27 May 2021: Animal Study
Acupuncture Can Play an Antidepressant Role by Regulating the Intestinal Microbes and Neurotransmitters in a Rat Model of Depression
Peng Li1E, Wenya Huang1E, Yi-ning Yan2B, Wenjing Cheng1C, Siyu Liu3C, Yang Huang1C, Wenjie Chen1C, Yi-ping Chen4D, Yuxun Gao1D, Weicheng Lu1B, Yijing Xu1B, Xianjun Meng1AG*DOI: 10.12659/MSM.929027
Med Sci Monit 2021; 27:e929027
Abstract
BACKGROUND: Acupuncture, which has many good effects and few adverse effects, is widely recognized as an alternative therapy for depression in clinical practice. This study aimed to explore the mechanism of acupuncture in antidepressant treatment.
MATERIAL AND METHODS: In this experiment, Sprague-Dawley rats were randomly divided into 4 groups: control, chronic unpredictable mild stress (CUMS), acupuncture, and fluoxetine groups. The CUMS, acupuncture, and fluoxetine groups were orphaned and subjected to chronic unpredictable stress for 6 weeks, and the acupuncture and fluoxetine groups were treated with their respective intervention in weeks 4-6. The body weight of rats was monitored weekly. After behavioral tests were completed, serum, feces, and hippocampal tissue of rats were collected.
RESULTS: The results showed that the acupuncture and fluoxetine treatments could alleviate the behavioral changes caused by CUMS. The treatments increased the total distance of rat crossing in the open-field test, prolonged the activity time of the open cross maze in the open arm, and improved the rate of sucrose consumption in the sucrose preference test. In addition, both the decreased level of dopamine (DA) and 5-hydroxytryptamine (5-HT) in serum and hippocampus caused by CUMS were improved after the treatments with acupuncture and fluoxetine, and the decreased expression of brain-derived neurotrophic factor signaling and the astrocytes in the hippocampus caused by CUMS were increased after the treatments with acupuncture and fluoxetine. Acupuncture and fluoxetine also decreased the β isoform of calmodulin-dependent protein kinase II in the hippocampus, which was increased by CUMS. Furthermore, acupuncture regulated intestinal microbial disorders caused by CUMS, which reduced the relative abundance ratio of Bacteroidetes/Firmicutes in rats.
CONCLUSIONS: Our experimental results indicate that acupuncture can alleviate depression-like performance in CUMS rats by regulating intestinal microbes and neurotransmitters.
Keywords: Acupuncture, Anti-N-Methyl-D-Aspartate Receptor Encephalitis, Acupuncture Therapy, Antidepressive Agents, Second-Generation, Behavior, Animal, Depression, fluoxetine, Hippocampus
Background
Depression is an affective disorder characterized by persistent sadness, feelings of inferiority and helplessness, and anxiety [1]. It has a high lifetime prevalence rate and recurrence [2], with an annual prevalence rate of 8% and a lifetime prevalence rate of 19%, and it can coexist with various medical diseases [3]. According to the latest data from the World Health Organization, there are more than 300 million people with depression in the world [4]. Depression has become one of the main causes of human health problems worldwide [5,6]. Antidepressants are the dominant form of clinical treatment for depression, and they include selective serotonin reuptake inhibitors, particularly fluoxetine [7]. Most antidepressants, including fluoxetine, have severe adverse effects and low efficacy, and at least one-third of patients do not obtain a therapeutic effect [8]. Acupuncture is an alternative therapy for depression, with fewer adverse effects and good therapeutic efficacy, as well as a low financial burden.
As a widely used alternative therapy, acupuncture for the treatment of depression has increasingly attracted attention in clinical and animal experimental research [9–11]. Some researchers suggest that the ability of acupuncture to regulate depression may be related to the corticostriatal reward circuit in the brain [12]. Studies on acupuncture in rat models of depression have shown that its effect on depression-like behavior may be associated with the hypothalamus-pituitary-adrenal axis [13]. Other studies have reported that acupuncture can regulate inflammatory factors such as interleukin-6 [14].
Studies have also shown that intestinal microbiota can play an important role in the pathogenesis and treatment of depression [15]. For example, continuous administration of
Thirteen “ghost” acupoints are commonly used to treat mental diseases in acupuncture and moxibustion in ancient China. We previously demonstrated that acupuncture at the Shangxing and Daling acupoints has an antidepressant effect [18], with both acupoints being among the 13 ghost acupoints of the traditional Chinese acupuncture theory system [19]. Based on previous experiments and the literature, we explored whether acupuncture at the Shangxing and Daling acupoints could have an antidepression effect through regulation of intestinal microbes and neurotransmitters.
Material and Methods
ANIMALS AND GROUPS:
Forty specific pathogen-free male Sprague-Dawley rats, approximately 5 weeks old and weighing 100–120 g, were provided by Shanghai SLAC Laboratory Animal Co., Ltd. (qualified no. SCXK 2017-0005, Shanghai, China). The animals were kept at the Animal Laboratory of Medical College of Xiamen University in a standard laboratory environment (temperature 22±2°C, 55±5% relative humidity, from 8: 00 AM to 8: 00 PM, simulated by alternating light cycles during the day and night. All rats were provided with free access to adequate water and food. All experimental operations were in line with international animal experimental ethics and requirements, and they were approved by the Animal Ethics Committee of Xiamen University (License No. XMULAC20170376).
All animals were fed adaptively in the laboratory for 1 week before the start of the formal experiment. The body weight of rats was measured weekly throughout the experiment, and the sucrose preference test and the open-field test were carried out to ensure that the baseline levels of all animals were the same. Eight rats with different baselines were excluded. Thirty-two rats with the same baseline were randomly divided into 4 groups: control (N=8), chronic unpredictable mild stress (CUMS; N=8), acupuncture and CUMS (AP; N=8), and fluoxetine and CUMS (Fx; N=8).
CUMS MODEL PROCEDURE AND TREATMENT:
The CUMS model, which was originally developed by Paul Willner, is widely used in the experimental study of depression in animals [20,21]. The rats in the control group were fed in cages and given free access to water and food. The rats in the other 3 groups were raised in a single cage, and the model was established for 6 weeks. The stresses were as follows: wet padding (24 h); food and water withheld (24 h); cold water swim (4°C, 5 min); restraint (3 h); noise stimulation (80 dB, 3 h); tail clamping (2 min); day and night inversion (12 h); and cage tilting (45°, 24 h).
The Fx group received fluoxetine intragastrically (2.1 mg/kg; PHR1394-1G, Sigma-Aldrich) every day 1 h before the CUMS in the fourth week [22]. The AP group was also treated with acupuncture every day 1 h before exposure to CUMS in the fourth week. Acupuncture was administered at the Shangxing and Daling acupoints by inserting steel needles into the 2 acupoints of the skin to 3- to 5-mm depth. All needles used were disposable aseptic steel needles with a specification of 0.18×13 mm (0.18-mm diameter; Hanyi, Beijing, China). At the Shangxing acupoint, the needle was inserted at an oblique angle (45°) to 3–5 mm, and at the Daling acupoint, the needle pierced the skin vertically to a depth of 2–3 mm. Needles were kept in place for 20 min. The acupuncture stimulation intensity was increased by rotating the needle clockwise (180°) and counterclockwise (180°) every 5 min.
After all behavioral tests, all rats were euthanized with pentobarbital combined with rapid bloodletting. Blood was collected from the abdominal aorta, placed at 4°C overnight, and centrifuged at 3000 rpm for 15 min, and the supernatant was extracted. Colons were cut open and feces were collected. The brain was removed from the skull, and the hippocampus was quickly separated. All operations were carried out on ice, and samples were quickly frozen in liquid nitrogen and then stored at −80°C. The procedures are shown in Figure 1.
BEHAVIORAL TESTS:
Behavioral tests, including the sucrose preference test and the open-field test, were performed 3 times. The first time was in the first week before CUMS modeling, and we used the results to exclude rats with different baselines or congenital depression. The second time was in the fourth week to eliminate rats with no significant change after 3 weeks of CUMS modeling, and the last time was in the seventh week.
OPEN-FIELD TEST:
The open-field test can be used to detect spontaneous activity and depression-like behavior of rats [23]. The rats were placed in a black open box (100×100×40 cm) in which they could run freely, and the bottom of the box was equally divided into 4×4 squares [24]. Each rat was placed in the center of the bottom of the open box and was then allowed to move freely for 5 min. An infrared camera was placed perpendicular to the top of the open box for recording the activity of the rats in all areas. Two experimenters who did not know the experimental grouping or the type of intervention recorded the number of squares crossed (horizontal score) and the number of upright explorations (vertical score), as well as the number of modification behaviors of rats and times that they defecated. At the end of each test, the box was cleaned with 75% ethanol for odor elimination to ensure that the experimental results of other rats would not be affected.
SUCROSE PREFERENCE TEST:
We describe a protocol for the measurement of anhedonia in mice [25]. Before the beginning of the formal sucrose experiment, we first carried out the adaptation part of the sucrose experiment. Each rat was provided with a bottle of pure water and a bottle of 1% sucrose solution at the same time for 48 h. The positions of the 2 water bottles were changed every 12 h. After that, water was withheld for 24 h. Then, we began the formal experiment, recorded the drinking of sucrose solution and pure water in each rat within 12 h, and then calculated the sucrose preference. Sucrose preference rate (%)=[sucrose solution consumption/(sucrose solution consumption+pure water consumption)]×100.
ELEVATED PLUS MAZE BEHAVIORAL TEST:
The elevated plus maze test is a widely used method to assess anxiety behavior of rodents [26]. The elevated cross labyrinth used a suspended device 1 m above the ground, consisting of 2 open arms (50×10×40 cm), a central area (10×10 cm), and 2 closed arms (50×10×40 cm). At the beginning of the test, the rats were placed in the central area with their heads facing the open arms. A high-resolution infrared camera suspended directly above was connected to the behavioral test system to record statistics, including the distance and time that rats were in the open and closed arms. After each rat experiment, the equipment was washed with 75% ethanol to eliminate odor.
ENZYME-LINKED IMMUNOSORBENT ASSAY:
The levels of dopamine (DA) and 5-hydroxytryptamine (5-HT) in serum and the hippocampus were measured by enzyme-linked immunosorbent assay (Elabscience, USA). All experimental operations were carried out following the manufacturer’s instructions for the use of the kit. First, 300 μL of washing solution was added to soak the enzyme plate for 30 s, the solution was then discarded, and the micropores were dried on the absorbent paper. Next, 50 μL of detection buffer and 50 μL of 2× diluted standard were added to the standard well on the plate. Then, 50 μL of detection buffer and 50 μL of standard dilution solution were added into the blank well, 80 μL of detection buffer and 20 μL of serum samples were added into the sample well, and 50 μL of diluted detection antibody was added to all wells. The plate was incubated at room temperature for 1.5 h, and the detection solution was discarded. Three hundred microliters of washing solution were added to each well, and the plate was washed 6 times and patted dry. Then, 100 μL of horseradish peroxidase (HRP)-labeled streptavidin was added to each well, and the plate was incubated at room temperature for 30 min. The wells were emptied, 300 μL of washing solution was added to each well to wash 6 times, and the plate was patted dry. Finally, 100 μL of chromogenic substrate was added to each well, the plate was incubated in darkness for 5 min, and 100 μL of stop solution was added to each well. Within 30 min, the optical density value at 450-nm wavelength was detected by the enzyme labeling instrument to determine the absorbance value of the sample.
REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION:
The mRNA coding sequences of calmodulin-dependent protein kinase II (β-CaMKII), N-methyl-d-aspartate receptor (NMDAR), and brain-derived neurotrophic factor (BDNF) were identified from the Consensus Coding Sequence database of the National Center for Biotechnology Information (NCBI). The polymerase chain reaction (PCR) primers were developed before the reverse transcription (RT)-PCR process.
The tissue samples of about 100 mg were weighed, ground with liquid nitrogen, and extracted with 1 mL of Trizol reagent at room temperature for 5 min. The samples were then centrifuged at 12 000 rpm for 10 min at 4°C, and the supernatant was collected. Next, 0.2 mL of chloroform was added to each 1 mL of the supernatant, and the mixture was incubated at room temperature for 3 min and centrifuged at 12 000 rpm for 10 min at 4°C. The sample was divided into 3 layers. The top layer was taken, 500 μL of the aqueous phase was absorbed, and 250 μL of anhydrous ethyl alcohol was added. The mixture was then transferred to the adsorption column. Next, 500 μL of resultant deproteinized solution was centrifuged at 12 000 rpm for 45 s, and 500 μL of rinse solution was added for 2 rinses, followed by centrifugation at 12 000 rpm for 45 s. The RNA was eluted, and the concentration was determined by agarose gel electrophoresis. One microgram was extracted from each sample for reverse transcription, and each sample was detected by β-actin and primers, respectively. Finally, 2 μL of PCR products was detected by agarose gel. The electrophoresis conditions were 140 V, 15 min, gel imaging. We analyzed the gel using ImageJ software (National Institutes of Health, Bethesda, MD).
WESTERN BLOT ANALYSIS:
The hippocampal tissue protein was put into RIPA buffer containing protease and phosphatase inhibitors and ground to a homogenate. This procedure carried out on ice. About 30 μg of the protein homogenate was placed in a tube, and 4× loading buffer was added before the tube was placed in a boiling water bath for 10 min. Afterward, the sample was centrifuged at 10 000 rpm for 10 min. The protein sample was separated by electrophoresis on a 10% SDS-PAGE gel, and the separated proteins were then transferred from the gel to a polyvinylidene fluoride (PVDF) membrane by electroporation. Afterward, the PVDF membrane was immersed in a blocking solution containing 5% skimmed milk powder and incubated at room temperature for 1 h. Then the membrane was washed in phosphate-buffered Tween (PBST) for 5 min, 3 times. Diluted anti-BDNF (1: 2000, Millipore, USA), anti-β-CaMKII (1: 2000, Santa Cruz, USA), and anti-NMDAR (1: 2000, Millipore, USA) antibodies were added to the membrane, which was subsequently incubated overnight at 4°C. The next day, the membrane was washed with PBST for 20 min, 3 times, and HRP-labeled second antibody (Proteintech, USA) was added, followed by incubation at room temperature for 1 h. Afterward, the membrane was washed in PBST for 10 min, 3 times. Finally, the PVDF membrane was reacted with freshly prepared enhanced chemiluminescent agent solution for 2 min, and the PVDF membrane was quickly exposed and developed in the darkroom.
IMMUNOFLUORESCENCE:
Brains were rapidly removed, frozen at −80 °C, and cut into 2-mm coronal slices in a rat brain matrix. After 10% formalin fixation, hippocampal tissue samples were processed into paraffin-embedded blocks and 5-μm-thick sections were cut for immunostaining. Hippocampal CA1 region sections were deparaffinized and treated with EDTA antigen repair buffer (pH 8.0). Antibodies used were anti-ionized calcium binding adaptor molecule 1 (IBA-1; anti-Iba1 rabbit pAb, Servicebio, HRP anti-rabbit IgG (HRP-conjugated goat anti-rabbit IgG, Servicebio), anti-glial fibrillary acidic protein (anti-GFAP; anti-GFAP rabbit pAb, Servicebio), and Cy3 anti-rabbit (Cy3-conjugated goat anti-rabbit IgG, Servicebio). Finally, the sections were sealed with the antifluorescence solution (G1401-5ML, Servicebio), and the immunofluorescence images were observed and recorded with fluorescence microscope (NIKON ECLIPSE C1, NIKON DS-U3).
16S RDNA BIOINFORMATICS ANALYSIS:
The genomic DNA was extracted from rat fecal samples using a DNA extraction kit (Magen, Guangzhou, China). The 16S rDNA V3–V4 region of the ribosomal RNA gene for each sample was amplified by PCR. Primers used for 16S rDNA were as follows: 341F: CCTACGGGNGGCWGCAG; 806R: GGACTACHVGGGTATCTAAT. Using the manufacturer’s instructions, the DNA quantified using ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (PE250) on an Illumina platform according to standard protocols. The raw reads were deposited into the NCBI Sequence Read Archive database. The analysis of the operational taxonomic units (OTUs) was performed under 97% similarity level, and multivariate analysis and tests for significance were also performed. A 16S rDNA analysis was performed utilizing the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database.
STATISTICAL ANALYSIS:
Each data set is expressed in the form of the mean±standard error of the mean. The software for statistical analysis was SPSS 21.0 for Windows 10. When each group of data accorded with normality and met the homogeneity of variance, one-way analysis of variance was carried out for intergroup statistics. In addition, the Games-Howell test was used for post hoc analyses. If the data did not conform to normality, we used nonparametric testing for different groups for statistical analysis.
Results
BEHAVIORAL TESTS:
As shown in Figure 2, there was a significant difference in body weight between the control group and the CUMS group (N=8). After the intervention treatment began, the AP and Fx groups both showed significant weight gain compared with the CUMS group. Compared with the Fx group, the AP group recovered more slowly, but the gap between groups gradually narrowed after the fifth week. In the sixth week, the AP group was close to the Fx group. Compared with the control group, the total distance in the open-field test of the CUMS group was reduced, as were the activity time of the open cross maze in the open arm, and the sucrose preference test. The AP and Fx treatment groups demonstrated a reversal of these behavioral changes.
THE EFFECTS OF ACUPUNCTURE ON THE LEVELS OF DA AND 5-HT:
As shown in Figure 3, compared with the control group, the 5-HT content in the hippocampus and serum of the CUMS group was decreased (P<0.001, P<0.001). After the rats received acupuncture treatment, the 5-HT content in the hippocampus and serum increased compared with the CUMS group (P<0.001, P<0.001). Fluoxetine treatment also led to increased 5-HT content in the hippocampus and serum compared with the CUMS group (P<0.001, P<0.001). Compared with the control group, the DA content in the hippocampus of the CUMS group decreased (P<0.05). The DA content in the serum also decreased, but not significantly (P>0.05). In both the AP and Fx groups, the DA content in the hippocampus and serum increased compared with the CUMS group (P>0.05), but the increases were not significant in either group (P>0.05).
THE EXPRESSION AT THE MRNA LEVEL OF β-CAMKII/NMDAR/BDNF:
As shown in Figure 4, in comparison with the control group (N=6), the expression of β-CaMKII mRNA in the hippocampus of the CUMS group was significantly increased (P<0.01), while BDNF and NMDAR mRNA expression decreased (P<0.05, P<0.05). In the AP group, compared with the CUMS group, the expression of β-CaMKII mRNA decreased (P<0.05) and NMDAR mRNA expression increased (P<0.05). The BDNF mRNA expression increased, but the difference was not statistically significant (P>0.05). Compared with the CUMS group, the expression of β-CaMKII mRNA in the rats in the Fx group was significantly decreased (P<0.01), while the expression of BDNF and NMDAR mRNA was significantly increased (P<0.01, P<0.05).
THE EXPRESSION OF β-CAMKII/NMDAR/BDNF SIGNAL:
The results shown in Figure 5 indicate that, compared with the control group (N=3), the expression of BDNF and NMDAR protein in the hippocampus of the CUMS group decreased (P<0.05), the expression of β-CaMKII protein increased significantly (P<0.05), after the acupuncture treatment, compared with the CUMS group, the BDNF and NMDAR protein expression increased (P<0.05, P<0.01), and the β-CaMKII protein expression decreased (P<0.01). For the Fx group, compared with the CUMS group, BDNF and NMDAR protein expression increased (P<0.01, P<0.05) and β-CaMKII protein expression decreased significantly (P<0.001).
EXPRESSION OF GFAP AND IBA-1:
Results shown in Figure 6 indicate that, compared with the control group (N=3), the content of astrocytes in the hippocampus of rats in the CUMS group was reduced (P<0.001). In the AP and Fx groups, the astrocyte content was increased compared with the CUMS group (P<0.01 and P<0.001, respectively). Compared with the control group, the content of microglia in the hippocampus of the CUMS group decreased (P<0.01). In the AP group, the microglia content was significantly higher than in the model group (P<0.05). For the Fx group, the microglia content was not significantly higher than that of the CUMS group (P>0.05).
ACUPUNCTURE REDUCES CUMS-INDUCED INTESTINAL FLORA IMBALANCE:
The effect of acupuncture on the diversity and richness of intestinal flora was assessed in CUMS rats (N=5). The Shannon diversity index was calculated to indicate the diversity in each group. Different samples and the relative abundance of intestinal microorganisms at the portal level were analyzed according to OTU Venn.
The results are shown in Figure 7. Based on relative abundance, Bacteroidetes and Firmicutes were the most dominant intestinal microbiota among the 3 groups. The ratio of Bacteroidetes/Firmicutes has been considered to be an important indicator that is positively correlated with depression. In our study, the abundance of Firmicutes was much lower in the CUMS group relative to the control and AP groups, and the abundance of Bacteroidetes was higher. The ratio of Bacteroidetes/Firmicutes was increased in CUMS group.
We conducted a KEGG analysis to compare the differences in the metabolic pathways between the 3 groups. The differences identified through the KEGG analysis were mainly manifested in cofactors and vitamins, cell growth, and death. In addition, we conducted a Kruskal-Wallis rank sum test for differences in the metabolic pathways. The differences were mainly manifested in metabolism of cofactors and vitamins, carbohydrate metabolism, and amino acid metabolism. The results are shown in Figure 8.
Discussion
The purpose of this study was to explore the mechanism of acupuncture in regulating depression in CUMS model rats. CUMS is widely used as a rodent depression model [27]. We previously carried out studies to confirm that acupuncture can reverse behavioral changes in depression model rats, affect the nitric oxide-cyclic guanosine monophosphate signaling pathway, and have an antidepressant effect [18]. We created a rat model of depression through CUMS standard procedures and established the effectiveness of the model through open-field and sucrose preference tests. These tests mainly assess core symptoms of depression, such as lack of pleasure. The experiment found that the depressive-like symptoms induced by CUMS, such as loss of weight and appetite and reduced activity, were alleviated in the AP and Fx groups [28]. The results at the molecular level showed that acupuncture and fluoxetine could alleviate the behavioral changes caused by CUMS. In addition, levels of both DA and 5-HT in serum and the hippocampus were improved, and the expression of BDNF signaling and the number of astrocytes in the hippocampus were increased.
Low levels of DA or 5-HT have been proved to be connected with depression [29,30]. Studies have shown that 5-HT and BDNF are closely related to regulation of neurogenesis, synaptic plasticity, and neuronal survival [31], and there is a certain connection between these 2 signals [32]. Our experimental results are consistent with the current evidence that 5-HT can alleviate depression. AP can significantly increase the content of 5-HT in the brain hippocampus and the serum of depression model rats, and AP upregulated the content of BDNF/NMDAR and the mRNA of BDNF/NMDAR in the hippocampus tissue of depression model rats. BDNF is a major neurotrophic factor in brain, which plays an important role in the survival and guidance of neurons [33]. BDNF is required for the survival and normal functioning of neurons in the brain [34]. Astrocyte cultures derived from the hippocampus have been shown to promote neurogenesis [35]. Astrocytes have a high level of GFAP expression, and GFAP has become one of the most frequently used astrocyte markers [36]. IBA-1, a protein that is specifically expressed in activated microglia, can also be an astrocyte marker [37]. Studies have shown that CUMS may cause neurological impairment [38]. We tested the activity of astrocytes and microglia in hippocampus by GFAP and IBA-1. The expression of astrocytes in the AP and FX groups was found to be significantly higher than in the CUMS group. Increased βCaMKII can play a role in blocking the BDNF receptor. β-CaMKII in the hippocampus of the CUMS group was overexpressed, and acupuncture was found to improve this phenomenon.
The present study shows that disruption of intestinal microorganisms reduces hippocampal neurotransmitter levels, such as 5-HT and BDNF [39,40]. Intestinal microflora disorders have been shown to be important factors associated with depression [41]. Many clinical studies and animal experiments have found that emotional responses are significantly correlated with changes in intestinal flora. Decreased fecal flora diversity has been observed in patients with depression [42,43]. Our study suggests that acupuncture can reduce depression behavior in CUMS rats by regulating intestinal microbes. According to the bioinformatics analysis of 16S rDNA sequencing, the abundance of intestinal microbes in CUMS depression model rats decreased, and acupuncture regulated the changes of intestinal microbial diversity induced by CUMS. The 16S rDNA analysis revealed that the bacterial biodiversity of the CUMS group was significantly lower than that of the control group, while that of the AP group was higher compared with the CUMS group. No significant changes in the composition of intestinal flora were found between the 3 groups.
In general, our study established that Shangxing and Daling acupoints possess antidepressant effect in the CUMS rat. Intestinal microbes and metabolites can affect the 5-HT and BDNF factors in the nervous system through a series of immune responses [47,48] and subsequently affect the entire nervous system. Acupuncture treatment can have an antidepressant effect through this route [49]. The present study provides new insights into the treatment of depression. However, there are some limitations that need to be improved. Specifically, just 2 acupoints may not be representative of all 13 ghost points; however, it is unrealistic to use acupuncture for all 13 ghost points in a single experiment. We aim to choose other points from among the 13 ghost points in our future research. In addition, more brain regions should be considered beyond the hippocampus, and the internal relationship between different indicators should be explored. Further research is essential to discover the exact mechanism underlying the regulation of gut microbiota and neurotransmitters and neurons, which can support the hypothesis more fully and thus make acupuncture more useful in the treatment of psychiatric disorders.
Conclusions
In conclusion, our research shows that acupuncture can reverse depression-like behavior in CUMS model rats. The antidepressant effect may arise from regulation of DA and 5-HT neurotransmitters and effects on β-CaMKII/NMDAR/BDNF signaling. Further, acupuncture also improves the intestinal microflora disorder caused by CUMS, which may be associated with the antidepressant effect of acupuncture.
Figures
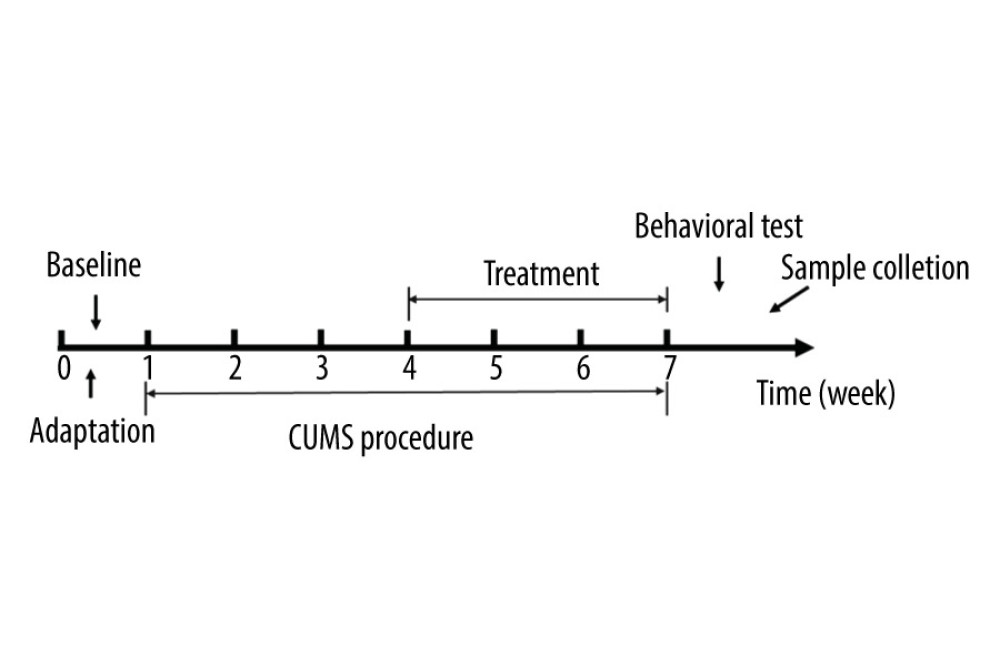 Figure 1. The experimental flow chart.
Figure 1. The experimental flow chart. 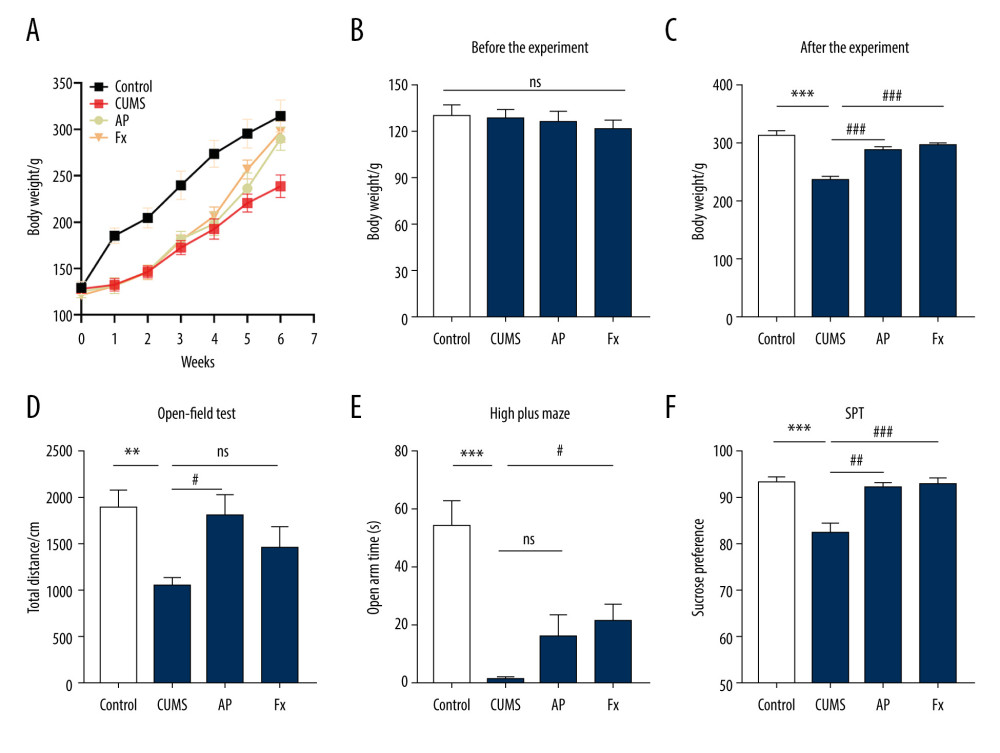 Figure 2. Behavior tests were conducted for the rats in 4 groups. (A) The body weight of rats in the 4 groups. (B) The body weight of rats before the chronic unpredictable mild stress (CUMS). (C) The body weight of rats after the treatment. (D) The results of the open-field test. (E) The results of the elevated cross maze test. (F) The results of the sucrose preference test experiment. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine.
Figure 2. Behavior tests were conducted for the rats in 4 groups. (A) The body weight of rats in the 4 groups. (B) The body weight of rats before the chronic unpredictable mild stress (CUMS). (C) The body weight of rats after the treatment. (D) The results of the open-field test. (E) The results of the elevated cross maze test. (F) The results of the sucrose preference test experiment. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine. 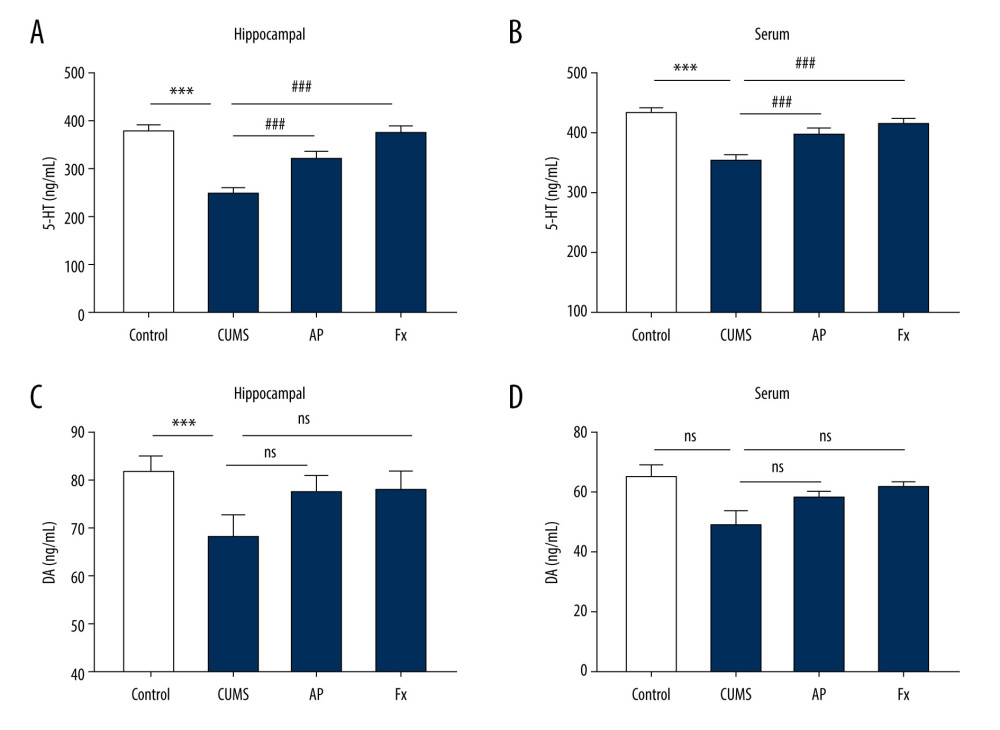 Figure 3. Statistical graph of 5-hydroxytryptamine (5-HT) and dopamine (DA) content in rat serum and hippocampus samples. (A) 5-HT content in hippocampus. (B) 5-HT content in serum. (C) DA content in hippocampus. (D) DA content in serum. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine.
Figure 3. Statistical graph of 5-hydroxytryptamine (5-HT) and dopamine (DA) content in rat serum and hippocampus samples. (A) 5-HT content in hippocampus. (B) 5-HT content in serum. (C) DA content in hippocampus. (D) DA content in serum. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine. 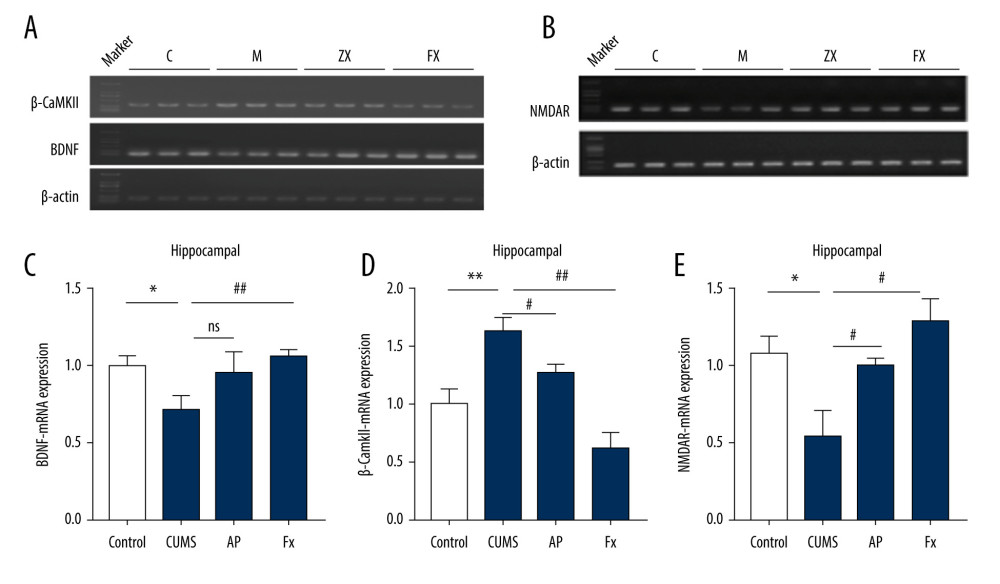 Figure 4. Statistical graph of the expression of β-CaMKII/NMDAR/BDNF mRNA in the hippocampus of rats. (A) Expression of BDNF and β-CaMKII mRNA in the hippocampus by reverse transcription polymerase chain reaction (RT-PCR). (B) Expression of NMDAR mRNA in the hippocampus by RT-PCR. (C) Analysis of BDNF mRNA. (D) Analysis of β-CaMKII mRNA. (E) Analysis of NMDAR mRNA. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor.
Figure 4. Statistical graph of the expression of β-CaMKII/NMDAR/BDNF mRNA in the hippocampus of rats. (A) Expression of BDNF and β-CaMKII mRNA in the hippocampus by reverse transcription polymerase chain reaction (RT-PCR). (B) Expression of NMDAR mRNA in the hippocampus by RT-PCR. (C) Analysis of BDNF mRNA. (D) Analysis of β-CaMKII mRNA. (E) Analysis of NMDAR mRNA. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor. 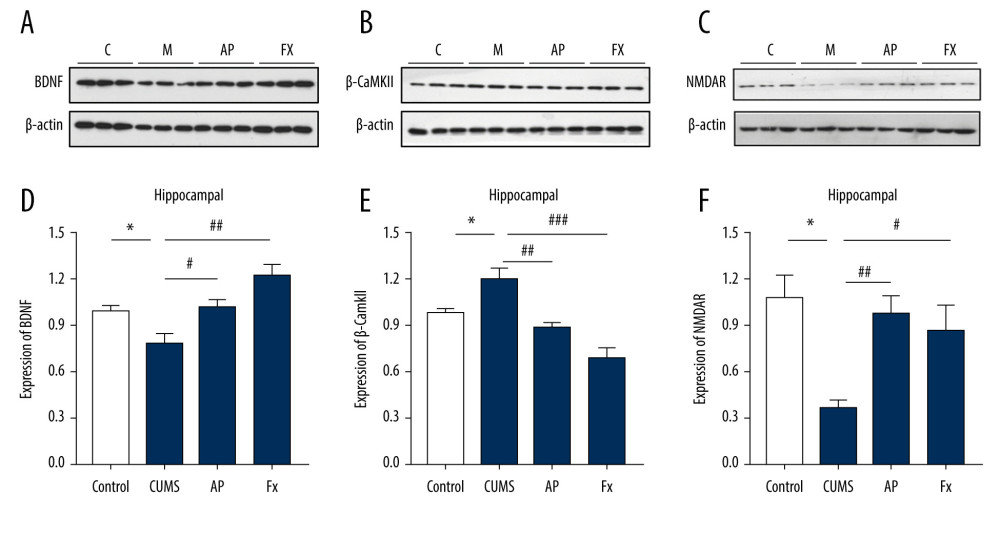 Figure 5. Statistical graph of β-CaMKII/NMDAR/BDNF protein expression in the rat hippocampus. (A) Expression of BDNF proteins in the hippocampus by western blotting. (B) Expression of β-CaMKII proteins in the hippocampus by western blotting. (C) Expression of NMDAR proteins in the hippocampus by western blotting. (D) Analysis of BDNF proteins. (E) Analysis of β-CaMKII proteins. (F) Analysis of NMDAR proteins. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor.
Figure 5. Statistical graph of β-CaMKII/NMDAR/BDNF protein expression in the rat hippocampus. (A) Expression of BDNF proteins in the hippocampus by western blotting. (B) Expression of β-CaMKII proteins in the hippocampus by western blotting. (C) Expression of NMDAR proteins in the hippocampus by western blotting. (D) Analysis of BDNF proteins. (E) Analysis of β-CaMKII proteins. (F) Analysis of NMDAR proteins. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor. 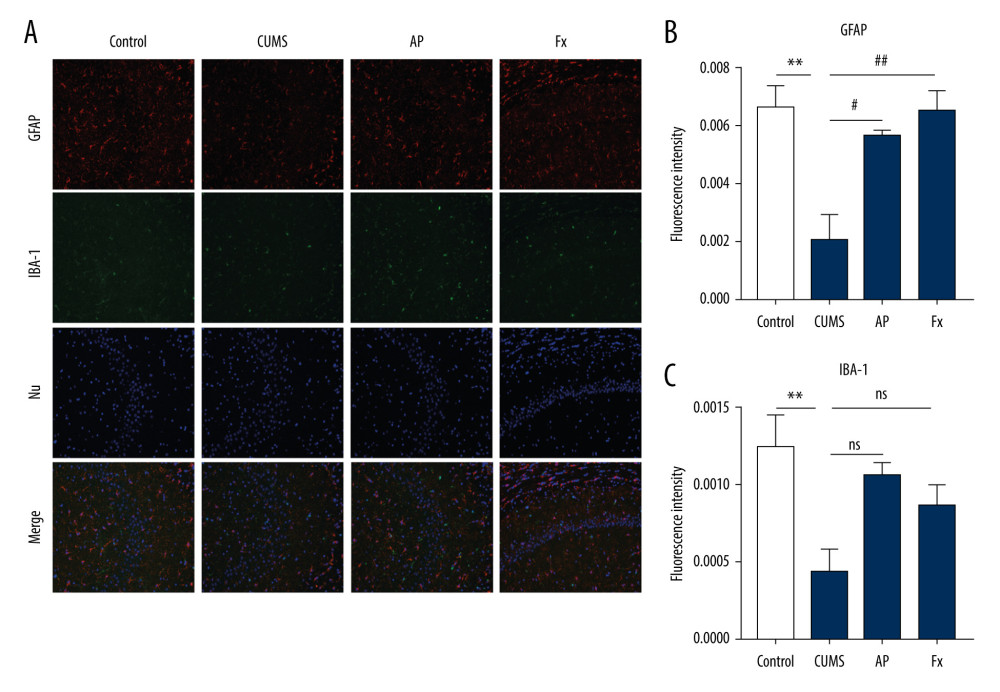 Figure 6. (A) Immunofluorescence confocal microscopy imaging hippocampus CA1 regions, magnification ×200. (B) Expression of GFAP in the hippocampus. (C) Expression of IBA-1 in the hippocampus. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine; GFAP – glial fibrillary acidic protein; IBA-1 – ionized calcium binding adaptor molecule 1.
Figure 6. (A) Immunofluorescence confocal microscopy imaging hippocampus CA1 regions, magnification ×200. (B) Expression of GFAP in the hippocampus. (C) Expression of IBA-1 in the hippocampus. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine; GFAP – glial fibrillary acidic protein; IBA-1 – ionized calcium binding adaptor molecule 1. 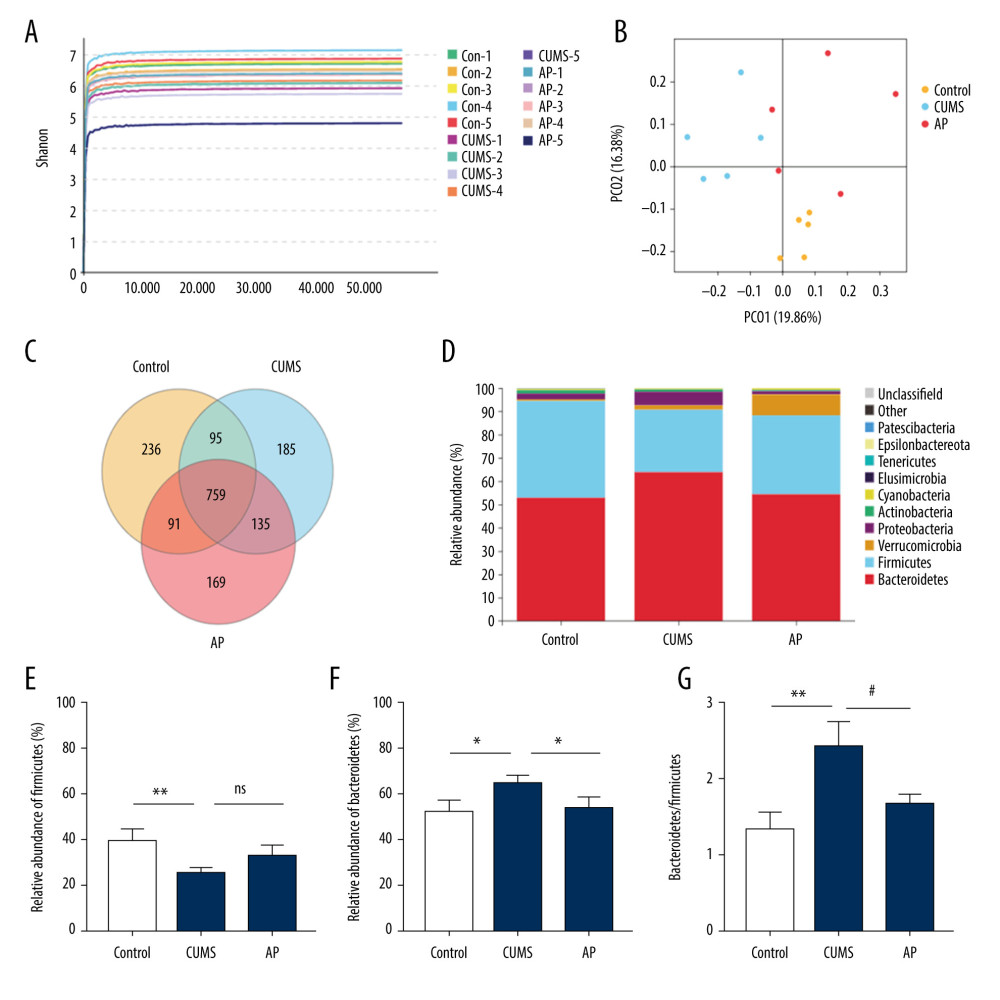 Figure 7. Effect of acupuncture on the diversity and richness of intestinal flora in chronic unpredictable mild stress (CUMS) rats. (A) The Shannon index value indicates the diversity of samples. (B) Principal coordinates analysis (PCoA) for the distance between samples (β diversity) calculated using unweighted UniFrac distance. (C) A Venn diagram of the operational taxonomic units (OTUs) analyzes samples of different groups. (D) Relative abundance analysis at the phylum level. (E) Relative abundance of Firmicutes at the phylum level. (F) Relative abundance of Bacteroidetes at the phylum level. (G) Relative abundance of Bacteroidetes/Firmicutes at the phylum level. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine group.
Figure 7. Effect of acupuncture on the diversity and richness of intestinal flora in chronic unpredictable mild stress (CUMS) rats. (A) The Shannon index value indicates the diversity of samples. (B) Principal coordinates analysis (PCoA) for the distance between samples (β diversity) calculated using unweighted UniFrac distance. (C) A Venn diagram of the operational taxonomic units (OTUs) analyzes samples of different groups. (D) Relative abundance analysis at the phylum level. (E) Relative abundance of Firmicutes at the phylum level. (F) Relative abundance of Bacteroidetes at the phylum level. (G) Relative abundance of Bacteroidetes/Firmicutes at the phylum level. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine group. 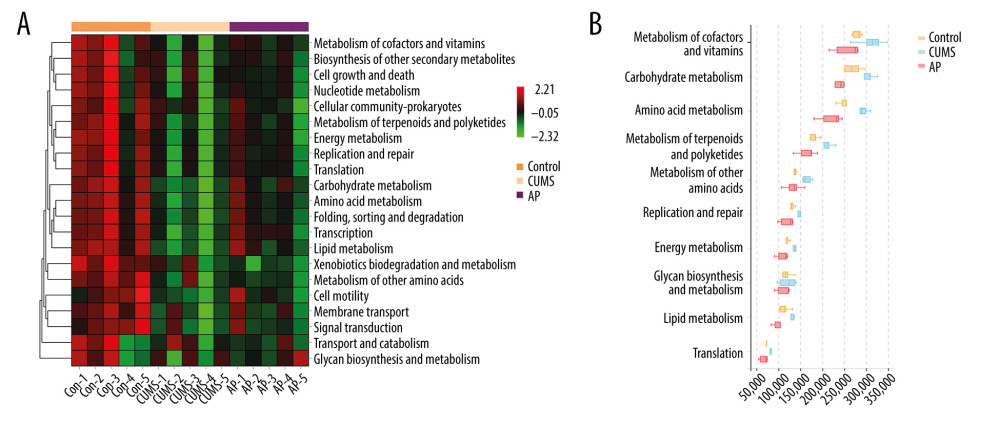 Figure 8. (A) The functional abundance heat map of the differential enrichment of intestinal microorganisms in each group. (B) Kruskal-Wallis rank sum test for differences in metabolic pathways. Con – control; CUMS – chronic unpredictable mild stress; AP – acupuncture group.
Figure 8. (A) The functional abundance heat map of the differential enrichment of intestinal microorganisms in each group. (B) Kruskal-Wallis rank sum test for differences in metabolic pathways. Con – control; CUMS – chronic unpredictable mild stress; AP – acupuncture group. References
1. Hedegaard H, Johnson RL: Natl Health Stat Report, 2019(125); 1-8
2. Nelson CJ, Cho C, Berk AR, Are gold standard depression measures appropriate for use in geriatric cancer patients? A systematic evaluation of self-report depression instruments used with geriatric, cancer, and geriatric cancer samples: J Clin Oncol, 2010; 28(2); 348-56
3. Kessler RC, Bromet EJ, The epidemiology of depression across cultures: Annu Rev Public Health, 2013; 34; 119-38
4. World Health Organization (WHO): Depression and other common mental disorders: Global health estimates, 2017, Geneva, WHO
5. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017: Lancet, 2018; 392(10159); 1789-858
6. Barnett R, Depression: Lancet, 2019; 393(10186); 2113
7. Berton O, Nestler EJ, New approaches to antidepressant drug discovery: Beyond monoamines: Nat Rev Neurosci, 2006; 7(2); 137-51
8. Kim J, Farchione T, Potter A, Esketamine for treatment-resistant depression – first FDA-approved antidepressant in a new class: N Engl J Med, 2019; 381(1); 1-4
9. Cao J, Tu Y, Orr SP, Analgesic effects evoked by real and imagined acupuncture: A neuroimaging study: Cereb Cortex, 2019; 29(8); 3220-31
10. Jorm AF, Christensen H, Griffiths KM, Effectiveness of complementary and self-help treatments for depression: Med J Aust, 2002; 176(10); S84-96
11. Kessler RC, Soukup J, Davis RB, The use of complementary and alternative therapies to treat anxiety and depression in the United States: Am J Psychiatry, 2001; 158(2); 289-94
12. Wang Z, Wang X, Liu J, Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder: J Psychiatr Res, 2017; 84; 18-26
13. Le JJ, Yi T, Qi L, Electroacupuncture regulate hypothalamic-pituitary-adrenal axis and enhance hippocampal serotonin system in a rat model of depression: Neurosci Lett, 2016; 615; 66-71
14. Lu J, Shao RH, Hu L, Potential antiinflammatory effects of acupuncture in a chronic stress model of depression in rats: Neurosci Lett, 2016; 618; 31-38
15. Valles-Colomer M, Falony G, Darzi Y, The neuroactive potential of the human gut microbiota in quality of life and depression: Nat Microbiol, 2019; 4(4); 623-32
16. Bharwani A, Mian MF, Surette MG: BMC Med, 2017; 15(1); 7
17. Li Y, Peng Y, Ma P: Front Pharmacol, 2018; 9; 967
18. Huang W, Meng X, Huang Y, Nitric oxide and cyclic guanosine monophosphate signaling mediates the antidepressant effects of acupuncture in the rat model of chronic unpredictable mild stress: Med Sci Monit, 2019; 25; 9112-22
19. Martin BR, Complementary medicine therapies that may assist with weight loss: A narrative review: J Chiropr Med, 2019; 18(2); 115-26
20. Willner P, Towell A, Sampson D, Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant: Psychopharmacology (Berl), 1987; 93(3); 358-64
21. Hu C, Luo Y, Wang H, Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress: PLoS One, 2017; 12(9); e0185129
22. Singh VP, Patil CS, Jain NK, Tramadol interaction with fluoxetine: An isobolographic analysis: Drug Dev Res, 2004; 61(2); 79-85
23. Kraeuter AK, Guest PC, Sarnyai Z, The open field test for measuring locomotor activity and anxiety-like behavior: Methods Mol Biol, 2019; 1916; 99-103
24. Takahashi A, Kato K, Makino J, Multivariate analysis of temporal descriptions of open-field behavior in wild-derived mouse strains: Behav Genet, 2006; 36(5); 763-74
25. Liu MY, Yin CY, Zhu LJ, Sucrose preference test for measurement of stress-induced anhedonia in mice: Nat Protoc, 2018; 13(7); 1686-98
26. Walf AA, Frye CA, The use of the elevated plus maze as an assay of anxiety-related behavior in rodents: Nat Protoc, 2007; 2(2); 322-28
27. Hill MN, Hellemans KG, Verma P, Neurobiology of chronic mild stress: Parallels to major depression: Neurosci Biobehav Rev, 2012; 36(9); 2085-117
28. Hains AB, Yabe Y, Arnsten AF, Chronic stimulation of alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress: Neurobiol Stress, 2015; 2; 1-9
29. Taylor AE, Munafò MR, Triangulating meta-analyses: The example of the serotonin transporter gene, stressful life events and major depression: BMC Psychol, 2016; 4(1); 23
30. Pascucci T, Ventura R, Latagliata EC, The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine: Cereb Cortex, 2007; 17(12); 2796-804
31. Luo L, Zhou WH, Cai JJ, Gene expression profiling identifies downregulation of the neurotrophin-MAPK signaling pathway in female diabetic peripheral neuropathy patients: J Diabetes Res, 2017; 2017 8103904
32. Mattson MP, Maudsley S, Martin B, BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders: Trends Neurosci, 2004; 27(10); 589-94
33. Wainwright SR, Galea LA, The neural plasticity theory of depression: Assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus: Neural Plast, 2013; 2013 805497
34. McAllister AK, Lo DC, Katz LC, Neurotrophins regulate dendritic growth in developing visual cortex: Neuron, 1995; 15(4); 791-803
35. Song H, Stevens CF, Gage FH, Astroglia induce neurogenesis from adult neural stem cells: Nature, 2002; 417(6884); 39-44
36. Eng LF, Ghirnikar RS, Lee YL, Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000): Neurochem Res, 2000; 25(9–10); 1439-51
37. Sun X, Xu M, Cao Q: Sci Rep, 2020; 10(1); 1038
38. Shen C, Cao K, Cui S, SiNiSan ameliorates depression-like behavior in rats by enhancing synaptic plasticity via the CaSR-PKC-ERK signaling pathway: Biomed Pharmacother, 2020; 124; 109787
39. Caviedes A, Lafourcade C, Soto C, BDNF/NF-κB signaling in the neurobiology of depression: Curr Pharm Des, 2017; 23(21); 3154-63
40. Liu S, Guo R, Liu F, Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway: Neuropsychiatr Dis Treat, 2020; 16; 859-69
41. Rogers GB, Keating DJ, Young RL, From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways: Mol Psychiatry, 2016; 21(6); 738-48
42. Chen JJ, Zheng P, Liu YY, Sex differences in gut microbiota in patients with major depressive disorder: Neuropsychiatr Dis Treat, 2018; 14; 647-55
43. Sarkar A, Lehto SM, Harty S, Psychobiotics and the manipulation of bacteria-gut-brain signals: Trends Neurosci, 2016; 39(11); 763-81
44. Huang X, Chen W, Yan C, Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its progression: Biomed Pharmacother, 2019; 118; 109258
45. Zinkernagel MS, Zysset-Burri DC, Keller I, Association of the intestinal microbiome with the development of neovascular age-related macular degeneration: Sci Rep, 2017; 7; 40826
46. Jiang H, Ling Z, Zhang Y, Altered fecal microbiota composition in patients with major depressive disorder: Brain Behav Immun, 2015; 48; 186-94
47. El Aidy S, Dinan TG, Cryan JF, Gut microbiota: The conductor in the orchestra of immune-neuroendocrine communication: Clin Ther, 2015; 37(5); 954-67
48. Guseva D, Holst K, Kaune B, Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract: Inflamm Bowel Dis, 2014; 20(9); 1516-29
49. Haroon E, Miller AH, Inflammation effects on brain glutamate in depression: Mechanistic considerations and treatment implications: Curr Top Behav Neurosci, 2017; 31; 173-98
Figures
 Figure 1. The experimental flow chart.
Figure 1. The experimental flow chart. Figure 2. Behavior tests were conducted for the rats in 4 groups. (A) The body weight of rats in the 4 groups. (B) The body weight of rats before the chronic unpredictable mild stress (CUMS). (C) The body weight of rats after the treatment. (D) The results of the open-field test. (E) The results of the elevated cross maze test. (F) The results of the sucrose preference test experiment. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine.
Figure 2. Behavior tests were conducted for the rats in 4 groups. (A) The body weight of rats in the 4 groups. (B) The body weight of rats before the chronic unpredictable mild stress (CUMS). (C) The body weight of rats after the treatment. (D) The results of the open-field test. (E) The results of the elevated cross maze test. (F) The results of the sucrose preference test experiment. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine. Figure 3. Statistical graph of 5-hydroxytryptamine (5-HT) and dopamine (DA) content in rat serum and hippocampus samples. (A) 5-HT content in hippocampus. (B) 5-HT content in serum. (C) DA content in hippocampus. (D) DA content in serum. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine.
Figure 3. Statistical graph of 5-hydroxytryptamine (5-HT) and dopamine (DA) content in rat serum and hippocampus samples. (A) 5-HT content in hippocampus. (B) 5-HT content in serum. (C) DA content in hippocampus. (D) DA content in serum. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine. Figure 4. Statistical graph of the expression of β-CaMKII/NMDAR/BDNF mRNA in the hippocampus of rats. (A) Expression of BDNF and β-CaMKII mRNA in the hippocampus by reverse transcription polymerase chain reaction (RT-PCR). (B) Expression of NMDAR mRNA in the hippocampus by RT-PCR. (C) Analysis of BDNF mRNA. (D) Analysis of β-CaMKII mRNA. (E) Analysis of NMDAR mRNA. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor.
Figure 4. Statistical graph of the expression of β-CaMKII/NMDAR/BDNF mRNA in the hippocampus of rats. (A) Expression of BDNF and β-CaMKII mRNA in the hippocampus by reverse transcription polymerase chain reaction (RT-PCR). (B) Expression of NMDAR mRNA in the hippocampus by RT-PCR. (C) Analysis of BDNF mRNA. (D) Analysis of β-CaMKII mRNA. (E) Analysis of NMDAR mRNA. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor. Figure 5. Statistical graph of β-CaMKII/NMDAR/BDNF protein expression in the rat hippocampus. (A) Expression of BDNF proteins in the hippocampus by western blotting. (B) Expression of β-CaMKII proteins in the hippocampus by western blotting. (C) Expression of NMDAR proteins in the hippocampus by western blotting. (D) Analysis of BDNF proteins. (E) Analysis of β-CaMKII proteins. (F) Analysis of NMDAR proteins. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor.
Figure 5. Statistical graph of β-CaMKII/NMDAR/BDNF protein expression in the rat hippocampus. (A) Expression of BDNF proteins in the hippocampus by western blotting. (B) Expression of β-CaMKII proteins in the hippocampus by western blotting. (C) Expression of NMDAR proteins in the hippocampus by western blotting. (D) Analysis of BDNF proteins. (E) Analysis of β-CaMKII proteins. (F) Analysis of NMDAR proteins. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; BDNF – brain-derived neurotrophic factor; β-CaMKII – β isoform of calcium/calmodulin-dependent protein kinase II; Fx – fluoxetine; NMDAR – N-methyl-d-aspartate receptor. Figure 6. (A) Immunofluorescence confocal microscopy imaging hippocampus CA1 regions, magnification ×200. (B) Expression of GFAP in the hippocampus. (C) Expression of IBA-1 in the hippocampus. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine; GFAP – glial fibrillary acidic protein; IBA-1 – ionized calcium binding adaptor molecule 1.
Figure 6. (A) Immunofluorescence confocal microscopy imaging hippocampus CA1 regions, magnification ×200. (B) Expression of GFAP in the hippocampus. (C) Expression of IBA-1 in the hippocampus. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the chronic unpredictable mild stress (CUMS) group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine; GFAP – glial fibrillary acidic protein; IBA-1 – ionized calcium binding adaptor molecule 1. Figure 7. Effect of acupuncture on the diversity and richness of intestinal flora in chronic unpredictable mild stress (CUMS) rats. (A) The Shannon index value indicates the diversity of samples. (B) Principal coordinates analysis (PCoA) for the distance between samples (β diversity) calculated using unweighted UniFrac distance. (C) A Venn diagram of the operational taxonomic units (OTUs) analyzes samples of different groups. (D) Relative abundance analysis at the phylum level. (E) Relative abundance of Firmicutes at the phylum level. (F) Relative abundance of Bacteroidetes at the phylum level. (G) Relative abundance of Bacteroidetes/Firmicutes at the phylum level. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine group.
Figure 7. Effect of acupuncture on the diversity and richness of intestinal flora in chronic unpredictable mild stress (CUMS) rats. (A) The Shannon index value indicates the diversity of samples. (B) Principal coordinates analysis (PCoA) for the distance between samples (β diversity) calculated using unweighted UniFrac distance. (C) A Venn diagram of the operational taxonomic units (OTUs) analyzes samples of different groups. (D) Relative abundance analysis at the phylum level. (E) Relative abundance of Firmicutes at the phylum level. (F) Relative abundance of Bacteroidetes at the phylum level. (G) Relative abundance of Bacteroidetes/Firmicutes at the phylum level. Compared with the control (Con) group, * P<0.05, ** P<0.01, *** P<0.001; compared with the CUMS group, # P<0.05, ## P<0.01, ### P<0.001. Data represent mean±standard error of the mean. AP – acupuncture; Fx – fluoxetine group. Figure 8. (A) The functional abundance heat map of the differential enrichment of intestinal microorganisms in each group. (B) Kruskal-Wallis rank sum test for differences in metabolic pathways. Con – control; CUMS – chronic unpredictable mild stress; AP – acupuncture group.
Figure 8. (A) The functional abundance heat map of the differential enrichment of intestinal microorganisms in each group. (B) Kruskal-Wallis rank sum test for differences in metabolic pathways. Con – control; CUMS – chronic unpredictable mild stress; AP – acupuncture group. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








