EACTS Guidelines on myocardial revascularization: Eur Heart J, 2019; 40(2); 87-165
3. Ibanez B, James S, Agewall S, 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC): Eur Heart J, 2018; 39(2); 119-77
4. Rabani V, Montange D, Meneveau N, Davani S, Impact of ticagrelor on P2Y1 and P2Y12 localization and on cholesterol levels in platelet plasma membrane: Platelets, 2018; 29(7); 709-15
5. Xu K, Ye S, Zhang S, Impact of platelet endothelial aggregation receptor-1 genotypes on platelet reactivity and early cardiovascular outcomes in patients undergoing percutaneous coronary intervention and treated with aspirin and clopidogrel: Circ Cardiovasc Interv, 2019; 12(5); e007019
6. Yang J, Chen X, Zhou J, Associations of candidate gene polymorphisms with poor responsiveness to aspirin: A meta-analysis: Clin Exp Pharmacol Physiol, 2018 [Epub ahead of print]
7. Shiotani A, Murao T, Fujita Y, Novel single nucleotide polymorphism markers for low dose aspirin-associated small bowel bleeding: PLoS One, 2013; 8(12); e84244
8. Zhang HZ, Kim MH, Jeong YH, Predictive values of post-clopidogrel platelet reactivity assessed by different platelet function tests on ischemic events in East Asian patients treated with PCI: Platelets, 2014; 25(4); 292-99
9. Machal J, Hlinomaz O, Kostolanska K, CYP2C19 and CYP3A4 activity and ADP-induced platelet reactivity in Prasugrel- or Ticagrelor-treated STEMI patients: Monocentric study in PRAGUE-18 trial participants: Xenobiotica, 2020 [Epub ahead of print]
10. Würtz M, Nissen PH, Grove EL, Genetic determinants of on-aspirin platelet reactivity: Focus on the influence of PEAR1: PLoS One, 2014; 9(10); e111816
11. Bjerre KP, Clemmensen TS, Berg K, Platelet aggregation and response to aspirin therapy in cardiac allograft vasculopathy: J Heart Lung Transplant, 2020; 39(4); 371-78
12. Ozkan H, Kiris I, Gulmen S, Frequency of development of aspirin resistance in the early postoperative period and inadequate inhibition of thromboxane A2 production after coronary artery bypass surgery: Turk Gogus Kalp Damar Cerrahisi Derg, 2018; 26(4); 536-43
13. Winter MP, Schneeweiss T, Cremer R, Platelet reactivity patterns in patients treated with dual antiplatelet therapy: Eur J Clin Invest, 2019 [Epub ahead of print]
14. Levine GN, Bates ER, Bittl JA, 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: J Thorac Cardiovasc Surg, 2016; 152(5); 1243-75
15. Mehta SR, Bassand JPCURRENT-OASIS 7 Investigators, Dose comparisons of clopidogrel and aspirin in acute coronary syndromes: N Engl J Med, 2010; 363(10); 930-42
Tables
 Table 1. Patients’ demographic data.
Table 1. Patients’ demographic data. Table 2. Comparison of the platelet aggregation rates of the patients with bleeding events and those without bleeding events.
Table 2. Comparison of the platelet aggregation rates of the patients with bleeding events and those without bleeding events. Table 3. Distribution of arachidonic acid-induced platelet aggregation rates in patients with reported bleeding events.
Table 3. Distribution of arachidonic acid-induced platelet aggregation rates in patients with reported bleeding events. Table 4. Distribution of arachidonic acid-induced platelet aggregation rates in patients without reported bleeding events.
Table 4. Distribution of arachidonic acid-induced platelet aggregation rates in patients without reported bleeding events. Table 5. Comparison of single-nucleotide polymorphisms between patients with and without reported bleeding events.
Table 5. Comparison of single-nucleotide polymorphisms between patients with and without reported bleeding events. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








