30 July 2023: Review Articles
Enhancing Bone Grafting Outcomes: A Comprehensive Review of Antibacterial Artificial Composite Bone Scaffolds
Yi Liu1AEF, Liuxing He1AFG, Lijia Cheng1AEFG*, Xinsong Li1EF, Min Gao1EF, Qinzhi Li1E, Jingjing Gao1EF, Murugan Ramalingam123FDOI: 10.12659/MSM.939972
Med Sci Monit 2023; 29:e939972
Abstract
ABSTRACT: Bone defects and dysfunctions are prevalent among patients, resulting from various causes such as trauma, tumors, congenital malformations, inflammation, and infection. The demand for bone defect repair materials is second only to blood transfusions. Artificial bone composites offer numerous advantages for bone damage repair, including their availability, absence of rejection or immune reactions, high malleability, exceptional mechanical strength, and outstanding biocompatibility. However, bacterial infections frequently occur during bone transplantation or on graft material structures, leading to severe complications such as osteomyelitis and osteoporosis. Moreover, existing osteogenic materials alone are inadequate to address the challenges posed by traumatic infections, presenting a significant hurdle for clinicians in reconstructing infectious bone defects. Consequently, it is crucial to functionalize artificial bone composites to facilitate effective bone repair and regeneration. Notably, antibacterial capabilities play a critical role in preventing and treating infectious bone defects, and current research is focusing on the interface between artificial bone composites and antibacterial treatments. This article provides an extensive review of the current state of artificial composite bone scaffolds with antibacterial properties for infection prevention in bone grafting.
Keywords: Anti-Bacterial Agents, Tissue Scaffolds, Multifunctional Nanoparticles, Anti-Infective Agents, Bone Diseases, Infectious, Humans, Bone Transplantation, Arthrodesis, Osteogenesis, Inflammation
Background
Bone is an important tissue and organ of the human body; once damage is done, it will seriously impact human life activities. Causes of bone damage or dysfunction include trauma, tumors, congenital malformations, and inflammatory infections [1]. Posttraumatic infectious bone defects are the focus of many orthopedic scientists [2], and severe contamination from open fractures is a common cause [3]. Cases can be attributed to postoperative infection or acute/chronic osteomyelitis. Lack of blood supply, soft-tissue loss, bone defect area, internal fixation surface, and scaffold surface that easily mediates bacterial adhesion and biofilm formation are all factors in bone defect infection [4–6]. Almost all types of bacteria and fungi can infect bone repair materials [7].
The treatment methods for infectious bone defects after trauma include bone transplantation, bone transport technology, osteoinductive membrane technology, and antibiotic composite sustained-release carrier implantation technology [11–15]. Among these, bone grafting is the most important clinical treatment of infectious bone defects. Because the operation is simple, there are few postoperative complications and good fracture healing, and bone grafting can be used to treat infectious bone defects with segmental and large bone defects [16]. In addition, when the transplant material fills the bone defect, it can control the infection through an efficient bacteriostatic strategy, induce the formation of fibrous membranes, provide good blood transport, and form a relatively independent and stable space at the bone defect, which is conducive to secondary bone reconstruction and bone healing, and effectively improves the osteogenesis of cancellous bone [17,18].
In the field of material engineering, bone defect repair materials are mainly divided into 3 categories: autologous bone, allogeneic bone, and artificial bone materials, with the following characteristics: 1) Autologous bone: due to the lack of immune response and rejection, autologous bone grafts have become the industry standard for clinical bone grafts, but their supply is scarce and they cannot be widely used in clinical practice. 2) Allogeneic bone: it is difficult to apply allogeneic bone grafts on a large scale due to the risk of failures such as incomplete absorption, immune rejection, disease transmission, and microcrack propagation. 3) Artificial bone materials: these have become the main source of bone grafts [19–21].
At present, from the perspective of material structure and properties, artificial biomaterials for bone repair include metal materials, inorganic non-metallic materials, polymer materials, and composite materials, which have the following characteristics: 1) Pure metals, titanium alloys, stainless steel, cobalt alloys, and other metal materials have excellent mechanical properties, and fatigue resistance [22]. 2) Inorganic non-metallic materials, such as bioactive ceramics and bone cement, have good stability, biocompatibility, bone conductivity, bone inducibility, and biodegradability [23–25]. Scaffolds made from the graphene family and its derivatives can enhance the mechanical properties of the material, and can also improve the osteogenic and antibacterial properties of the original material [26]. 3) Polymer materials are divided into natural polymer materials and synthetic polymer materials. Compared with the former 2 materials, polymer materials have low density, more convenient manufacturing, good degradation, and less environmental pollution. Metal bases, polymer bases, and ceramic bases are the 3 categories into which composite materials can be separated. Metal inclusions have the best machining and mechanical qualities of all of these. Polymer matrix compounds have a high density, a unique slow denaturation, and a high special strength. Ceramic matrix compounds are increasingly appreciated because of their high biological activity [27]. However, in clinical applications, when the above materials are used alone, they have certain defects, such as the biological inertness of metals, which can easily cause rejection and inflammation in the body. Long-term immersion of scaffolds in body fluids can release a large number of metal ions, which are toxic and harmful to human tissues, and even loosen and break metal stents [28]. Ceramic materials have drawbacks as well, including brittleness and fracturing, insufficient plasticity and low ductility, uncontrollable degradation rates, and the need to be combined with other materials that have high mechanical strength and a quick degradation rate in order to improve these properties. When employed alone, synthetic and natural polymers have a lower elastic modulus than metallic and ceramic materials, which results in a relatively inferior load-carrying capability [29].
As a result, composite materials have attracted considerable interest in the treatment of bone defects, often through a combination of multiple technologies, such as 3D printing, nanotechnology, freeze-drying, the foaming method, and electrospinning [30–33], to reduce the defects of materials used alone, to investigate the development of more ideal artificial bone composite materials with both plasticity and a certain mechanical strength, osteoinductive activity, and biocompatibility. Composites currently under development include the following: The Clinoptilolite-Hydroxyapatite (CPT-HA) composite has good bioactivity and extremely high bioactivity in the repair of bone defects [34]. The novel graphene oxide-incorporated silicate-doped nano-hydroxyapatite composite material can improve the bone regeneration ability and achieve bone repair with the treatment of bone defects [35]. The porous scaffold composed of bioactive glass containing purified boron oxide permeated by cellulose acetate-gelatin solution can improve the porosity, mechanical properties, and biological activity of raw materials [36]. The 3D porous composite scaffold prepared with boron-doped hydroxyapatite and baghdadite can improve the biodegradation rate of raw materials and increase the matching similarity with bone tissue [37]. Finally, a composite scaffold of cellulose acetate/oxidized pullulan/gelatin (CA/ox-PULL/GEL) three-dimensional scaffolds with tubular morphology modified with boron for dentin regeneration also improved the osteogenic properties [38].
However, in addition to providing certain osteogenic properties, materials for the treatment of infected bone defects also require special characteristics, such as efficient antibacterial properties, which not only prevent implant-related infections, but also destroy bacterial biofilms and avoid developing osteomyelitis, and exert efficient antibacterial effects (Figure 1). To develop artificial bone composites with efficient antibacterial and osteoinductive properties to provide viable treatment options for infected bone defects, it is necessary to combine the fields of microbiology and biomaterials engineering [4]. This article aims to review the current status of artificial composite bone scaffolds to prevent infection in bone grafting.
Antibacterial Strategy of Artificial Bone Composites
At present, antibacterial agents used in antibacterial artificial bone scaffold materials include antibiotics, inorganic antibacterial agents, and natural antibacterial agents [39,40]. Antibiotics are the best antibacterial agents for the clinical treatment of bone implant-related diseases. Because of its good antibacterial performance and low development cost, it has been widely used in clinics. Inorganic antibacterial strategy has become a research hotspot because it is not easy to induce drug resistance, mainly including ion-mediated strategy, physical strategy, and nanoparticles (NPs)-mediated strategy. The natural antibiotic-induced strategy is not easily rejected by the immune system and has good cell compatibility. Natural antibiotics include flavonoids, alkaloids, phenolic compounds, proteins, and organic acids. According to the source, it can be divided into animal-derived antibacterial agents and plant-derived antibacterial agents. The combined bacteriostasis strategy combines the above steps, which is the best way to overcome the single bacteriostasis strategy’s weakness and improve the bacteriostasis effect (Figure 2).
Progress in Research on Antibiotics-Induced Artificial Bone Scaffolds
Antibiotics can play a bactericidal role by inhibiting the synthesis of the bacterial cell wall, interacting with the cell membrane, interfering with protein synthesis, and inhibiting transcription and replication of nucleic acids [41–43]. Antibiotics can be widely used in the repair of bone defect infection, but unreasonable use of antibiotics will lead to drug-resistant strains [44]. With the continuous development of local sustained-release systems in bone tissue engineering, this problem has been solved (Figure 3). Studies have shown that a variety of antibiotics can be effectively combined with artificial bone scaffolds to release drugs for bone infection defects. Antibiotics or specific drugs can not only improve the antibacterial ability of the material scaffold, but due to the structural characteristics of the material scaffold, the combined composite scaffold can also effectively control the release of antibiotics or specific drugs, thereby achieving true local and targeted therapeutic effects [45]. Typical examples of antibiotics-induced scaffolds with antimicrobial properties are listed below.
Levofloxacin, a quinolone, was widely used in the treatment of bone implant-related infections because of its ability to penetrate the trabecular bone. Kuang et al have shown that the combination of levofloxacin and mesoporous silica to make a composite scaffold could significantly improve the sustained release performance of drugs. Its composite scaffold inhibited the adsorption and adhesion of bacteria, thereby achieving an antibacterial effect [46]. Vancomycin is a glycopeptide widely used in the treatment of bone implant-related infections because of its obvious antibacterial effect on drug-resistant
Therefore, the combination of antibiotics and artificial bone to produce composite scaffolds can more precisely control the release of drugs and greatly improve the utilization rate of antibiotics. The new antibiotics-induced strategy is efficient and less dangerous, but there are still many issues with the drug and stent combination that need to be taken into account, including the physical and chemical properties of drugs, encapsulation and delivery efficiency, preparation, material composition, and scaffold structure [45,50,51]. The key to the antibacterial effect of antibiotics-induced antibacterial artificial bone scaffolds is to successfully match the antibacterial delivery system to the 3D structure of the scaffold.
Progress in Research on Inorganic Antibacterial Artificial Bone Scaffolds
Long-term use of antibiotic artificial bone implantation can lead to generation of bacterial resistance. Therefore, the search for other antibacterial artificial bone scaffolds has become a focus of research. Inorganic antibacterial artificial bone scaffolds are an important topic of current research. Some methods include metal ion-mediated, photocatalyst physical activation, and nanoparticle catalytic antibacterial activity. Metal ions (eg, Ag+, Zn2+, and Cu2+), oxide photocatalysis (eg, titanium dioxide [TiO2] and zinc oxide [ZnO]), nanoscale metal antibacterial materials (eg, molybdenum sulfide [MoS2] and copper sulfide [CuS]), and carbon-based nanocomposites (eg, graphene derivatives and carbon nanotubes) are currently widely used in artificial bone scaffolds.
Ion-Mediated Antibacterial Artificial Bone Scaffolds
Ion-mediated antibacterial artificial bone scaffolds are stents prepared by using ions to mediate sterilization or bacteriostatic, mainly manifested as the incorporation of functional elements, such as Ag+, Zn2+, and Cu2+ doping into the composite scaffolds. These elements can be doped, combined, or coated into composite artificial bone scaffolders in the form of oxides, nanoparticles, or ions. The released ions can kill bacteria through the degradation behavior of scaffolds.
Ag can be incorporated into composite scaffolds in the form of ions or oxidation complexes due to its obvious antibacterial effect, which significantly improves the antibacterial ability of scaffolds [52–54]. Zhang et al have shown that the composite material produced by the incorporation of Ag+ significantly improves the activity of alkaline phosphatase (ALP) and the expression of genes related to osteogenesis under the condition of a good antibacterial effect [55]. Jiang et al designed a porous hydroxyapatite/polyurethane composite scaffold that effectively inhibited
Because of its good antibacterial activity, Cu has an obvious antibacterial effect on gram-positive and gram-negative bacteria. However, if the concentration of Cu2+ is too high, it induces apoptosis of cells, resulting in a series of toxic reactions [57–60], which limits its further clinical application. To make up for this defect. Wu et al have shown that the copper-containing mesoporous bioactive glass (MBG) scaffolds produced by the incorporation of Cu2+ can release Cu2+ through the scaffolds and also has an obvious antibacterial effect. It also further controlled the release concentration of Cu2+ by adding different concentrations of Cu2+-MBG scaffolds, to achieve a good antibacterial effect within a safe range [61].
Zn has good osteogenic, vascularization, and antibacterial capabilities. It is mainly derived from zinc metal, zinc salt compounds, and zinc oxide. Zn2+ released from the scaffold can penetrate the cell wall, causing the cell contents to flow out, inactivating nucleic acids and enzymes, and activating reactive oxygen species (ROS) to kill bacteria [62–65]. Felice et al prepared polycaprolactone (PCL): HA: ZnO and PCL: ZnO scaffolds to evaluate their antibacterial activity against
Therefore, the key to achieve antibacterial performance of the composite scaffold prepared by ion incorporation method is to control the doping and degradation behavior of functional ions, control the ion concentration released by the scaffold within the safe range, and greatly improve the bioavailability of ions. It is necessary to modify composite artificial bone scaffolds to improve their ability to treat infection.
Physical Antibacterial Artificial Bone Scaffolds
Physical antibacterial artificial bone scaffolds can improve the antibacterial ability of scaffolds by endowing them with physical properties such as surface charge and external stimulation, which can change the microenvironment around bacteria and effectively kill bacteria [53,66–69]. This is another important inorganic antibacterial strategy, including photocatalytic reaction, piezoelectric effect, and physical thermal effect. Meanwhile, nanomaterials and nanostructures have unique physical and chemical properties and may play an important role in the physical activation of antibacterial strategies [70–72].
Under the action of strong light, the photocatalyst will form an electron-hole pair. When the substrate comes into contact with air, a series of redox reactions will occur with the substrate at the interface, thereby generating ROS, which can effectively kill bacteria [66,69]. Some studies have designed a graphdiyne (GDY)-modified TiO2-rich fiber scaffold that could enhance photocatalytic activity and can be used for the prevention of implant infection [73]. Under the irradiation of ultraviolet light with a wavelength of 365 nm, the electron spin resonance spectrum would display ROS signals, and GDY would be ionized in this case, so that TiO2 was activated after photocatalysis was transmitted to the material surface and the ROS generation ability of TiO2 was improved. Zhang et al showed that the incorporation of fluorine (F), ytterbium (Yb), and holmium (Ho) could significantly improve the photocatalytic activity of TiO2, and after 15 min of laser irradiation with a wavelength of 1060 nm, more reactive oxygen species could be produced to participate in a series of redox reactions, effectively killing the biofilm of
Under the piezoelectric effect, a positive charge is generated on the surface of piezoelectric ceramics, which can also induce antibacterial activity [74,45]. Sugimoto et al developed a lead-free piezoelectric (Ba, Ca) (Ti, Zr) O3 scaffold with enhanced antibacterial properties that could be used for bone tissue engineering. The charged surface of the scaffold showed good antibacterial activity, which was related to the micro-electric field formed by the surface charge around the material and the reactive oxygen species generated by decomposition of the surrounding solution [76].
The physical thermal effect is a method of killing bacteria that can absorb energy through light, electricity, and other ways to generate heat energy [68]. When combined with artificial bone scaffolds, they have a good bacteriocidal effect [68,77]. It has been shown that the composite scaffolds of forsterite and bioceramic scaffolds with photothermal activity produced by 3D printing technology can significantly improve the antibacterial effect [74] becauseforsterite can produce free carbon under strong light and high heat. Under infrared light conditions, this free carbon has a strong photothermal action and can have a good antibacterial effect against
Nanoparticles-Mediated Antibacterial Artificial Bone Scaffolds
Nanoparticles are metastable substances intermediate between solids and liquids. Nanomaterials have the advantages of surface effects, volume effects, quantum size effects, and macroscopic quantum tunneling effects, which can show special physical and chemical characteristics and be safe for the environment [78]. Because of the nanoscale metal ions’ excellent high specific surface area, microorganisms can be better absorbed, resulting in improved antibacterial and catalytic activity. Another advantage of the antibacterial activity of nanoparticles is their positive charge [79]. Slavin et al have shown that positively charged nanoparticles can alter the function of electron transport chains in bacteria, and bacteria in contact with these nanoparticles produce ROS, which induces oxidative stress [80], thereby inactivating bacteria by direct interaction with ubiquitin or its function in the electron transfer chain. Copper sulfide nanocomposites and carbon-based nanocomposites are also typical antibacterial materials with physical thermal effects [81].
The bactericidal mechanisms of nanoparticles include ROS generation, cation release, biomolecular destruction, membrane interaction, and ATP depletion [82]. Nanoparticles seem to activate multiple pathways at the same time, although there is no research to prove this conjecture and elucidate the activation pathways, which may be why nanoparticles have strong antibacterial activity, but this needs further study. ROS production, gene regulatory changes, cell wall permeation, and metabolite binding are challenges for bacteria to adapt to the environment and survive because they cannot establish defense mechanisms against these interactions at the same time (Figure 4). On the other hand, due to the similarity of biomolecules, these mechanisms are also toxic to human cells, but potential treatments for bacterial infections can be targeted by using specific ligands and bacterial cell receptors.
Jiang et al used silver nanoparticles (Ag-NPs) attached to a 3D-printed polyether ether ketone (PEEK) scaffold (Figure 5) [56]. After incubating the artificial bone scaffold material with
Progress in Research on Natural Antibiotic-Induced Antibacterial Artificial Bone Scaffolds
Compared with manufactured antibiotics, natural antibiotics can also inhibit bacteria and reduce inflammation, but the burden on the human body is less or even beneficial to the body. Studies have found that natural antibiotics can destroy the structure of bacterial membranes, in addition to sterilization, but also contain a variety of vitamins, minerals, and bioactive factors, can enhance the body’s immune function and resist the invasion of foreign bacteria and viruses [84,85]. Natural antibiotics (eg, flavonoids, alkaloids, phenolic compounds, proteins, and organic acids) come from nature and have many advantages. They can be classified into animal-derived and plant-derived antimicrobials [86].
Animal-Derived Antibacterial Agents
Animal-derived antibacterial agents incorporate amino acids, natural peptides, and high-molecular sugars. Xiao et al focused on the antibacterial movement of amino acids throughout their subjective and quantitative antibacterial experiments against
Natural peptide antibacterial agents, such as lysozyme and lactoperoxidase, have multi-target single or synergistic antibacterial effects on cells and have been an important research topic in animal-derived antibacterial agents [88]. Lysozyme is safe and has no adverse effects on the human body, and its mechanism of action is to destroy the β-1,4 glycosidic bond. Additionally, Yu et al found that lysozymes from the hemolymph of 3 species of lepidopteran insect hatchlings (
Protamine is a simple globular protein that exists in the sperm of fish. It has a strong inhibitory effect on
Chitosan (CS) is a kind of high-molecular sugar that is the deacetylation product of chitin. It can be dissolved in many dilute acids. It has become focus research on antibacterial artificial bones due to its good biocompatibility and strong antibacterial activity. Microscopically, contrasted with
Nonetheless, the unfortunate solubility and low mechanical properties of CS in water limit their biomedical applications. For this reason, recent studies have chemically modified CS, such as by quaternization, carboxymethylation, and cationization, and provided new directions for the further development of new materials with better physicochemical and biological properties [94]. For example, Yang et al used quaternary ammonium formation of hydroxypropyl trimethyl ammonium chloride (HACC) and poly (L-lactide-glycolide) and hydroxyapatite (HA) to graft CS onto polylactide-glycolide (PLGA) and hydroxyapatite (HA) 3D printing supports. The complicated antibacterial bone platforms containing HACC can decrease bacterial attachment and biofilm arrangement in vitro and in vivo. In addition, an ATP leakage assay showed that HACC-immobilized scaffolds can effectively destroy bacterial biofilm [95].
Plant-Derived Antibacterial Agents
Plant antimicrobials are the most commonly used natural antimicrobial agents. Chinese traditional herbal medicine has a long history and great potential for developing such antibacterial agents. Andrographis paniculata, Garlic, Buckwheat, Bitterwood, Coptis Chinensis, Berberine, Houttuynia, and Houttuynia cordata are commonly used botanical antibacterial agents. Plant extracts contain many bioactive substances, so they can effectively inhibit the growth of microorganisms, and have strong broad-spectrum antibacterial activity [96]. However, research on the antibacterial mechanisms of plant-natural antibacterial agents is limited.
Azeem et al reported the antibacterial properties of quercetin extracted from Gracilaria, including the antibacterial properties against drug-resistant
Progress in Research on Combined Antibacterial Strategies and Composite Scaffolds
Although antibiotic induction, ion mediation, physical capability activation, and nano-catalysis, combined with natural biological antimicrobial agents, will significantly improve the antibacterial effect, each antibacterial strategy has its own limitations. For example, antibiotic drugs can induce bacteria to develop drug resistance, metal ions have biocompatibility limitations, the input of light-stimulated signals in physical strategies and the existing research on the mechanism of photothermal antibacterial strategies are insufficient, and the local controllable release of natural antibacterial agents and material degradation need to be addressed. Clinical data show that the ideal antibacterial effect cannot generally be achieved by a single treatment. Combined antibacterial artificial bone scaffolds are made by combining 2 or more antimicrobials with different mechanisms, such as antibiotics, ions, photocatalysts, nanoparticles, animal antimicrobials, and plant antimicrobials. However, this creates additional requirements for stent design and material selection, which requires reasonable and effective integration of different antibacterial agents with stent materials and structures. Some typical combined antibacterial strategies for artificial bone composite scaffolds are listed below.
Some studies found that the composite scaffold made by combining levofloxacin-loaded and rubidium-doped methylene blue sensitized gelatin scaffolds could significantly improve the antibacterial effect by destroying the cell membrane of bacteria [100]. The composite scaffold of CS-containing polyethylene oxide and zinc oxide hydrogel produced by 3D printing technology had a bacteriostatic rate of 99.9%, and the antibacterial effect was significantly enhanced [101]. Huang et al coated berberine-containing CS on a composite artificial bone scaffold made of nano-hydroxyapatite/polyamide 66 (n-HA/PA66). In a sustained-release experiment, the sustained release of berberine in phosphate-buffered saline was 150 h, and it was confirmed by sterilization experiments that the delivery system also had a strong antibacterial effect. In addition, in vitro biological evaluation showed that the coated scaffolds could serve as a good substrate for human osteosarcoma cells (MG63) to adhere, move, grow, and proliferate, indicating that the antimicrobial delivery system was non-cytotoxic [102]. Researchers have also incorporated calcium phosphate cement (CPC) with bone morphogenetic protein and gentamicin into bulk recombinant bovine xenografts (MRBX), and then obtained CPC-MRBX after CPC solidification. A large-segment infected femoral defect was then created in a rabbit model to test the repair capacity of CPC-MRBX. Over time, the bone callus around the graft gradually increased, the external fixation could be removed, and the function returned to normal. Experiments have shown that CPC-MRBX could be used to repair large-segment infected bone defects, and had excellent anti-infection effects [103]. The reinfection rates of the control and experimental groups in the vancomycin-loaded polymethyl methacrylate (VCM-PMMA) experiments were 58.3% and 16.7%, respectively. It was demonstrated that the use of VCM-PMMA can significantly control joint infection and reduce the incidence of reinfection [104].
Therefore, a combined antibacterial strategy consisting of 2 or more antibacterial methods with different mechanisms not only does not interfere with the mutual antibacterial effect, but also can enhance the mutual antibacterial effect. Many studies have demonstrated that combining different antibacterial strategies can make up for the disadvantages of adopting a single antibacterial strategy, and each material has complementary advantages, thus exerting antibacterial and bone formation effects to a large extent. In addition, single antibacterial strategies may have limited antibacterial activity against specific bacteria, while combined antibacterial strategies can confer broad-spectrum antibacterial properties on scaffolds. The synergistic effect of combined antibacterial strategies can greatly improve antibacterial performance. The combination of multiple antibacterial strategies and composite scaffolds is the best antibacterial strategy at present, and the current research prospect is good.
Discussion
Artificial bone composites can reduce material defects when used alone [105]. For example, metal-matrix composites can provide mechanical support, providing superior machining and mechanical quality for scaffolding [55]. Polymer matrix composites can effectively improve the specific strength and modulus of the scaffold so that it has excellent toughness. Ceramic matrix composites can improve the bioactivity, degradability, bone inductance, and bone conductivity of scaffolds, and promote cell migration and nutrient transport [106]. The active ingredients of bioceramics promote cell differentiation and proliferation, which in turn promotes osteogenesis and angiogenesis, and ultimately contributes to bone healing and regeneration. However, it is difficult to meet the complex treatment needs of infected bone defects with only osteogenic and regenerative grafting materials, and bacterial infection may occur during bone repair or on artificial bone scaffolds. Osteomyelitis, osteoporosis, and other diseases caused by postoperative infection are serious sequelae of orthopedic diseases.
At present, an effective strategy for treating infectious bone defects after trauma is to combine antibacterial strategies based on different antibacterial mechanisms with various artificial bone composite materials and to develop a variety of artificial bone composite scaffolds with antibacterial functions as bone graft materials. The antibacterial function induced by antibiotics, mediated by metal ions, physical antibacterial function, nano-catalysis function, and natural antibacterial agent induced antibacterial function described in detail in this paper all have certain advantages under certain circumstances, but there are some limitations in their application to artificial bone composites. Although antibiotic induction is the most effective and widely employed antibacterial strategy in clinical practice, its extensive use can lead to the production of drug-resistant strains [107]. Therefore, the main challenge at present is to design alternative antibacterial strategies. While the continuous release of metal ions can achieve good antibacterial efficacy, the ions used are typically heavy metals, which pose biosafety risks and significant obstacles in clinical applications [108,109]. Physically activated antibacterial strategies have the potential to address the issue of drug-resistant bacteria, but further research is required to deepen our understanding. Additionally, nano-mediated antibacterial strategies and the incorporation of natural antibacterial agents also impact their biocompatibility [110]. Moreover, the antibacterial mechanism of most substances of natural antibacterial agents needs further study.
The development of infection-resistant artificial bone composites must be adapted to their specific clinical applications to achieve better resistance to infection while having safe biocompatibility [111]; therefore, it is important to strike a balance between antibacterial power and biocompatibility. In addition, the combined antibacterial strategy is a good way to enhance the antibacterial effect through the synergistic effect with a variety of strategies, and can effectively overcome the shortcomings of a single method with a comprehensive antibacterial [112]. However, due to the multiple strategies involved, it is challenging to design and prepare artificial bone scaffolders without compromising their bone-origin properties and high antibacterial efficiency.
Future Directions
In-depth research on the antibacterial mechanism of biomaterials and scaffold loading principles may yield significant breakthroughs in the treatment of tissue infections. Simultaneously, considering the synergistic effects of ion doping and release, drug loading, and release, as well as physical effects such as light, heat, and sound induction, is necessary to meet the requirements of practical applications. Additionally, the development of new antibacterial materials and conducting in vitro, in vivo, and clinical evaluations may provide valuable insights. Undoubtedly, endowing artificial bone composite material scaffolds with excellent antibacterial performance and biocompatibility will play a pivotal role in treating bone implant-related infections and ultimately promote bone repair.
Conclusions
This review examined the latest advancements and challenges in the realm of artificial composite bone scaffolds for preventing bone graft infection. By conducting a thorough analysis of diverse research findings, we presented a comprehensive scientific classification of antimicrobial strategies employed in artificial bone composites (antibiotics-induced strategy, inorganic antibacterial strategy, natural antibiotic-induced strategy, and combined bacteriostasis strategy), and also summarized the antibacterial strategies and activities associated with each category of artificial bone composites, as well as discussing the limitations and deficiencies of antibacterial strategies of artificial bone composites. This filling of knowledge gaps concerning antibacterial artificial bone scaffolds contributes to a more profound understanding and serves as a scientific foundation and a source of innovative ideas for the extensive development of ideal bone tissue regeneration and repair materials possessing effective antibacterial properties.
Figures
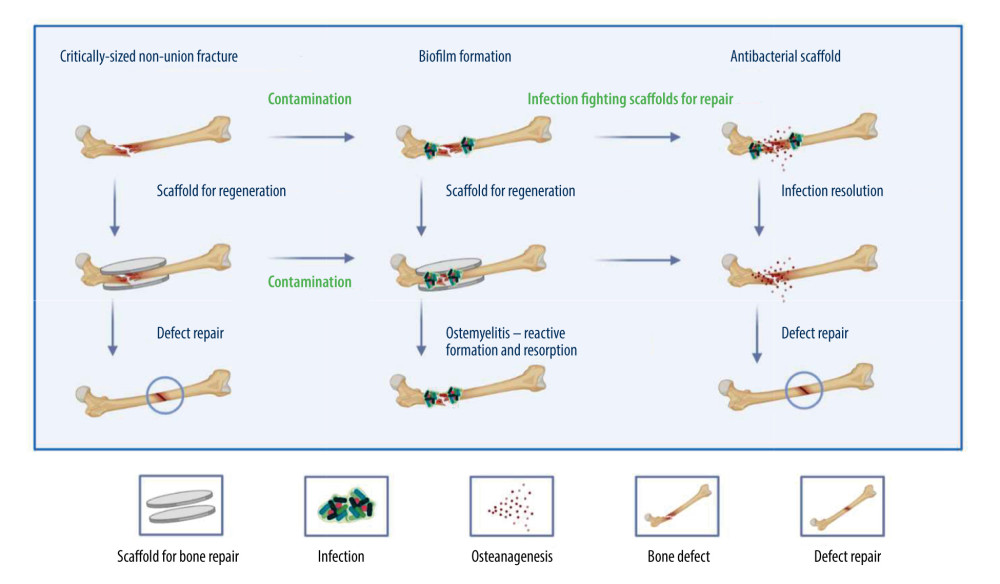 Figure 1. The therapeutic effectiveness of bone scaffolds is evaluated using different types of bone defects. Before or after the stent is placed in place, bacteria may invade to cause infection, and without antimicrobial treatment, osteomyelitis will develop. Osteomyelitis, which is characterized by bone resorption and reactive bone growth. Originally designed to stop bacterial colonization, infection-resistant scaffolds can also be used to remove existing biofilms and promote defect healing. Created with BioRender.com.
Figure 1. The therapeutic effectiveness of bone scaffolds is evaluated using different types of bone defects. Before or after the stent is placed in place, bacteria may invade to cause infection, and without antimicrobial treatment, osteomyelitis will develop. Osteomyelitis, which is characterized by bone resorption and reactive bone growth. Originally designed to stop bacterial colonization, infection-resistant scaffolds can also be used to remove existing biofilms and promote defect healing. Created with BioRender.com. 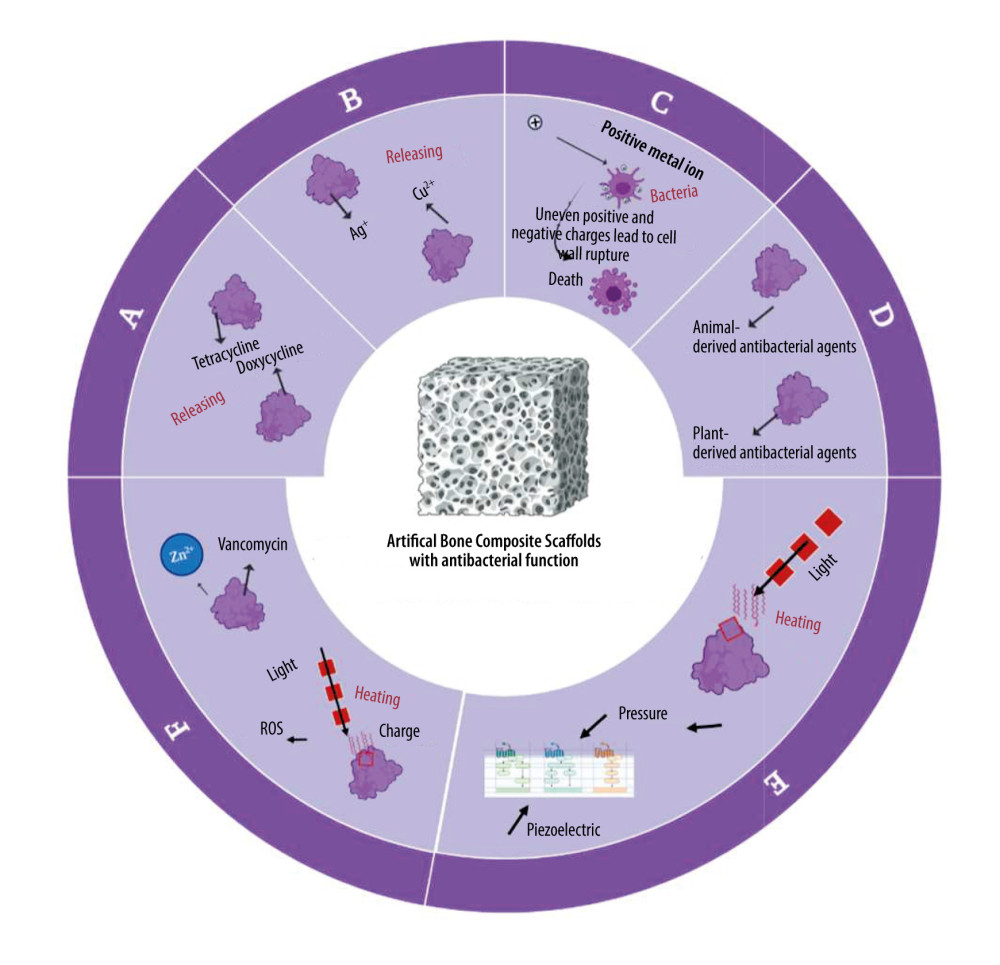 Figure 2. Antimicrobial systems for artificial bone composites depend on different antimicrobial strategies. A: antibiotics-induced strategy; B: ion-mediated strategy; C: physical strategy; D: natural antibiotic-induced strategy; E: NPs-mediated strategy; F: combined bacteriostasis strategy. Created with BioRender.com.
Figure 2. Antimicrobial systems for artificial bone composites depend on different antimicrobial strategies. A: antibiotics-induced strategy; B: ion-mediated strategy; C: physical strategy; D: natural antibiotic-induced strategy; E: NPs-mediated strategy; F: combined bacteriostasis strategy. Created with BioRender.com. 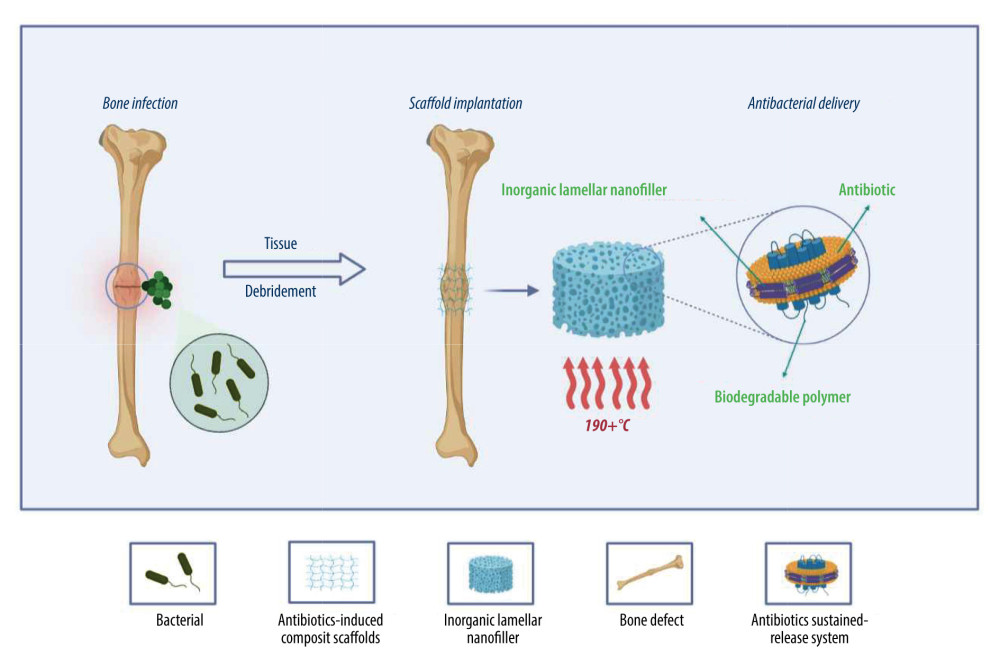 Figure 3. Simple antibacterial mechanism diagram and model of antibiotics-induced composite scaffolds with local sustained release system. Created with BioRender.com.
Figure 3. Simple antibacterial mechanism diagram and model of antibiotics-induced composite scaffolds with local sustained release system. Created with BioRender.com. 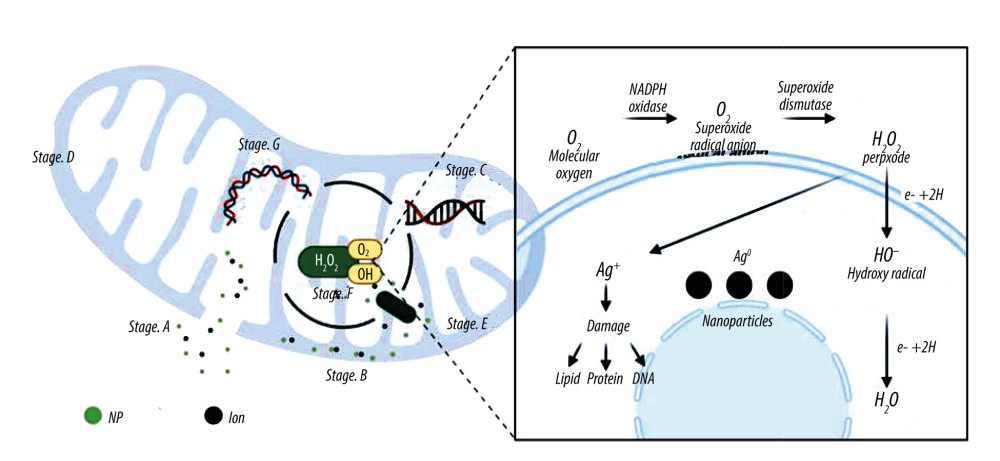 Figure 4. A model showing the cellular mechanism of Ag-NPs exposure to Gram-negative Escherichia coli. Stage A: The disintegration of the cell wall makes the intracellular components leave the cell; Stage B: Ag-NPs entered the cytoplasmic space and began to separate the cytoplasmic fluid from the cell membrane; Stage C: The interaction between Ag-NPs and DNA. Inhibition can lead to ROS production; Stage D: Cell pits appear after exposure; Stage E: Inhibition of normal ribosomal function leads to ROS production protein malformation or inhibition and improper DNA function; Stage F: Production of ROS; G: Interaction with proteins, specific cysteine. Created with BioRender.com.
Figure 4. A model showing the cellular mechanism of Ag-NPs exposure to Gram-negative Escherichia coli. Stage A: The disintegration of the cell wall makes the intracellular components leave the cell; Stage B: Ag-NPs entered the cytoplasmic space and began to separate the cytoplasmic fluid from the cell membrane; Stage C: The interaction between Ag-NPs and DNA. Inhibition can lead to ROS production; Stage D: Cell pits appear after exposure; Stage E: Inhibition of normal ribosomal function leads to ROS production protein malformation or inhibition and improper DNA function; Stage F: Production of ROS; G: Interaction with proteins, specific cysteine. Created with BioRender.com. ![Artificial bone scaffolds containing silver nanoparticles (Ag-NPs) were prepared by 3D printing (An experimental process, refer to[56]). Created with BioRender.com.](https://jours.isi-science.com/imageXml.php?i=medscimonit-29-e939972-g005.jpg&idArt=939972&w=1000) Figure 5. Artificial bone scaffolds containing silver nanoparticles (Ag-NPs) were prepared by 3D printing (An experimental process, refer to[56]). Created with BioRender.com.
Figure 5. Artificial bone scaffolds containing silver nanoparticles (Ag-NPs) were prepared by 3D printing (An experimental process, refer to[56]). Created with BioRender.com. 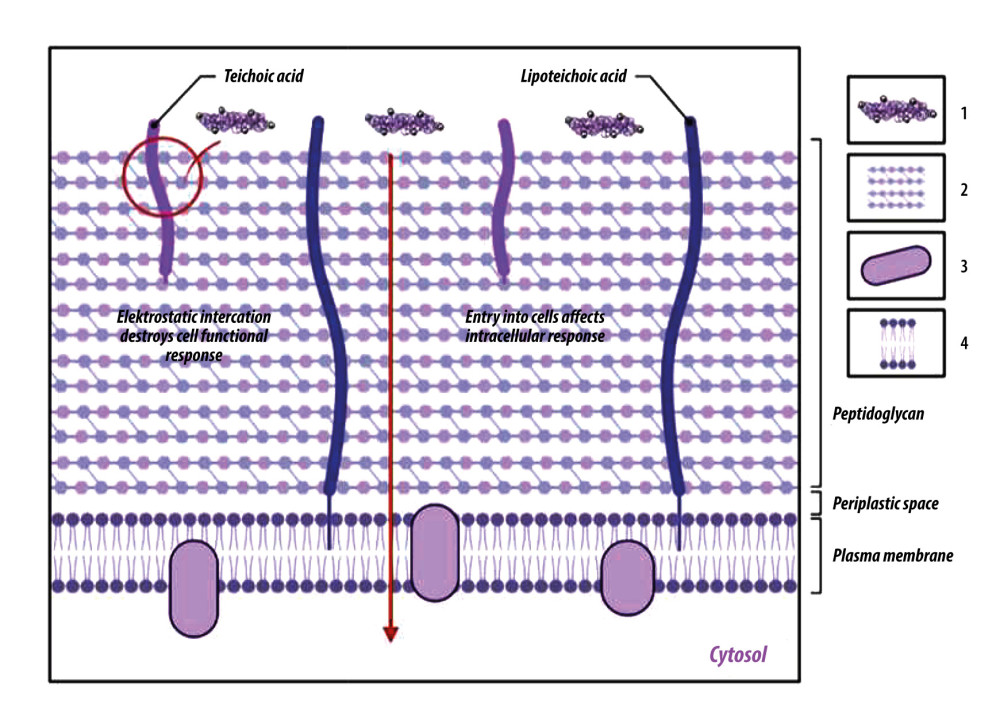 Figure 6. Chitosan antibacterial mechanism model. One is that chitosan particles contain a large amount of positively charged ammonia. These can be electrostatic connected with the negatively charged substances in the outer layer of bacterial cells, adsorbed on the cell surface to form a polymer film, prevent nutrients from entering the cell, and make the negative charge on the cell wall and membrane unbalanced, thus inhibiting cell growth. The other is to penetrate the cell, absorb the anionic substances in the cell, hinder the transcription of genetic material and protein translation, disturb the normal physiological activities of the cell, to achieve the antibacterial effect. 1: Chitosan; 2: Peptidoglycan layer; 3: Membrane protein; 4: Phospholipid. Created with BioRender.com.
Figure 6. Chitosan antibacterial mechanism model. One is that chitosan particles contain a large amount of positively charged ammonia. These can be electrostatic connected with the negatively charged substances in the outer layer of bacterial cells, adsorbed on the cell surface to form a polymer film, prevent nutrients from entering the cell, and make the negative charge on the cell wall and membrane unbalanced, thus inhibiting cell growth. The other is to penetrate the cell, absorb the anionic substances in the cell, hinder the transcription of genetic material and protein translation, disturb the normal physiological activities of the cell, to achieve the antibacterial effect. 1: Chitosan; 2: Peptidoglycan layer; 3: Membrane protein; 4: Phospholipid. Created with BioRender.com. References
1. Oryan A, Monazzah S, Bigham-Sadegh A, Bone injury and fracture healing biology: Biomed Environ Sci, 2015; 28(1); 57-71
2. Zhou CH, Ren Y, Song HJ, One-stage debridement and bone transport versus first-stage debridement and second-stage bone transport for the management of lower limb post-traumatic osteomyelitis: J Orthop Translat, 2021; 28; 21-27
3. Trampuz A, Widmer AF, Infections associated with orthopedic implants: Curr Opin Infect Dis, 2006; 19(4); 349-56
4. Johnson CT, García AJ, Scaffold-based anti-infection strategies in bone repair: Ann Biomed Eng, 2015; 43(3); 515-28
5. Riool M, de Boer L, Jaspers V: Acta Biomater, 2014; 10(12); 5202-12
6. Zimmerli W, Clinical presentation and treatment of orthopaedic implant-associated infection: J Intern Med, 2014; 276(2); 111-19
7. Darouiche RO, Treatment of infections associated with surgical implants: N Engl J Med, 2004; 350(14); 1422-29
8. Ramage G, Tunney MM, Patrick S: Biomaterials, 2003; 24(19); 3221-27
9. Lew DP, Waldvogel FA, Osteomyelitis: Lancet, 2004; 364(9431); 369-79
10. Lazzarini L, Mader JT, Calhoun JH, Osteomyelitis in long bones: J Bone Joint Surg Am, 2004; 86(10); 2305-18
11. Wang X, Wang Z, Fu J, Huang K, Xie Z, Induced membrane technique for the treatment of chronic hematogenous tibia osteomyelitis: BMC Musculoskelet Disord, 2017; 18(1); 33
12. Tong K, Zhong Z, Peng Y, Masquelet technique versus Ilizarov bone transport for reconstruction of lower extremity bone defects following posttraumatic osteomyelitis: Injury, 2017; 48(7); 1616-22
13. Helbig L, Bechberger M, Aldeeri R, Initial peri- and postoperative antibiotic treatment of infected nonunions: results from 212 consecutive patients after mean follow-up of 34 months: Ther Clin Risk Manag, 2018; 14; 59-67
14. Bari M, Shahidul I, Huda SN, Rahman M, Management of forearm bone gap non-unions by Ilizarov technique: Orthopedics and Rheumatology Open Access Journals, 2016; 3(3); 39-41
15. Yin P, Zhang L, Zhang L, Ilizarov bone transport for the treatment of fibular osteomyelitis: A report of five cases: BMC Musculoskelet Disord, 2015; 16; 242
16. Shuaishuai W, Tongtong Z, Dapeng W, Implantable biomedical materials for treatment of bone infection: Front Bioeng Biotechnol, 2023; 11; 1081446
17. Kasis AG, Jensen C, Dharmadhikari R, Novel bone grafting technique in stand-alone ALIF procedure combining allograft and autograft (‘Northumbria Technique’) –fusion rate and functional outcomes in 100 consecutive patients: Eur Spine J, 2021; 30(5); 1296-302
18. Heidt ST, Louie PK, Khan JM, Comparing allografts to autografts for maintenance of cervical sagittal parameters and clinical outcomes following anterior cervical discectomy and fusion with anterior cervical plating: Neurospine, 2019; 16(3); 618-25
19. Flifl MAS, Marzook H, Denewar M, Elsheikh HA, Biological impact of alloplastic bone graft vs bovine xenograft and allograft materials in bone healing: An experimental study: J Contemp Dent Pract, 2022; 23(5); 482-91
20. Freischmidt H, Armbruster J, Rothhaas C, Efficacy of an antibiotic loaded ceramic-based bone graft substitute for the treatment of infected non-unions: Biomedicines, 2022; 10(10); 2513
21. Deng C, Chang J, Wu C, Bioactive scaffolds for osteochondral regeneration: J Orthop Translat, 2019; 17; 15-25
22. Parai R, Bandyopadhyay-Ghosh S, Engineered bio-nanocomposite magnesium scaffold for bone tissue regeneration: J Mech Behav Biomed Mater, 2019; 96; 45-52
23. Lee DH, Tripathy N, Shin JH, Enhanced osteogenesis of β-tricalcium phosphate reinforced silk fibroin scaffold for bone tissue biofabrication: Int J Biol Macromol, 2017; 95; 14-23
24. Jodati H, Yılmaz B, Evis Z, A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features: Ceramics International, 2020; 46(10); 15725-39
25. Zhao C, Liu W, Zhu M, Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review: Bioact Mater, 2022; 18; 383-98
26. Jodati H, Yilmaz B, Evis Z, In vitro and in vivo properties of graphene-incorporated scaffolds for bone defect repair: Ceramics International, 2021; 47(21); 29535-49
27. Zhang J, Feng Y, Zhou X, Research status of artificial bone materials: International Journal of Polymeric Materials and Polymeric Biomaterials, 2021; 70(1); 37-53
28. Parai R, Bandyopadhyay-Ghosh S, Engineered bio-nanocomposite magnesium scaffold for bone tissue regeneration: J Mech Behav Biomed Mater, 2019; 96; 45-52
29. Wenzhan H, Hongjie L, Yongliang M, Jianrong X, Preparation and in vitro degradation behavior of Mg-based foam biomaterials for bone tissue engineering: Rare Metal Materials and Engineering, 2019; 48(10); 3081-87
30. Gu X, Xie X, Li N, Zheng Y, Qin L, In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal: Acta Biomater, 2012; 8(6); 2360-74
31. Witte F, Fischer J, Nellesen J, In vivo corrosion and corrosion protection of magnesium alloy LAE442: Acta Biomater, 2010; 6(5); 1792-99
32. Danoux CB, Barbieri D, Yuan H, In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration: Biomatter, 2014; 4(1); e27664
33. Moonesi Rad R, Pazarçeviren E, Ece Akgün E, In vitro performance of a nanobiocomposite scaffold containing boron-modified bioactive glass nanoparticles for dentin regeneration: J Biomater Appl, 2019; 33(6); 834-53
34. Alshemary AZ, Pazarçeviren AE, Keskin D, Porous clinoptilolite-nano biphasic calcium phosphate scaffolds loaded with human dental pulp stem cells for load bearing orthopedic applications: Biomed Mater, 2019; 14(5); 055010
35. Dalgic AD, Alshemary AZ, Tezcaner A, Silicate-doped nano-hydroxyapatite/graphene oxide composite reinforced fibrous scaffolds for bone tissue engineering: J Biomater Appl, 2018; 32(10); 1392-405
36. Moonesi Rad R, Alshemary AZ, Evis Z, Cellulose acetate-gelatin-coated boron-bioactive glass biocomposite scaffolds for bone tissue engineering: Biomed Mater, 2020; 15(6); 065009
37. Jodati H, Evis Z, Tezcaner A, 3D porous bioceramic based boron-doped hydroxyapatite/baghdadite composite scaffolds for bone tissue engineering: J Mech Behav Biomed Mater, 2023; 140; 105722
38. Moonesi Rad R, Atila D, Akgün EE, Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics: Mater Sci Eng C Mater Biol Appl, 2019; 100; 928-48
39. Morejón Alonso L, Fernández Torres I, Zayas Tamayo Á M, Antibacterial effect of acrylic bone cements loaded with drugs of different action’s mechanism: J Infect Dev Ctries, 2019; 13(6); 487-95
40. Cobb LH, McCabe EM, Priddy LB, Therapeutics and delivery vehicles for local treatment of osteomyelitis: J Orthop Res, 2020; 38(10); 2091-103
41. Abushaheen MA, Fatani AJ, Alosaimi M, Antimicrobial resistance, mechanisms and its clinical significance: Dis Mon, 2020; 66(6); 100971
42. Vázquez-Laslop N, Mankin AS, How macrolide antibiotics work: Trends Biochem Sci, 2018; 43(9); 668-84
43. Otsuka Y, Potent antibiotics active against multidrug-resistant gram-negative bacteria: Chem Pharm Bull (Tokyo), 2020; 68(3); 182-90
44. Lu H, Liu Y, Guo J, Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects: Int J Mol Sci, 2016; 17(3); 334
45. Vallet-Regí M, Lozano D, González B, Izquierdo-Barba I, Bone infections: Biomaterials against bone infection: Adv Healthc Mater, 2020; 9(13); 2070039
46. Kuang Z, Dai G, Wan R, Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold: Genes Dis, 2021; 8(2); 193-202
47. Manchón A, Alkhraisat MH, Rueda-Rodriguez C, Silicon bioceramic loaded with vancomycin stimulates bone tissue regeneration: J Biomed Mater Res B Appl Biomater, 2018; 106(6); 2307-15
48. Bakhsheshi-Rad H, Hamzah E, Ismail A, Novel nanostructured baghdadite-vancomycin scaffolds: In-vitro drug release, antibacterial activity and biocompatibility: Materials Letters, 2017; 209; 369-72
49. Semyari H, Salehi M, Taleghani F, Fabrication and characterization of collagen-hydroxyapatite-based composite scaffolds containing doxycycline via freeze-casting method for bone tissue engineering: J Biomater Appl, 2018; 33(4); 501-13
50. Kargozar S, Montazerian M, Hamzehlou S, Mesoporous bioactive glasses: Promising platforms for antibacterial strategies: Acta Biomaterialia, 2018; 81; 1-19
51. Unnithan AR, Arathyram R, Kim CS, Scaffolds with antibacterial properties: Nanotechnology Applications for Tissue Engineering, 2015; 103-23
52. Kumar Saini R, Prasad Bagri L, Bajpai AK, Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications: Colloids Surf B Biointerfaces, 2019; 177; 211-18
53. Shuai C, Xu Y, Feng P, Wang G, Xiong S, Peng S, Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver: Chemical Engineering Journal, 2019; 374; 304-15
54. Yazdimamaghani M, Vashaee D, Assefa S, Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering: J Biomed Nanotechnol, 2014; 10(6); 911-31
55. Zhang Y, Zhai D, Xu M, 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity: Biofabrication, 2017; 9(2); 025037
56. Jiang J, Li L, Li K, Antibacterial nanohydroxyapatite/polyurethane composite scaffolds with silver phosphate particles for bone regeneration: J Biomater Sci Polym Ed, 2016; 27(16); 1584-98
57. Erol M, Mouriňo V, Newby P, Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering: Acta Biomaterialia, 2012; 8(2); 792-801
58. Azeena S, Subhapradha N, Selvamurugan N, Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering: Mater Sci Eng C Mater Biol Appl, 2017; 71; 1156-65
59. Baino F, Potestio I, Vitale-Brovarone C, Production and physicochemical characterization of Cu-doped silicate bioceramic scaffolds: Materials, 2018; 11(9); 1524
60. Kaya S, Cresswell M, Boccaccini AR, Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications: Mater Sci Eng C Mater Biol Appl, 2018; 83; 99-107
61. Wu C, Zhou Y, Xu M, Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity: Biomaterials, 2013; 34(2); 422-33
62. Alavi M, Nokhodchi A, An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers: Carbohydr Polym, 2020; 227; 115349
63. Li C, Ai F, Miao X, “The return of ceramic implants”: Rose stem inspired dual layered modification of ceramic scaffolds with improved mechanical and anti-infective properties: Mater Sci Eng C Mater Biol Appl”, 2018; 93; 873-79
64. Felice B, Sánchez MA, Socci MC, Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity: Mater Sci Eng C Mater Biol Appl, 2018; 93; 724-38
65. Applerot G, Lipovsky A, Dror R, Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury: Advanced Functional Materials, 2009; 19(6); 842-52
66. Zhang Z, Wang Y, Teng W, An orthobiologics-free strategy for synergistic photocatalytic antibacterial and osseointegration: Biomaterials, 2021; 274; 120853
67. Saxena V, Hasan A, Pandey LM, Effect of Zn/ZnO integration with hydroxyapatite: A review: Materials Technology, 2018; 33(2); 79-92
68. Zhu T, Zhu M, Zhu Y, Fabrication of forsterite scaffolds with photothermal-induced antibacterial activity by 3D printing and polymer-derived ceramics strategy: Ceramics International, 2020; 46(9); 13607-14
69. Bigham A, Aghajanian AH, Behzadzadeh S: Mater Sci Eng C Mater Biol Appl, 2019; 99; 83-95
70. Wiedmer D, Cui C, Weber F, Antibacterial surface coating for bone scaffolds based on the dark catalytic effect of titanium dioxide: ACS Appl Mater Interfaces, 2018; 10(42); 35784-93
71. Hu D, Deng Y, Jia F, Jin Q, Ji J, Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms: Acs Nano, 2019; 14(1); 347-59
72. Wang Y, Yang Y, Shi Y, Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives: Adv Mater, 2020; 32(18); 1904106
73. Wang R, Shi M, Xu F, Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection: Nat Commun, 2020; 11(1); 4465
74. Zhang G, Yang Y, Shi J, Near-infrared light II-assisted rapid biofilm elimination platform for bone implants at mild temperature: Biomaterials, 2021; 269; 120634
75. Yao T, Chen J, Wang Z, The antibacterial effect of potassium-sodium niobate ceramics based on controlling piezoelectric properties: Colloids Surf B Biointerfaces, 2019; 175; 463-68
76. Sugimoto H, Biggemann J, Fey T, Lead-free piezoelectric (Ba, Ca)(Ti, Zr) O3 scaffolds for enhanced antibacterial property: Materials Letters, 2021; 297; 129969
77. Zhao B, Wang H, Dong W, A multifunctional platform with single-NIR-laser-triggered photothermal and NO release for synergistic therapy against multidrug-resistant Gram-negative bacteria and their biofilms: J Nanobiotechnology, 2020; 18(1); 59
78. Kawano N, Miura H, Kamo YStudies on the components of thumb. II. On root components: Yakugaku Zasshi, 1967; 87(9); 1146-48 [in Japanese]
79. Long YM, Zhao XC, Clermont AC, Negatively charged silver nanoparticles cause retinal vascular permeability by activating plasma contact system and disrupting adherens junction: Nanotoxicology, 2016; 10(4); 501-11
80. Slavin YN, Asnis J, Hńfeli UO, Bach H, Metal nanoparticles: Understanding the mechanisms behind antibacterial activity: J Nanobiotechnology, 2017; 15; 65
81. Xu J-W, Yao K, Xu Z-K, Nanomaterials with a photothermal effect for antibacterial activities: An overview: Nanoscale, 2019; 11(18); 8680-91
82. Kim JS, Kuk E, Yu KN, Antimicrobial effects of silver nanoparticles: Nanomedicine, 2007; 3(1); 95-101
83. Niu W, Chen M, Guo Y, A multifunctional bioactive glass-ceramic nanodrug for post-surgical infection/cancer therapy-tissue regeneration: ACS Nano, 2021; 15(9); 14323-37
84. Gao X, Ding J, Liao C, Defensins: The natural peptide antibiotic: Adv Drug Deliv Rev, 2021; 179; 114008
85. Browne K, Chakraborty S, Chen R, A new era of antibiotics: The clinical potential of antimicrobial peptides: Int J Mol Sci, 2020; 21(19); 7047
86. Xiao L, Li L, Li L, Exploitation of natural antimicrobial agents: China Surfactant Deterg Cosmet, 2002; 78-81
87. Xiao H, Li Y, Lin Q, Advances in multiple targets mechanism of antimicrobial peptides: J Food Sci Biotechnol, 2022; 5; 11-19
88. Chen Y, Xu J, Qv Y, Xi G, Zhang M, Study on bacteria-inhibiting effect of amino acids: Chin J Biochem Pharm, 2001; 29-30
89. Yu KH, Kim KN, Lee JH: Dev Comp Immunol, 2002; 26(8); 707-13
90. Lockey TD, Ourth DD: Biochem Biophys Res Commun, 1996; 220(3); 502-8
91. Hu T, Abbah SA, Wang M, Novel protamine-based polyelectrolyte carrier enhances low-dose rhBMP-2 in posterolateral spinal fusion: Spine, 2015; 40(9); 613-21
92. Hu L, Xing R, Liu S, Qin Y, Chen X, Research progress on chitosan derivatives and its antibacterial activity: Plant Prot, 2015; 41; 16-18
93. Yilmaz Atay H, Antibacterial activity of chitosan-based systems: Functional Chitosan: Drug Delivery and Biomedical Applications, 2019; 457-89
94. Khattak S, Wahid F, Liu L-P, Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives: Appl Microbiol Biotechnol, 2019; 103; 1989-2006
95. Yang Y, Yang S, Wang Y, Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan: Acta Biomaterialia, 2016; 46; 112-28
96. Efenberger-Szmechtyk M, Nowak A, Czyzowska A, Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products: Crit Rev Food Sci Nutr, 2021; 61(1); 149-78
97. Azeem M, Hanif M, Mahmood K, An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review: Polymer Bull (Berl), 2023; 80(1); 241-62
98. Salerno A, Dieguez S, Diaz-Gomez L, Synthetic scaffolds with full pore interconnectivity for bone regeneration prepared by supercritical foaming using advanced biofunctional plasticizers: Biofabrication, 2017; 9(3); 035002
99. Wang W, Yang J, Zhang J, An Arabidopsis secondary metabolite directly targets expression of the bacterial type III secretion system to inhibit bacterial virulence: Cell Host Microbe, 2020; 27(4); 601-13e7
100. Huang D, Zuo Y, Zou Q, Antibacterial chitosan coating on nano-hydroxyapatite/polyamide66 porous bone scaffold for drug delivery: J Biomater Sci Polym Ed, 2011; 22(7); 931-44
101. Sun X, Zhao L, Hu YStudy on in vivo drug delivery and repairing large segmental infected bony defect with massive reconstituted bovine xenograft aided by calcium phosphate cement drug core: Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2005; 19(3); 165-69 [in Chinese]
102. Wang Z, Wang A, Tang GEffect of vancomycin-loaded polymethylmethacrylate on one-stage revision arthroplasty in treating experimental hemiprosthetic hip infections of rabbits: Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2006; 20(6); 634-39 [in Chinese]
103. He X, Liu Y, Tan Y, Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity: Mater Sci Eng C Mater Biol Appl, 2019; 105; 110155 [Retracted article]
104. Ramesh S, Kovelakuntla V, Meyer AS, Rivero IV, Three-dimensional printing of stimuli-responsive hydrogel with antibacterial activity: Bioprinting, 2021; 24; e00106
105. Zhang W, Sun T, Zhang J, Construction of artificial periosteum with methacrylamide gelatin hydrogel-Wharton’s jelly based on stem cell recruitment and its application in bone tissue engineering: Mater Today Bio, 2023; 18; 100528
106. Tajbakhsh S, Hajiali F, A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering: Mater Sci Eng C Mater Biol Appl, 2017; 70(Pt 1); 897-912
107. Händel N, Schuurmans JM, Brul S, ter Kuile BH: Antimicrob Agents Chemother, 2013; 57(8); 3752-62
108. Ning C, Wang X, Li L, Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism: Chem Res Toxicol, 2015; 28(9); 1815-22
109. Tang X, Hu W, Ke X, Antibacterial and desalting behavior of forward osmosis membranes engineered with metal ions: Desalination, 2022; 530; 115655
110. Khan S, Cho WC, Jaragh-Alhadad LA, Nano-bio interaction: An overview on the biochemical binding of DNA to inorganic nanoparticles for the development of anticancer and antibacterial nano-platforms: Int J Biol Macromol, 2023; 225; 544-56
111. Campoccia D, Montanaro L, Arciola CR, A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces: Biomaterials, 2013; 34(33); 8018-29
112. Blair M, Côté JM, Cotter A, Nephrotoxicity from vancomycin combined with piperacillin-tazobactam: A comprehensive review: Am J Nephrol, 2021; 52(2); 85-97
Figures
 Figure 1. The therapeutic effectiveness of bone scaffolds is evaluated using different types of bone defects. Before or after the stent is placed in place, bacteria may invade to cause infection, and without antimicrobial treatment, osteomyelitis will develop. Osteomyelitis, which is characterized by bone resorption and reactive bone growth. Originally designed to stop bacterial colonization, infection-resistant scaffolds can also be used to remove existing biofilms and promote defect healing. Created with BioRender.com.
Figure 1. The therapeutic effectiveness of bone scaffolds is evaluated using different types of bone defects. Before or after the stent is placed in place, bacteria may invade to cause infection, and without antimicrobial treatment, osteomyelitis will develop. Osteomyelitis, which is characterized by bone resorption and reactive bone growth. Originally designed to stop bacterial colonization, infection-resistant scaffolds can also be used to remove existing biofilms and promote defect healing. Created with BioRender.com. Figure 2. Antimicrobial systems for artificial bone composites depend on different antimicrobial strategies. A: antibiotics-induced strategy; B: ion-mediated strategy; C: physical strategy; D: natural antibiotic-induced strategy; E: NPs-mediated strategy; F: combined bacteriostasis strategy. Created with BioRender.com.
Figure 2. Antimicrobial systems for artificial bone composites depend on different antimicrobial strategies. A: antibiotics-induced strategy; B: ion-mediated strategy; C: physical strategy; D: natural antibiotic-induced strategy; E: NPs-mediated strategy; F: combined bacteriostasis strategy. Created with BioRender.com. Figure 3. Simple antibacterial mechanism diagram and model of antibiotics-induced composite scaffolds with local sustained release system. Created with BioRender.com.
Figure 3. Simple antibacterial mechanism diagram and model of antibiotics-induced composite scaffolds with local sustained release system. Created with BioRender.com. Figure 4. A model showing the cellular mechanism of Ag-NPs exposure to Gram-negative Escherichia coli. Stage A: The disintegration of the cell wall makes the intracellular components leave the cell; Stage B: Ag-NPs entered the cytoplasmic space and began to separate the cytoplasmic fluid from the cell membrane; Stage C: The interaction between Ag-NPs and DNA. Inhibition can lead to ROS production; Stage D: Cell pits appear after exposure; Stage E: Inhibition of normal ribosomal function leads to ROS production protein malformation or inhibition and improper DNA function; Stage F: Production of ROS; G: Interaction with proteins, specific cysteine. Created with BioRender.com.
Figure 4. A model showing the cellular mechanism of Ag-NPs exposure to Gram-negative Escherichia coli. Stage A: The disintegration of the cell wall makes the intracellular components leave the cell; Stage B: Ag-NPs entered the cytoplasmic space and began to separate the cytoplasmic fluid from the cell membrane; Stage C: The interaction between Ag-NPs and DNA. Inhibition can lead to ROS production; Stage D: Cell pits appear after exposure; Stage E: Inhibition of normal ribosomal function leads to ROS production protein malformation or inhibition and improper DNA function; Stage F: Production of ROS; G: Interaction with proteins, specific cysteine. Created with BioRender.com.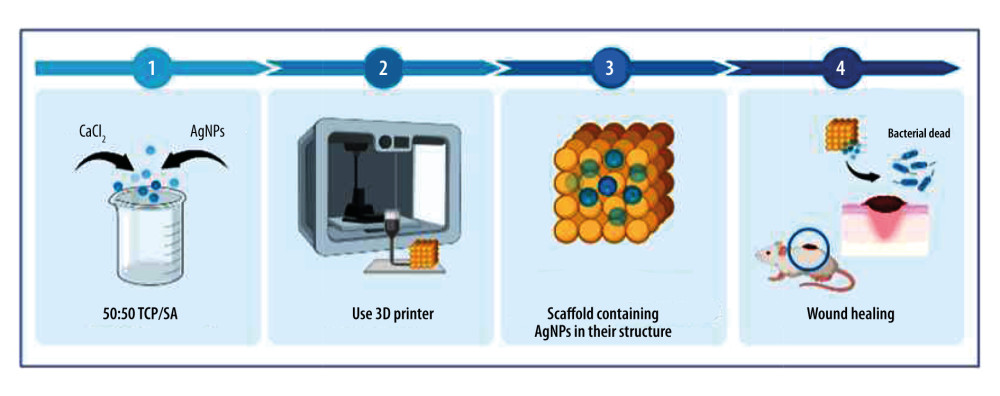 Figure 5. Artificial bone scaffolds containing silver nanoparticles (Ag-NPs) were prepared by 3D printing (An experimental process, refer to[56]). Created with BioRender.com.
Figure 5. Artificial bone scaffolds containing silver nanoparticles (Ag-NPs) were prepared by 3D printing (An experimental process, refer to[56]). Created with BioRender.com. Figure 6. Chitosan antibacterial mechanism model. One is that chitosan particles contain a large amount of positively charged ammonia. These can be electrostatic connected with the negatively charged substances in the outer layer of bacterial cells, adsorbed on the cell surface to form a polymer film, prevent nutrients from entering the cell, and make the negative charge on the cell wall and membrane unbalanced, thus inhibiting cell growth. The other is to penetrate the cell, absorb the anionic substances in the cell, hinder the transcription of genetic material and protein translation, disturb the normal physiological activities of the cell, to achieve the antibacterial effect. 1: Chitosan; 2: Peptidoglycan layer; 3: Membrane protein; 4: Phospholipid. Created with BioRender.com.
Figure 6. Chitosan antibacterial mechanism model. One is that chitosan particles contain a large amount of positively charged ammonia. These can be electrostatic connected with the negatively charged substances in the outer layer of bacterial cells, adsorbed on the cell surface to form a polymer film, prevent nutrients from entering the cell, and make the negative charge on the cell wall and membrane unbalanced, thus inhibiting cell growth. The other is to penetrate the cell, absorb the anionic substances in the cell, hinder the transcription of genetic material and protein translation, disturb the normal physiological activities of the cell, to achieve the antibacterial effect. 1: Chitosan; 2: Peptidoglycan layer; 3: Membrane protein; 4: Phospholipid. Created with BioRender.com. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








