10 October 2023: Lab/In Vitro Research
Diagnostic Value of Synovial Fluid Biomarkers for Periprosthetic Joint Infection: A Prospective, Double-blind Trial
Ying Xu123BCDE, Xueting Ma4BCD, Haoran Guo135CF, Hairong Tang1BF, Jiayu Liu1CD, Chengbin Wang163AG*, Chi Wang73ADFDOI: 10.12659/MSM.940842
Med Sci Monit 2023; 29:e940842
Abstract
BACKGROUND: This prospective, double-blind study investigated the clinical diagnostic value of synovial fluid S100 calcium-binding protein A8 (S100A8) and S100 calcium-binding protein A9 (S100A9) in periprosthetic joint infection (PJI) and investigated the subtypes of a-defensin that have diagnostic value for PJI.
MATERIAL AND METHODS: Synovial fluid samples were collected from 82 patients with suspected PJI after total joint arthroplasty. Patients were divided into a PJI group (n=39) and non-PJI group (n=43). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was used to determine S100A8, S100A9, α-defensin, and internal reference standards in synovial fluid. The receiver operating characteristic (ROC) curve was used to analyze the diagnostic efficiency of S100A8, S100A9, and α-defensin for PJI, as well as the diagnostic value in combination with common biomarkers of infection.
RESULTS: S100A8, 3 variants of S100A9, and 3 α-defensins (human neutrophil peptides [HNP]1-3) in synovial fluid were significantly higher in the PJI group than in the non-PJI group (P<0.001). The sensitivity, specificity, and the area under ROC curve (AUC) for diagnosing PJI were 97.4%, 86.0%, and 0.964 (95% CI: 0.929-0.998), respectively, for synovial fluid S100A8; 87.2%, 88.4% and 0.902 (95% CI: 0.823-0.980), respectively, for S100A9; and 89.7%, 83.7%, and 0.933 (95% CI: 0.884-0.982), respectively, for HNP1-3. The diagnostic efficiency was improved when combined with synovial fluid white blood cell count and percentage of polymorphonuclear neutrophils.
CONCLUSIONS: Synovial fluid S100A8, S100A9, and HNP1-3 have satisfactory diagnostic efficiency for the diagnosis of PJI, which will help clinicians to accurately diagnose PJI.
Keywords: alpha-Defensins, Arthroplasty, Replacement, Bacterial Infections, S100A9 Protein, Human, S100A8 Protein, Human, Humans, Synovial Fluid, Prosthesis-Related Infections, Prospective Studies, Double-Blind Method, biomarkers, Arthritis, Infectious, Calcium-Binding Proteins, Sensitivity and Specificity, Arthroplasty, Replacement, Hip
Background
Periprosthetic joint infection (PJI) is one of the most challenging and devastating complications after total joint arthroplasty The incidences of PJI were reported as 0.3% to 1.7% after total hip joint arthroplasty and 0.8% to 1.9% after total knee joint arthroplasty; moreover, the incidence of reinfection was 14% [1]. Typical postoperative symptoms of PJI include pain, fever, swelling, prolonged postoperative wound effusion, and impaired joint function [2]. However, the symptoms of patients with early and intermediate infections (<3 months or 3–12 months after surgery) are often not apparent [2].
Accurate and early diagnosis of PJI is essential to guide treatment, but no single diagnostic tool currently exists to independently diagnose all cases of PJI [3]. Diagnosis of PJI usually relies on a combination of culture of synovial fluid or tissue, clinical symptoms, and changes in blood and synovial fluid biomarkers [4]. An elevated synovial fluid white blood cell count (SF-WBC), percentage of polymorphonuclear neutrophil (SF-PMN), erythrocyte sedimentation rate (ESR), and serum C-reactive protein (CRP) level have recently been suggested as useful biomarkers for the diagnosis of PJI [5]. In addition, novel biomarkers with potential diagnostic efficiency for PJI, such as D-lactate [6], lipocalin-2 [7], and synovial fluid myeloperoxidase [8], are also under exploration. Therefore, investigation of novel biomarkers with higher diagnostic efficiency is crucial for the accurate diagnosis of PJI.
In our previous study, we applied the proteomics of synovial fluid through liquid chromatography-tandem mass spectrometry (LC-MS/MS) and found that differentially expressed proteins, including S100 calcium-binding protein A8 (S100A8) and S100 calcium-binding protein A9 (S100A9), could be promising biomarkers for the diagnosis of PJI [9]. S100A8 and S100A9 are members of the S100 calcium-binding protein family and are mainly expressed on neutrophils and monocytes/macrophages [10]. S100A8 and S100A9 are involved in inflammatory regulation, and their expression levels are associated with acute and chronic inflammatory diseases [11]. This study will further explore the diagnostic value of synovial fluid S100A8 and S100A9 for PJI.
Recent evidence suggests that α-defensin shows an increasing trend in patients with PJI [12]. There are 6 subtypes of α-defensin, 4 of which are mainly released by human neutrophils and are also known as human neutrophil peptides (HNP) 1–4, while the release of α-defensin 5 and α-defensin 6 is associated with Paneth cells [13]. Currently, few studies have explored the subtypes of α-defensin implicated in the diagnosis of PJI, so this study will make a preliminary exploration in this field.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) is a “top-down” mass spectrometry technique that analyzes intact proteins in samples that have not been digested, which allows for the detection of protein variants, such as the location and identity of post-translational modifications [14]. MALDI-TOF-MS detects proteins in samples based on differences in the mass-to-charge ratio (m/z) of proteins and generates mass spectrum, and semi-quantitative detection of proteins can be achieved by adding internal reference standards [15].
Therefore, in this study, we first examined S100A8, S100A9, and α-defensin in the synovial fluid of patients diagnosed with PJI and non-PJI after total joint arthroplasty using MALDI-TOF-MS and investigated the presence of variants of these 3 proteins using the unique advantages of MALDI-TOF-MS. We aimed to investigate the clinical diagnostic value of synovial fluid S100A8 and S100A9 for PJI, as well as that of the subtypes of α-defensin that play a key role in the diagnosis of PJI, in order to provide more valuable biomarkers for the early diagnosis of PJI.
Material and Methods
STUDY DESIGN:
This prospective, double-blind trial was conducted at the First Medical Center of Chinese PLA General Hospital, Beijing, China. This study was approved by the Ethics Committee of the Chinese PLA General Hospital (S2022-271-01), and all participants signed an informed consent form.
STUDY PARTICIPANTS:
Patients diagnosed with PJI and non-PJI after total joint arthroplasty at the First Medical Center of Chinese PLA General Hospital from July 2021 to March 2022 were included in this study. Each enrolled patient was numbered in a double-blind manner. The patients were randomly selected according to the number of cases required for the study, and their clinical samples and data were collected.
INCLUSION AND EXCLUSION CRITERIA:
The inclusion criteria were as follows: (1) age >18 years; and (2) patients after hip and knee arthroplasty with suspected PJI based on signs, symptoms, and test results. Exclusion criteria were as follows: (1) patients with systemic or other infections; (2) patients with gout, rheumatoid arthritis, ankylosing spondylitis, and other metabolic and autoimmune diseases; and (3) patients with liver and kidney disease, lung disease, and recent active heart disease.
PJI DIAGNOSTIC CRITERIA:
Criteria proposed by the Musculoskeletal Infection Society (MSIS) in 2011 were used as the criterion standard for the diagnosis of PJI [16]: (1) There is a sinus tract communicating with the prosthesis; or (2) a pathogen is isolated by culture from at least 2 separate tissue or fluid samples obtained from the affected prosthetic joint; or (3) four of the following 6 criteria were met: elevated ESR and serum CRP concentration; elevated SF-WBC; elevated SF-PMN; presence of purulence in the affected joint; isolation of a microorganism in 1 culture of periprosthetic tissue or fluid; greater than 5 neutrophils per high-power field in 5 high-power fields observed from histologic analysis of periprosthetic tissue at ×400 magnification. Patients who met the criteria were assigned to the PJI group, and those who did not meet the criteria were assigned to the non-PJI group.
COLLECTION OF SAMPLES:
Patients’ data were collected through the hospital information system of our hospital, including patients’ age, sex, medical history, and other information. Synovial fluid and peripheral blood samples were collected from patients who met the inclusion and exclusion criteria and were numbered following the double-blind principle.
Synovial fluid samples were divided into 3 parts. The first part was centrifuged at 3000 rpm for 5 min, and the supernatant was dispensed into tubes and stored at −80°C for MALDI-TOF-MS detection. The second part was injected into blood culture bottles for aerobic, anaerobic, and fungal culture. Finally, the third part was used to test the SF-WBC and SF-PMN.
Peripheral blood was collected using 1: 4 sodium citrate anticoagulation tubes, EDTA-K3 anticoagulation tubes, and 1: 9 sodium citrate anticoagulation tubes for the detection of ESR, white blood cell count (WBC), CRP, plasma interleukin-6, plasma D-dimer, and plasma fibrinogen, respectively.
MALDI-TOF-MS DETECTION:
The synovial fluid S100A8, S100A9, and α-defensin were measured by MALDI-TOF-MS (Intelligene Biosystems (Qingdao) Co., Ltd., Beijing, China). The S100A8-His recombinant protein (11138-H08B, Sino Biological, Beijing, China) was chosen as the internal reference standards, m/z=12249. Erucic acid matrix was provided by Intelligene Biosystems. First, the internal reference standards stock solution was diluted 10-fold with 25 mM NH4HCO3. Then, 2 μL synovial fluid and 5 μL internal reference standards diluent were added to a 96-well plate, and after mixing, 2 μL of the mixture was added to a new 96-well plate. Then, 5 μL erucic acid matrix was added, mixed well, and 2 μL of the new mixture was added to a 96-well hydrophobic target plate for MALDI-TOF-MS detection. These steps were performed through an automatic pre-processing system. The “peak of target protein/peak of internal reference standards” was recorded as the test results of S100A8, S100A9, and α-defensin. Each sample was tested in triplicate, and the mean value was used as the final test result.
STATISTICAL ANALYSIS:
SPSS 26.0 and GraphPad Prism 8.3.0 were used for statistical analysis. GPower 3.1 was used to perform power analysis. The Kolmogorov-Smirnov test was used to test the normality of all data. The data with a normal distribution were expressed as mean±standard deviation, and the
Results
GENERAL INFORMATION:
Power analysis showed that at least 17 patients were included in each group. We randomly selected 90 patients who met the inclusion criteria, but 8 of these patients were excluded because they met the exclusion criteria. Finally, a total of 82 patients (42 men and 40 women) after total joint arthroplasty were included in this study. Patients were divided into groups according to the MSIS criteria, with 39 patients in the PJI group and 42 in the non-PJI group. There was no significant difference in age, sex, body mass index (BMI), and replacement site between the 2 groups (P>0.05; Table 1). Among all the enrolled patients, 27 of 39 PJI patients had positive synovial fluid culture results (sensitivity of synovial fluid culture was 69.23%). The results of microbiological culture of synovial fluid showed Staphylococcus (14 cases), Streptococcus (4 cases), Candida (3 cases), Klebsiella (2 cases), Proteus (2 cases), and Pseudomonas (1 case). Among these, 2 patients had mixed microbial infection (Staphylococcus aureus, Bacillus cereus, and Bacillus pumilus; Staphylococcus lugdunensis and Staphylococcus auricularis).
DETECTION OF SYNOVIAL FLUID S100A8, S100A9, AND α-DEFENSIN:
We used MALDI-TOF-MS to detect synovial fluid in both groups, and the spectrogram showed 1 peak of S100A8, 3 peaks of S100A9, and 3 peaks of α-defensin. By comparing with the database and referring to the relevant literature [17,18], we tentatively classified the peaks near m/z=10 833 as S100A8, m/z=12 688 as des-MTCKM S100A9, m/z=13151 as des-M S100A9, and m/z=13 273 as S-nitrosylated S100A9 (Figure 1). Both des-MTCKM S100A9 and des-M S100A9 were truncated variants of S100A9. Des-MTCKM S100A9 was truncated for the first 5 amino acids and acetylated for the sixth amino acid of S100A9; and des-M S100A9 was truncated for the first amino acid and acetylated for the second amino acid of S100A9. In addition, 3 peaks could be observed near m/z=3000. By referring to the database and relevant literature [19,20], we classified m/z=3374 as HNP1, m/z=3345 as HNP2, and m/z=3489 as HNP3 (Figure 1).
DIAGNOSTIC ACCURACY OF SYNOVIAL FLUID S100A8, S100A9 AND HNP1-3 FOR PJI:
A “10 833 peak/12 249 peak” was considered as the detection result of S100A8, “12 688+13 151+13 273 peak/12 249 peak” as the detection result of S100A9, and “3374+3445+3489 peak/12 249 peak” as the detection result of HNP1-3. Of the entire cohort, the PJI group showed a significantly higher synovial fluid S100A8 level, of 1.57 (IQR 0.48–4.17), than did the non-PJI group, which was 0.00 (IQR 0.00–0.05) (Z=−7.221, P<0.001). The same trend was found for synovial fluid S100A9 (PJI group: 0.74 [IQR 0.29–1.70]; non-PJI group: 0.05 [IQR 0.00 to 0.10], Z=−6.255, P <0.001) and HNP1-3 (PJI group: 4.49 [IQR 1.59 to 16.39]; and non-PJI group: 0.04 [IQR 0.00–0.40], Z=−6.746, P<0.001) (Figure 2A–2C). The receiver operating characteristic (ROC) curve analysis (Figure 3) showed that synovial fluid S100A8, S100A9, and HNP1-3 were efficient for the diagnosis of PJI, and the area under the ROC curve (AUC) values were 0.964 (95% CI: 0.929–0.998), 0.902 (95% CI: 0.823–0.980), and 0.933 (95% CI: 0.884–0.982), respectively. The cut-off values of synovial fluid S100A8, S100A9, and HNP1-3 for the diagnosis of PJI were 0.104, 0.150, and 0.851, respectively; the sensitivities were 97.4%, 87.2%, and 89.7%, respectively; and the specificities were 86.0%, 88.4%, and 83.7%, respectively (Table 2).
SYNOVIAL FLUID S100A8, S100A9 AND HNP1-3 IN CULTURE-POSITIVE AND -NEGATIVE PJI:
To investigate whether synovial fluid culture results affect the levels of synovial fluid S100A8, S100A9, and HNP1-3, we divided the 39 PJI patients into culture-positive (n=27) and culture-negative (n=12) subgroups based on synovial fluid culture results. There were no significant differences in age, sex, replacement site, BMI, and time to onset between the 2 groups (P>0.05). Synovial fluid S100A8, S100A9, and HNP1-3 levels were compared between the culture-positive and culture-negative subgroups. Synovial fluid S100A8, S100A9, and HNP1-3 had no significant difference between these 2 subgroups (P>0.05; Table 3).
SYNOVIAL FLUID S100A8, S100A9 AND HNP1-3 IN ACUTE, DELAYED AND CHRONIC PJI:
To investigate whether the levels of synovial fluid S100A8, S100A9, and HNP1-3 were affected by the stage of PJI onset, we also divided PJI patients into an acute PJI group (<3 months, n=10), delayed PJI group (3 to 12 months, n=11), and chronic PJI group (>12 months, n=18) according to the period of onset of PJI [21]. There were no statistically significant differences in age, sex, replacement site, and BMI among the groups (P>0.05). The levels of S100A8, S100A9, and HNP1-3 in synovial fluid were compared among the 3 groups, and the results showed that there were no significant differences in the levels of synovial fluid S100A8, S100A9, and HNP1-3 between these 3 subgroups (P>0.05; Table 3).
COMPARISON OF S100A8, S100A9, HNP1-3 WITH COMMON BIOMARKERS OF INFECTION:
Except for D-dimer, biomarkers in blood and synovial fluid were significantly higher in the PJI group than in the non-PJI group (P<0.05; Table 4). Synovial fluid S100A8 had the highest diagnostic efficiency for PJI (AUC: 0.964 [95% CI: 0.929–0.998]), followed by synovial fluid HNP1-3 (AUC: 0.933 [95% CI: 0.884–0.982]), SF-PMN (AUC: 0.931 [95% CI: 0.870–0.992]), SF-WBC (0.923 (95% CI: 0.884–0.982]), and S100A9 (AUC: 0.902 [95% CI: 0.823–0.980]). S100A8 had the highest sensitivity for the diagnosis of PJI, at 97.4% (Table 5, Figure 4). Moreover, we analyzed the value of the combination of these biomarkers in the diagnosis of PJI through a binary logistic regression model. S100A8 combined with SF-WBC and SF-PMN in the diagnosis of PJI increased the AUC value (0.975 [95% CI: 0.943–1.000]) and specificity (93.9%). S100A9 combined with SF-WBC and SF-PMN in the diagnosis of PJI increased the AUC value (0.953 [95% CI: 0.898–1.000]), sensitivity (89.2%) and specificity (97.0%). HNP1-3 combined with SF-WBC and SF-PMN in the diagnosis of PJI increased the AUC value (0.973 [95% CI: 0.938–1.000]) and specificity (100%) (Table 5, Figure 4).
Discussion
PJI is the third most common and serious complication after total joint arthroplasty, accounting for 15.3% of cases, after aseptic loosening (36.5%) and prosthesis dislocation (17.7%) [22]. The diagnosis of PJI has always been a major difficulty for orthopedic surgeons, and an accurate and economical diagnostic method is still lacking. Although MSIS criteria already exist to help clinicians diagnose PJI, sinus tracts are found at a low rate, with only 4 of 39 patients with PJI included in this study having sinus tracts. Moreover, PJI in patients with negative culture results is mainly attributed to low-virulence microorganisms’ infection, premature antibiotic therapy, and incapable adoption of enriched medium culture [23]. The prevalence of culture-negative PJI has been reported to vary between 5% and 42% [24]. Similar to the previous reports, the sensitivity of synovial fluid cultures in our present study was only 69.23%. Furthermore, the secondary criteria for MSIS usually rely on a combination of multiple blood and synovial fluid biomarker tests, cultures, and histological techniques, making the diagnosis quite complex. To improve the diagnosis rate of PJI, an array of new laboratory methods was introduced in recent years, such as molecular biological diagnosis, antigen and antibody assays, and tests for biomarkers in blood and synovial fluid [25].
S100A8 and S100A9 are 2 subunits of calprotectin. It has been reported that calprotectin can be used as a biomarker to assist in the diagnosis of PJI, but there are few studies on the diagnostic value of S100A8 and S100A9 for PJI [26]. S100A8 and S100A9 proteins are associated with multiple human diseases, including infection, and have become the focus of current research [12,27–29]. At the onset of PJI, S100A8 and S100A9 are released from activated neutrophils and monocytes/macrophages by activating toll-like receptor 4, acting as endogenous damage-associated molecular pattern molecules, resulting in significantly higher local concentrations and prompting upregulation of various pro-inflammatory cytokine levels [30,31]. Our study showed that synovial fluid S100A8 and S100A9 were detected as monomers and three S100A9 variants were found. Both synovial fluid S100A8 and S100A9 have outstanding diagnostic value for PJI.
α-Defensin is an antimicrobial peptide consisting of 29 to 35 amino acids and is released predominantly by neutrophils in the presence of pathogens [32]. As a component of the innate immune system, α-defensin has broad-spectrum antimicrobial activity against gram-positive bacteria, gram-negative bacteria, mycobacteria, fungi, and viruses [33]. When PJI is present, it is secreted into synovial fluid as a response to joint infection, and it promotes depolarization of the cell membrane by interacting with or binding to negatively charged cell membranes, thereby inducing rapid death of invading pathogens [34]. There are 2 types of α-defensin detection methods currently available: the enzyme-linked immunosorbent assay (ELISA) and the lateral flow test [35]. However, the common drawback of these 2 approaches is that they can only quantitatively detect α-defensin captured by antibodies and cannot provide insight into the subtypes of α-defensin that play a key role in diagnosing PJI. In our study, we detected 3 subtypes of α-defensins and tentatively suggested that HNP1-3 plays a major role in the diagnosis of PJI. Probably due to the low level of HNP4, which is lower than 2% of HNP1-3 [36], HNP4 was not detected by MALDI-TOF-MS. Furthermore, α-defensin 5 and α-defensin 6, also known as human enteric defensin, play a significant role in regulating intestinal microbiota and maintaining intestinal homeostasis, mainly at high concentrations in small intestinal crypts and intestinal mucus [37]. This may be the reason why we could not detect α-defensin 5 and α-defensin 6.
Since many of the available biomarkers may not produce appreciable content changes in culture-negative PJI and early PJI, these biomarkers will not be able to identify these 2 types of PJI [38]. Thus, we analyzed S100A8, S100A9, and HNP1-3 levels in synovial fluid from culture-positive and culture-negative PJI as well as from acute, delayed, and chronic PJI. Our results showed that the levels of these 3 proteins were not affected by culture results or the onset stages of PJI. In addition, these 3 proteins have diagnostic value for PJI that is comparable to or even higher than that of common infectious biomarkers, and their diagnostic efficiency could be improved when combined with SF-WBC and SF-PMN.
MALDI-TOF-MS was used to detect synovial fluid S100A8, S100A9, and α-defensin since it has the following advantages when compared with ELISA [39,40]. First, MALDI-TOF-MS has a simple operation, which means that it can directly detect samples without treatment of enzyme digestion, thus greatly reducing detection time and labor. Second, with the advantages of high throughput and automation, MALDI-TOF-MS can process and test large volumes of samples using an automated pre-processing system, testing 96 samples within 1 h. Third, it has high accuracy. ELISA quantifies proteins by absorbance, and all substances that bind to antibodies are involved in quantification, making it prone to false positives. In contrast, MALDI-TOF-MS can directly display peaks of interest by mass spectrometry, making their quantification more accurate. This approach allows us to investigate various variant forms of the target protein, as well as the types of proteins that function.
There were some limitations in the present study. This study involved the samples from a single center, so the sample size was relatively small. Therefore, a larger cohort study is warranted. Furthermore, due to the matrix competition effect of MALDI-TOF-MS [41], only semi-quantitative detection of proteins could be performed in this study. Subsequently, we will develop the mass spectrometric immunoassays method, which is the absolute quantitative detection of the target protein by MALDI-TOF-MS after enrichment of target proteins with the corresponding antibodies [42].
In conclusion, this study exploited the unique advantages of MALDI-TOF-MS to identify for the first time S100A8, three S100A9 variants, and 3 subtypes of α-defensin (HNP1-3) that were elevated in the synovial fluid of PJI patients. The sensitivity and specificity of these 3 proteins in the diagnosis of PJI were higher than those of common infectious biomarkers, and their combination with SF-WBC and SF-PMN could improve their diagnostic value. In addition, these 3 proteins were not affected by culture results and disease stage and could accurately diagnose culture-negative PJI and PJI at various stages. Our study provided clinicians with more valuable biomarkers for the diagnosis of PJI and an in-depth analysis of possible variants and subtypes of these proteins. In addition, MALDI-TOF-MS overcomes the shortcomings of traditional diagnostic methods, and has high automation, faster detection speed, and simpler operation, which is suitable for the rapid detection of a large number of clinical samples. This study laid a solid foundation for the development of absolute quantitative mass spectrometric immunoassay methods, which will provide reliable new methods for the early diagnosis of PJI.
Conclusions
We detected synovial fluid S100A8, S100A9, and α-defensin by MALDI-TOF-MS for the first time, and found that these 3 proteins have high diagnostic efficiency and clinical application value for PJI and therefore can be considered as biomarkers to assist the diagnosis of PJI. In this study, we determined the increased level of these 3 proteins in the synovial fluid of patients with PJI through semi-quantitative tests, which laid a solid foundation for the development of subsequent absolute quantification methods.
Figures
 Figure 1. Synovial fluid S100A8, S100A9 and α-defensin were detected by MALDI-TOF-MS in PJI and non-PJI groups. PJI – periprosthetic joint infection; m/z=3374, HNP1; m/z=3345, HNP2; m/z=3489, HNP3; m/z=10833, S100A8; m/z=12688, des-MTCKM S100A9; m/z=13151, des-M S100A9; m/z=13273, S-nitrosylated S100A9; m/z=12249; IFS – internal reference standards. This figure was generated using QuanPRO protein quantitation mass spectrometer, Intelligene Biosystems (Qingdao) Co., Ltd., Beijing, China.
Figure 1. Synovial fluid S100A8, S100A9 and α-defensin were detected by MALDI-TOF-MS in PJI and non-PJI groups. PJI – periprosthetic joint infection; m/z=3374, HNP1; m/z=3345, HNP2; m/z=3489, HNP3; m/z=10833, S100A8; m/z=12688, des-MTCKM S100A9; m/z=13151, des-M S100A9; m/z=13273, S-nitrosylated S100A9; m/z=12249; IFS – internal reference standards. This figure was generated using QuanPRO protein quantitation mass spectrometer, Intelligene Biosystems (Qingdao) Co., Ltd., Beijing, China.  Figure 2. Scatterplot analysis of synovial fluid S100A8, S100A9, HNP1-3 levels in PJI and non-PJI groups. (A) Scatterplot analysis of synovial fluid S100A8 levels in PJI and non-PJI groups. (B) Scatterplot analysis of synovial fluid S100A9 levels in PJI and non-PJI groups. (C) Scatterplot analysis of synovial fluid HNP1-3 levels in PJI and non-PJI groups. PJI – periprosthetic joint infection. * Significance difference between the groups, P<0.001. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA.
Figure 2. Scatterplot analysis of synovial fluid S100A8, S100A9, HNP1-3 levels in PJI and non-PJI groups. (A) Scatterplot analysis of synovial fluid S100A8 levels in PJI and non-PJI groups. (B) Scatterplot analysis of synovial fluid S100A9 levels in PJI and non-PJI groups. (C) Scatterplot analysis of synovial fluid HNP1-3 levels in PJI and non-PJI groups. PJI – periprosthetic joint infection. * Significance difference between the groups, P<0.001. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA.  Figure 3. ROC analysis of synovial fluid S100A8, S100A9, and HNP1-3 in diagnosing periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA.
Figure 3. ROC analysis of synovial fluid S100A8, S100A9, and HNP1-3 in diagnosing periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA. 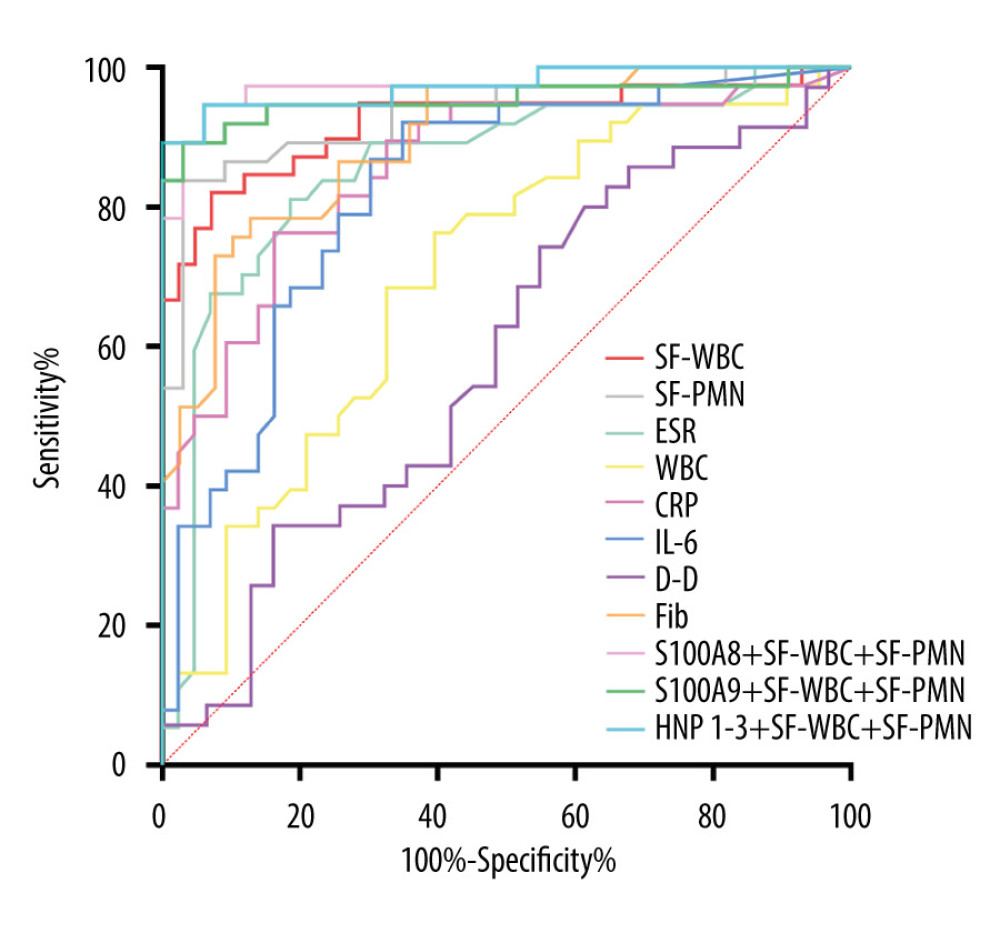 Figure 4. ROC analysis of common infection biomarkers for the diagnosis of periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides; SF-WBC – synovial fluid white blood cells count; SF-PMN – percentage of polymorphonuclear neutrophil in the synovial fluid; ESR – erythrocyte sedimentation rate; WBC – whole blood white blood cell count; CRP – whole blood C-reactive protein; IL-6 – plasma interleukin-6; D-D – plasma D-dimer; Fib – plasma fibrinogen. This figure was generated using GraphPad Prism 8.3.0, Graphpad Software Company, USA.
Figure 4. ROC analysis of common infection biomarkers for the diagnosis of periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides; SF-WBC – synovial fluid white blood cells count; SF-PMN – percentage of polymorphonuclear neutrophil in the synovial fluid; ESR – erythrocyte sedimentation rate; WBC – whole blood white blood cell count; CRP – whole blood C-reactive protein; IL-6 – plasma interleukin-6; D-D – plasma D-dimer; Fib – plasma fibrinogen. This figure was generated using GraphPad Prism 8.3.0, Graphpad Software Company, USA. Tables
Table 1. General clinical information of enrolled patients.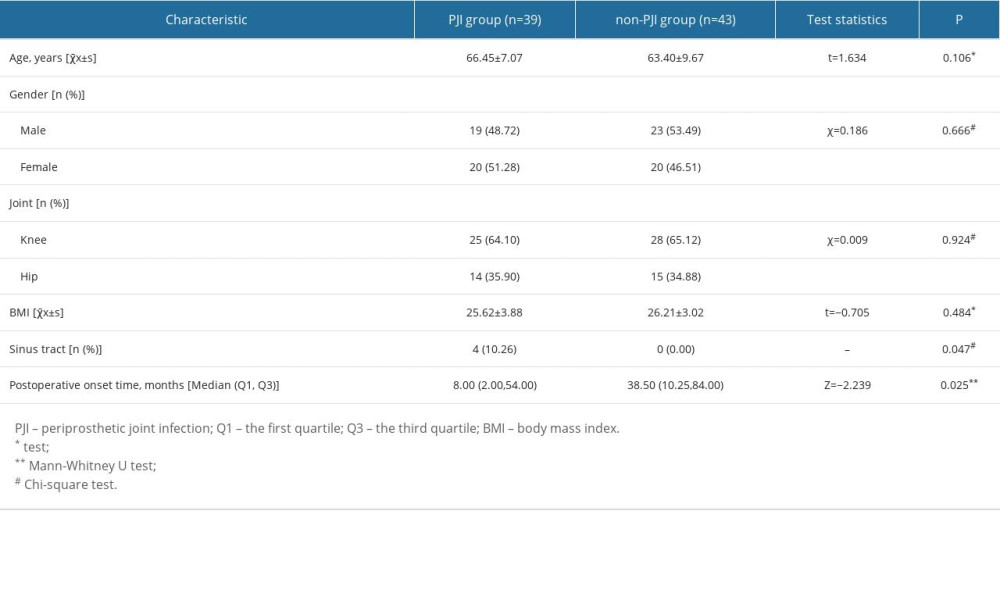 Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection.
Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection.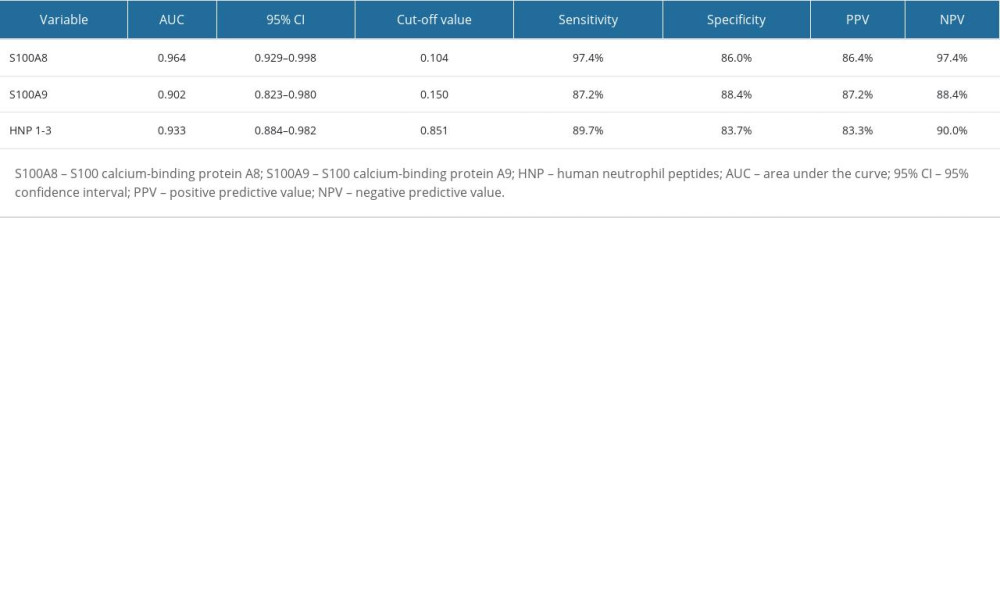 Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels.
Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels.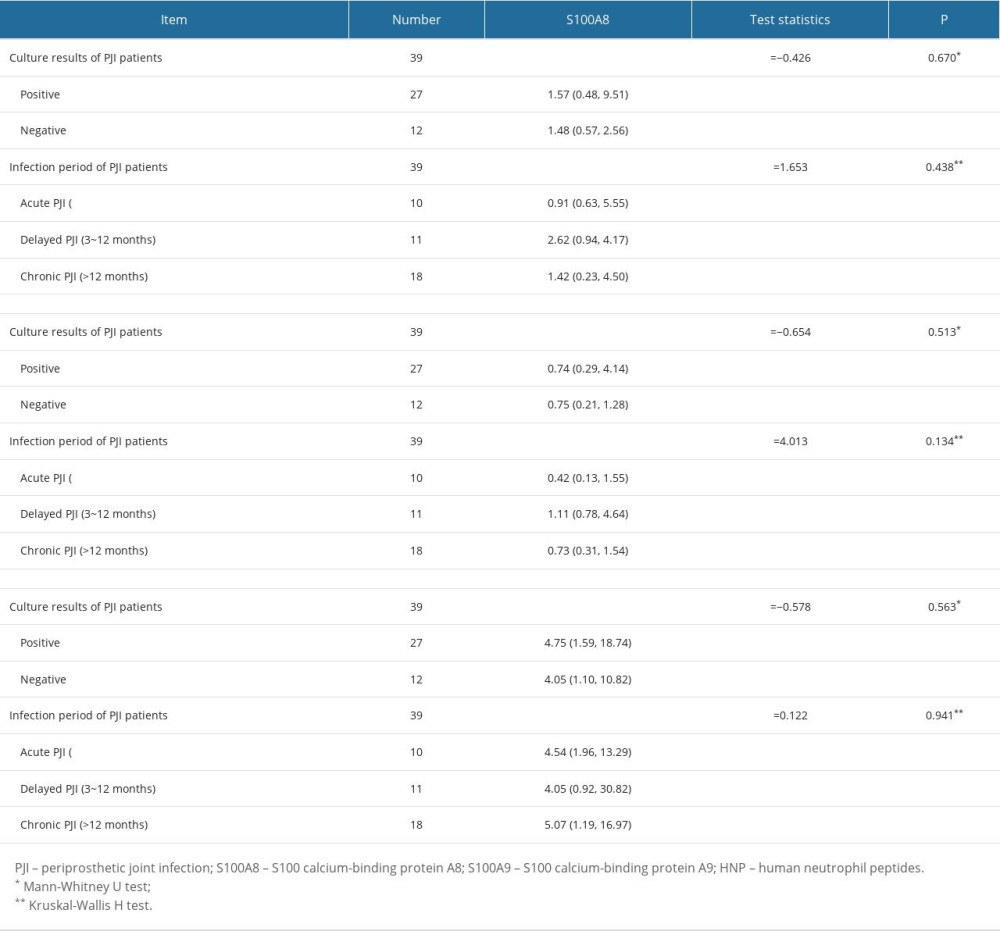 Table 4. The levels of common infection biomarkers in PJI and non-PJI groups.
Table 4. The levels of common infection biomarkers in PJI and non-PJI groups.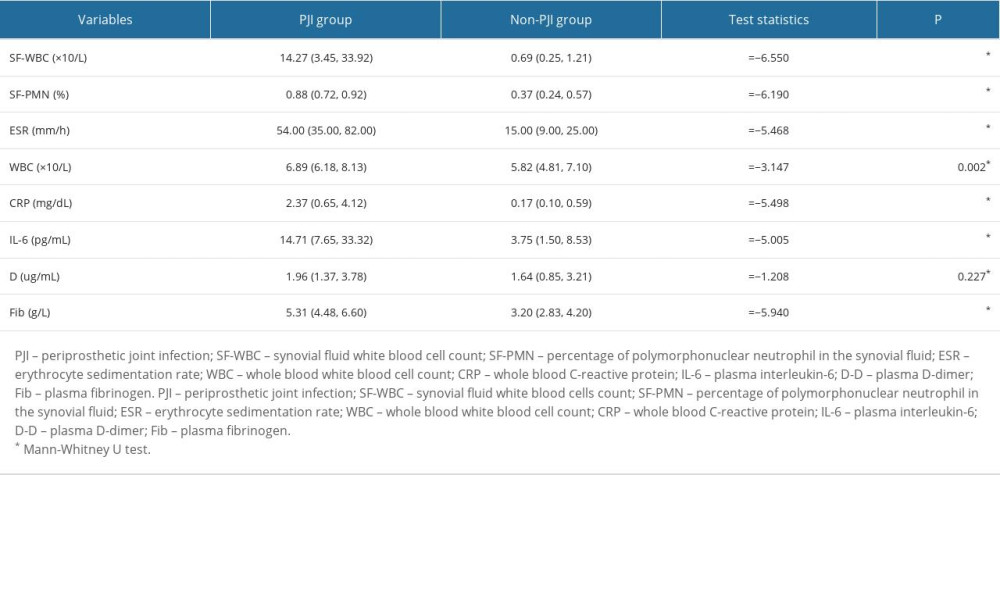 Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection.
Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection.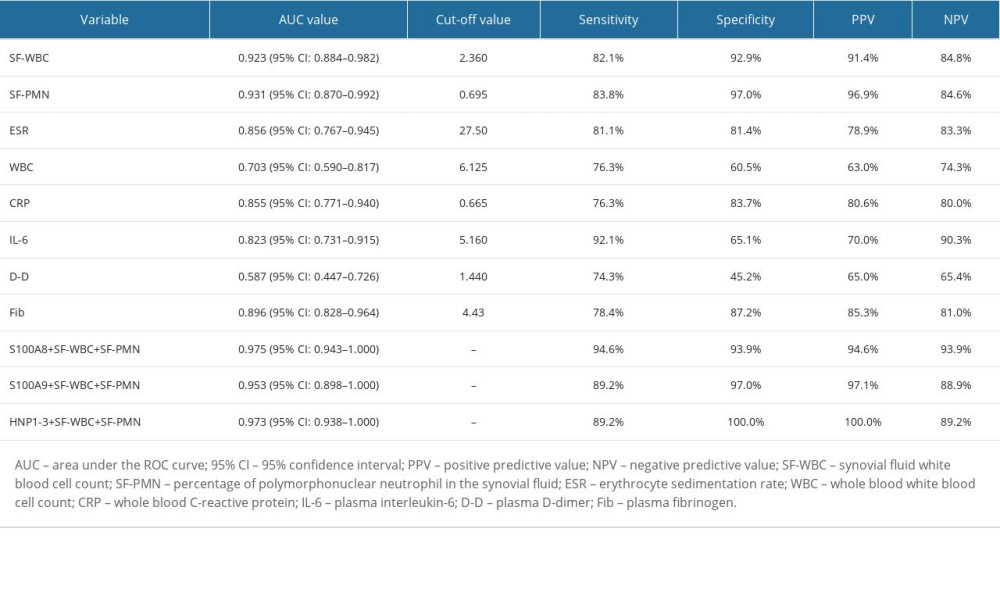
References
1. Izakovicova P, Borens O, Trampuz A, Periprosthetic joint infection: current concepts and outlook: EFORT Open Rev, 2019; 4; 482-94
2. Wang K, Li W, Liu H, Progress in prevention, diagnosis, and treatment of periprosthetic joint infection: Evid Based Complement Alternat Med, 2021; 2021; 3023047
3. Chisari E, Parvizi J, Accuracy of blood-tests and synovial fluid-tests in the diagnosis of periprosthetic joint infections: Expert Rev Anti Infect Ther, 2020; 18; 1135-42
4. Masters TL, Bhagwate AV, Dehankar MK, Human transcriptomic response to periprosthetic joint infection: Gene, 2022; 825; 146400
5. Solarino G, Bizzoca D, Moretti L, What’s new in the diagnosis of periprosthetic joint infections: Focus on synovial fluid biomarkers: Trop Med Infect Dis, 2022; 7; 355
6. Fuchs M, Faschingbauer M, Riklin-Dold M, D-lactate is a promising biomarker for the diagnosis of periprosthetic joint infection: Front Surg, 2022; 9; 1082591
7. Vergara A, Fernandez-Pittol MJ, Munoz-Mahamud E, Evaluation of Lipocalin-2 as a biomarker of periprosthetic joint infection: J Arthroplasty, 2019; 34; 123-25
8. Ikeda S, Uchiyama K, Minegishi Y, Evaluation of myeloperoxidase in synovial fluid as a biomarker for chronic periprosthetic joint infection: Int Orthop, 2020; 44; 1915-20
9. Wang C, Wang Q, Li R, LTF, PRTN3, and MNDA in synovial fluid as promising biomarkers for periprosthetic joint infection: Identification by quadrupole orbital-trap mass spectrometry: J Bone Joint Surg Am, 2019; 101; 2226-34
10. Lee JS, Lee NR, Kashif A, S100A8 and S100A9 promote apoptosis of chronic eosinophilic leukemia cells: Front Immunol, 2020; 11; 1258
11. Donato R, Cannon BR, Sorci G, Functions of S100 proteins: Curr Mol Med, 2013; 13; 24-57
12. Li Z, Zhang Q, Shi L, alpha-Defensin versus leukocyte esterase in periprosthetic joint infection: An updated meta-analysis: Biomed Res Int, 2020; 2020; 3704285
13. Zhao L, Lu W, Defensins in innate immunity: Curr Opin Hematol, 2014; 21; 37-42
14. Cupp-Sutton KA, Wu S, High-throughput quantitative top-down proteomics: Mol Omics, 2020; 16; 91-9
15. Greco V, Piras C, Pieroni L, Applications of MALDI-TOF mass spectrometry in clinical proteomics: Expert Rev Proteomics, 2018; 15; 683-96
16. Parvizi J, Zmistowski B, Berbari EF, New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society: Clin Orthop Relat Res, 2011; 469; 2992-94
17. Gao J, Meyer K, Borucki K, Multiplex immuno-MALDI-TOF MS for targeted quantification of protein biomarkers and their proteoforms related to inflammation and renal dysfunction: Anal Chem, 2018; 90; 3366-73
18. Lim SY, Raftery M, Cai H, S-nitrosylated S100A8: Novel anti-inflammatory properties: J Immunol, 2008; 181; 5627-36
19. Preiano M, Maggisano G, Lombardo N, Influence of storage conditions on MALDI-TOF MS profiling of gingival crevicular fluid: Implications on the role of S100A8 and S100A9 for clinical and proteomic based diagnostic investigations: Proteomics, 2016; 16; 1033-45
20. Pisano E, Cabras T, Montaldo C, Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS: Eur J Oral Sci, 2005; 113; 462-68
21. Fernandez-Sampedro M, Farinas-Alvarez C, Garces-Zarzalejo C, Accuracy of different diagnostic tests for early, delayed and late prosthetic joint infection: BMC Infect Dis, 2017; 17; 592
22. Otto-Lambertz C, Yagdiran A, Wallscheid F, Periprosthetic infection in joint replacement: Dtsch Arztebl Int, 2017; 114; 347-53
23. Watanabe S, Kobayashi N, Tomoyama A, Clinical characteristics and risk factors for culture-negative periprosthetic joint infections: J Orthop Surg Res, 2021; 16; 292
24. Palan J, Nolan C, Sarantos K, Culture-negative periprosthetic joint infections: EFORT Open Rev, 2019; 4; 585-94
25. Arvieux C, Common H, New diagnostic tools for prosthetic joint infection: Orthop Traumatol Surg Res, 2019; 105; S23-S30
26. Xing J, Li J, Yan Z, Diagnostic accuracy of calprotectin in periprosthetic joint infection: A diagnostic meta-analysis: J Orthop Surg Res, 2022; 17; 11
27. Averill MM, Kerkhoff C, Bornfeldt KE, S100A8 and S100A9 in cardiovascular biology and disease: Arterioscler Thromb Vasc Biol, 2012; 32; 223-29
28. Koh HM, Lee HJ, Kim DC, High expression of S100A8 and S100A9 is associated with poor disease-free survival in patients with cancer: A systematic review and meta-analysis: Transl Cancer Res, 2021; 10; 3225-35
29. Defrene J, Berrazouane S, Esparza N, Deletion of S100a8 and S100a9 enhances skin hyperplasia and promotes the Th17 response in imiquimod-induced psoriasis: J Immunol, 2021; 206; 505-14
30. Vogl T, Gharibyan AL, Morozova-Roche LA, Pro-inflammatory S100A8 and S100A9 proteins: Self-assembly into multifunctional native and amyloid complexes: Int J Mol Sci, 2012; 13; 2893-917
31. Grassi M, Salari P, Farinelli L, Synovial biomarkers to detect chronic periprosthetic joint infection: A pilot study to compare calprotectin rapid test, calprotectin ELISA immunoassay and leukocyte esterase test: J Arthroplasty, 2022; 37; 781-86
32. Xie K, Qu X, Yan M, Procalcitonin and alpha-Defensin for diagnosis of periprosthetic joint infections: J Arthroplasty, 2017; 32; 1387-94
33. Pupaibool J, Fulnecky EJ, Swords RL, alpha-Defensin-novel synovial fluid biomarker for the diagnosis of periprosthetic joint infection: Int Orthop, 2016; 40; 2447-52
34. Yuan J, Yan Y, Zhang J, Diagnostic accuracy of alpha-defensin in periprosthetic joint infection: A systematic review and meta-analysis: Int Orthop, 2017; 41; 2447-55
35. Bonanzinga T, Ferrari MC, Tanzi G, The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: A literature review: EFORT Open Rev, 2019; 4; 10-13
36. Holly MK, Diaz K, Smith JG, Defensins in viral infection and pathogenesis: Annu Rev Virol, 2017; 4; 369-91
37. Yang E, Shen J, The roles and functions of Paneth cells in Crohn’s disease: A critical review: Cell Prolif, 2021; 54; e12958
38. Goh GS, Parvizi J, Diagnosis and treatment of culture-negative periprosthetic joint infection: J Arthroplasty, 2022; 37; 1488-93
39. Zhong J, Sun Y, Xie M, Proteoform characterization based on top-down mass spectrometry: Brief Bioinform, 2021; 22; 1729-50
40. Nickerson JL, Baghalabadi V, Rajendran S, Recent advances in top-down proteome sample processing ahead of MS analysis: Mass Spectrom Rev, 2023; 42; 457-95
41. Wang J, Qiu S, Chen S, MALDI-TOF MS imaging of metabolites with a N-(1-naphthyl) ethylenediamine dihydrochloride matrix and its application to colorectal cancer liver metastasis: Anal Chem, 2015; 87; 422-30
42. Trenchevska O, Nelson RW, Nedelkov D, Mass spectrometric immunoassays in characterization of clinically significant proteoforms: Proteomes, 2016; 4; 13
Figures
 Figure 1. Synovial fluid S100A8, S100A9 and α-defensin were detected by MALDI-TOF-MS in PJI and non-PJI groups. PJI – periprosthetic joint infection; m/z=3374, HNP1; m/z=3345, HNP2; m/z=3489, HNP3; m/z=10833, S100A8; m/z=12688, des-MTCKM S100A9; m/z=13151, des-M S100A9; m/z=13273, S-nitrosylated S100A9; m/z=12249; IFS – internal reference standards. This figure was generated using QuanPRO protein quantitation mass spectrometer, Intelligene Biosystems (Qingdao) Co., Ltd., Beijing, China.
Figure 1. Synovial fluid S100A8, S100A9 and α-defensin were detected by MALDI-TOF-MS in PJI and non-PJI groups. PJI – periprosthetic joint infection; m/z=3374, HNP1; m/z=3345, HNP2; m/z=3489, HNP3; m/z=10833, S100A8; m/z=12688, des-MTCKM S100A9; m/z=13151, des-M S100A9; m/z=13273, S-nitrosylated S100A9; m/z=12249; IFS – internal reference standards. This figure was generated using QuanPRO protein quantitation mass spectrometer, Intelligene Biosystems (Qingdao) Co., Ltd., Beijing, China. Figure 2. Scatterplot analysis of synovial fluid S100A8, S100A9, HNP1-3 levels in PJI and non-PJI groups. (A) Scatterplot analysis of synovial fluid S100A8 levels in PJI and non-PJI groups. (B) Scatterplot analysis of synovial fluid S100A9 levels in PJI and non-PJI groups. (C) Scatterplot analysis of synovial fluid HNP1-3 levels in PJI and non-PJI groups. PJI – periprosthetic joint infection. * Significance difference between the groups, P<0.001. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA.
Figure 2. Scatterplot analysis of synovial fluid S100A8, S100A9, HNP1-3 levels in PJI and non-PJI groups. (A) Scatterplot analysis of synovial fluid S100A8 levels in PJI and non-PJI groups. (B) Scatterplot analysis of synovial fluid S100A9 levels in PJI and non-PJI groups. (C) Scatterplot analysis of synovial fluid HNP1-3 levels in PJI and non-PJI groups. PJI – periprosthetic joint infection. * Significance difference between the groups, P<0.001. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA. Figure 3. ROC analysis of synovial fluid S100A8, S100A9, and HNP1-3 in diagnosing periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA.
Figure 3. ROC analysis of synovial fluid S100A8, S100A9, and HNP1-3 in diagnosing periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides. This figure was generated using GraphPad Prism 8.3.0, GraphPad Software Company, USA. Figure 4. ROC analysis of common infection biomarkers for the diagnosis of periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides; SF-WBC – synovial fluid white blood cells count; SF-PMN – percentage of polymorphonuclear neutrophil in the synovial fluid; ESR – erythrocyte sedimentation rate; WBC – whole blood white blood cell count; CRP – whole blood C-reactive protein; IL-6 – plasma interleukin-6; D-D – plasma D-dimer; Fib – plasma fibrinogen. This figure was generated using GraphPad Prism 8.3.0, Graphpad Software Company, USA.
Figure 4. ROC analysis of common infection biomarkers for the diagnosis of periprosthetic joint infection. S100A8 – S100 calcium-binding protein A8; S100A9 – S100 calcium-binding protein A9; HNP – human neutrophil peptides; SF-WBC – synovial fluid white blood cells count; SF-PMN – percentage of polymorphonuclear neutrophil in the synovial fluid; ESR – erythrocyte sedimentation rate; WBC – whole blood white blood cell count; CRP – whole blood C-reactive protein; IL-6 – plasma interleukin-6; D-D – plasma D-dimer; Fib – plasma fibrinogen. This figure was generated using GraphPad Prism 8.3.0, Graphpad Software Company, USA. Tables
 Table 1. General clinical information of enrolled patients.
Table 1. General clinical information of enrolled patients. Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection.
Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection. Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels.
Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels. Table 4. The levels of common infection biomarkers in PJI and non-PJI groups.
Table 4. The levels of common infection biomarkers in PJI and non-PJI groups. Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection.
Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection. Table 1. General clinical information of enrolled patients.
Table 1. General clinical information of enrolled patients. Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection.
Table 2. Diagnostic efficiency of synovial fluid S100A8, S100A9, and HNP1-3 for periprosthetic joint infection. Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels.
Table 3. Effect of various influencing factors on synovial fluid S100A8, S100A9, and HNP1-3 levels. Table 4. The levels of common infection biomarkers in PJI and non-PJI groups.
Table 4. The levels of common infection biomarkers in PJI and non-PJI groups. Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection.
Table 5. Diagnostic efficiency of common infection biomarkers for periprosthetic joint infection. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








