13 August 2020: Lab/In Vitro Research
Mixed Lineage Kinase Domain-Like Protein Promotes Human Monocyte Cell Adhesion to Human Umbilical Vein Endothelial Cells Via Upregulation of Intercellular Adhesion Molecule-1 Expression
Fen Cai12ABC, Jia-Li Wang13BC, Yi-Lin Wu1C, Yan-Wei Hu14AEG*, Qian Wang1GDOI: 10.12659/MSM.924242
Med Sci Monit 2020; 26:e924242
Abstract
BACKGROUND: Atherosclerosis is a progressive inflammatory disease that involves a variety of inflammatory and proinflammatory factors, including intercellular adhesion molecule (ICAM)-1. ICAM-1 plays an important role in atherosclerosis by promoting cell adhesion. Mixed lineage kinase domain-like (MLKL), a critical regulator of necroptotic cell death, is indicated to play an important role in atherosclerosis. This study investigated the effects of MLKL on ICAM-1 expression and cell adhesion, thus providing a new direction for the research of atherosclerosis pathogenesis.
MATERIAL AND METHODS: siRNA-MLKL and pcDNA-MLKL were designed, and the expression of MLKL and ICAM-1 were estimated by real-time polymerase chain reaction at the mRNA level and Western blotting at the protein level. The adhesion of human monocyte cells (THP-1) to human umbilical vein endothelial cells (HUVECs) was examined under immunofluorescence microscopy, and the ability of cell adhesion was evaluated by ImageJ software.
RESULTS: Overexpression of MLKL greatly enhanced ICAM-1 expression in HUVECs and the adherence of THP-1 cells to HUVECs. Knockdown of MLKL by siRNA dramatically inhibited the expression of ICAM-1 and the adherence of THP-1 cells to HUVECs. MLKL could promote THP-1 adhesion to HUVECs by activating ICAM-1 expression in HUVECs.
CONCLUSIONS: MLKL can promote THP-1 cell adhesion to HUVECs through up-regulation of ICAM-1 expression in HUVECs. Thus, MLKL might be a useful target for reducing adhesion of monocytes to endothelial cells and atherosclerosis.
Keywords: Activated-Leukocyte Cell Adhesion Molecule, atherosclerosis, Cell Adhesion, Down-Regulation, Endothelium, Vascular, Human Umbilical Vein Endothelial Cells, Intercellular Adhesion Molecule-1, Monocytes, Protein Kinases, Real-Time Polymerase Chain Reaction
Background
Atherosclerosis is a progressive, lipid-driven inflammatory disease that is a primary cause of cardiovascular diseases [1]. Cell adhesion is an important step in atherosclerosis progression, which is dependent on inflammation. Previous studies have revealed that infiltration and activation of monocytes induced by adhesion are characteristic of human atherosclerotic lesions [2]. Nevertheless, the mechanisms involved in the process still need further investigation. ICAM-1 is a glycoprotein that is mainly expressed on cell membranes by endothelial cells (ECs) and immune cells, among others. It is a member of immunoglobulin superfamily, and through CDII/CD18 ligand complexes on leukocytes, it mediates the recruitment and binding of monocytes and neutrophils in the vascular wall. This causes leukocytes to adhere to the subcutaneous layer and eventually become foam cells [3]. ICAM-1 plays an important role in the early stages of atherosclerosis [4]. Studies have demonstrated that most extracellular functional regions of ICAM-1 can combine with ligands, thus reflecting ICAM-1 expression on the cell surface. ICAM-1 can initiate a local inflammatory reaction of blood vessels and cause vascular wall damage, and it is one of the markers of inflammatory reaction [5]. The level of ICAM-1 is closely related to the activation degree of vascular endothelial cells (VECs). Previous research found that endothelial ICAM-1 expression is increased in atherosclerotic lesions [6]. Microvessels in lipid-rich plaques express ICAM-1, which promotes the migration of transendothelial inflammatory cells to the plaque [7]. ICAM-1 was found to be related to the clinical prognosis and pathogenesis of coronary heart disease [8,9].
In programmed cell death, it has recently been found that necroptosis refers to a process related to inflammatory diseases. MLKL is considered a key executor of necroptosis because it can send out intracellular molecules, inducing inflammatory responses. Research on MLKL biological functions has revealed that it is closely related to inflammation and autophagy [10]. Recently, an increase of MLKL expression was shown in patients who had carotid atherosclerosis instability [11]. In addition, MLKL phosphorylation, often found in advanced atheromas, is significant for necroptosis [12]. So far, no data are available on the effect of MLKL in monocyte adhesion to human umbilical vein endothelial cells (HUVECs), and the potential mechanism of MLKL in atherosclerosis remains poorly understood. This study found that MLKL could enhance ICAM-1 expression in HUVECs, thereby improving the adhesion of THP-1 human monocyte cells to HUVECs.
Material and Methods
REAGENTS:
Fetal bovine serum (FBS), RPMI1640 medium, and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Gibco. Antibodies against rabbit MLKL (A5579) and ICAM-1 were obtained from Abcam.
CELL CULTURE:
HUVECs and THP-1 cells were obtained from ATCC (Manassas, VA, USA). THP-1 cells were maintained in RPMI1640 medium containing 10% FBS, and HUVECs were maintained in DMEM containing 10% FBS. Cells were seeded up to 70–80% confluency in 6- or 12-well plates prior to use.
RNA ISOLATION AND QUANTITATIVE REAL-TIME PCR:
All RNA from cultured cells was extracted with TRIzol reagent (Invitrogen Corporation) and reverse-transcribed. Real-time polymerase chain reaction (PCR; TaKaRa Bio Corporation) was used to analyze gene expression. Assays were determined in triplicate. The primers of MLKL were
the primers of ICAM-1 were
and the primers of GAPDH were
The ΔΔCt method and GAPDH as a reference were used to analyze data.
WESTERN BLOT ANALYSIS:
Cell protein samples were obtained according to established methods. The extracted samples were separated with 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, which were blocked with a 5% nonfat milk solution. After incubation with the primary antibodies at 4°C for 12 h, samples were incubated with secondary antibodies (goat anti-rabbit). The proteins were observed by electrochemiluminescence. The gray levels of resolved proteins were measured by ImageJ (National Institutes of Health, Bethesda, MD, USA).
TRANSFECTION WITH SIRNA:
HUVECs were transfected with 50 nmol/L of RNA with small interfering RNA (siRNA) and irrelevant control siRNA (negative control). Negative control siRNA (siRNA-NC) was an equal concentration with a nontargeting control mimic sequence. MLKL-siRNA, ICAM-1-siRNA, and siRNA-NC were obtained from Heyuan (Guangzhou,China). The target sequence for MLKL siRNA was 5′-GGTGTGAAGAGATGAAATA-3′, while the target sequence for ICAM-1 siRNA was 5′-GCAAGAAGATAGCCAACCA-3′. The qRT-PCR and Western blot analysis were performed to check MLKL and ICAM-1 transfection efficacy.
RECOMBINANT PLASMID CONSTRUCTION:
Plasmid with full-length MLKL cDNA was obtained from OriGene (Rockville, MD, USA). PCR was used to amplify MLKL cDNA, which was subcloned in the pcDNA3.1 (+) vector. The right recombinant plasmid MLKL cDNA (pcDNA-MLKL) sequence was confirmed by sequencing.
CELL ADHESION ASSAY:
Transfected HUVECs were seeded in 48-well plates up to 80% confluency. Each treatment group included 3 wells. THP-1 cells were marked with 5 mmol/L carboxyfluorescein succinimidyl ester (CFSE, Abcam, Shanghai, China) for 10 min at 37°C. After being washed 3 times with PBS, cell pellets were resuspended in complete medium at 90% confluence, and THP-1 cells were then cultured as endothelial monolayers at 37°C. After 4 h, PBS was used to wash co-cultured cells. The adhesion of THP-1 cells to HUVECs was examined under immunofluorescence microscopy (Olympus, Tokyo, Japan) within 10 randomly selected microscopic fields of view in order to count cells. The number of fluorescently labeled cells in each well was calculated using ImageJ software.
STATISTICAL ANALYSIS:
Means±standard deviation (SD) are used to express the results. GraphPad Prism 7.0 software and SPSS version 21.0 (Chicago, IL, USA) were used to analyze data. The
Results
DOWN-REGULATING MLKL INHIBITED ICAM-1 EXPRESSION IN HUVECS:
Previous study found that MLKL is closely related to inflammation and ICAM-1 is a proinflammatory cytokine in HUVECs. This study investigated the correlation between MLKL and ICAM-1. HUVECs were transfected with siRNA-MLKL, and then Western blotting and qRT-PCR were used to determine MLKL and ICAM-1 expression in HUVECs. MLKL treated with siRNA significantly downregulated MLKL mRNA expression (Figure 1A) and protein levels (Figure 1B, 1C), and siRNA-mediated MLKL silencing reduced ICAM-1 mRNA expression (Figure 1D) and protein levels (Figure 1E, 1F).
UPREGULATION OF MLKL ENHANCED ICAM-1 EXPRESSION IN HUVECS:
The pcDNA-MLKL was utilized to upregulate MLKL expression, and it significantly upregulated MLKL mRNA expression (Figure 2A) and protein levels (Figure 2B, 2C). In addition, pcDNA-MLKL increased ICAM-1 mRNA (Figure 2D) and protein (Figure 2E, 2F) expression in HUVECs. The above results suggest that MLKL was related to the expression of ICAM-1 in HUVECs.
MLKL WAS INVOLVED IN THP-1 CELL ADHESION TO HUVECS:
Previous results showed that MLKL promoted ICAM-1 expression in HUVECs. Direct interaction between ICAM-1 and molecules on monocyte surface affected cell adhesion [13]. Here, we investigated the correlation between MLKL and cell adhesion. MLKL siRNA and recombinant plasmids overexpressing MLKL were used to investigate the effects of MLKL on the adherence of fluorescent-labeled THP-1 monocytes to HUVECs. Our results showed that MLKL siRNA significantly decreased the adhesion of THP-1 cells to HUVECs (Figure 3A, 3B). By contrast, THP-1 cell adhesion to HUVECs was promoted by recombinant plasmid-mediated MLKL overexpression (Figure 3C, 3D). The results indicated that MLKL was related to THP-1 monocyte adherence to HUVECs.
MLKL PROMOTED THP-1 CELL ADHESION TO HUVECS VIA UPREGULATING ICAM-1 EXPRESSION:
Previous results have shown that ICAM-1 expression is enhanced by overexpression of MLKL. The process was restrained by ICAM-1 siRNA-mediated silencing (Figure 4A–4C). The THP-1 cell adhesion to HUVECs through transfection with pcDNA-MLKL was suppressed by treatment with siRNA-ICAM-1 (Figure 4G, 4I). By contrast, siRNA-MLKL could reduce ICAM-1 mRNA and protein expression in HUVECs. This effect was further inhibited by treatment with ICAM-1 siRNA (Figure 4D–4F). In addition, the THP-1 cell adhesion to HUVECs by treatment with siRNA-MLKL was further inhibited by treatment with siRNA-ICAM-1 (Figure 4H, 4J). These results indicate that MLKL increases the cell adhesion of THP-1 to HUVECs through upregulating ICAM-1 expression.
Discussion
Atherosclerosis is a complex inflammatory disorder of the vasculature in which endothelium lining the vessels becomes damaged in response to cardiovascular risk factors. Through expression of surface adhesion molecules such as ICAM-1, vascular cell adhesion molecule-1, dysfunctional ECs facilitate vascular inflammation [14]. ICAM-1 continues to be expressed on the surface of VECs and participates in signal transduction and cytoskeleton rearrangement in inflammation [15]. ICAM-1 expression is constitutively low and weakly detectable on ECs under noninflammatory conditions, while increased ICAM-1 expression has been found in coronary artery disease, stenosis, myocardial infarction, and arteriosclerosis [16]. Existing evidence indicates that ICAM-1 is significant for atherosclerosis pathogenesis, and many risk factors may induce atherosclerosis through their effect on ICAM-1. This study shows that changes in MLKL expression have an impact on subsequent ICAM-1 expression, and it is the first report of MLKL mediation on ICAM-1 expression in HUVECs. Prior research found that lipopolysaccharides enhanced ICAM-1expression through the MYD88-BLT2-ERK pathway [17]. Whether MLKL regulates ICAM-1 via this pathway or an entirely different pathway needs to be investigated further.
Necroptosis involves necrosome formation, which includes receptor-interacting protein kinase (RIPK) 1, RIPK3, and MLKL. MLKL is organized into an N-terminal helical bundle domain and a C-terminal pseudokinase domain that contains a RIPK3 phosphorylation site. These domains are connected by an auto-inhibitory support region, which can transfer the structural changes caused by phosphorylation well. The C-terminal domain activates the N-terminal helix bundle [18]. When MLKL is phosphorylated by RIPK3, oligomerized MLKL results in plasma membrane rupture via direct destruction of the membrane bilayer [19]. MLKL is expressed in the spleen, colon, thymus, intestine, lung, and living body [20,21], and it is a marker of critical illness and sepsis [22]. The expression of phosphorylated MLKL is increased in humans with advanced atheromas [23]. No data are available on the relationship between MLKL and cell adhesion. The present study found that MLKL could promote ICAM-1 expression and monocyte adhesion to HUVECs. The siRNA targeting MLKL significantly inhibited the expression of ICAM-1 mRNA and protein. MLKL overexpression promoted the expression of ICAM-1 in HUVECs. Furthermore, MLKL overexpression promoted THP-1 cell adhesion to HUVECs, while the adhesion of HUVECs to THP-1 monocytes by MLKL overexpression was inhibited by treatment with siRNA-ICAM-1. These data together indicate that MLKL could promote THP-1 cell adhesion to HUVECs via increasing ICAM-1 expression. Prior research showed that NF-κB activation was related to ICAM-1 expression [24]. Whether MLKL promotes ICAM-1 through the NF-κB pathway needs to be further investigated. In addition, the underlying mechanisms of MLKL effects on ICAM-1 expression and monocyte adhesion to ECs also need further investigation.
Conclusions
We conclude that MLKL can promote THP-1 cell adhesion to HUVECs through upregulating ICAM-1 expression in HUVECs. Thus, MLKL might be a useful target for reducing adhesion of monocytes to ECs and atherosclerosis.
Figures
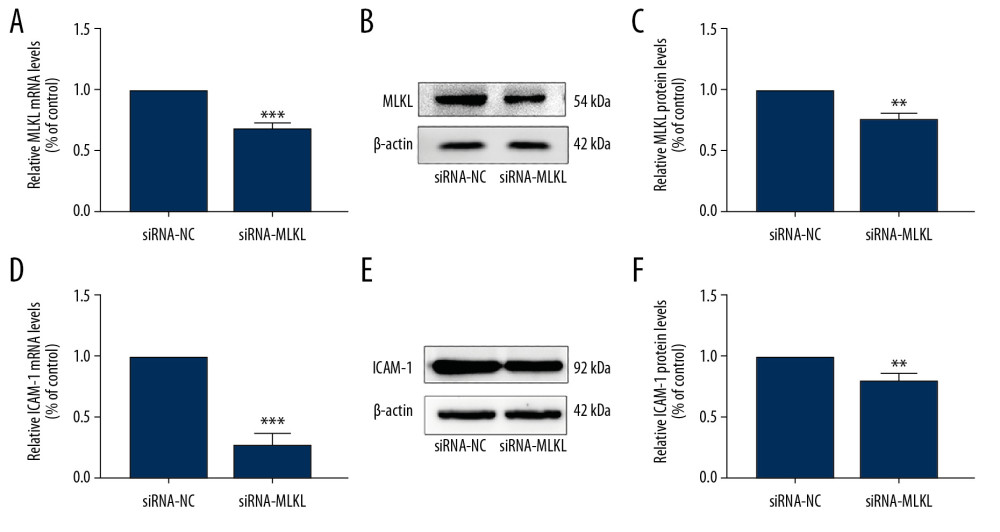 Figure 1. Knockdown of MLKL downregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either negative control or siRNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blotting and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Data are given as mean±SD of 3 independent assays, with each experiment repeated in triplicate. ** P<0.01,*** P<0.001 vs. controls.
Figure 1. Knockdown of MLKL downregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either negative control or siRNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blotting and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Data are given as mean±SD of 3 independent assays, with each experiment repeated in triplicate. ** P<0.01,*** P<0.001 vs. controls. 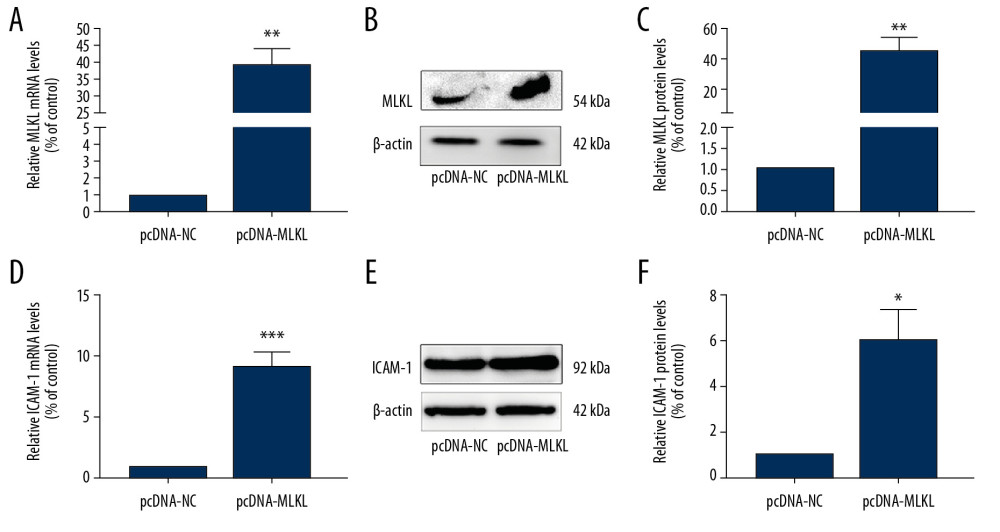 Figure 2. Overexpression of MLKL upregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either control vector pcDNA or pcDNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blot analysis and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Three independent assays (3 replicates for each experiment) were performed (mean±SD). * P<0.05, ** P<0.01, *** P<0.001 vs. controls.
Figure 2. Overexpression of MLKL upregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either control vector pcDNA or pcDNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blot analysis and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Three independent assays (3 replicates for each experiment) were performed (mean±SD). * P<0.05, ** P<0.01, *** P<0.001 vs. controls. 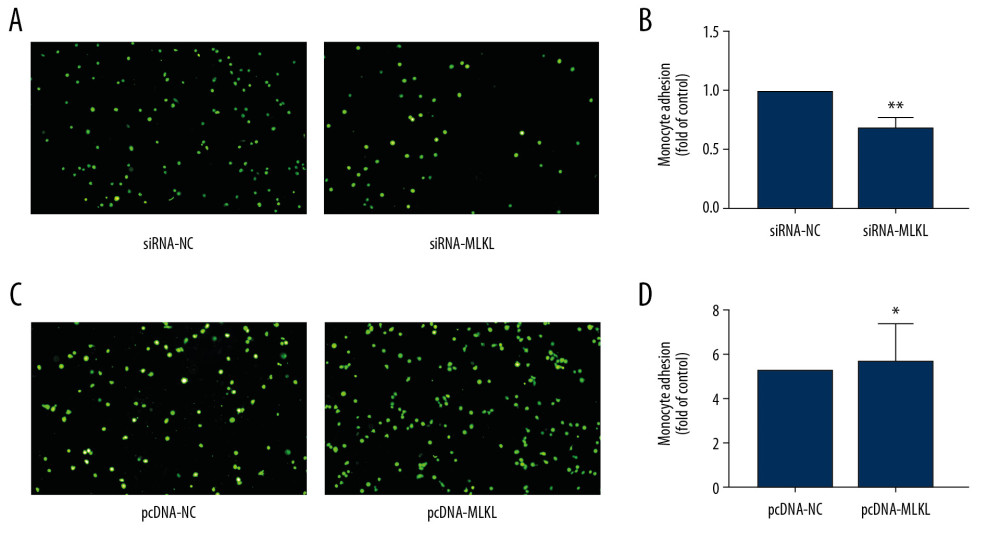 Figure 3. MLKL was involved in THP-1 cell adhesion to HUVECs. (A–D) HUVECs were transfected with siRNA-NC or siRNA-MLKL for 24 h, and then cultured together with CFSE-labeled THP-1 cells for 4 h. The number of adherent monocytes was evaluated by fluorescence microscopy. (A, C) Representative images for adherence of THP-1 cells to transfected HUVECs. (B, D) Column charts show the fold differences in THP-1 cell adhesion to transfected HUVECs after standardization against the corresponding controls. Experiments were repeated 3 times. Values are the mean of 3 replicates. * P<0.05, ** P<0.01 vs. Control.
Figure 3. MLKL was involved in THP-1 cell adhesion to HUVECs. (A–D) HUVECs were transfected with siRNA-NC or siRNA-MLKL for 24 h, and then cultured together with CFSE-labeled THP-1 cells for 4 h. The number of adherent monocytes was evaluated by fluorescence microscopy. (A, C) Representative images for adherence of THP-1 cells to transfected HUVECs. (B, D) Column charts show the fold differences in THP-1 cell adhesion to transfected HUVECs after standardization against the corresponding controls. Experiments were repeated 3 times. Values are the mean of 3 replicates. * P<0.05, ** P<0.01 vs. Control. 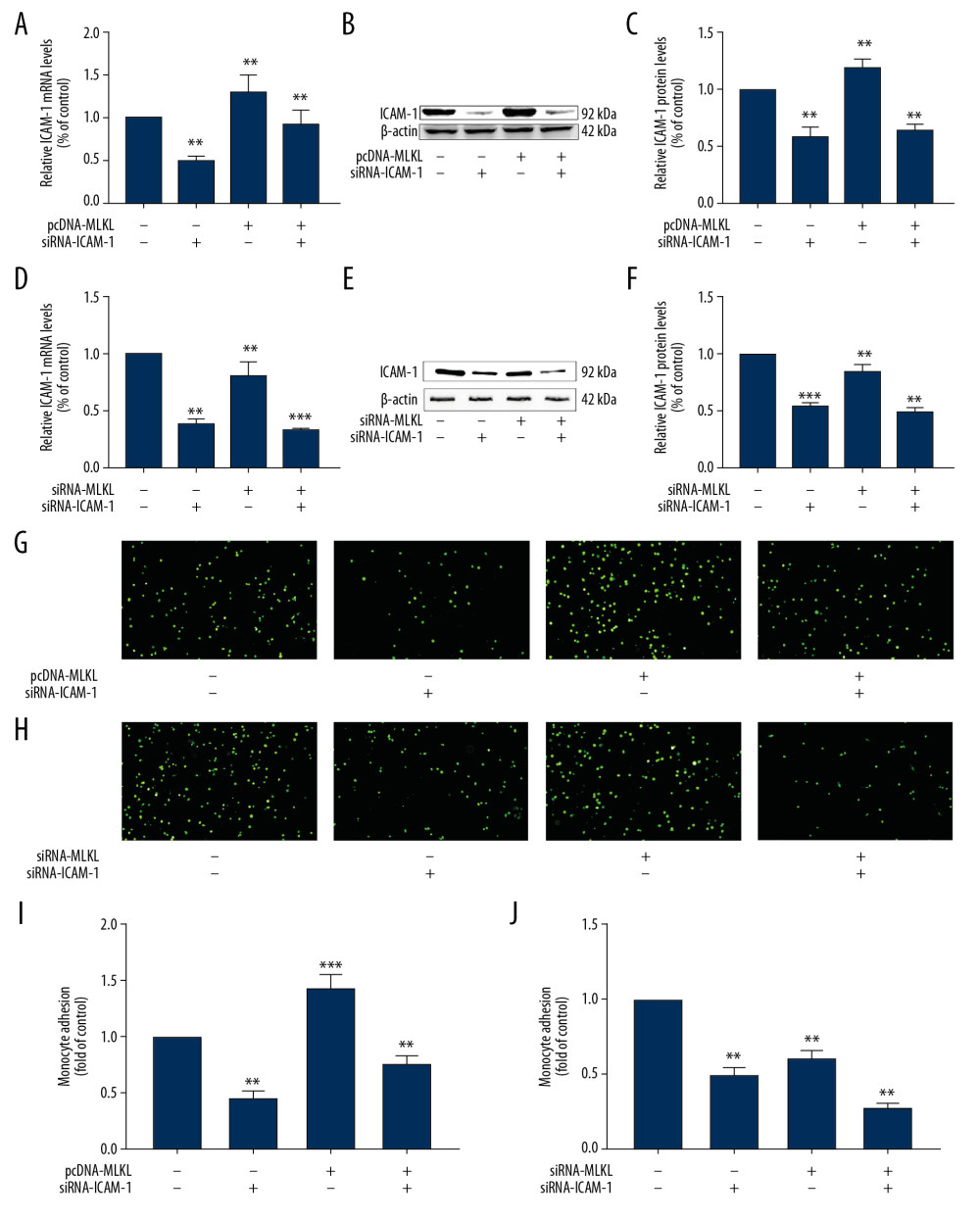 Figure 4. MLKL induced adhesion of THP-1 cells to HUVECs via regulating ICAM-1. (A–C) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (A) ICAM-1 mRNA expression was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (B) ICAM-1 protein levels were measured by analysis of Western blot and representative Western blot images. (C) ICAM-1protein levels were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (D–F) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (D) The expression of ICAM-1 mRNA was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (E) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. (F) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (G, I) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. The number of adherent monocytes was examined by fluorescence microscopy. (G) Representative images for adherence of THP-1 cells to transfected HUVECs. (I) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. (H, J) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (H) Representative images for adherence of THP-1 cells to transfected HUVECs. (J) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. Data are presented as mean±SD of 3 independent assays, each performed in triplicate. ** P<0.01, *** P<0.001 vs. controls.
Figure 4. MLKL induced adhesion of THP-1 cells to HUVECs via regulating ICAM-1. (A–C) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (A) ICAM-1 mRNA expression was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (B) ICAM-1 protein levels were measured by analysis of Western blot and representative Western blot images. (C) ICAM-1protein levels were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (D–F) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (D) The expression of ICAM-1 mRNA was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (E) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. (F) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (G, I) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. The number of adherent monocytes was examined by fluorescence microscopy. (G) Representative images for adherence of THP-1 cells to transfected HUVECs. (I) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. (H, J) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (H) Representative images for adherence of THP-1 cells to transfected HUVECs. (J) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. Data are presented as mean±SD of 3 independent assays, each performed in triplicate. ** P<0.01, *** P<0.001 vs. controls. References
1. Labarrere CA, DiCarlo HL, Bammerlin E, Failure of physiologic transformation of spiralarteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta: Am J Obstet Gynecol, 2017; 216; 281-87
2. Huang B, Hu P, Hu A, Naringenin attenuates carotid restenosis in rats after balloon injury through its anti-inflammation and anti-oxidative effects via the RIP1-RIP3-MLKL signaling pathway: Eur J Pharmacol, 2019; 855; 167-74
3. Xu YJ, Li P, Zheng L, Forkhead Box C2 attenuates lipopolysaccharide-induced cell adhesion via suppression of intercellular adhesion molecule-1 expression in human umbilical vein endothelial cells: DNA Cell Biol, 2019; 38; 583-91
4. Lv JX, Kong Q, Ma X, Current advances in circulating inflammatory biomarkers in atherosclerosis and related cardio-cerebrovascular diseases: Chronic Dis Transl Med, 2017; 3; 207-12
5. Soeki T, Sata M, Inflammatory biomarkers and atherosclerosis: Int Heart J, 2016; 57; 134-39
6. Jolivel V, Bicker F, Binamé F, Perivascular microglia promote blood vessel disintegration in the ischemic penumbra: Acta Neuropathol, 2015; 129(2); 279-95
7. Shi MJ, Yuan H, Hu HQ, Relationship between intercellular cell adhesion molecule-1 and angiogenesis in mice: Chin J Clin Pharmacol, 2017; 244; 1348-50
8. Wang SX, Tan L, Wang J, Zhong JQ, Effect of levocarnitine on TIMP-1, ICAM-1 expression of rats with coronary heart disease and its myocardial protection effect: Asian Pac J Trop Med, 2016; 9; 269-73
9. Chou CH, Ueng KC, Liu YF, Impact of intercellular adhesion molecule-1 genetic polymorphisms on coronary artery disease susceptibility in Taiwanese subjects: Int J Med Sci, 2015; 12; 510-16
10. Ogasawara M, Yano T, Tanno M, Suppression of autophagic flux contributes to cardiomyocyte death by activation of necroptotic pathways: J Mol Cell Cardiol, 2017; 108; 203-13
11. Guo FX, Wu Q, Li P, The role of the LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling: Cell Death Differ, 2019; 26; 1670-87
12. Li YQ, Wang JY, Qian ZQ, Osthole inhibits intimal hyperplasia by regulating the NF-κB and TGF-β1/Smad2 signalling pathways in the rat carotid artery after balloon injury: Eur J Pharmacol, 2017; 811; 232-39
13. Mruk DD, Xiao X, Lydka M, Intercellular adhesion molecule 1: Recent findings and new concepts involved in mammalian spermatogenesis: Semin Cell Dev Biol, 2014; 129; 43-54
14. Hansson GK, Hermansson A, The immune system in atherosclerosis: Nat Immunol, 2011; 12; 204-12
15. Pradhan AD, Shrivastava S, Cook NR, Symptomatic peripheral arterial disease in women: nontraditional biomarkers: Circulation, 2008; 117; 823-31
16. Cybulsky MI, Iiyama K, Li H, A major role for VCAM-1, but not ICAM-1, in early atherosclerosis: J Clin Invest, 2001; 107; 1255-62
17. Park G, Kim J, LPS up-regulates ICAM-1 expression in breast cancer cells by stimulating a MyD88-BLT2-ERK-linked cascade, which promotes adhesion to monocytes: Mol Cell, 2015; 38; 821-28
18. Grootjans S, Vanden Berghe T, Vandenabeele P, Initiation and execution mechanisms of necroptosis: An overview: Cell Death Differ, 2017; 24; 1184-95
19. Huang D, Zheng X, Wang ZA, The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process: Mol Cell Biol, 2017; 37; e00497-16
20. Alvarez-Diaz S, Dillon CP, Lalaoui N, The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis: Immunity, 2016; 45; 513-26
21. Zhang X, Fan C, Zhang H, MLKL and FADD are critical for suppressing progressive lymphoproliferative disease and activating the NLRP3 inflammasome: Cell Rep, 2016; 16; 3247-59
22. Vucur M, Roderburg C, Kaiser L, Elevated serum levels of mixed lineage kinase domain-like protein predict survival of patients during intensive care unit treatment: Dis Markers, 2018; 2018 1983421
23. Zhe-Wei S, Li-Sha G, Yue-Chun L, The role of necroptosis in cardiovascular disease: Front Pharmacol, 2018; 9; 721
24. Guichet PO, Guelfi S, Teigell M, Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells: Stem Cells, 2015; 33; 21-34
Figures
 Figure 1. Knockdown of MLKL downregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either negative control or siRNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blotting and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Data are given as mean±SD of 3 independent assays, with each experiment repeated in triplicate. ** P<0.01,*** P<0.001 vs. controls.
Figure 1. Knockdown of MLKL downregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either negative control or siRNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blotting and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Data are given as mean±SD of 3 independent assays, with each experiment repeated in triplicate. ** P<0.01,*** P<0.001 vs. controls. Figure 2. Overexpression of MLKL upregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either control vector pcDNA or pcDNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blot analysis and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Three independent assays (3 replicates for each experiment) were performed (mean±SD). * P<0.05, ** P<0.01, *** P<0.001 vs. controls.
Figure 2. Overexpression of MLKL upregulated ICAM-1 expression in HUVECs. (A–F) HUVECs were transfected with either control vector pcDNA or pcDNA-MLKL for 24 h. (A, D) mRNA levels of MLKL and ICAM-1 were evaluated using qRT-PCR. Column chart showed the fold differences in the MLKL and ICAM-1mRNA after standardization against the GAPDH mRNA. (B, E) The protein levels of MLKL and ICAM-1 were evaluated by Western blot analysis and representative Western blot images of MLKL and ICAM-1. (C, F) Protein levels of MLKL and ICAM-1 were evaluated using Western blotting. Column charts show the fold differences in the MLKL and ICAM-1 band intensities after standardization against the band intensity of β-actin. Three independent assays (3 replicates for each experiment) were performed (mean±SD). * P<0.05, ** P<0.01, *** P<0.001 vs. controls. Figure 3. MLKL was involved in THP-1 cell adhesion to HUVECs. (A–D) HUVECs were transfected with siRNA-NC or siRNA-MLKL for 24 h, and then cultured together with CFSE-labeled THP-1 cells for 4 h. The number of adherent monocytes was evaluated by fluorescence microscopy. (A, C) Representative images for adherence of THP-1 cells to transfected HUVECs. (B, D) Column charts show the fold differences in THP-1 cell adhesion to transfected HUVECs after standardization against the corresponding controls. Experiments were repeated 3 times. Values are the mean of 3 replicates. * P<0.05, ** P<0.01 vs. Control.
Figure 3. MLKL was involved in THP-1 cell adhesion to HUVECs. (A–D) HUVECs were transfected with siRNA-NC or siRNA-MLKL for 24 h, and then cultured together with CFSE-labeled THP-1 cells for 4 h. The number of adherent monocytes was evaluated by fluorescence microscopy. (A, C) Representative images for adherence of THP-1 cells to transfected HUVECs. (B, D) Column charts show the fold differences in THP-1 cell adhesion to transfected HUVECs after standardization against the corresponding controls. Experiments were repeated 3 times. Values are the mean of 3 replicates. * P<0.05, ** P<0.01 vs. Control. Figure 4. MLKL induced adhesion of THP-1 cells to HUVECs via regulating ICAM-1. (A–C) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (A) ICAM-1 mRNA expression was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (B) ICAM-1 protein levels were measured by analysis of Western blot and representative Western blot images. (C) ICAM-1protein levels were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (D–F) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (D) The expression of ICAM-1 mRNA was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (E) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. (F) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (G, I) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. The number of adherent monocytes was examined by fluorescence microscopy. (G) Representative images for adherence of THP-1 cells to transfected HUVECs. (I) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. (H, J) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (H) Representative images for adherence of THP-1 cells to transfected HUVECs. (J) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. Data are presented as mean±SD of 3 independent assays, each performed in triplicate. ** P<0.01, *** P<0.001 vs. controls.
Figure 4. MLKL induced adhesion of THP-1 cells to HUVECs via regulating ICAM-1. (A–C) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (A) ICAM-1 mRNA expression was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (B) ICAM-1 protein levels were measured by analysis of Western blot and representative Western blot images. (C) ICAM-1protein levels were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (D–F) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (D) The expression of ICAM-1 mRNA was evaluated using qRT-PCR. Column charts show the fold differences in the ICAM-1 mRNA after standardization against the GAPDH mRNA. (E) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. (F) The protein levels of ICAM-1 were evaluated by Western blot analysis and representative Western blot images. Column charts show the fold differences in the ICAM-1 band intensities after standardization against the band intensity of β-actin. (G, I) HUVECs were transfected by pcDNA-MLKL at 90% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. The number of adherent monocytes was examined by fluorescence microscopy. (G) Representative images for adherence of THP-1 cells to transfected HUVECs. (I) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. (H, J) HUVECs were transfected by siRNA-MLKL at 70% confluence for 6 h, and then treated with siRNA-ICAM-1 for 24 h. (H) Representative images for adherence of THP-1 cells to transfected HUVECs. (J) Column charts show the fold differences in the adherence of THP-1 cells to transfected HUVECs after standardization against the corresponding controls. Data are presented as mean±SD of 3 independent assays, each performed in triplicate. ** P<0.01, *** P<0.001 vs. controls. In Press
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
09 May 2024 : Review article
A Review of the Current Status of Disease-Modifying Therapies and Prevention of Alzheimer’s DiseaseMed Sci Monit In Press; DOI: 10.12659/MSM.945091
09 Apr 2024 : Clinical Research
Correlation between Thalamocortical Tract and Default Mode Network with Consciousness Levels in Hypoxic-Isc...Med Sci Monit In Press; DOI: 10.12659/MSM.943802
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








