28 September 2020: Animal Study
Ability of Post-treatment Glycyrrhizic Acid to Mitigate Cerebral Ischemia/Reperfusion Injury in Diabetic Mice
Yuan Li12BCD, Na Yao3ACF, Ting Zhang3D, Fengying Guo4B, Xiangying Niu5F, Zhihui Wu3E, Shaozhang Hou3AEFG*DOI: 10.12659/MSM.926551
Med Sci Monit 2020; 26:e926551
Abstract
BACKGROUND: Diabetes aggravates cerebral ischemia/reperfusion (I/R) injury by increasing inflammatory reactions, but its specific mechanism is currently unclear.
MATERIAL AND METHODS: Diabetes was induced in mice with a high-fat diet combined with streptozotocin. These mice were subjected to transient middle cerebral artery occlusion (tMCAO) for 60 min, followed by reperfusion for 24–72 h and post-treatment glycyrrhizic acid (GA). Control and diabetic mice were randomly allocated to 8 groups of 18 mice each. Blood glucose, brain infarction, brain edema, and neurological function were monitored. Necrosis was determined by Nissl staining, loss of neurons by immunofluorescent (IF) staining for NeuN, and activation of inflammatory microglia by IF staining for Iba-1. Levels of HMGB1, TLR4, Myd88, and NF-κB mRNA and protein in ischemic brain were determined by qRT-PCR and western blotting, respectively, and serum concentrations of IL-1β, IL-6, and TNF-α by ELISA.
RESULTS: Infarction volume, brain edema, and neurological function after tMCAO were significantly aggravated in diabetes, but ameliorated by post-treatment GA. GA also reduced neuronal loss and microglial activation. Cerebral Myd88 level showed a positive correlation with neurological scores. GA suppressed the expression of Myd88 and a proinflammatory pathway that included Myd88, HMGB1, TLR4, and NF-κB, as well as reducing serum concentrations of IL-1β, IL-6, and TNF-α.
CONCLUSIONS: Post-treatment inhibited inflammatory responses and provided therapeutic benefits in diabetic mice with cerebral I/R injury, suggesting that GA may be a candidate drug to suppress cerebral I/R in diabetic patients.
Keywords: Brain Ischemia, Diabetes Mellitus, Type 2, Glycyrrhizic Acid, Myeloid Differentiation Factor 88, Cerebrovascular Disorders, Diabetes Complications, Diabetes Mellitus, Experimental, Gene Expression Regulation
Background
Stroke is the second leading cause of death in the world [1]. Most (87%) strokes are ischemic, caused by blockage of cerebral arteries, which eventually leads to hypoxic ischemic brain damage [2]. Although thrombolytic therapy within 3 hours of the onset of ischemic stroke remains the treatment of choice [3], thrombolytic therapy may cause ischemia/reperfusion (I/R) injury to the brain, which can worsen patient prognosis [4]. Diabetes has been shown to aggravate cerebral I/R injury [5]. Although tight glycemic control is the principal therapeutic strategy for diabetes, it is often not effective in improving outcomes after I/R injury, because inflammation remains even under euglycemic conditions [6]. Therefore, treatment that reduces inflammatory responses is essential for diabetic patients with cerebral I/R.
Glycyrrhizic acid (GA, C42H62O16) is the most efficacious component of the roots of licorice plants. GA has been shown to attenuate inflammatory responses during acute phases of cerebral I/R by down-regulating expression of nuclear factor kappa B (NF-κB) [7]. The anti-inflammatory compound N-palmitoylethanolamide-oxazoline (PEA-OXA) has also been shown useful in improving function in cerebral I/R injury-related disorders in diabetic rats [8]. Because reduction of inflammatory responses during cerebral I/R was found to alleviate brain edema and infarction size [9], we hypothesized that GA could reduce cerebral I/R injury in diabetic patients by suppressing inflammatory responses.
Inflammation is regulated by various humoral factors and cytokines [10,11]. To evaluate the effects of GA on intracellular signal pathways regulating inflammatory response, we focused on the myeloid differentiation primary response gene 88 (Myd88), an essential adaptor protein for innate immunity and pathogen-associated molecular pattern recognition. All Toll-like receptors (TLRs), except for TLR3, have been reported to be dependent on Myd88 in down-stream signal transduction [12]. Myd88 has been shown to contribute to neuroinflammation in cerebral I/R [13,14] and to directly trigger NF-κB activation when compared with HMGB1 and TLRs [15]. Micronized palmitoylethanolamide (PEA-m), which inhibits the iba-1 and NF-κB inflammatory pathways involved in Myd88-associated inflammatory signaling, has been found to be neuroprotective in diabetic mice [16]. We therefore developed a mouse model combining diabetes with cerebral I/R injury. The molecular mechanisms of action of GA on cerebral infarction in diabetes were evaluated by assessing the effects of GA treatment on HMGB1/TLR4/Myd88/NF-κB signaling in this mouse model.
Material and Methods
ANIMALS AND AGENTS:
The experimental protocol, which complied with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publications No.8023, revised 1978), was approved by the institutional animal ethics committee of Ningxia Medical University (No.2018-031). Three-week-old male C57BL/6J mice, weighing 9–12 g, were obtained from Vital River Laboratory Animal Technology (Beijing, China) and maintained under a 12-h light-dark cycle with a relative humidity of 50–60% and a room temperature of 24±1°C. All mice had free access to food and water.
DIABETES MODEL:
Diabetes was induced in mice with a high-fat diet (60.2% carbohydrates, 13.8% proteins, 26% fats; HFKBIO, Beijing, China) for 6 weeks and injections of 65 mg/kg (pH 4.1–4.5) streptozotocin (STZ; Sigma, Darmstadt, Germany) [17]. Control mice were fed a normal diet (60% carbohydrates, 31.4% proteins, 8.6% fats; HFKBIO) for 6 weeks. Blood glucose concentrations were measured once weekly for 3 weeks (i.e. at ages 10, 11 and 12 weeks); if all 3 measurements showed blood glucose concentrations above 11.1 mmol/L, the mice were considered diabetic. The control and diabetic mice were randomly allocated to 8 groups of 18 mice each: Untreated Control (C), Control+24 h-tMCAO (C-24), Diabetes+24 h-tMCAO (D-24), Diabetes+24 h-tMCAO+GA (D-24+GA), Diabetes+72 h-tMCAO (D-72), Diabetes+72 h-tMCAO+GA (D-72 + GA), Diabetes+24 h-tMCAO+NBP (D-24+NBP), and Diabetes+24 h-tMCAO+TAK-242 (D-24+ TAK-242).
TMCAO:
The tMCAO procedure was performed as previously described [18]. The stump of the external carotid artery was closed with an electric coagulator to induce all blood to flow from the common carotid artery to the internal carotid artery. The internal carotid artery was sutured 12 mm from the carotid bifurcation and the sutures maintained for 60 minutes. Control mice underwent the same procedure without suturing. During the tMCAO procedure, body temperature was maintained at 36.5±0.5°C with a feedback-controlled heating pad (RWD Life Science Company, Shenzhen, China). Mice without infarction or with intracranial hemorrhage were excluded from this study.
POST-TREATMENT:
GA (purity ≥98%; TCI, Tokyo, Japan) was diluted in 0.5% Tween-80, and was injected intraperitoneally at a dose of 20 mg/kg BW (body weight) 30 min after suture removal [7]. Mice maintained for 72h after tMCAO received 0.5% Tween-80 with or without GA every 12 hours. Positive control mice were treated with dl-3-N-butylphthalide (NBP, purity ≥99%, 75 mg/kg BW, SYJT, Shijiazhuang, China) or resatorvid (TAK-242, 3 mg/kg BW, MedChemExpress, Monmouth Junction, NJ, USA) every 12 hours, as both reagents had been shown to effectively exacerbate stroke injury [19,20].
VENOUS BLOOD GLUCOSE:
Blood samples were collected from the lateral tail vein, and blood glucose concentrations were measured every 12 hours using a glucometer (Accu-Chek Performa; Roche, Indianapolis, IN, USA).
NEUROLOGICAL SCORES:
Neurological deficits were assessed blindly by 2 investigators 24 h after cerebral ischemia using a previously described grading system [21], with normal scored as grade 0, forelimb flexion when lifted by the tail scored as grade 1, circling to the contralateral side when held by the tail on a flat surface despite normal posture at rest scored as grade 2, leaning to the contralateral side at rest scored as grade 3, and no spontaneous motor reaction scored as grade 4.
MEASUREMENT OF INFARCT VOLUME AND BRAIN EDEMA:
Infarction volume was evaluated by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Freshly removed brains were cut into 2-mm-thick coronal slices using a mice brain matrix. The slices were stained with 2% TTC (Sigma-Aldrich) for 30 min at 37°C in the dark, followed by fixation with 10% formalin. The stained slices were scanned and analyzed with Image J software (NIH, Bethesda, MD, USA).
Wet weight of removed brains was measured immediately after resection and dry weight was measured after brains were dried at 95°C for 24 hours in an oven. Brain Water Content (%) was calculated as [(wet weight-dry weight)/wet weight]×100 [22].
WESTERN BLOTTING:
Western blotting of proteins of freshly collected brains was performed as described [23,24]. The primary antibodies were polyclonal rabbit anti-β-actin (Proteintech, 20536-1-AP, 1: 1000, Rosemont, IL, USA), polyclonal rabbit anti-TLR4 (Abcam, ab13556, 1: 500, Cambridge, UK), polyclonal rabbit anti-Myd88 (Abcam, ab2064, 1: 500), polyclonal rabbit anti-HMGB1 (Abcam, ab18256, 1: 200) and polyclonal rabbit anti-NF-κB (Cell Signaling Technology, CST, #8242, 1: 500, Danvers, MA, USA), all diluted with phosphate buffered saline (PBS). The membranes were washed with PBS and incubated with goat anti-rabbit IgG secondary antibody (ZSJQ, ZB-2308, Beijing, China). Relative protein expression was assessed densitometrically with a BD imaging system (Becton Dickinson, Franklin Lakes, NJ, USA).
REAL TIME QUANTITATIVE POLYMERASE CHAIN REACTION ANALYSIS:
Real time quantitative polymerase chain reactions were performed as described [25]. In brief, mice were sacrificed under deep anesthesia and their brains were dissected out immediately. Total RNA was isolated using Sepasol-RNA I Super G (Nakalai Tesque, Kyoto, Japan), according to the manufacturer’s instructions. Quantitative reverse transcription PCR (RT-PCR) was performed using StepOne Plus (Applied Biosystems, Foster City, CA, USA). The quantity of each mRNA was normalized to that of GADPH mRNA. The primers used in these experiments were:
ELISA:
Serum samples were collected from each group of mice. All reagents were equilibrated to room temperature, and the concentrations of TNF-α, IL-6 and IL-1β were measured using ELISA kits (Abcam, Cambridge, UK), according to the manufacturer’s instructions.
HISTOLOGICAL EXAMINATION:
Six mice from each group were anesthetized and perfused with ice-cold 0.9% NaCl followed by 4% paraformaldehyde (PFA, Leagene, Beijing, China). Their brains were removed, fixed in 4% PFA for 5–7 days at 4°C, and embedded in paraffin using standard methods. Coronal sections were cut to 4-μm thickness and immunohistologically stained as described with polyclonal rabbit anti-Myd88 antibody (Abcam, ab2064, 1: 100) [26]. The percentages of positive cells in ischemic areas were determined using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
Immunofluorescent staining was performed as described [27]. Brain sections were permeabilized with 0.1% Triton X-100 for 2 hours and incubated with primary antibodies, including polyclonal rabbit anti-NeuN (CST, #24307, 1: 100, Boston, MA, USA), polyclonal goat anti-Iba-1(Abcam, ab5076, 1: 500), and polyclonal rabbit anti-NF-κB (Cell Signaling Technology, CST, #8242, 1: 100). The sections were washed and incubated with Alexa 488-labeled anti-donkey IgG secondary antibody (Abbkine, Wuhan, China). Brain sections were double-stained with 2-(4-amidinophenyl)-6-indolecarbamidine (DAPI, ZSJQ, Beijing, China). Immunofluorescent images were analyzed with a confocal laser scanning microscope (Nikon, Tokyo, Japan).
Nissl staining was performed as described using a Nissl staining kit (Solarbio, Beijing, China) [28]. Briefly, brain slices were dehydrated in an ethanol gradient, washed in ultra-pure water, immersed in xylene, and stained with thionine. Cell morphology was observed by microscopy.
STATISTICAL ANALYSIS:
GraphPad Prism 8.0 software was used for all statistical analyses. Results were reported as mean±SD, and inter-group differences compared by one-way analysis of variance (ANOVA), followed by Turkey’s test. Correlations were determined with Spearman’s ρ. P values <0.05 were considered statistically significant.
Results
GA SUPPRESSED EXPRESSION OF MYD88 AND OTHER FACTORS INVOLVED IN HMGB1/TLR4/MYD88/NF-κB SIGNALING:
The percentage of Myd88-positive cells was higher in the D-24 than in the C-24 group, but was significantly lower in the D-24+GA than in the D-24 group. The effects of GA were further confirmed by comparing the D-72 and D-72+GA groups (Figure 1A, 1B). The percentage of Myd88-positive cells correlated positively with neurological score in both the D-24+GA (p=0.0069) and D-72+GA (p=0.0024) groups (Figure 1C), a finding confirmed by the correlation between Myd88 protein level and neurological score in the D-24+GA (p=0.0069) and D-72+GA (p=0.0036) groups (Figure 1D, 1E). These observations indicated that diabetes aggravated I/R injury through MyD88 expression, both of which were suppressed by GA administration.
Previous studies have indicated that MyD88 is involved in the pathway promoting proinflammatory reactions, in which TLR4, HMGB1 and NF-κB play important roles [11]. Post-treatment with GA reduced the expression of TLR4 and HMGB1 in both the D-24+GA and D-72+GA groups (Figure 2A–2C). In addition, tMCAO increased the number of NF-κB-positive cells in diabetic mice, an increased reduced by post-treatment with GA (Figure 2D, 2E).
GA INHIBITED THE INFLAMMATORY RESPONSE INDUCED BY CEREBRAL I/R INJURY IN DIABETIC MICE:
The effects of GA on the serum concentrations of inflammatory molecules stimulated by cerebral I/R were determined by ELISA. Serum TNF-α, IL-6 and IL-1β concentrations were increased after I/R injury. These concentrations were higher in the D-24 than in the C-24 group, but were significantly lower in the D-24+GA than in the D-24 group. The effect of GA was further confirmed by comparing the D-72 and D-72+GA groups (Figure 2F–2H).
GA AFTER TREATMENT AMELIORATED CEREBRAL INJURY IN DIABETIC MICE:
When compared with the C-24 group, mice in the D-24 group showed significantly greater infarct volume, neurological score and brain water content (Figure 3A–3D). Comparisons of the D-24+GA and D-72+GA groups with the D-24 and D-72 groups, respectively, showed that treatment with GA significantly reduced infarct volume, neurological score and brain water content (Figure 3A–3D). Despite GA having beneficial effects on cerebral infarction, it did not ameliorate hyperglycemia in diabetic mice (Figure 3E), indicating that, in diabetic mice, post-treatment GA improved cerebral I/R injury without reducing blood glucose level.
GA AFTER TREATMENT DECREASED NEURONAL LOSS AND MICROGLIAL ACTIVATION:
To assess the effects of GA on brain tissue, brain tissues of mice were examined histologically by Nissl staining and immunohistochemically for expression of NeuN (a marker for neurons) and Iba-1 (a marker for activated microglia). Compared with the C-24 group, smaller numbers of Nissl bodies and NeuN-positive cells and a higher percentage of Iba-1-positive microglia were observed in the D-24 group, indicating that tMCAO reduced the number of neurons and increased the number of activated microglia. Comparisons of the D-24+GA and D-24 groups showed that post-treatment GA ameliorated the loss of neurons during I/R (Figure 4A–4D) and significantly reduced the percentage of Iba-1-positive microglia in diabetic mice (Figure 4E, 4F).
GA SHOWED GREATER BETTER ANTI-INFLAMMATORY EFFECTS THAN TAK-242 AND NBP ON HMGB1/TLR4-MYD88/NF-κB SIGNALING IN DIABETIC MICE:
NBP has been approved and marketed as an anti-stroke drug [29], and TAK-242 has been shown to have protective effects in rats with cerebral I/R injury [20]. GA treatment, however was a more potent inhibitor of the signaling pathway involving HMGB1, TLR4, Myd88 and NF-κB than NBP or TAK-242 in diabetic mice with cerebral I/R injury (Figure 5).
Discussion
This study clearly showed that diabetes aggravated cerebral I/R injury in mice, as shown by the larger infarct volume, more severe brain edema, and poorer neurological function after tMCAO in diabetic mice. Moreover, diabetes enhanced expressive HMGB1/TLR4/Myd88/NF-κB signaling in these mice. Uncontrolled inflammation can exacerbate brain damage after cerebral I/R [30]. Diabetes has been reported to cause continuous microglial polarization in the brain, which may exacerbate inflammation [31]. Accumulated inflammatory responses may promote cytokine release and neutrophil infiltration, leading to tissue damage and neurological deficits [32].
Because GA has been used to regulate exacerbated inflammation in several disease statuses [33,34], it was expected to effectively reduce cerebral I/R injury in diabetic animals. This study found that GA after tMCAO edema in diabetic mice improved the viability of neurons and reduced inflammatory microglia, which resulted in reduced infarction volume and brain. Further, as cerebral Myd88 level was positively correlated with neurological scores, GA likely ameliorated I/R injury by inactivating a proinflammatory pathway that included Myd88. Myd88 has been regarded as a key molecule connecting signals from TLR4 to NF-κB, stimulating the expression of various proinflammatory cytokines, including TNF-α. GA treatment reduced TLR4 and NF-κB expression, along with Myd88, suggesting that GA inactivated the entire pathway. Proinflammatory cytokines expressed through the activation of this pathway were probably responsible for cerebral inflammation, which eventually caused poor outcomes of I/R injury in diabetic mice. Similarly, an inhibitor of TLR4 effectively ameliorated stroke in diabetes, mainly through inhibition of Myd88 [35]. The translocation of NF-κB p65 in several signaling pathways has been found to exaggerate inflammatory responses, and palmitoylethanolamide (PEA) was also shown to relieve injury by regulating NF-κB [36–38].
Anti-HMGB1 antibody has been found to reduce the leakiness of the blood brain barrier in diabetic mice subjected to tMCAO, suggesting a potential therapeutic strategy for patients with ischemic stroke [39]. Although treatment with anti-HMGB1 is logical and promising, GA has 2 possible advantages over anti-HMGB1. First, GA may have multiple molecular targets, as shown in this study, whereas anti-HMGB1 antibody inhibits HMGB1 alone. Second, GA and its pharmaceutical preparations have been approved as safe for clinical application to patients [40,41].
The potential mechanisms by which post-treatment GA ameliorates cerebral I/R injury in diabetic mice may be associated with its anti-inflammatory properties. Expression of the signaling pathway that involves HMGB1, TLR4, Myd88, and NF-κB was found to be higher in diabetic than in non-diabetic mice following cerebral I/R injury. GA, however, effectively inhibited this pathway, suggesting that GA plays a neuroprotective role against cerebral I/R injury. Because GA is already used in clinical practice, further studies assessing the effects of GA on ischemic stroke in diabetic patients are warranted.
Conclusions
Diabetes aggravates cerebral I/R injury by enhancing the expression level of the HMGB1/TLR4-Myd88/NF-κB p65 signaling pathway, an effect relieved by post-treatment application of GA. GA may ameliorate cerebral I/R injury in diabetes and have greater anti-inflammatory activity than NBP and TAK-242.
Figures
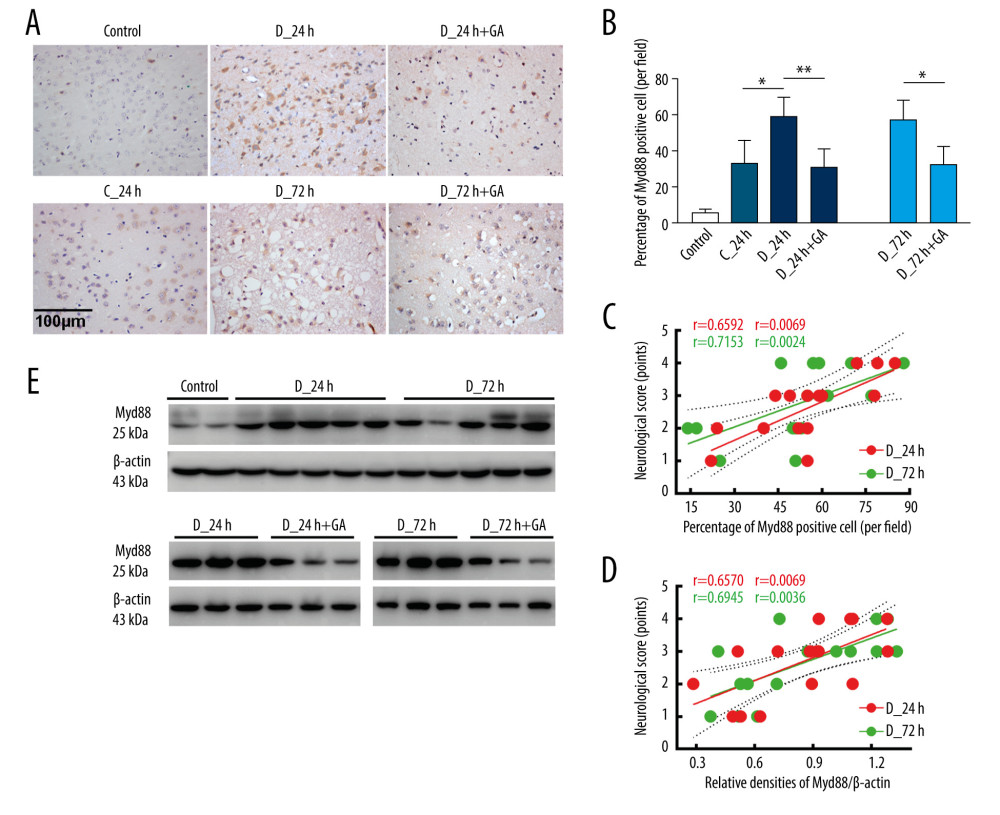 Figure 1. Myd88 expression correlated with neurological scores and was suppressed by GA. (A, B) Immunohistochemical staining of cerebral Myd88 in each group of mice. (C) Correlation between the percentage of Myd88 positive cells and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0024) groups (n=16 each). (D, E) Correlation between cerebral Myd88 protein level, as shown by western blotting, and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0036) groups (n=16 each). Data are shown as mean±SD. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001.
Figure 1. Myd88 expression correlated with neurological scores and was suppressed by GA. (A, B) Immunohistochemical staining of cerebral Myd88 in each group of mice. (C) Correlation between the percentage of Myd88 positive cells and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0024) groups (n=16 each). (D, E) Correlation between cerebral Myd88 protein level, as shown by western blotting, and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0036) groups (n=16 each). Data are shown as mean±SD. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001. 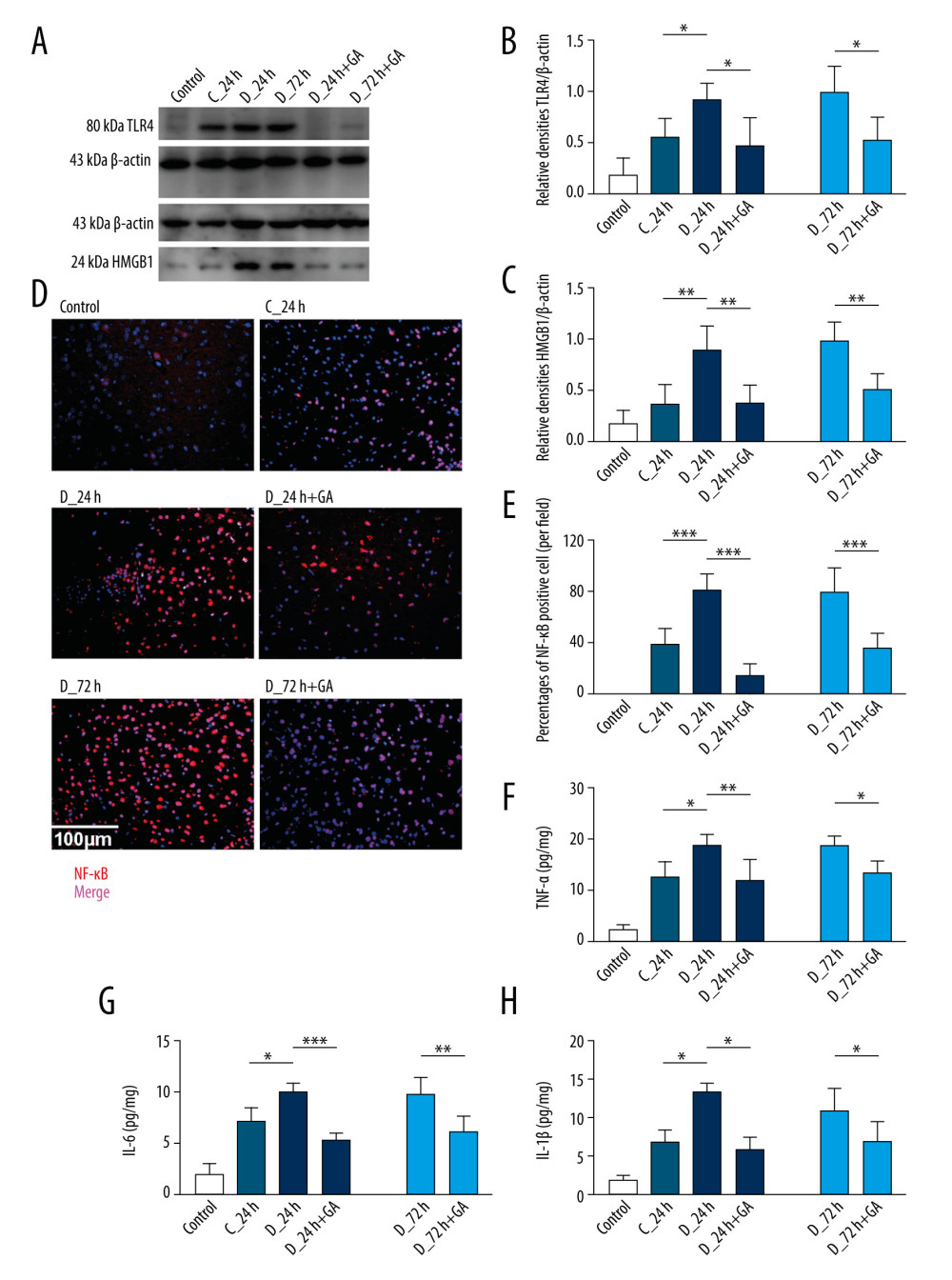 Figure 2. GA suppressed expression of Myd88 and other factors involved in inflammatory reactions. (A) TLR4 and HMGB1 protein expression, as shown by western blotting. (B, C) Quantitation of the results in A. (D). Immunofluorescence staining of NF-κB; the merged area indicates active transcription. (E) Percentages of NF-κB positive cells. (F–H) Serum concentrations of TNF-α, IL-6 and IL-1β. Each point is representative of 6 mice. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001, *** p<0.0001.
Figure 2. GA suppressed expression of Myd88 and other factors involved in inflammatory reactions. (A) TLR4 and HMGB1 protein expression, as shown by western blotting. (B, C) Quantitation of the results in A. (D). Immunofluorescence staining of NF-κB; the merged area indicates active transcription. (E) Percentages of NF-κB positive cells. (F–H) Serum concentrations of TNF-α, IL-6 and IL-1β. Each point is representative of 6 mice. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001, *** p<0.0001. 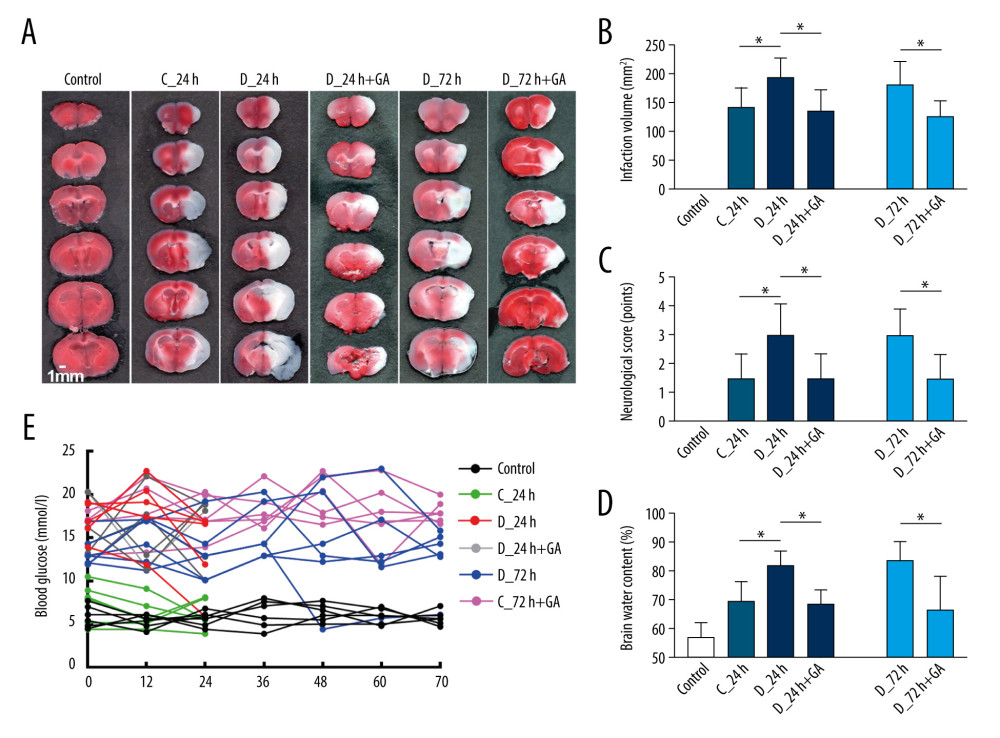 Figure 3. Post-treatment GA ameliorated diabetes-aggravated cerebral injury. Groups of mice were sacrificed 0 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h after tMCAO, A. TTC staining. (B) Cerebral infarction volume. (C) Neurological scores. (D) Percentage of brain water content. (E) Blood glucose concentrations. Results are presented as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05.
Figure 3. Post-treatment GA ameliorated diabetes-aggravated cerebral injury. Groups of mice were sacrificed 0 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h after tMCAO, A. TTC staining. (B) Cerebral infarction volume. (C) Neurological scores. (D) Percentage of brain water content. (E) Blood glucose concentrations. Results are presented as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05. 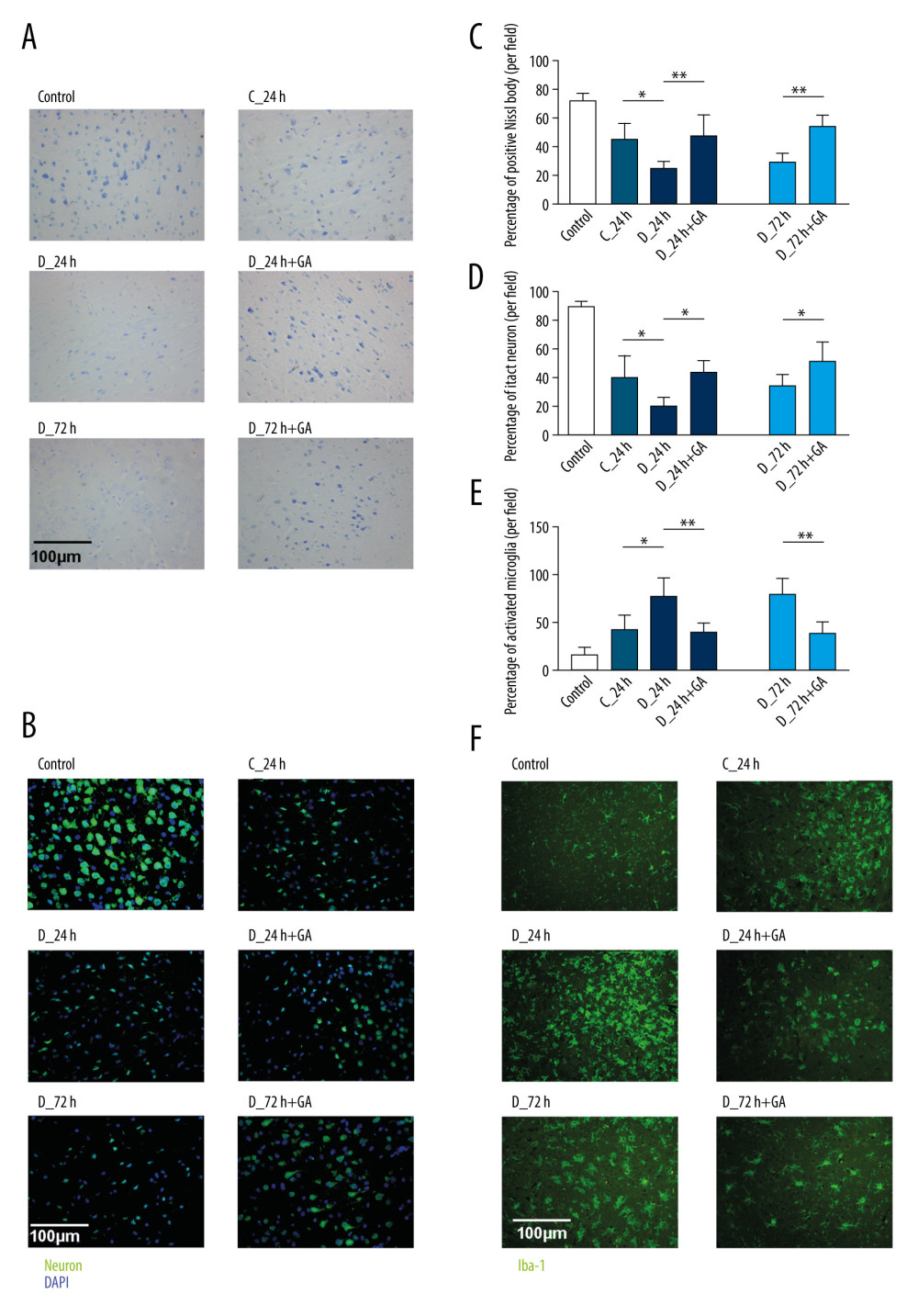 Figure 4. Post-treatment GA decreased neuronal loss and microglial activation. (A) Nissl staining. (B) Immunofluorescence staining of neurons. (C, D) Percentages of cells positive for Nissl staining and positive for neuronal cell markers. (E) Immunofluorescence staining of iba-1 labeled microglial. (F) Percentages of cells positive for iba-1. Data are shown as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05, ** p<0.001.
Figure 4. Post-treatment GA decreased neuronal loss and microglial activation. (A) Nissl staining. (B) Immunofluorescence staining of neurons. (C, D) Percentages of cells positive for Nissl staining and positive for neuronal cell markers. (E) Immunofluorescence staining of iba-1 labeled microglial. (F) Percentages of cells positive for iba-1. Data are shown as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05, ** p<0.001. 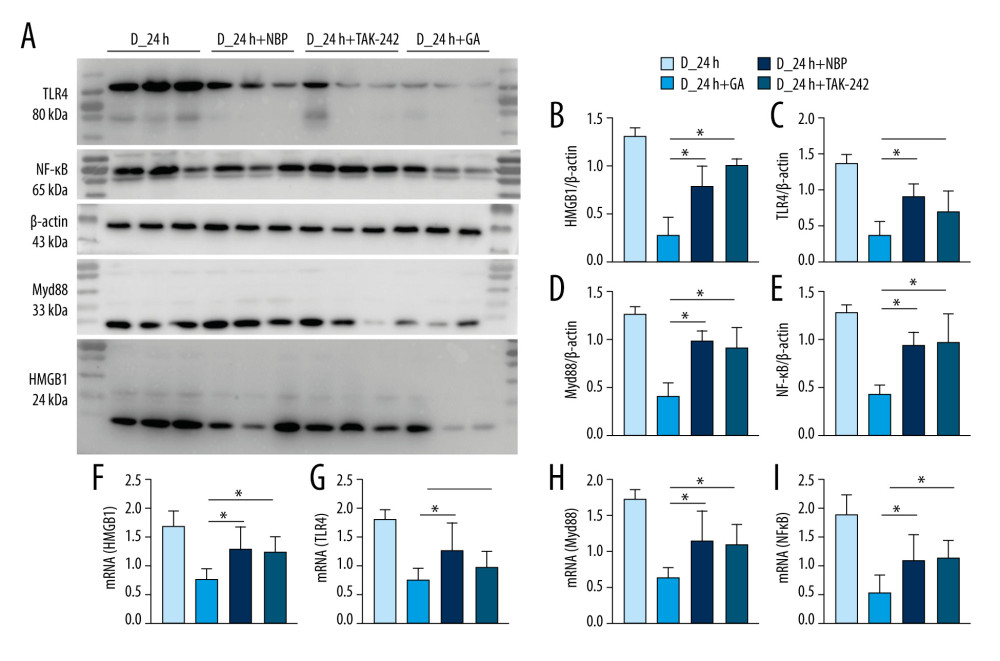 Figure 5. GA had more potent anti-inflammatory effects on HMGB1/TLR4-Myd88/NF-κB signaling than TAK-242 and NBP. (A) Western blotting showing expression of HMGB1, TLR4, Myd88, and NF-κB in groups of mice. (B–E) Quantitation of HMGB1 (B), TLR4 (C), Myd88 (D), and NF-κB (E) proteins. (F–I) Quantitation of HMGB1 (F), TLR4 (G), Myd88 (H), and NF-κB (I) mRNAs. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05.
Figure 5. GA had more potent anti-inflammatory effects on HMGB1/TLR4-Myd88/NF-κB signaling than TAK-242 and NBP. (A) Western blotting showing expression of HMGB1, TLR4, Myd88, and NF-κB in groups of mice. (B–E) Quantitation of HMGB1 (B), TLR4 (C), Myd88 (D), and NF-κB (E) proteins. (F–I) Quantitation of HMGB1 (F), TLR4 (G), Myd88 (H), and NF-κB (I) mRNAs. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05. References
1. Lozano R, Naghavi M, Foreman K, Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010: Lancet, 2012; 380(9859); 2095-128
2. Papanagiotou P, White CJ, Endovascular reperfusion strategies for acute stroke: JACC Cardiovasc Interv, 2016; 9(4); 307-17
3. Grotta JC, tPA for stroke: Important progress in achieving faster treatment: JAMA, 2014; 311(16); 1615-17
4. Fukuta T, Asai T, Yanagida Y, Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke: FASEB J, 2017; 31(5); 1879-90
5. Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ, Diabetes, hyperglycaemia, and acute ischaemic stroke: Lancet Neurol, 2012; 11(3); 261-71
6. Gregg EW, Sattar N, Ali MK, The changing face of diabetes complications: Lancet Diabetes Endocrinol, 2016; 4(6); 537-47
7. Hou SZ, Li Y, Zhu XL, Ameliorative effects of diammonium glycyrrhizinate on inflammation in focal cerebral ischemic-reperfusion injury: Brain Res, 2012; 1447; 20-27
8. Fusco R, Scuto M, Cordaro M, N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway: Int J Mol Sci, 2019; 20(19); 4845
9. Mizuma A, Yenari MA, Anti-inflammatory targets for the treatment of reperfusion injury in stroke: Front Neurol, 2017; 8; 467
10. Graeber MB, Neuroinflammation: No rose by any other name: Brain Pathol, 2014; 24(6); 620-22
11. Albornoz EA, Woodruff TM, Gordon R, Inflammasomes in CNS diseases: Exp Suppl, 2018; 108; 41-60
12. Di Padova F, Quesniaux VFJ, Ryffel B, MyD88 as a therapeutic target for inflammatory lung diseases: Expert Opin Ther Targets, 2018; 22(5); 401-8
13. Wang LQ, Zhou HJ, LncRNA MALAT1 promotes high glucose-induced inflammatory response of microglial cells via provoking MyD88/IRAK1/TRAF6 signaling: Sci Rep, 2018; 8(1); 8346
14. Ye X, Kong D, Wang J, MyD88 contributes to neuroinflammatory responses induced by cerebral ischemia/reperfusion in mice: Biochem Biophys Res Commun, 2016; 480(1); 69-74
15. Bernard NJ, O’Neill LA, Mal, more than a bridge to MyD88: IUBMB Life, 2013; 65(9); 777-86
16. Impellizzeri D, Peritore AF, Cordaro M, The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice: FASEB J, 2019; 33(10); 11364-80
17. Rees DA, Alcolado JC, Animal models of diabetes mellitus: Diabet Med, 2005; 22(4); 359-70
18. Lin X, Miao P, Wang J, Surgery-related thrombosis critically affects the brain infarct volume in mice following transient middle cerebral artery occlusion: PLoS One, 2013; 8(9); e75561
19. Yan RY, Wang SJ, Yao GT, The protective effect and its mechanism of 3-n-butylphthalide pretreatment on cerebral ischemia reperfusion injury in rats: Eur Rev Med Pharmacol Sci, 2017; 21(22); 5275-82
20. Rice TW, Wheeler AP, Bernard GR, A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis: Crit Care Med, 2010; 38(8); 1685-94
21. Longa EZ, Weinstein PR, Carlson S, Cummins R, Reversible middle cerebral artery occlusion without craniectomy in rats: Stroke, 1989; 20(1); 84-91
22. Traber PG, Ganger DR, Blei AT, Brain edema in rabbits with galactosamine-induced fulminant hepatitis. Regional differences and effects on intracranial pressure: Gastroenterology, 1986; 91(6); 1347-56
23. Burnette WN, “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate – polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A: Anal Biochem, 1981; 112(2); 195-203
24. Fusco R, D’amico R, Cordaro M, Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis: Oncotarget, 2018; 9(59); 31355-66
25. Fleige S, Pfaffl MW, RNA integrity and the effect on the real-time qRT-PCR performance: Mol Aspects Med, 2006; 27(2–3); 126-39
26. Schiffer D, Giordana MT, Cavalla P, Immunohistochemistry of glial reaction after injury in the rat: Double stainings and markers of cell proliferation: Int J Dev Neurosci, 1993; 11(2); 269-80
27. Antanitus DS, Choi BH, Lapham LW: Brain Res, 1975; 89(2); 363-67
28. Shipley MT, Ennis M, Behbehani MM, Acetylcholinesterase and Nissl staining in the same histological section: Brain Res, 1989; 504(2); 347-53
29. Abdoulaye IA, Guo YJ, A review of recent advances in neuroprotective potential of 3-N-butylphthalide and its derivatives: Biomed Res Int, 2016; 2016 5012341
30. Venkat P, Chopp M, Chen J, Blood-brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke: J Am Heart Assoc, 2017; 6(6); e005819
31. Venkat P, Chopp M, Chen J, Cell-based and exosome therapy in diabetic stroke: Stem Cells Transl Med, 2018; 7(6); 451-55
32. Khoshnam SE, Winlow W, Farzaneh M, Pathogenic mechanisms following ischemic stroke: Neurol Sci, 2017; 38(7); 1167-86
33. Kao TC, Shyu MH, Yen GC, Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation: J Agric Food Chem, 2010; 58(15); 8623-29
34. Yu JY, Ha JY, Kim KM, Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver: Molecules, 2015; 20(7); 13041-54
35. Abdul Y, Abdelsaid M, Li W, Inhibition of toll-like receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: Possible role of vascular endothelial TLR-4: Mol Neurobiol, 2019; 56(3); 1607-17
36. Cordaro M, Impellizzeri D, Siracusa R, Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia: Toxicol Appl Pharmacol, 2017; 329; 231-40
37. Gugliandolo E, Fusco R, D’Amico R, Anti-inflammatory effect of ATB-352, a H2S-releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats: Pharmacol Res, 2018; 132; 220-31
38. D’amico R, Fusco R, Gugliandolo E: Phytomedicine, 2019; 54; 27-42
39. Wang C, Jiang J, Zhang X, Inhibiting HMGB1 reduces cerebral ischemia reperfusion injury in diabetic mice: Inflammation, 2016; 39(6); 1862-70
40. Musumeci D, Roviello GN, Montesarchio D, An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies: Pharmacol Ther, 2014; 141(3); 347-57
41. Omar HR, Komarova I, El-Ghonemi M, Licorice abuse: Time to send a warning message: Ther Adv Endocrinol Metab, 2012; 3(4); 125-38
Figures
 Figure 1. Myd88 expression correlated with neurological scores and was suppressed by GA. (A, B) Immunohistochemical staining of cerebral Myd88 in each group of mice. (C) Correlation between the percentage of Myd88 positive cells and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0024) groups (n=16 each). (D, E) Correlation between cerebral Myd88 protein level, as shown by western blotting, and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0036) groups (n=16 each). Data are shown as mean±SD. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001.
Figure 1. Myd88 expression correlated with neurological scores and was suppressed by GA. (A, B) Immunohistochemical staining of cerebral Myd88 in each group of mice. (C) Correlation between the percentage of Myd88 positive cells and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0024) groups (n=16 each). (D, E) Correlation between cerebral Myd88 protein level, as shown by western blotting, and neurological scores in the D-24+GA (p=0.0069) and D-72+GA (p=0.0036) groups (n=16 each). Data are shown as mean±SD. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001. Figure 2. GA suppressed expression of Myd88 and other factors involved in inflammatory reactions. (A) TLR4 and HMGB1 protein expression, as shown by western blotting. (B, C) Quantitation of the results in A. (D). Immunofluorescence staining of NF-κB; the merged area indicates active transcription. (E) Percentages of NF-κB positive cells. (F–H) Serum concentrations of TNF-α, IL-6 and IL-1β. Each point is representative of 6 mice. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001, *** p<0.0001.
Figure 2. GA suppressed expression of Myd88 and other factors involved in inflammatory reactions. (A) TLR4 and HMGB1 protein expression, as shown by western blotting. (B, C) Quantitation of the results in A. (D). Immunofluorescence staining of NF-κB; the merged area indicates active transcription. (E) Percentages of NF-κB positive cells. (F–H) Serum concentrations of TNF-α, IL-6 and IL-1β. Each point is representative of 6 mice. The 2 ends of each horizontal line are the 2 groups being compared, * p<0.05, ** p<0.001, *** p<0.0001. Figure 3. Post-treatment GA ameliorated diabetes-aggravated cerebral injury. Groups of mice were sacrificed 0 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h after tMCAO, A. TTC staining. (B) Cerebral infarction volume. (C) Neurological scores. (D) Percentage of brain water content. (E) Blood glucose concentrations. Results are presented as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05.
Figure 3. Post-treatment GA ameliorated diabetes-aggravated cerebral injury. Groups of mice were sacrificed 0 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h after tMCAO, A. TTC staining. (B) Cerebral infarction volume. (C) Neurological scores. (D) Percentage of brain water content. (E) Blood glucose concentrations. Results are presented as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05. Figure 4. Post-treatment GA decreased neuronal loss and microglial activation. (A) Nissl staining. (B) Immunofluorescence staining of neurons. (C, D) Percentages of cells positive for Nissl staining and positive for neuronal cell markers. (E) Immunofluorescence staining of iba-1 labeled microglial. (F) Percentages of cells positive for iba-1. Data are shown as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05, ** p<0.001.
Figure 4. Post-treatment GA decreased neuronal loss and microglial activation. (A) Nissl staining. (B) Immunofluorescence staining of neurons. (C, D) Percentages of cells positive for Nissl staining and positive for neuronal cell markers. (E) Immunofluorescence staining of iba-1 labeled microglial. (F) Percentages of cells positive for iba-1. Data are shown as mean±SD. The images are representative of 6 independent experiments. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05, ** p<0.001. Figure 5. GA had more potent anti-inflammatory effects on HMGB1/TLR4-Myd88/NF-κB signaling than TAK-242 and NBP. (A) Western blotting showing expression of HMGB1, TLR4, Myd88, and NF-κB in groups of mice. (B–E) Quantitation of HMGB1 (B), TLR4 (C), Myd88 (D), and NF-κB (E) proteins. (F–I) Quantitation of HMGB1 (F), TLR4 (G), Myd88 (H), and NF-κB (I) mRNAs. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05.
Figure 5. GA had more potent anti-inflammatory effects on HMGB1/TLR4-Myd88/NF-κB signaling than TAK-242 and NBP. (A) Western blotting showing expression of HMGB1, TLR4, Myd88, and NF-κB in groups of mice. (B–E) Quantitation of HMGB1 (B), TLR4 (C), Myd88 (D), and NF-κB (E) proteins. (F–I) Quantitation of HMGB1 (F), TLR4 (G), Myd88 (H), and NF-κB (I) mRNAs. The 2 ends of each horizontal line are the 2 groups being compared. * p<0.05. In Press
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








