08 July 2023: Clinical Research
The Triglyceride Glucose (TyG) Index as a Sensible Marker for Identifying Insulin Resistance and Predicting Diabetic Kidney Disease
Hui Fang Li1ABCDEFG*, Xia Miao1B, Ying Li2AGDOI: 10.12659/MSM.939482
Med Sci Monit 2023; 29:e939482
Abstract
BACKGROUND: Patients with insulin-resistant diabetes have the highest risk of kidney disease. The triglyceride glucose (TyG) index is considered a reliable and simple marker of insulin resistance. We studied the relationship between the TyG index, diabetic kidney disease (DKD), and related metabolic disorders in patients with type 2 diabetes.
MATERIAL AND METHODS: This retrospective study included a consecutive case series from January 2021 to October 2022 in the Department of Endocrinology at Hebei Yiling Hospital. In total, 673 patients with type 2 diabetes met the inclusion criteria. The TyG index was calculated by napierian logarithmic (ln) (fasting triglyceride×fasting glucose /2). Patient demographic and clinical indicators were obtained from medical records, and statistical analysis was conducted using SPSS version 23.
RESULTS: The TyG index was significantly related to metabolic indicators (low-density lipoprotein, high-density lipoprotein, alanine aminotransferase, plasma albumin, serum uric acid, triglyceride, and fasting glucose) and urine albumin (P<0.01) but not with serum creatinine and estimated glomerular filtration rate. In multiple regression analysis, an increase in the TyG index was revealed to be an independent risk factor for DKD (OR: 1.699, P<0.001).
CONCLUSIONS: The TyG index was independently related to DKD and related metabolic disorders; therefore, the TyG index can be used as an early sensitive target for clinical guidance in the treatment of DKD with insulin resistance.
Keywords: Diabetes Mellitus, Type 2, Diabetic Nephropathies, Insulin Resistance, Humans, Glucose, Retrospective Studies, Triglycerides, Uric Acid, Blood Glucose, biomarkers, Risk Factors
Background
The incidence and prevalence of end-stage kidney disease (ESKD) is increasing worldwide and is becoming a heavy economic burden for society. Diabetic kidney disease (DKD) is the major cause of chronic kidney disease (CKD) and ESKD [1]. Over the past decades, despite improving blood glucose control and renal protection, the incidence of ESKD related to diabetes has not been significantly reduced. The major characteristics of type 2 diabetes are insulin resistance (IR) and β-cell dysfunction, although there is wide variation in clinical practice. To solve the complexity of diabetes, new subgroups or subtypes of diabetes have recently been proposed. Among them, diabetes with severe IR has the highest risk of kidney disease due to its clinical characteristics. Although the importance of IR in the pathogenesis of diabetes is well known, IR indicators have not been widely used in clinical practice, which may be related to the following factors. First the cost, time, and process of measuring insulin are complex. The normal blood glucose clamp test is considered the criterion standard for measuring IR, although it is complex and difficult to use. Due to the wide range of “normal” values of fasting plasma insulin, the homeostasis model assessment is unpreferable, being more variable than the clamp method, as a normal steady-state relationship between plasma glucose and insulin levels in patients with diabetes no longer exists. The triglyceride glucose (TyG) index is a non-insulin-based IR index that combines triglycerides and fasting blood glucose (FBG) [2]. It has been identified as a reliable biomarker of IR, with better sensitivity than the homeostasis model assessment [3]. The TyG index is simple to use, economical, and reliable, serving as an alternative indicator of IR. Moreover, it has been used to study metabolic syndrome, nonalcoholic fatty liver disease, atherosclerosis, and type 2 diabetes mellitus [4], with findings confirming that a high TyG index can be independently related to the incidence of atherosclerotic cardiovascular disease, heart failure, stroke, and other major vascular diseases [5,6]. Research has shown that IR occurs 20 years before type 2 diabetes mellitus diagnosis; therefore, IR has been confirmed to be an independent predictor of cardiovascular disease without diabetes.
Some prospective small sample studies have reported that IR can precede microalbuminuria in patients with diabetes [7,8]. IR interrelated with hypertension, central obesity, and dyslipidemia is aggravated by the occurrence of DKD, suggesting that reduced insulin sensitivity is involved in the pathogenesis and may be a potential treatment target. Moreover, the improvement of IR in patients with diabetes is related to the protection of the heart and kidney [9]. However, the research between TyG and DKD is still scarce. Therefore, this study used the TyG index level as a breakthrough point and explored the correlation between the TyG index level and DKD risk through a case-control study. Research shows that the incidence of diabetes is concentrated in 40 to 59 year olds [10]. However, we studied type 2 diabetes kidney disease, so the minimum threshold of age for enrollment was 40 years old. The focus of this study was diabetes nephropathy, which is characterized by micro-albuminuria at first, and a large amount of albuminuria (>0.5 g/24 h) may occur in the late stage [11]. In addition, the study object was not a renal pathological result, but a grouping of diabetes nephropathy and non-diabetes nephropathy based on proteinuria. In 321 cases of diabetes patients with renal biopsy, 179 patients were without DKD (55.8%), and membranous nephropathy was the most common non-diabetic renal disease in patients with diabetes [12,13]. Therefore, this study excluded patients with an albumin creatinine ratio (ACR) >1.0 g.
Material and Methods
PARTICIPANTS:
This was a retrospective study of patients with type 2 diabetes hospitalized in the Department of Endocrinology, Hebei Yiling Hospital Affiliated to Hebei Medical University (Shijiazhuang, Hebei, China) from January 2021 to October 2022. The inclusion criteria were as follows: (1) type 2 diabetes was diagnosed according to the World Health Organization [14] and the Chinese diabetes Society [15]; or the diagnosis was confirmed, and the hypoglycemic treatment was more than 6 months; and (2) age ≥40 years. The exclusion criteria were as follows: (1) patients without type 2 diabetes; (2) type 2 diabetes patients with lactic acid poisoning, or ketoacidosis, or severe infection, or critical patients, including those with conditions such as organ failure, acute myocardial infarction, and cerebral infarction; and (3) the laboratory inspection data was incomplete. For patients with DKD, urinary ACR (UACR) greater than 1.0 g and estimated glomerular filtration rate (eGFR) <45 mL4n.1.73 m2 were additional exclusion criteria. The study was approved by the Ethics Committee of Hebei Yiling Hospital Affiliated to Hebei Medical University and was conducted in accordance with the Declaration of Helsinki. Appropriate consent was obtained from all participants.
CLINICAL AND BIOCHEMICAL PARAMETERS:
Anthropometric parameters included blood pressure assessments. Blood and urine samples were collected in the morning after participants fasted from eating, drinking, and smoking for at least 8 h. All blood and urine samples were tested immediately after collection. The plasma levels of parameters were measured using an automatic biochemical analyzer (7600-020; Hitachi Inc, Tokyo, Japan). The UACR is a dry chemical method used to measure urinary protein and creatinine (Korea Cyber Reader 720.). Some patients with diabetes complicated with hypertension and patients with cardiovascular and cerebrovascular diseases were being treated with statins.
DEFINITIONS:
The eGFR was calculated from a serum creatinine and age study equation (CKD-EPI) [16].The TyG index was calculated as follows: TyG=napierian logarithmic (ln) (fasting TG [mg/dL]×FBG [mg/dL]/2) [17]. DKD was defined as UACR ≥30 mg/g at least twice in the consecutive morning urine collections.
STATISTICAL ANALYSIS:
SPSS 23.0 software (IBM Corp, Armonk, NY, USA) was used to analyze the data. The data are expressed as the mean±median standard deviation (interquartile interval) for continuous variables and percentage for categorical variables. The
Results
VARIABLES OF PATIENTS WITH TYPE 2 DIABETES AND DKD:
A total of 328 patients without DKD (non-DKD) and 345 patients with DKD with average age of 63.89 years and a median disease duration of 10.71 years were included. A total of 51.26% (345/673) of the patients with type 2 diabetes were identified as having DKD. Compared with IR in the non-DKD group, IR in patients with DKD was shown by the TyG index (P<0.001). Similarly, patients with DKD had a significantly longer course of disease (P=0.02), older age (P<0.001), and higher serum creatinine (Cre; P<0.001), uric acid (UA; P=0.033), total triglycerides (TG; P=0.023), and FPB levels (P<0.001) than those in the non-DKD group. In addition, lower levels of free triiodothyronine (FT3; P=0.006), albumin (ALB; P<0.001), glutamyl aminotransferase (GGT; P=0.003), high-density lipoprotein (HDL; P=0.014), and eGFR (P<0.001) were observed. However, there was no difference in thyroid stimulating hormone, free thyroxine, platelets, low-density lipoprotein cholesterol (LDL-C), and alanine aminotransferase (ALT) levels between the groups, and the DKD group showed serious glucose and lipid metabolism disorders (Table 1).
TYG INDEX IN THE EARLY STAGES OF DKD:
Correlation analysis shows that TyG index was positively correlated with UA (r=0.164#), UACR (r=0.172), LDL (r=0.277), and ALT (r=0.184; P<0.001 for each) but negatively correlated with HDL levels (r=−0.269, P<0.001). However, the TyG index was not significantly correlated with Cre level (r=0.075, P=0.051) or eGFR (r=−0.018, P=0.639). The results showed that IR was involved in early metabolic disorders of DKD (Table 2).
TYG WAS AN INDEPENDENT RISK FACTOR FOR DKD:
Univariate and multivariate regression analyses were performed to identify independent risk factors for DKD (Table 3). The results showed that DKD risk factors included the TyG index (OR=1.521, 95% CI: 1.247–1.854), age (OR=1.028, 95% CI: 1.013–1.044), ALB (OR=0.891, 95% CI: 0.858–0.925), and FPB (OR=1.096, 95% CI: 1.049–1.145). After adjusting for confounders, such as disease course, FT3, GGT, HDL, and TG, the results still showed that the TyG index remained strongly associated with DKD (OR=1.693, 95% CI: 1.022–2.803), indicating that the TyG index was an independent risk factor for DKD.
FITTED EQUATIONS AND ROC FOR IDENTIFICATION RISK FACTOR FOR DKD:
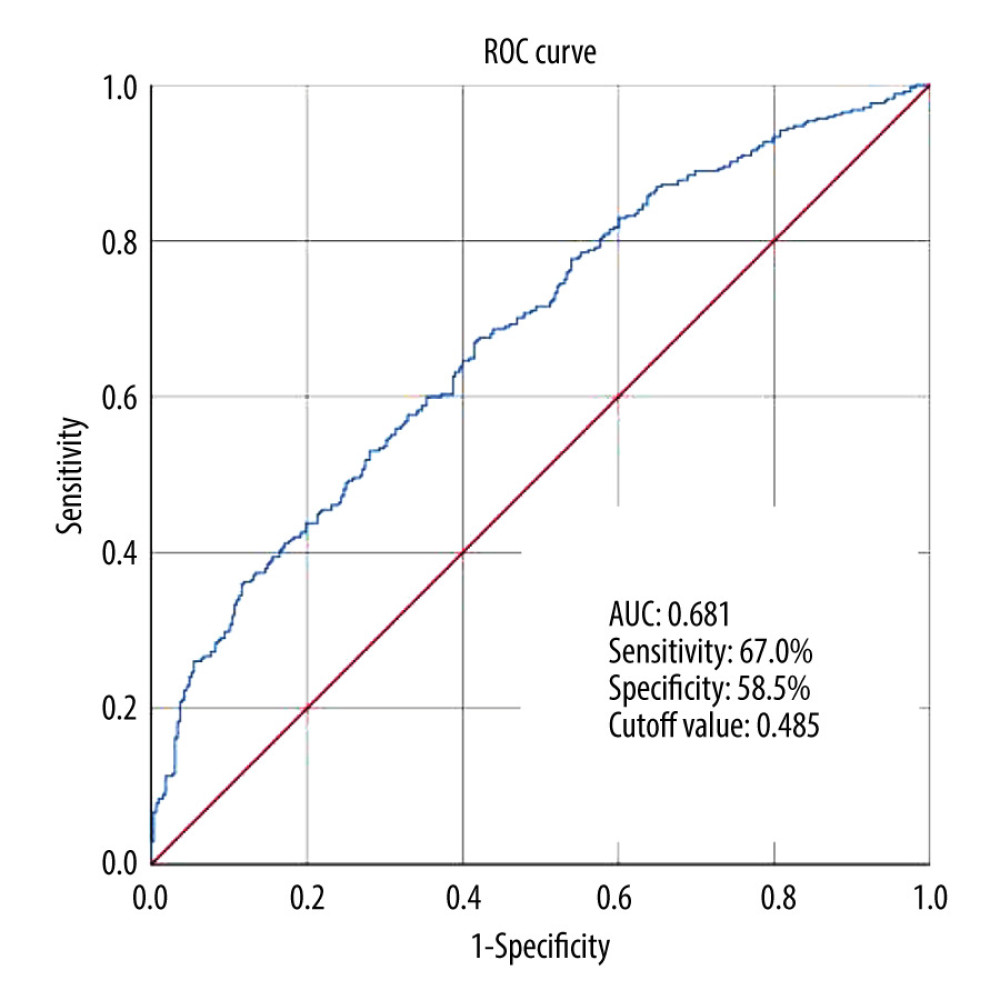
Adjusted for confounding factors of course, age, FT3, ALB, GGT, Cre, UA, HDL, TG, FPB and eGFR, the results showed that TyG was still a risk factor for DKD, and for every 1 unit increase in TyG, the risk of DKD increased by 1.699 times. According to the regression analysis results, the fitted equations is −1.441× 0.025age× −0.123ALB × 0.53TyG (R2: 0.107; Table 4). ROC analysis was used to evaluate the performance of TyG in identifying the risk of DKD in patients. As shown in Figure 1, the AUC for predicting DKD was 0.681 (95% CI: 0.641–0.72), the cut-off value was 0.485, and the corresponding sensitivity and specificity were 67.0% and 58.5%, respectively.
INCREASE IN THE TYG INDEX REDUCED EGFR AND PROMOTED DKD PROGRESSION:
First, a stratification analysis was conducted according to the TyG median. The patients were divided into 2 groups, a low TyG group (TyG ≤9.249) and high TyG group (TyG >9.25). Simultaneously, eGFR and age were stratified. The results showed that TyG ≤9.249 showed no statistical significance in the eGFR2 (60–89.9 mL/min/1.73 m2) stage and age (>71 years old) subgroup (P=0.674, P=0.57, respectively). However, TyG >9.25 showed significant differences in the eGFR2 (60–89.9 mL/min/1.73 m2) stage and age (>71 years old) subgroup between the non-DKD and DKD groups (P<0.001, P=0.003, respectively; Table 5).
Discussion
We applied the insulin resistance biomarker TyG to study the relationship between insulin resistance and DKD. We demonstrated that the TyG index was closely related to DKD (
Treating DKD is difficult, especially in patients with phase III diabetes and ESKD. Therefore, early identification and attention to the management of high-risk patients with DKD can be an effective way to reduce the incidence rate of DKD. IR is a pathological reaction of hyperinsulinemia and impaired glucose tolerance caused by the decreased insulin sensitivity of target tissues (such as liver, muscle, and adipose tissue). IR can lead to hyperglycemia, hypertension, dyslipidemia, visceral obesity, hyperuricemia, and other conditions. Moreover, IR and its consequences are the basis of the typical pathological and clinical characteristics of type 2 diabetes. Therefore, researching the relationship between IR and DKD will help to identify novel and effective treatments to improve the prognosis of type 2 diabetes.
The results of this study showed that the TyG index in the DKD group was significantly higher than that in the non-DKD group (
Research data on the correlation between the TyG index and DKD are limited. Our study showed that the TyG index was positively correlated with UA, UACR, LDL-C, and FBG (
Epidemiological studies have shown that inflammatory reactions can lead to the occurrence of type 2 diabetes mellitus by participating in IR and further aggravating it in the presence of hyperglycemia, thus promoting multiple long-term diabetes complications [22]. The TyG index is also considered a reliable marker of systemic inflammation, which can be an inflammatory factor in the progression of DKD [23]. The multivariable analysis adjusts for potential confounding factors and establishes the fitting equation, while the ROC curve clarifies the sensitivity and specificity of the model, indicating that a high TyG index plays an important role in the pathogenesis of DKD. The TyG index can be easily calculated using TG and FPG; therefore, the TyG index is expected to be widely used in clinical practice to identify patients at high risk of DKD and promptly intervene.
There are different factors influencing the TyG index on eGFR, in view of the decrease in eGFR with age. In the present study, the subgroup analysis of eGFR and age showed that when the TyG index was >9.25, differences in eGFR and age were statistically significant between the 2 groups. This suggests that the TyG index increases as the risk of DKD increases and eGFR decreases. Another study showed that there was a significant correlation between the TyG index and DKD in patients with an eGFR ≥90 mL/min/1.73 m2; however, in patients with an eGFR <90 mL/min/1.73m2, this correlation was not significant [24]. A large-scale cohort study to elucidate the exact impact of the TyG index on eGFR is necessary [25].
This study had several limitations. First, this was a cross-sectional observational study; therefore, a causal relationship could not be obtained. Second, we collected the data of all inpatients in the Endocrinology Department of the third-level grade-A traditional Chinese medicine hospital in the past 22 months, including those hospitalized for type 2 diabetes. Moreover, most patients had multiple complications of diabetes at the same time, which may have caused data bias.
Conclusions
We found that there was a significant correlation between the increase in TyG index and DKD. TyG is an important factor in the pathophysiology of DKD and may be an important target for treatment and prevention. Prospective research is needed to explore the effect of TyG on DKD progression.
Tables
Table 1. Characteristics of patients with type 2 diabetes and with and without DKD.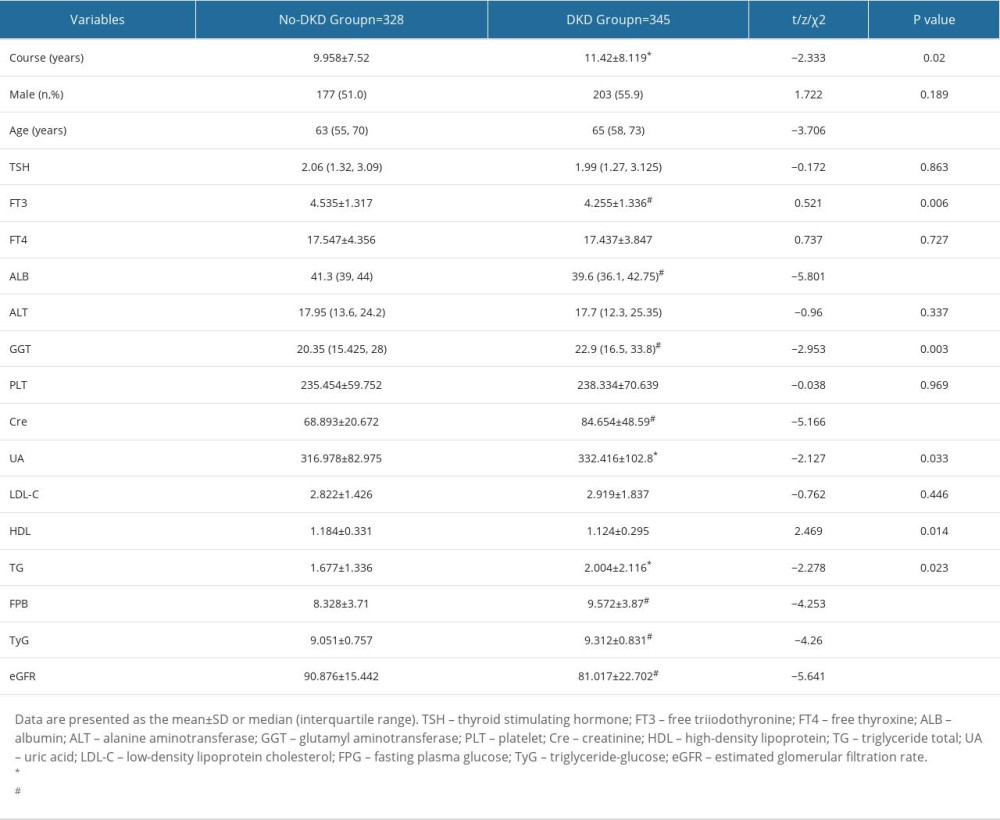 Table 2. Correlation analysis of TyG index with renal function and metabolic factor.
Table 2. Correlation analysis of TyG index with renal function and metabolic factor.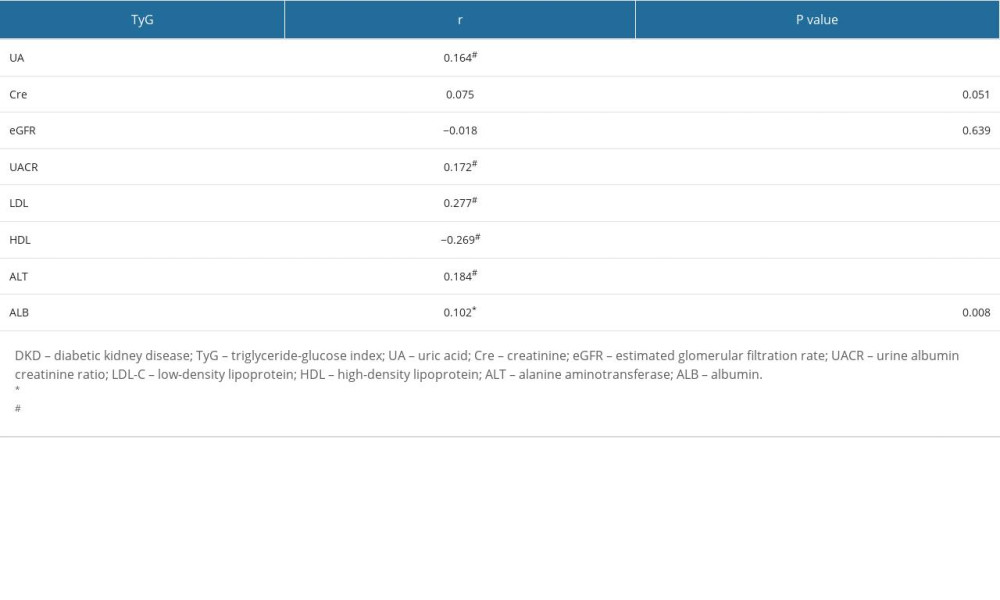 Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease.
Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease.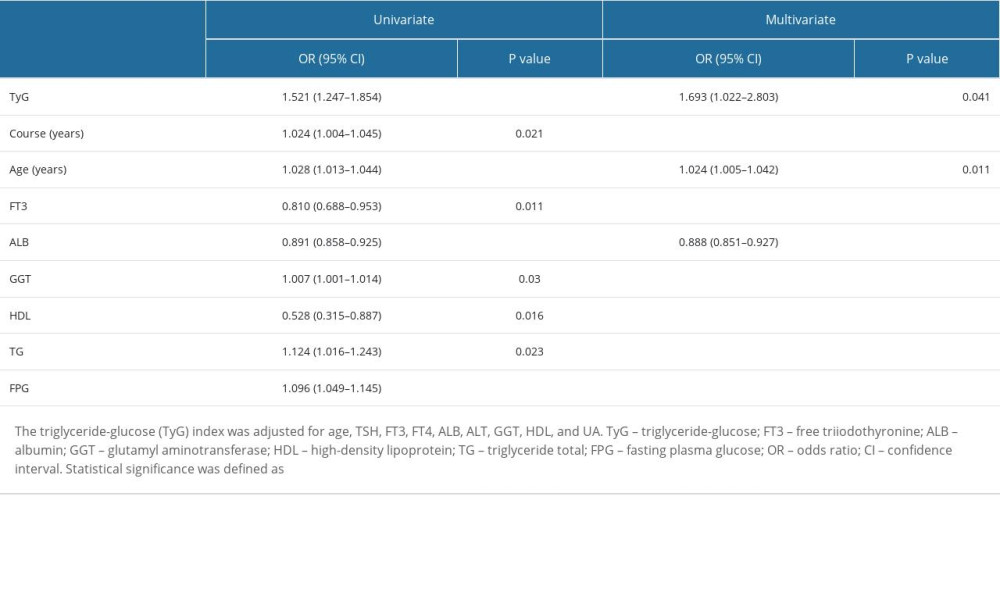 Table 4. Predictors of diabetes nephropathy.
Table 4. Predictors of diabetes nephropathy.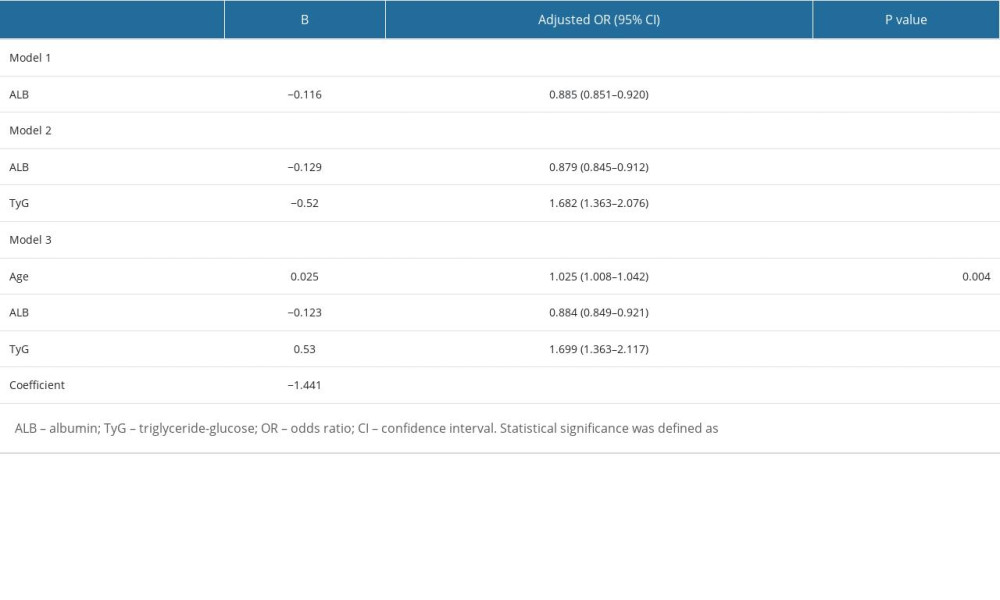 Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR.
Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR.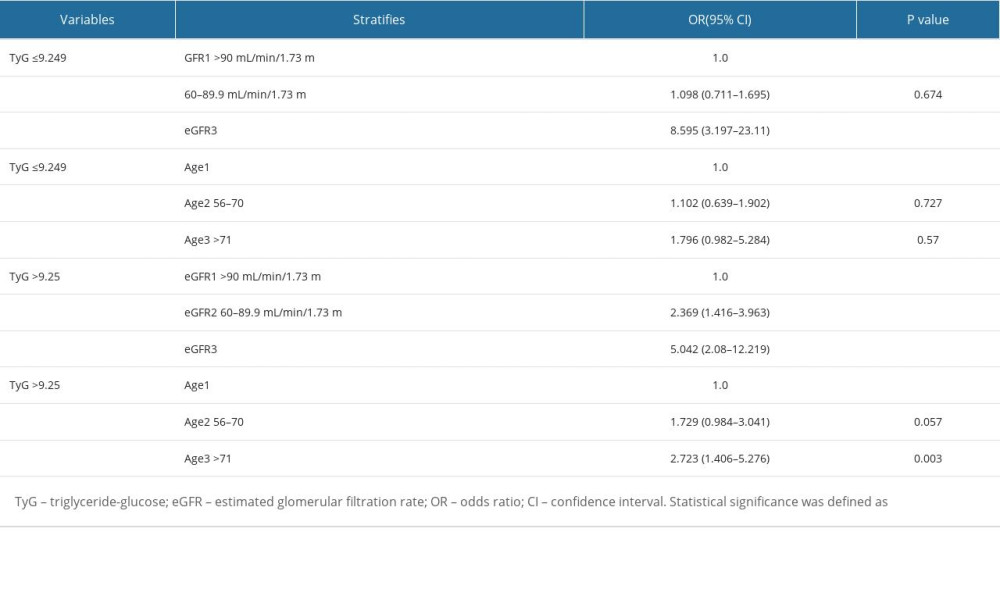
References
1. León-Jiménez D, Miramontes-González JP, Márquez-López L, Basal insulin analogues in people with diabetes and chronic kidney disease: Diabet Med, 2022; 39(2); e14679
2. Li M, Zhan A, Huang X, Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: The China H-type hypertension registry study: Cardiovasc Diabetol, 2020; 19; 139
3. Khan SH, Sobia F, Niazi NK, Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance: Diabetol Metab Syndr, 2018; 10; 74
4. Zimmet P, Alberti KG, Shaw J, Global and societal implications of the diabetes epidemic: Nature, 2001; 414; 782-87
5. Lee EY, Yang HK, Lee J, Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes: Lipids Health Dis, 2016; 15; 155
6. Jin JL, Sun D, Cao YX, Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: A nested case-control study: Ann Med, 2018; 50; 576-86
7. Blendea MC, Jacobs D, Stump CS, Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II over expression: Am J Physiol Endocrinol Metab, 2005; 288(2); 353-59
8. Thorn LM, Forsblom C, Fagerudd J, Metabolic syndrome in type 1 diabetes: Association with diabetic nephropathy and glycemic control (the Finn Diane study): Diabetes Care, 2005; 28; 2019-24
9. Liang S, Cai GY, Chen XM, Clinical and pathological factors associated with progression of diabetic nephropathy: Nephrology (Carlton), 2017; 22(Suppl 4); 14-19
10. Cho NH, Shaw JE, Karuranga S, IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045: Diabetes Res Clin Pract, 2018; 138; 271-81
11. John EE, Roy S, Eapen JJ, When to suspect non-diabetic kidney disease in a diabetic patient?: Cureus, 2022; 14(8); e28091
12. Shadab S, Mittal P, Barwad A, Characterizing predictors of non-diabetic kidney disease (NDKD) in diabetic patients: Int Urol Nephrol, 2022; 54(6); 1303-9
13. Yang Z, Feng L, Huang Y, A differential diagnosis model for diabetic nephropathy and non-diabetic renal disease in patients with type 2 diabetes complicated with chronic kidney disease: Diabetes Metab Syndr Obes, 2019; 12; 1963-72
14. Alberti KG, Zimmet PZ, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation: Diabet Med, 1998; 15; 539-53
15. National guidelines for the prevention and control of diabetes in primary care (2018): Zhonghua Nei Ke Za Zhi, 2018; 57; 885-93 [in Chinese]
16. Zafari N, Churilov L, MacIsaac RJ, Diagnostic performance of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation at estimating glomerular filtration rate in adults with diabetes mellitus: A systematic review and meta-analysis protocol: BMJ Open, 2019; 9(8); e031558
17. Ramdas Nayak VK, Satheesh P, Shenoy MT, Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance: J Pak Med Assoc, 2022; 72(5); 986-88
18. Zhao L, Zou Y, Bai L, Prognostic value of metabolic syndrome in renal structural changes in type 2 diabetes: Int Urol Nephrol, 2022; 54(8); 2005-14
19. McCormick N, O’Connor MJ, Yokose C, Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization: Arthritis Rheumatol, 2021; 73(11); 2096-104
20. Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A, Hyperuricemia-induced endothelial insulin resistance: The nitric oxide connection: Pflugers Arch, 2022; 474(1); 83-98
21. Adeva-Andany MM, Fernández-Fernández C, Funcasta-Calderón R, Insulin resistance is associated with clinical manifestations of diabetic kidney disease (glomerular hyperfiltration, albuminuria, and kidney function decline): Curr Diabetes Rev, 2022; 18(7); e171121197998
22. Ding Q, Gao Z, Chen K, Inflammation-related epigenetic modification: The bridge between immune and metabolism in type 2 diabetes: Front Immunol, 2022; 13; 883410
23. Ruan GT, Xie HL, Zhang HY, A novel inflammation and insulin resistance related indicator to predict the survival of patients with cancer: Front Endocrinol (Lausanne), 2022; 13; 905266
24. Iwai T, Miyazaki M, Yamada G, Diabetes mellitus as a cause or comorbidity of chronic kidney disease and its outcomes: The Gonryo-study: Clin Exp Nephrol, 2018; 22(2); 328-36
25. Liu L, Xia R, Song X, Association between the triglyceride-glucose index and diabetic nephropathy in patients with type 2 diabetes: A cross-sectional study: J Diabetes Investig, 2021; 12(4); 557-65
Tables
 Table 1. Characteristics of patients with type 2 diabetes and with and without DKD.
Table 1. Characteristics of patients with type 2 diabetes and with and without DKD. Table 2. Correlation analysis of TyG index with renal function and metabolic factor.
Table 2. Correlation analysis of TyG index with renal function and metabolic factor. Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease.
Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease. Table 4. Predictors of diabetes nephropathy.
Table 4. Predictors of diabetes nephropathy. Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR.
Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR. Table 1. Characteristics of patients with type 2 diabetes and with and without DKD.
Table 1. Characteristics of patients with type 2 diabetes and with and without DKD. Table 2. Correlation analysis of TyG index with renal function and metabolic factor.
Table 2. Correlation analysis of TyG index with renal function and metabolic factor. Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease.
Table 3. Univariate and multivariate analyses of patients with type 2 diabetes and with and without diabetic kidney disease. Table 4. Predictors of diabetes nephropathy.
Table 4. Predictors of diabetes nephropathy. Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR.
Table 5. TyG index in patients with type 2 diabetes stratified by age, eGFR. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952