05 June 2021: Animal Study
Anti-Psoriatic Effects of Middle Fragment of Chlamydial Plasmid-Encoded Protein pGP3 in an Imiquimod-Induced Psoriasis Mouse Model
Yiming Zhang1A, Miaomiao Ma2A, Jun Li3D, Yingye Wu2C, Lu Xue2C, Rongrong Zhao2B, Lu Wang2E, Shuping Hou2G, Huiping Wang2G*DOI: 10.12659/MSM.929781
Med Sci Monit 2021; 27:e929781
Abstract
BACKGROUND: Previously, we demonstrated that the chlamydial protein pGP3 forms a stable complex with LL-37 to neutralize its proinflammatory activity during the pathogenesis of psoriasis. The middle domain of pGP3 (pGP3M) is critical for the binding and neutralization of LL-37. Here, we further examined the mechanism underlying pGP3-mediated inhibition of psoriasis progression and evaluated the inhibitory effect of pGP3M on the development of psoriasis-like skin lesions in mice.
MATERIAL AND METHODS: Stock solutions of pGP3M and pGP3 (100 μg/mL) were prepared using sterile ultrapure water and intramuscularly injected into the left leg of the imiquimod (IMQ)-induced psoriasis mouse model. The severity of skin lesions was evaluated based on the psoriasis area and severity index score and ear skin thickness. The skin biopsy and blood samples were collected on the 8th day for histological analysis and inflammatory cytokines detection.
RESULTS: Erythema, scaling, and thickening were observed on the dorsal skin and the right ear skin of IMQ-treated mice. Treatment with pGP3 and pGP3M alleviated the IMQ-induced erythema, inflammatory cell infiltration, and scaly plaques. Compared with IMQ-treated and PBS-treated mice, pGP3- and PGP3M-treated mice had less inflammatory cell infiltration in skin tissues and had significantly reduced IL-17A, IFN-γ, and IL-22 levels in serum.
CONCLUSIONS: The anti-psoriatic efficacy of exogenous pGP3M was similar to that of pGP3. This indicated that pGP3M attenuated the IMQ-induced inflammatory and psoriatic symptoms in mice by binding and inhibiting LL-37. Further research is needed to examine the toxicity of pGP3 and pGP3M before clinical trial evaluation.
Keywords: cathelicidins, Chlamydia, Psoriasis, Anti-Inflammatory Agents, Antigens, Bacterial, Antimicrobial Cationic Peptides, Bacterial Proteins, Carrier Proteins, Plasmids, Skin
Background
Psoriasis is an immune-mediated chronic skin disease with a complex etiology, which involves the interaction between genetic risk factors and environmental triggers [1,2]. Globally, the incidence of psoriasis is approximately 1–3% [3]. The quality of life of patients with psoriasis, which is a lifelong disease, markedly decreases. The major type of psoriasis is chronic plaque psoriasis, which accounts for approximately 85–90% of all psoriasis cases. Chronic plaque psoriasis is characterized by markedly demarcated, red, and scaly plaques, mostly on the extension side of the trunk [4]. Additionally, chronic plaque psoriasis is associated with inflammation of the joints (psoriasis arthritis) and multiple comorbidities [5], such as increased insulin resistance and cardiovascular risk, which increase the risk of mortality. In recent years, with the elucidation of the pathogenesis of psoriasis, development of targeted therapeutic drugs for psoriasis has become an important research topic.
The etiology of psoriasis onset is poorly understood. LL-37, a human cathelicidin antimicrobial peptide, is reported to trigger inflammation in psoriasis [6]. The production of LL-37 in keratinocytes is triggered upon infection with various bacteria [7]. In addition to its antimicrobial activity in humans, LL-37 was found to be related to proinflammatory cytokines in patients with psoriasis [8]. The levels of LL-37 in psoriatic plaques are higher than those in healthy skin. LL-37 forms a complex with self-DNA when the dead cells undergo degradation, which results in the activation of Toll-like receptor (TLR). Consequently, various inflammatory cytokines, such as IL-17, IFN-γ, and IL-22, are secreted, which promote the production of LL-37 in the keratinocytes [9,10]. These pathological changes in the skin lesions result in psoriasis dermatitis.
In a previous study, it was found that chlamydial plasmid-encoded protein pGP3 can neutralize the antichlamydial activity of LL-37 peptide by binding. PGP3 comprises a C-terminal domain (133–260), a middle domain (67–66), and an N-terminal domain (1–66). The binding domain of LL-37 was mapped to the pGP3 middle region (pGP3M), which forms triple helixes that are flexible in interacting with other molecules. It was suggested that pGP3M has potential therapeutic effects for LL-37-involved non-genital-tract diseases, such as psoriasis [11]. We previously demonstrated that pGP3 exhibits anti-psoriatic activity in the imiquimod (IMQ)-induced psoriasis-like dermatitis mouse model and that LL-37 may be a therapeutic target for psoriasis [12]. Thus, we hypothesised that pGP3M mediates the alleviation of LL-37-induced pathological changes in psoriasis. This study was designed to evaluate the inhibitory effect of pGP3M on the development of psoriasis-like skin lesions using the IMQ-induced psoriasis-like dermatitis mouse model, whose phenotype is similar to that of human plaque-type psoriasis [13].
Material and Methods
CHEMICALS AND REAGENT PREPARATION:
IMQ cream (Aldara™ 5%) was purchased from 3M Pharmaceuticals. PGP3M was synthesised by GenScript (Nanjing, China). The protocols for the expression and purification of pGP3 have been described elsewhere [13].
ANIMAL MODEL OF PSORIASIS:
BALB/c female mice (6–8 weeks old) were purchased from Tianjin Oid Laboratory Products (Tianjin, China). The animals were housed in cages with wood-shavings bedding material under the following conditions at the Laboratory Animal House of the Tianjin Medical University: temperature, 21±1°C; relative humidity, 50–70%; circadian cycle, 12-h light/dark cycle. The size of the cages was 320×210×160 mm and each cage housed 5 mice. The mice were allowed to acclimatize for 5 days and were fed a commercial rodent diet (Troufei Feed Technology Co., Ltd., Nantong, Jiangsu) and water ad libitum before the experiment. The food and water intake, bodyweight, and behavioural parameters of mice were monitored before and during the experimental period. The animal experiment was approved by the Animal Care and Ethics Committee of Tianjin Medical University (Approval number: TMUaMEC 2018025).
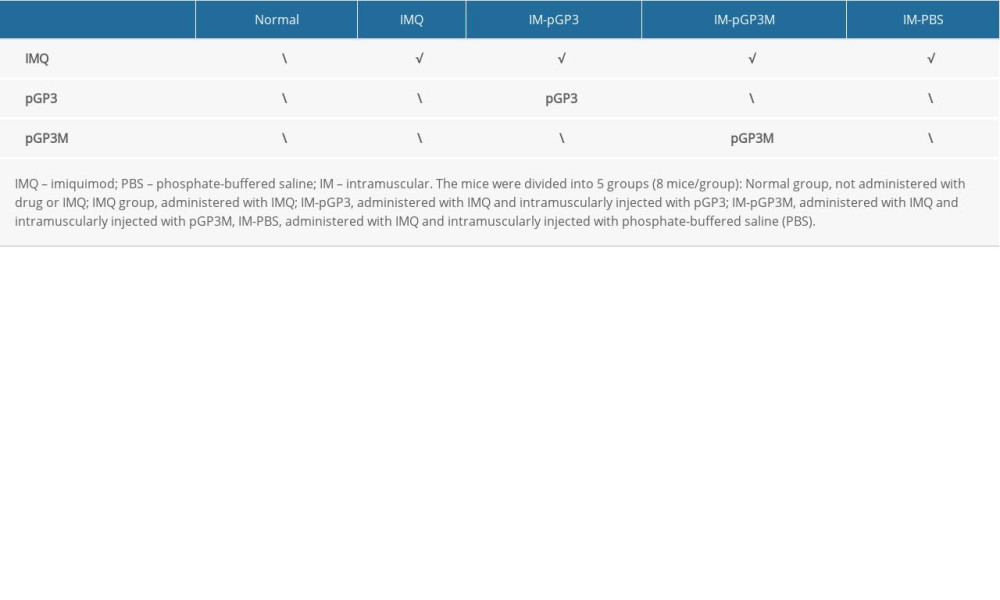
The histological and clinical manifestations, such as erythema, scaly skin, and inflammation, of IMQ-induced lesions in mice are similar to those of psoriasis in humans. Psoriasis-like dermatitis was induced in mice by IMQ cream topically applied on the shaved dorsal skin (62.5 mg/day) and the right ear of mice (20 mg/cm2) for 7 consecutive days [14,15]. The mice were divided into the following 5 experimental groups (8 mice/group): the Normal group, not administered drug or IMQ; the IMQ group, administered IMQ; the IM-pGP3 group, administered IMQ and intramuscularly injected with pGP3; the IM-pGP3M group, administered IMQ and intramuscularly injected with pGP3M; and the IM-PBS group, administered IMQ and intramuscularly injected with phosphate-buffered saline (PBS). The mice were treated as shown in Table 1.
PEPTIDE TREATMENT:
PBS, pGP3M, and pGP3 were freshly prepared before administration to the mice. The stock solutions of pGP3M and pGP3 (100 μg/mL) were prepared using either sterile ultrapure water or PBS. The left leg of the mice received 100 μl pGP3M, pGP3, or PBS for 7 consecutive days. IMQ cream was topically administered to the shaved skin area and right ear of the IMQ, IM-PBS, IM-pGP3, and pGP3M groups in the morning, while PBS, pGP3, and pGP3M were administered in the evening.
ASSESSING SEVERITY OF SKIN LESIONS:
The degree of erythema, skin thickening, and scaling on the affected area was quantified using the 4-point scale psoriasis area and severity index (PASI) (0=none; 1=slight; 2=moderate; 3=marked; 4=very marked). The severity of skin psoriasis-like dermatitis was measured based on the combined scores (erythema score+scaling score+thickening score), which were in the range of 0–12. On alternate days, ear thickness was measured using digital callipers (BEC, China) to examine the degree of epidermal proliferation.
TISSUE AND SERUM SAMPLE COLLECTION:
The mice were sacrificed on the 8th day. The central dorsal skin tissue (approximately 1 cm2) was excised for histological and cytokine analyses. Additionally, the retro-orbital blood samples were collected for cytokine detection.
HISTOPATHOLOGICAL AND CYTOKINE ANALYSES:
The excised samples were first fixed in 10% neutral-buffered formalin solution and then embedded in paraffin blocks. Afterwards, the samples were sectioned into 4-μm-thick sections using a rotary microtome. The sections were stained with hematoxylin-eosin (HE) stain and observed under a microscope.
The levels of psoriasis dermatitis-related inflammatory cytokines were measured using enzyme-linked immunosorbent assay. The retro-orbital blood samples were centrifuged at 3000
STATISTICAL ANALYSIS:
The PASI score and ear thickness were analysed using one-way analysis of variance. The differences were considered statistically significant when
Results
IMQ APPLICATION INDUCES PSORIASIS-LIKE DERMATITIS IN MICE:
On the second or third day of IMQ application, erythema, scaling, and thickening appeared on the dorsal skin and right ear pinna of mice. The skin lesions in the IMQ and IM-PBS groups were observed until the 8th or 9th day and the severity of lesions increased each day. The severity of psoriasis dermatitis in the IMQ and IM-PBS groups decreased after the 8th day. Hence, the total experimental period was 8 days. The IMQ and IM-PBS groups exhibited typical psoriatic symptoms and the severity increased each day until the 8th day.
EFFECT OF PGP3 OR PGP3M TREATMENT ON IMQ-INDUCED PSORIASIS:
The IM-pGP3 and IM-pGP3M groups exhibited typical signs of erythema, skin scaling, and thickening after the first 4 days of IMQ application, which improved rapidly after the 6th day. Compared with the IMQ and IM-PBS groups, the IM-pGP3 and IM-pGP3M groups exhibited milder skin lesions and shorter healing time (Figure 1).
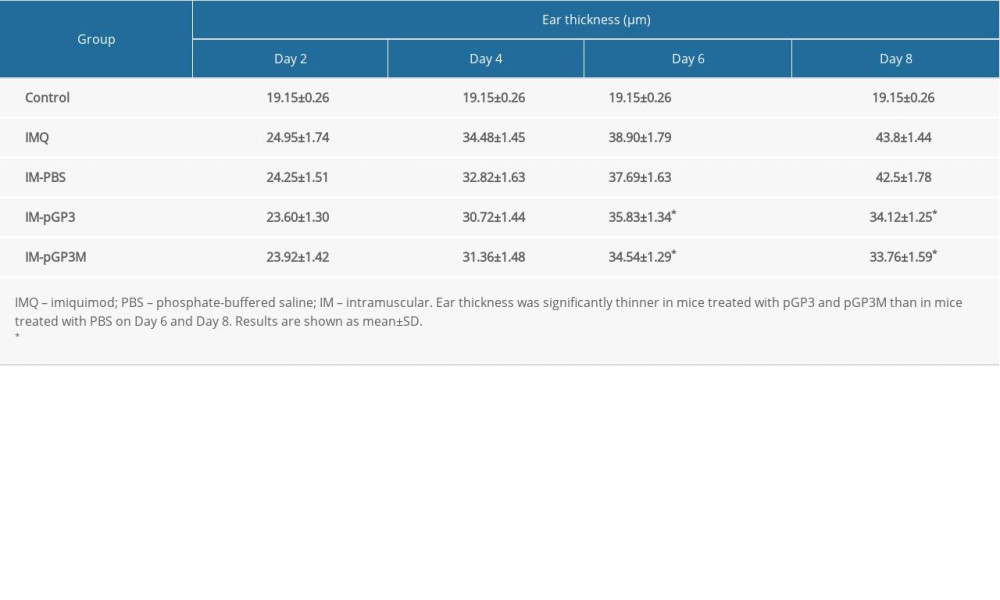
The ear skin thickness was examined to evaluate the degree of epidermal proliferation in each group of mice. After the 2nd day, the IMQ group exhibited thick and hard ear skin, with the capillaries and scales visible on the ear skin surface (Figure 2). As shown in Figure 3 and Table 2, the ear skin thickness in the IMQ group was significantly higher than that in the normal group from the 2nd day. Compared with that in the IM-PBS groups, the rate of increase in the ear skin thickness was slower in the pGP3 and pGP3M groups. Additionally, the rate of increase in the ear skin thickness started to decline in the IM-pGP3 and IM-pGP3M groups from the 5th or 6th day, which was earlier than that in the IMQ and IM-PBS groups.
A line chart was used to represent the PASI scores of different treatment groups. The PASI scores of IMQ and IM-PBS groups increased with time and reached a maximum score of 4, whereas those of the IM-pGP3 and IM-pGP3M groups increased for the first 6 days and rapidly decreased thereafter (Figure 4).
HISTOPATHOLOGICAL ANALYSIS:
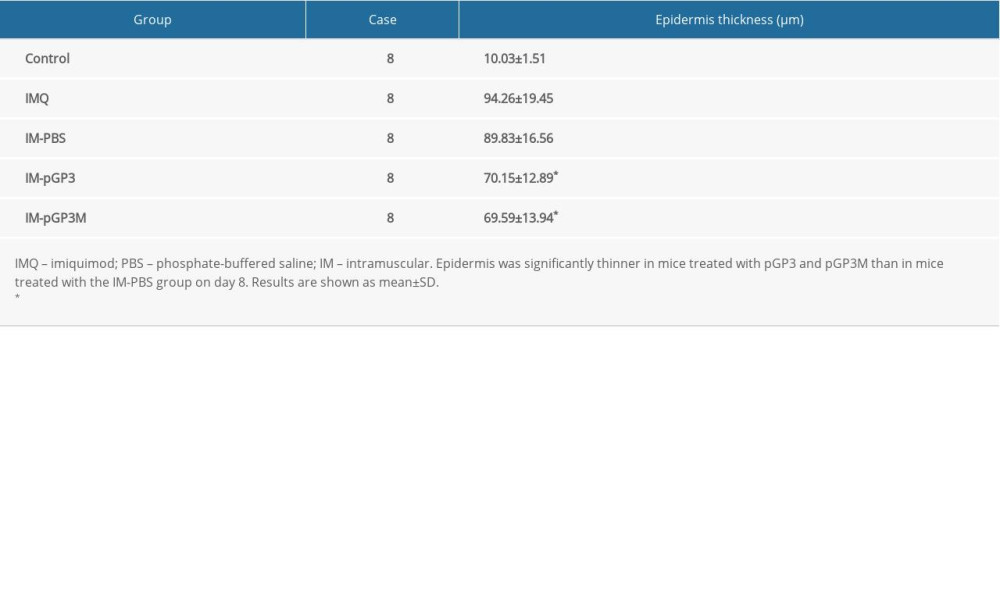
The histological features of the HE-stained dorsal skin sections of the IMQ group were consistent with the skin manifestations and PASI scores. The samples of the IMQ group exhibited significant acanthosis, hyperkeratosis, and inflammatory cell infiltration in the dermis. However, the analysis of dorsal skin sections of the normal group revealed no pathological changes in the epidermis and dermis. Compared with those in the IM-PBS groups, the epidermis thickness, acanthosis, and hyperkeratosis were markedly lower in the IM-pGP3 and IM-pGP3M groups (Figure 5, Table 3).
EFFECT OF THE PGP3 AND PGP3M ON INFLAMMATORY CYTOKINE PRODUCTION:
Compared with those in the normal group, the IL-17A, IFN-γ, and IL-22 levels in the serum and dorsal skin were significantly upregulated in the IMQ group. However, the skin tissue and serum levels of IL-17A, IFN-γ, and IL-22 in the IM-pGP3 and IM-pGP3M groups were significantly lower than those in the IMQ and IM-PBS groups. The serum and skin tissue levels of cytokines were not significantly different between the IM-pGP3 and IM-pGP3M groups, which was consistent with phenotypic manifestations and histopathological results (Figure 6).
Discussion
Previously, we reported that pGP3, a chlamydial plasmid-encoded protein, can alleviate the severity and delay the progression of psoriatic skin dermatitis in an IMQ-induced psoriasis mouse model by neutralizing LL-37 [12]. LL-37, a therapeutic target for psoriasis, forms a complex with self-DNA to activate plasmacytoid dendritic cells (DCs) through a TLR-dependent mechanism [10]. The activation of plasmacytoid DCs increases the production of type I IFN, which promotes myeloid DC activation, Th1/Th17 differentiation, and keratinocyte activation, and consequently upregulation of various cytokines [16].
The levels of IL-17A and IL-22, which are downstream effectors of the LL-37 pathway, are upregulated in the psoriatic skin lesions and systemic circulation [17]. The circulating LL-37 appeared to affect the production of INF-γ and IL-22, which are 2 key cytokines in the pathogenesis of psoriasis [18,19]. These cytokines further stimulate the release of LL-37 and promote inflammatory cell infiltration in the keratinocytes, which enhance local inflammation and keratinocyte proliferation [18]. Thus, drugs targeting cytokines and signaling pathways involved in the pathogenesis of psoriasis are effective in treatment of plaque psoriasis [20].
As pGP3M binds to LL-37 [12], we examined the role of this middle domain in mediating the anti-psoriatic effects of pGP3. The levels of cytokines in the psoriatic tissue lesions and retro-orbital blood samples, PASI scores, ear skin thickness, and histological features were examined in all groups. IMQ, which is used for condyloma acuminatum treatment, activates TLR-7/8 [21]. Additionally, IMQ induces psoriasis-like lesions in BALB/c mice through the IL-23/IL-17 axis. The IMQ-induced psoriasis mouse model was used in our study as it can stably replicate the clinical and pathological features of human plaque-type psoriasis [22]. The ear skin thickness of mice was analyzed as a parameter of skin inflammation. Our study demonstrated that protein pGP3 or pGP3M significantly alleviated IMQ-induced psoriasis-like lesions. The serum and dorsal skin tissue levels of IL-17A, IFN-γ, and IL-22 were downregulated in the IM-pGP3M and IM-pGP3 groups. Thus, we speculated that pGP3M exhibits anti-proliferative and anti-inflammatory activities by binding to LL-37.
Antimicrobial peptides play a vital role in the immune response against microbial infection. The inhibition of antimicrobial peptides may increase the risk of infection from other bacteria. Therefore, future studies must examine the effect of antimicrobial peptides on the physiological function of IL-37.
Conclusions
This study demonstrates that the efficacy of pGP3M to attenuate the inflammatory and psoriatic symptoms was similar to that of pGP3 in the IMQ-induced-psoriasis mouse model. This preclinical evidence suggests that pGP3M may be an effective therapeutic strategy for patients with psoriasis. Further investigations are needed to examine the toxicity of pGP3M in animal models before clinical trial evaluation.
Figures
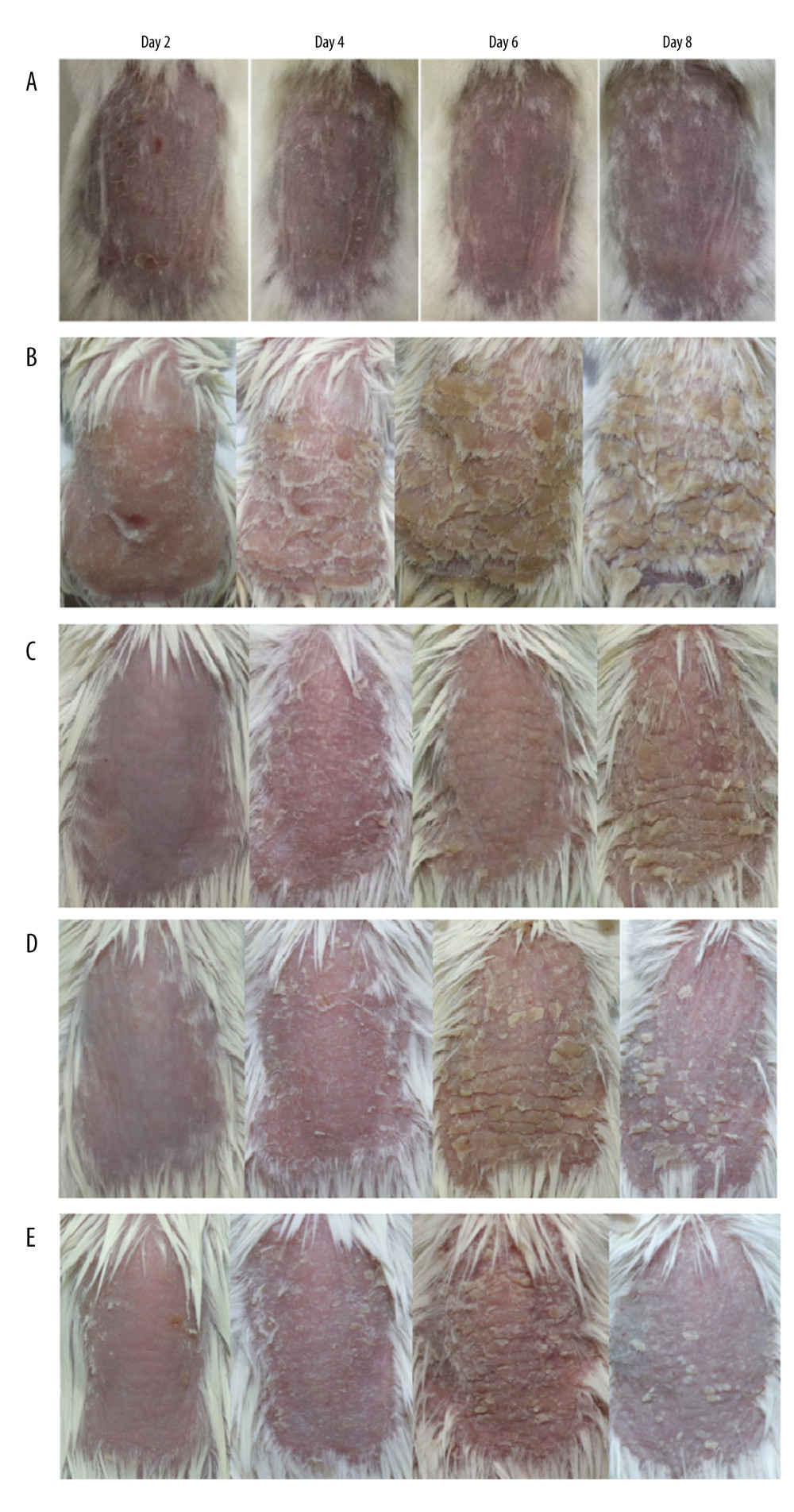 Figure 1. The phenotype of dorsal skin in different treatment groups observed on days 2, 4, 6, and 8. (A) Normal, (B) imiquimod (IMQ), (C) phosphate-buffered saline intramuscular injection (IM-PBS), (D) pGP3 intramuscular injection (IM-pGP3), and (E) pGP3M intramuscular injection (IM-pGP3M) groups. The IMQ, IM-PBS, IM-pGP3, and IM-pGP3M groups exhibited psoriasis-like dermatitis signs including erythema, skin scaling, and thickening after IMQ application. The typical signs of psoriasis-like dermatitis decreased after the sixth day in the IM-pGP3, and IM-pGP3M groups. Compared with the IMQ and IM-PBS groups, the IM-pGP3 and IM-pGP3M groups exhibited milder skin lesions and shorter healing time.
Figure 1. The phenotype of dorsal skin in different treatment groups observed on days 2, 4, 6, and 8. (A) Normal, (B) imiquimod (IMQ), (C) phosphate-buffered saline intramuscular injection (IM-PBS), (D) pGP3 intramuscular injection (IM-pGP3), and (E) pGP3M intramuscular injection (IM-pGP3M) groups. The IMQ, IM-PBS, IM-pGP3, and IM-pGP3M groups exhibited psoriasis-like dermatitis signs including erythema, skin scaling, and thickening after IMQ application. The typical signs of psoriasis-like dermatitis decreased after the sixth day in the IM-pGP3, and IM-pGP3M groups. Compared with the IMQ and IM-PBS groups, the IM-pGP3 and IM-pGP3M groups exhibited milder skin lesions and shorter healing time. 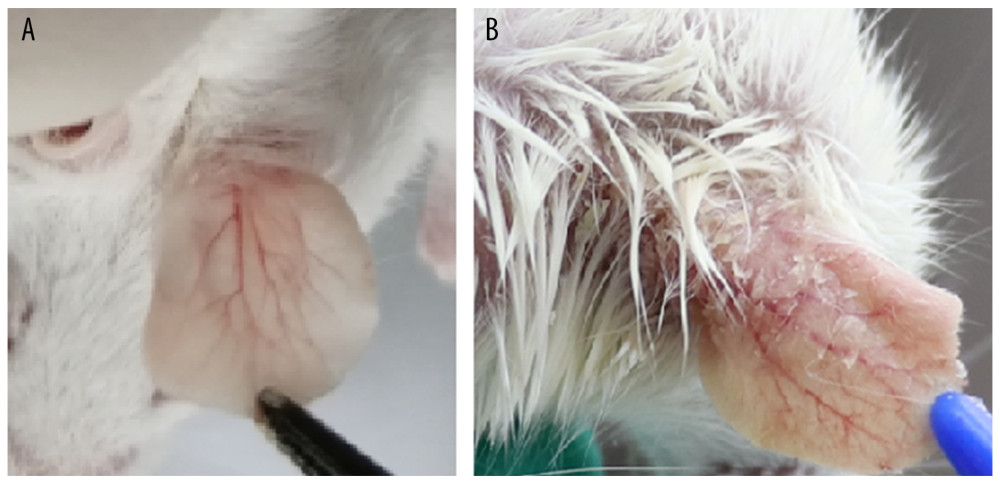 Figure 2. (A) Right ear skin of normal and (B) IMQ groups. The IMQ group exhibited thicker ear skin, increased scaling, and erythema than the normal group.
Figure 2. (A) Right ear skin of normal and (B) IMQ groups. The IMQ group exhibited thicker ear skin, increased scaling, and erythema than the normal group. 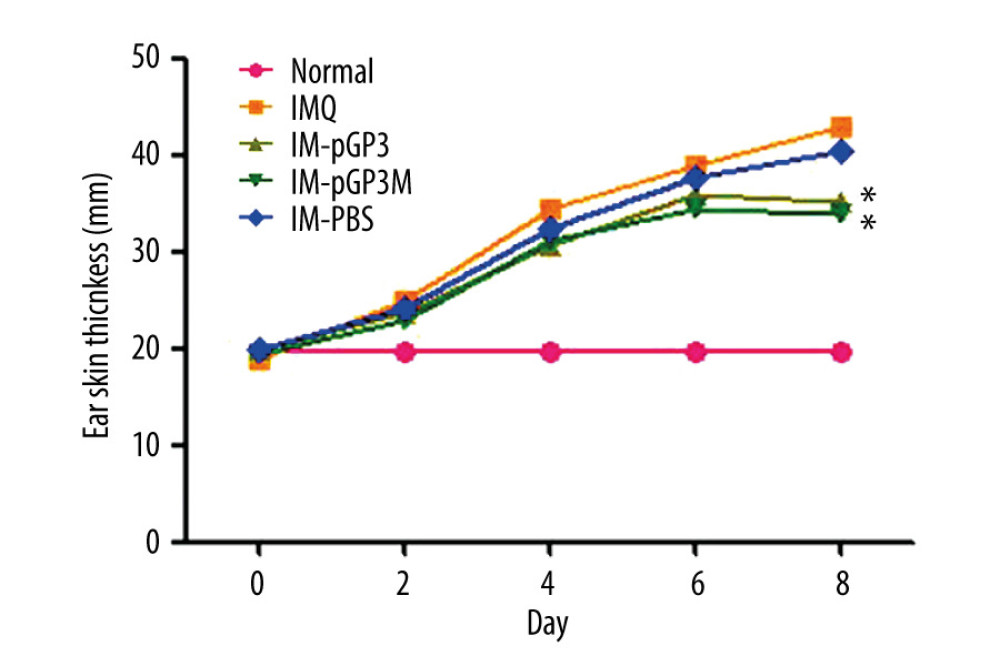 Figure 3. Right ear pinna thickness of the mice. After the 6th day, the ear pinna thickness of pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups was significantly thinner than that of PBS and IMQ groups. * P<0.05 compared with the IM-PBS group.
Figure 3. Right ear pinna thickness of the mice. After the 6th day, the ear pinna thickness of pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups was significantly thinner than that of PBS and IMQ groups. * P<0.05 compared with the IM-PBS group. 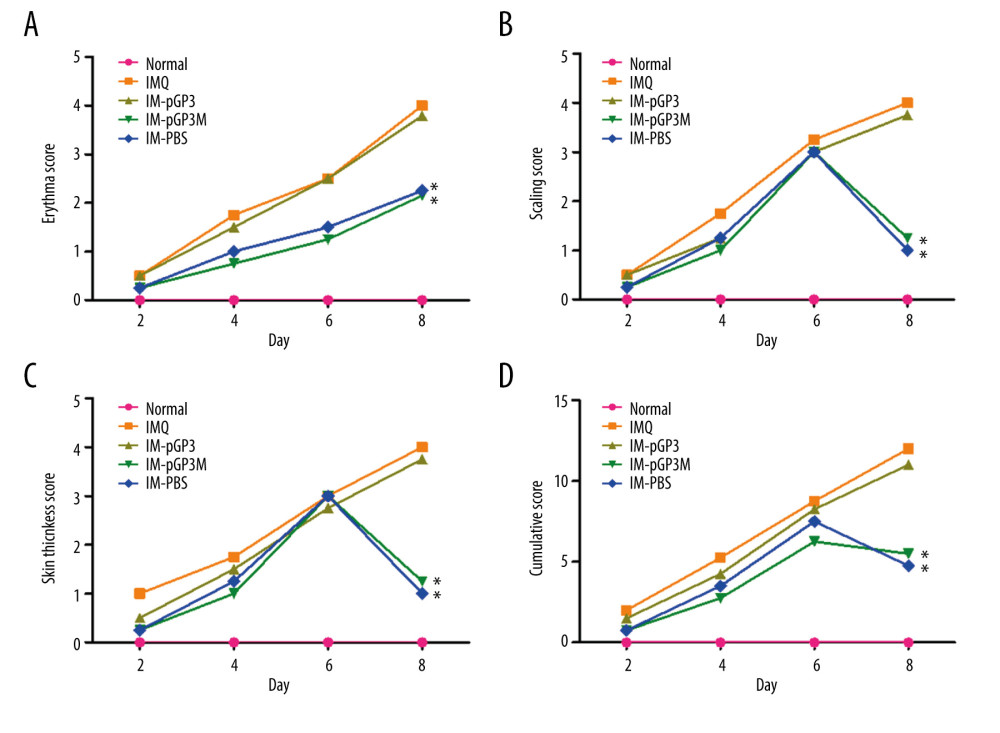 Figure 4. PASI scores were used to evaluate the degree of (A) erythema, (B) thickness, and (C) scaling of mouse dorsal skin in the normal, imiquimod (IMQ), phosphate-buffered saline intramuscular injection (IM-PBS), pGP3 intramuscular injection (IM-pGP3), and pGP3M intramuscular injection (IM-pGP3M) groups. PASI was scored on a 0–4-point scale. (D) Cumulative score (erythema score+thickness score+scaling score). * P<0.05 compared with the IM-PBS group.
Figure 4. PASI scores were used to evaluate the degree of (A) erythema, (B) thickness, and (C) scaling of mouse dorsal skin in the normal, imiquimod (IMQ), phosphate-buffered saline intramuscular injection (IM-PBS), pGP3 intramuscular injection (IM-pGP3), and pGP3M intramuscular injection (IM-pGP3M) groups. PASI was scored on a 0–4-point scale. (D) Cumulative score (erythema score+thickness score+scaling score). * P<0.05 compared with the IM-PBS group. 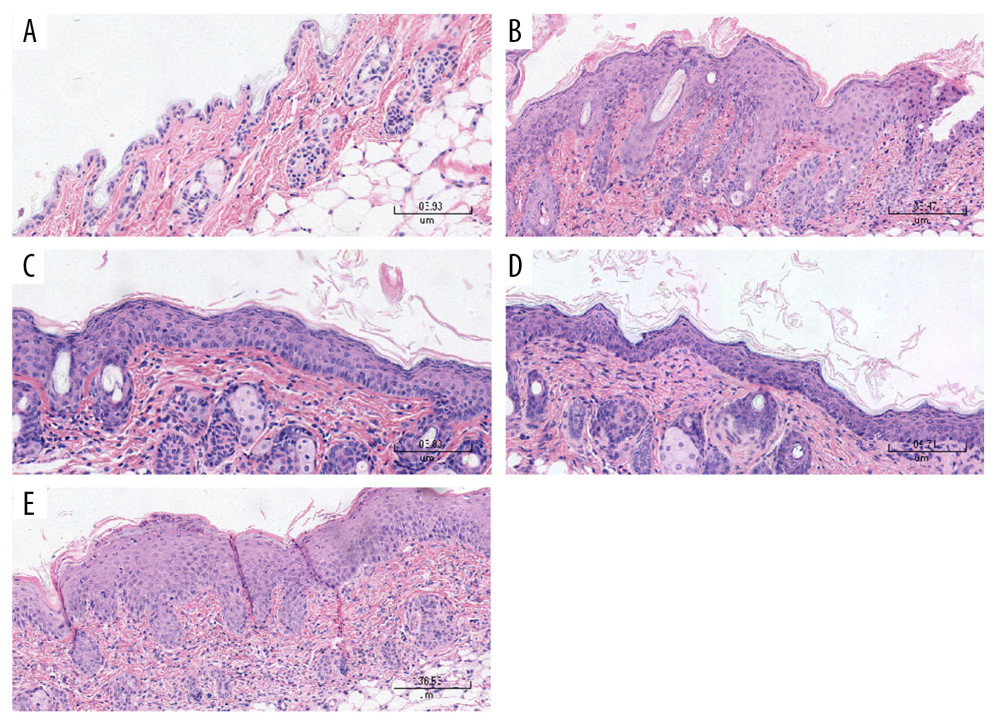 Figure 5. Histological examinations of the skin sections stained with hematoxylin-eosin (Magnification: 200×). (A) Normal mice. (B) The skin sections of the imiquimod (IMQ) group exhibited epidermal thickening, hyperkeratosis, parakeratosis, and inflammation in the dermis. (C, D) The hyperkeratosis and acanthosis in the pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups were milder than that in the IMQ group and (E) the phosphate-buffered saline intramuscular injection group.
Figure 5. Histological examinations of the skin sections stained with hematoxylin-eosin (Magnification: 200×). (A) Normal mice. (B) The skin sections of the imiquimod (IMQ) group exhibited epidermal thickening, hyperkeratosis, parakeratosis, and inflammation in the dermis. (C, D) The hyperkeratosis and acanthosis in the pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups were milder than that in the IMQ group and (E) the phosphate-buffered saline intramuscular injection group. 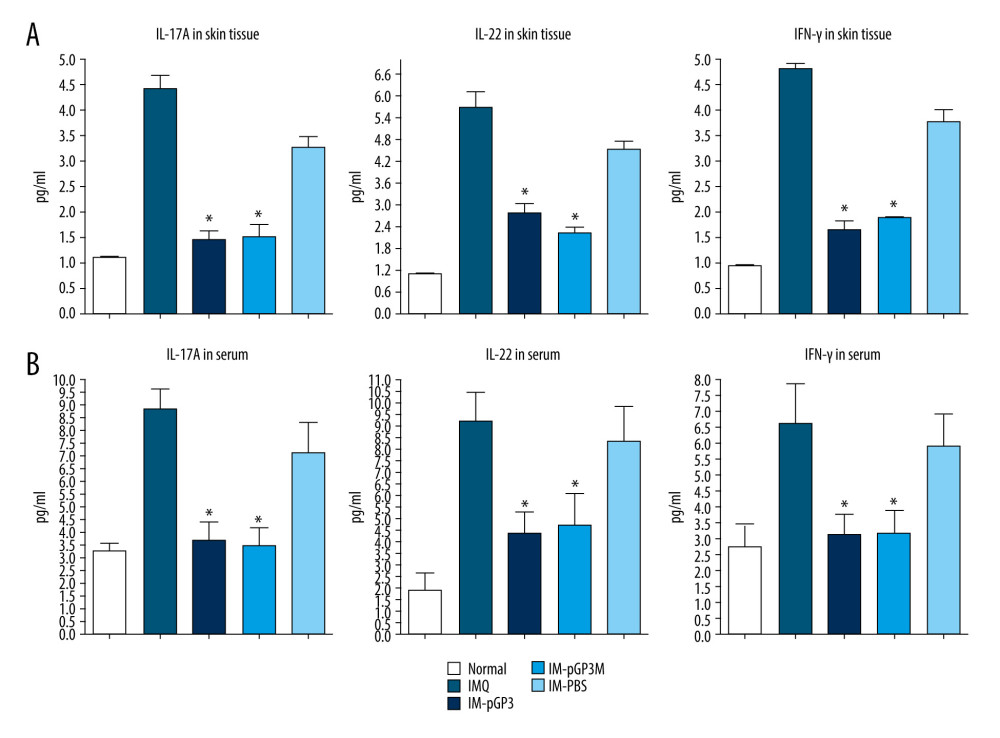 Figure 6. The cytokines levels in the serum and skin tissue were measured using ELISA. Data are presented as mean±standard deviation (8 mice/group). (A) The levels of IL-17A, IL-22, and IFN-γ in the skin lesions. * P<0.05 compared with the IM-PBS group. (B) The levels of IL-17A, IL-22, and IFN-γ in the serum. * P<0.05 compared with the IM-PBS group.
Figure 6. The cytokines levels in the serum and skin tissue were measured using ELISA. Data are presented as mean±standard deviation (8 mice/group). (A) The levels of IL-17A, IL-22, and IFN-γ in the skin lesions. * P<0.05 compared with the IM-PBS group. (B) The levels of IL-17A, IL-22, and IFN-γ in the serum. * P<0.05 compared with the IM-PBS group. References
1. Barrea L, Nappi F, Di Somma C, environmental risk factors in psoriasis: The point of view of the nutritionist: Int J Environ Res Public Health, 2016; 13(5); 743
2. Saelee C, Thongrakard V, Tencomnao T, Effects of Thai medicinal herb extracts with anti-psoriatic activity on the expression on NF-κB signaling biomarkers in HaCaT keratinocytes: Molecules, 2011; 16(5); 3908-32
3. Weger W, Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents: Br J Pharmacol, 2010; 160(4); 810-20
4. Hermann H, Runnel T, Aab A, miR-146b probably assists miRNA-146a in the suppression of keratinocyte proliferation and inflammatory responses in psoriasis: J Invest Dermatol, 2017; 137(9); 1945-54
5. Vachatova S, Andrys C, Krejsek J, Metabolic syndrome and selective inflammatory markers in psoriatic patients: J Immunol Res, 2016; 2016; 5380792
6. Lande R, Gregorio J, Facchinetti V, Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide: Nature, 2007; 449(7162); 564-69
7. Gregorio J, Meller S, Conrad C, Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons: J Exp Med, 2010; 207(13); 2921-30
8. Kanda N, Ishikawa T, Kamata M, Increased serum leucine, leucine-37 levels in psoriasis: Positive and negative feedback loops of leucine, leucine-37 and pro- or anti-inflammatory cytokines: Hum Immunol, 2010; 71(12); 1161-71
9. Lande R, Botti E, Jandus C, The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis: Nat Commun, 2014; 5; 5621
10. Chamilos G, Gregorio J, Meller S, Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37: Blood, 2012; 120(18); 3699-707
11. Hou S, Dong X, Yang Z, Chlamydial plasmid-encoded virulence factor Pgp3 neutralizes the antichlamydial activity of human cathelicidin LL-37: Infect Immun, 2015; 83(12); 4701-9
12. Hou S, Xu R, Zhu C, Chlamydial plasmid-encoded protein pGP3 inhibits development of psoriasis-like lesions in mice: Med Sci Monit, 2018; 24; 5159-67
13. Li Z, Chen D, Zhong Y, The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells: Infect Immun, 2008; 76(8); 3415-28
14. Sun J, Zhao Y, Hu J, Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1 beta and IL-6 production in mice: PLoS One, 2013; 8(6); e67078
15. Na Takuathung M, Wongnoppavich A, Panthong A, Antipsoriatic effects of wannachawee recipe on imiquimod-induced psoriasis-like dermatitis in BALB/c mice: Evid Based Complement Alternat Med, 2018; 2018; 7931031
16. Morizane S, Gallo RL, Antimicrobial peptides in the pathogenesis of psoriasis: J Dermatol, 2012; 39(3); 225-30
17. Van Belle AB, de Heusch M, Lemaire MM, IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice: J Immunol, 2012; 188(1); 462-69
18. Cai Y, Fleming C, Yan J, New insights of T cells in the pathogenesis of psoriasis: Cell Mol Immunol, 2012; 9(4); 302-9
19. Hwang YJ, Jung HJ, Kim MJ, Serum levels of LL-37 and inflammatory cytokines in plaque and guttate psoriasis: Mediators Inflamm, 2014; 2014; 268257
20. Rendon A, Schäkel K, Psoriasis pathogenesis and treatment: Int J Mol Sci, 2019; 20(6); 1475
21. Flutter B, Nestle FO, TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis: Eur J Immunol, 2013; 43(12); 3138-46
22. Van der Fits L, Mourits S, Voerman JS, Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis: J Immunol, 2009; 182(9); 5836-45
Figures
 Figure 1. The phenotype of dorsal skin in different treatment groups observed on days 2, 4, 6, and 8. (A) Normal, (B) imiquimod (IMQ), (C) phosphate-buffered saline intramuscular injection (IM-PBS), (D) pGP3 intramuscular injection (IM-pGP3), and (E) pGP3M intramuscular injection (IM-pGP3M) groups. The IMQ, IM-PBS, IM-pGP3, and IM-pGP3M groups exhibited psoriasis-like dermatitis signs including erythema, skin scaling, and thickening after IMQ application. The typical signs of psoriasis-like dermatitis decreased after the sixth day in the IM-pGP3, and IM-pGP3M groups. Compared with the IMQ and IM-PBS groups, the IM-pGP3 and IM-pGP3M groups exhibited milder skin lesions and shorter healing time.
Figure 1. The phenotype of dorsal skin in different treatment groups observed on days 2, 4, 6, and 8. (A) Normal, (B) imiquimod (IMQ), (C) phosphate-buffered saline intramuscular injection (IM-PBS), (D) pGP3 intramuscular injection (IM-pGP3), and (E) pGP3M intramuscular injection (IM-pGP3M) groups. The IMQ, IM-PBS, IM-pGP3, and IM-pGP3M groups exhibited psoriasis-like dermatitis signs including erythema, skin scaling, and thickening after IMQ application. The typical signs of psoriasis-like dermatitis decreased after the sixth day in the IM-pGP3, and IM-pGP3M groups. Compared with the IMQ and IM-PBS groups, the IM-pGP3 and IM-pGP3M groups exhibited milder skin lesions and shorter healing time. Figure 2. (A) Right ear skin of normal and (B) IMQ groups. The IMQ group exhibited thicker ear skin, increased scaling, and erythema than the normal group.
Figure 2. (A) Right ear skin of normal and (B) IMQ groups. The IMQ group exhibited thicker ear skin, increased scaling, and erythema than the normal group. Figure 3. Right ear pinna thickness of the mice. After the 6th day, the ear pinna thickness of pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups was significantly thinner than that of PBS and IMQ groups. * P<0.05 compared with the IM-PBS group.
Figure 3. Right ear pinna thickness of the mice. After the 6th day, the ear pinna thickness of pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups was significantly thinner than that of PBS and IMQ groups. * P<0.05 compared with the IM-PBS group. Figure 4. PASI scores were used to evaluate the degree of (A) erythema, (B) thickness, and (C) scaling of mouse dorsal skin in the normal, imiquimod (IMQ), phosphate-buffered saline intramuscular injection (IM-PBS), pGP3 intramuscular injection (IM-pGP3), and pGP3M intramuscular injection (IM-pGP3M) groups. PASI was scored on a 0–4-point scale. (D) Cumulative score (erythema score+thickness score+scaling score). * P<0.05 compared with the IM-PBS group.
Figure 4. PASI scores were used to evaluate the degree of (A) erythema, (B) thickness, and (C) scaling of mouse dorsal skin in the normal, imiquimod (IMQ), phosphate-buffered saline intramuscular injection (IM-PBS), pGP3 intramuscular injection (IM-pGP3), and pGP3M intramuscular injection (IM-pGP3M) groups. PASI was scored on a 0–4-point scale. (D) Cumulative score (erythema score+thickness score+scaling score). * P<0.05 compared with the IM-PBS group. Figure 5. Histological examinations of the skin sections stained with hematoxylin-eosin (Magnification: 200×). (A) Normal mice. (B) The skin sections of the imiquimod (IMQ) group exhibited epidermal thickening, hyperkeratosis, parakeratosis, and inflammation in the dermis. (C, D) The hyperkeratosis and acanthosis in the pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups were milder than that in the IMQ group and (E) the phosphate-buffered saline intramuscular injection group.
Figure 5. Histological examinations of the skin sections stained with hematoxylin-eosin (Magnification: 200×). (A) Normal mice. (B) The skin sections of the imiquimod (IMQ) group exhibited epidermal thickening, hyperkeratosis, parakeratosis, and inflammation in the dermis. (C, D) The hyperkeratosis and acanthosis in the pGP3 (IM-pGP3) and pGP3M (IM-pGP3M) intramuscular injection groups were milder than that in the IMQ group and (E) the phosphate-buffered saline intramuscular injection group. Figure 6. The cytokines levels in the serum and skin tissue were measured using ELISA. Data are presented as mean±standard deviation (8 mice/group). (A) The levels of IL-17A, IL-22, and IFN-γ in the skin lesions. * P<0.05 compared with the IM-PBS group. (B) The levels of IL-17A, IL-22, and IFN-γ in the serum. * P<0.05 compared with the IM-PBS group.
Figure 6. The cytokines levels in the serum and skin tissue were measured using ELISA. Data are presented as mean±standard deviation (8 mice/group). (A) The levels of IL-17A, IL-22, and IFN-γ in the skin lesions. * P<0.05 compared with the IM-PBS group. (B) The levels of IL-17A, IL-22, and IFN-γ in the serum. * P<0.05 compared with the IM-PBS group. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952