25 September 2023: Clinical Research
Impact of Nonselective and Selective α−1 Adrenergic Blockers on the Sedative Efficacy of Dexmedetomidine in Urologic Surgery: A Prospective, Observational Study
Jiseok BaikDOI: 10.12659/MSM.941614
Med Sci Monit 2023; 29:e941614
Abstract
BACKGROUND: This study aimed to compare the impact of a-1 adrenergic blockers – nonselective (alfuzosin, doxazosin, and terazosin) and selective (silodosin and tamsulosin) – on the sedative effects of the α-2 adrenergic agonist dexmedetomidine (DMT) in patients undergoing urologic surgery. The primary outcome was the sedative effect of DMT as determined by the bispectral index (BIS) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores.
MATERIAL AND METHODS: One hundred eighteen patients undergoing elective urologic surgery with spinal anesthesia were recruited. Patients were assigned based on their medication status to group N (no medication; n=33), group NS (nonselective α-1 blocker; n=27), or group S (selective α-1 blocker; n=58). Mean blood pressure (MBP), heart rate (HR), oxygen saturation (SpO₂), BIS, and MOAA/S scale scores were recorded at 5-minute (min) intervals after DMT administration.
RESULTS: Group NS had significantly higher BIS scores than groups N and S at 25 min (P=0.045) and 30 min (P=0.030) after DMT administration, indicating lower sedation levels. MBP significantly differed between the 3 groups at all time points, with group N experiencing a lower MBP than groups NS and S. No significant differences were found between the groups in MOAA/S scale scores, SpO₂, or HR.
CONCLUSIONS: Nonselective α-1 adrenergic blockers can reduce the sedative effects of DMT. Consequently, there may be a need for individualized anesthesia management considering the specific subtype of α-1 adrenergic blocker medication.
Keywords: Adrenergic alpha-1 Receptor Antagonists, Conscious Sedation, Dexmedetomidine, Anesthesia, Spinal, Urologic Surgical Procedures, Male, Humans, Prospective Studies, Hypnotics and Sedatives
Background
Benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS) are the most common urological conditions in older men that have negative effects on a patient’s quality of life [1,2]. α−1 adrenergic blockers have been shown to be effective in treating these conditions by relaxing the smooth muscles of the prostate and bladder neck via inhibition of sympathetic pathways [3,4]. Prazosin was the first selective α−1 adrenergic blocker, but 5 FDA-approved, long-acting α−1 adrenergic blockers have largely replaced it. Alfuzosin, doxazosin, and terazosin are considered to be nonselective (they block the α1A, α1B, and α1D adrenergic receptor subtypes equally), while silodosin and tamsulosin are considered to be selective for the α1A-adrenergic receptor subtype [5–7]. These α−1 adrenergic blockers represent first-line pharmacological treatment for symptomatic BPH and LUTS [3,8].
Despite medical treatment with α−1 adrenergic blockers, many patients still experience symptoms that prompt intervention by surgeries such as holmium laser resection of the prostate, transurethral resection of the prostate, and transurethral resection of bladder tumors. Although these surgical interventions are relatively simple, as always, they carry anesthetic and surgical risks [9,10]. To manage these risks and enhance patient comfort, various types of anesthesia are used based on the specific procedure. Local anesthesia is often sufficient for minor procedures, whereas regional anesthesia, including spinal anesthesia, and general anesthesia are used for surgeries on the lower body and more extensive operations, respectively [11]. Spinal anesthesia provides several advantages, such as rapid onset, excellent muscle relaxation, reduced blood loss, and avoidance of general anesthesia-related risks. It is commonly indicated for lower abdominal or pelvic surgeries. However, spinal anesthesia can lead to potential issues like headaches, hypotension, and urinary retention and may not be suitable for patients with coagulation problems or infections at the injection site [12,13]. Sedatives are frequently used in combination with spinal anesthesia to enhance the patient’s comfort and reduce anxiety.
Among the sedatives used, dexmedetomidine (DMT) for sedation after spinal anesthesia during urological surgical interventions has several advantages, including improved patient comfort, enhanced surgical conditions, shorter recovery times, and fewer adverse effects [14,15]. DMT, a selective α−2 adrenergic agonist, was first approved for use in the United States in 1999. It is used to provide sedation and anxiolysis in combination with regional anesthesia techniques in surgical settings. The mechanism of action of DMT involves the activation of α−2 adrenergic receptors in the central nervous system, particularly in the locus coeruleus, which is related to the modulation of sleep regulation and respiratory control. This leads to decreased sympathetic and increased parasympathetic outflow, resulting in sedation, anxiolysis, and analgesia [14,16,17].
Studies have shown that the sedative effects of DMT are influenced by other drugs, including opioids, benzodiazepines, and beta-blockers [18,19]. Moreover, as proven in several studies, the long-term use of α−1 adrenergic blockers can affect α−2 adrenergic receptors and thus may affect the sedative properties of DMT [20–22]. However, no study has compared its effects in the presence of nonselective vs selective α−1 adrenergic blockers.
This study aimed to compare the impact of nonselective α−1 adrenergic blockers (alfuzosin, doxazosin, and terazosin), selective α−1 adrenergic blockers (silodosin and tamsulosin), and no use of α−1 adrenergic blocker on the sedative effects of the α−2 adrenergic agonist DMT in 118 patients undergoing urologic surgery.
Material and Methods
ETHICAL STATEMENT:
This prospective, single-center, observational study was conducted at the Pusan National University Hospital. The study was approved by the Institutional Review Board of our hospital (approval no: H-1804-014-066) and was registered with the Clinical Research Informational Service (KCT0002967,
PATIENTS SELECTION AND GROUPING:
We recruited patients who had American Society of Anesthesiologists (ASA) physical status I or II, were 45–80 years of age, and were scheduled for elective urological surgery under spinal anesthesia and sedation using DMT. The following patients were excluded: patients who were obese (body mass index ≥33 kg/m2); those with contraindications to spinal anesthesia or who refused sedation during surgery; those with a psychiatric illness, any medical or surgical history involving the brain, or any neurological disorder; and those who were chronically administered sedatives or opioids. We initially considered 131 patients for enrollment, and 118 patients were ultimately enrolled. Those enrolled were assigned to 1 of 3 groups according to their usual α−1 adrenergic blocker medication status before surgery: group N (no α−1 adrenergic blocker use), group NS (nonselective α−1 adrenergic blocker use – alfuzosin, doxazosin, or terazosin), and group S (selective α−1 adrenergic blockers use – silodosin or tamsulosin). Figure 1 is the study flow diagram.
ANESTHESIA AND MANAGEMENT:
All the patients received routine preoperative care without premedication. On arrival in the operating room, standard monitoring began, including mean blood pressure (MBP), heart rate (HR), oxygen saturation (SpO2), and electrocardiography. Crystalloid at 10 mL/kg was administered intravenously before spinal anesthesia to prevent spinal anesthesia-induced hypotension. Spinal anesthesia was induced in the lateral decubitus position using a 25-gauge Quincke spinal needle and 10 mg of hyperbaric 0.5% bupivacaine injected into the L3/L4 intervertebral space. The patient was then placed in the supine position with oxygen at 3 L/min via a nasal cannula and bispectral index (BIS) monitoring began (A-2000 BIS monitor; Aspect Medical Systems Inc., Natick, MA, USA). After the achievement of an adequate sensory block, all groups received a loading dose of DMT at 1.0 μg/kg administered intravenously for 10 min followed by an infusion of DMT at 1.0 μg/kg/h. Intraoperative hypotension was defined as MBP <50 mmHg and bradycardia as HR <50 bpm. When hypotension occurred, the patient was treated with ephedrine 5 mg and crystalloid 300 mL, and bradycardia was treated with atropine 0.5 mg. Patients with 3 or more episodes of hypotension or bradycardia despite appropriate treatment were excluded from the study.
OUTCOMES ASSESSMENT:
Starting after administration of the loading dose of DMT, variables were manually recorded at 5-min intervals (at 0, 5, 10, 15, 20, 25, and 30 min following loading dose administration) in the following systematic manner: SpO2, HR, and MBP were recorded first, followed by the BIS score, then the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale score, in that order. The primary outcome of the study was the sedative properties of DMT as assessed through the BIS and MOAA/S scale scores, where sedation depth was evaluated using the BIS score (Table 1A), while the level of consciousness was assessed using the MOAA/S scale score (Table 1B) [23,24].
STATISTICAL ANALYSIS:
Our primary endpoint was comparing the sedative properties of DMT according to the medication type, especially the difference in BIS score at 30 min between group NS and group S. According to the results of our study, which included an effect size of 0.677, 27 patients in the NS group, 58 patients in the S group, and a Bonferroni-adjusted level of 0.05/3, the post hoc power of our study was calculated to be 65.7% using G*Power (ver. 3.1.9.6) software.
All analyses were performed using IBM SPSS Statistics (version 25; IBM Corporation, Armonk, NY), MedCalc (version 20; MedCalc Software, Ltd, Ostend, Belgium), and ARTool (version 1.6.2; University of Washington;
For repeatedly measured and non-parametrically distributed variables (BIS, MOAA/S, SpO2, HR, and MBP), we performed an aligned rank transformed ANOVA (ART-RMANOVA) using ARTool to analyze the interaction between time and group [25].
Δ BIS, Δ MOAA/S, Δ SpO2, Δ HR, and Δ MBP were used to evaluate the changes from baseline (ex: Δ BIS 5 min=BIS at 5 min–BIS at baseline); for these variables, Jonckheere-Terpstra trend tests were conducted to evaluate the trend according to the degree of α adrenergic block, as our experimental groups were ordered based on the degree of α adrenergic block (ie, group N, group S, group NS). Categorical data were analyzed with Fisher’s exact test. Two-sided
Results
PARTICIPANTS AND DEMOGRAPHIC DATA:
One hundred thirty-one patients scheduled for elective urological surgery were assessed for eligibility. Eleven patients were excluded because of inappropriate spinal anesthesia (group N, 2; group NS, 2; and group S, 8), and 1 patient in group N was excluded because of sedation refusal. Thus, 118 patients assigned to 3 groups (group N, n=33; group NS, n=27; and group S, n=58) completed the study (Figure 1). Demographic data, types of α−1 adrenergic blockers used, and duration of use are shown in Table 2. There were no significant differences among the 3 groups in ASA physical status, age, height, weight, medication duration, or type of surgery.
OUTCOMES:
There were statistically significant differences in the BIS scores between the 3 groups at 25 and 30 min. Group NS had significantly higher BIS scores than groups N and S at 25 min (group NS: 63.0 [54.0, 70.5] vs group N: 51.0 [46.0, 63.3] vs group S: 55.0 [46.0, 63.0]; P=0.045) and 30 min (group NS: 62.0 [51.0, 65.8] vs group N: 52.0 [47.0, 62.5] vs group S: 49.0 [45.0, 62.0]; P=0.030), but there was no significant difference between groups N and S (Figure 2A). There were no statistically significant differences in the MOAA/S scale scores, SpO2, or HR between the 3 groups measured from administration of the loading dose of DMT to 30 min later (Figure 2B–2D). However, except at baseline (0 min), there were statistically significant differences in MBP among the 3 groups. Group N had significantly lower MBP than groups NS and S at 5 min (P=0.008), 10 min (P=0.002), 15 min (P<0.001), 20 min (P=0.001), 25 min (P=0.001), and 30 min (P<0.001), but there was no significant difference between groups NS and S at any time (Figure 2D). Table 3 shows the change (Δ) between the baseline (0 min) and each time point (5, 10, 15, 20, 25, and 30 min) in the BIS score (Table 3A), MOAA/S scale score (Table 3B), oxygen saturation (SpO2) (Table 3C), heart rate (HR) (Table 3D), and mean blood pressure (MBP) (Table 3E). Group NS had statistically significantly less BIS score change than did groups N and S at 25 and 30 min. Group N had a statistically significant, considerably greater MBP change than did groups NS and S at 10, 15, 20, 25, and 30 min.
Discussion
This study aimed to determine the differential effect of α−1 adrenergic blockers categorized by their receptor subtype selectivity on the sedative properties of DMT during urological surgery under spinal anesthesia. Previously, studies have been conducted on whether the sedative action of dexmedetomidine, an α−2 adrenergic agonist, can be enhanced by the α−1 adrenergic blocker, but they did not compare the effects of the subtypes of α−1 adrenergic blockers [21,22]. We demonstrated that patients taking nonselective α−1 adrenergic blockers (alfuzosin, doxazosin, and terazosin) achieved a lesser degree of sedation in response to DMT, as measured by BIS scores, than patients taking selective α−1 adrenergic blockers (silodosin and tamsulosin) or not using α−1 adrenergic blockers. This suggests an interaction between the subtype of α−1 adrenergic blocker used and the sedative properties of DMT.
In perioperative anesthesia management, sedation is essential to provide patient comfort and improve the surgical conditions. DMT, a selective α−2 adrenergic agonist, is frequently employed due to its anxiolytic and analgesic properties [14,16]. Our study confirmed that DMT maintained effective sedation, as shown by the BIS and MOAA/S scale scores. However, BIS scores were significantly higher in group NS at the 25- and 30-min marks, indicating a lesser sedative effect of DMT in this group than in groups N and S. The higher BIS score in group NS might be due to the broad antagonistic action of nonselective α−1 adrenergic blockers, which could affect the function of α−2 adrenergic receptors and thus affect the sedative properties of DMT. This mechanism is not fully understood, but it is thought that widespread inhibition in the body of α1-adrenergic receptor subtypes by nonselective blockers could affect the functioning of the α−2 adrenergic receptors involved in DMT’s sedative properties [26,27]. This contrasts with selective blockers that primarily inhibit the α−1A adrenergic receptor subtype, potentially causing less interference with α−2 adrenergic receptors. This difference could be significant given the distribution of α−1 adrenergic receptor subtypes in the body. For instance, α−1A adrenergic receptors are present predominantly in the prostate and are associated with LUTS, whereas α−1B and α−1D receptors are implicated in systemic vascular resistance and are distributed in the spinal cord, brain, and vas deferens [7,28]. In contrast, there was no significant difference in the BIS scores of groups N and S, suggesting that the selectivity of these blockers for the α−1A adrenergic receptor subtype may limit their interference with α−2 adrenergic receptors, yielding no significant effect on the sedative properties of DMT.
Interestingly, despite significant differences between groups of BIS scores, which objectively measure sedation depth by monitoring electrical brain activity, scores for the MOAA/S scale, a subjective measure of consciousness levels [29,30], were not significantly different among the groups. The disparity between the BIS and MOAA/S results suggests that nonselective α−1 adrenergic blockers affect the sedative potency of DMT in terms of brain electrical activity without altering observable consciousness levels. The results, therefore, emphasize the need for careful interpretation of BIS scores when evaluating the depth of sedation in patients on nonselective α−1 adrenergic blockers.
Our study found no significant differences in MOAA/S scale scores, SpO2, or HR among the 3 groups, highlighting the significant impact of α−1 adrenergic blockers on MBP. In contrast, group N had significantly lower MBP values than the other 2 groups at each time point up to 30 min, except for at baseline. This is consistent with the known hypotensive mechanism of DMT, by which its action on central α−2 adrenergic receptors reduces sympathetic outflow and increases parasympathetic activity, thereby lowering blood pressure [14,17]. The reduced hypotensive response in groups NS and S could be related to the drugs’ action in blocking α−1 adrenergic receptors, which mediates vasoconstriction; therefore, blockade of these receptors could mitigate the hemodynamic effects of DMT [8,28,31]. Our results emphasize the importance of careful hemodynamic monitoring of patients receiving DMT for sedation, especially those not on α−1 adrenergic blockers. However, these results may reflect a more complex process than outlined here, and further research is necessary to confirm these findings and elucidate the underlying mechanisms.
Our study has several limitations. First, the small sample size and single-center nature of the study limit the generalizability of our results. Second, the observational design detects associations rather than causal relationships, and these may be influenced by confounding factors such as patient comorbidities, underlying diseases, and concurrent medications. Fourth, we did not control for the duration of use of α−1 adrenergic blockers or assess their plasma concentrations, either of which could have significantly affected the observed results. Furthermore, we did not explore the impact of different DMT dosing regimens, nor did it account for the potential influence of other concurrently used medications. Despite these limitations, our findings raise intriguing questions about the interaction of α−1 adrenergic blockers and DMT, particularly concerning its sedative effect during surgery. Future large-scale, multicenter, randomized controlled trials are required to confirm our results, assess potential long-term effects, and elucidate the precise mechanisms underlying these observed associations. It would also be beneficial to explore the potential impacts of the duration of α−1 blocker use on postoperative recovery and patient satisfaction.
Conclusions
Our findings suggest that nonselective α−1 adrenergic blockers can reduce the sedative effects of DMT, while selective α−1 blockers do not seem to impact DMT’s sedation levels significantly. Furthermore, α−1 blockers can moderate DMT-induced hypotension. These findings emphasize the necessity for personalized anesthesia strategies based on a patient’s specific α−1 blocker type, vigilant monitoring of vital signs during sedation with DMT, and possible dose adjustment for those on nonselective α−1 blockers. Additional research is warranted to fully understand these interactions and refine sedation approaches in urologic surgery patients.
Figures
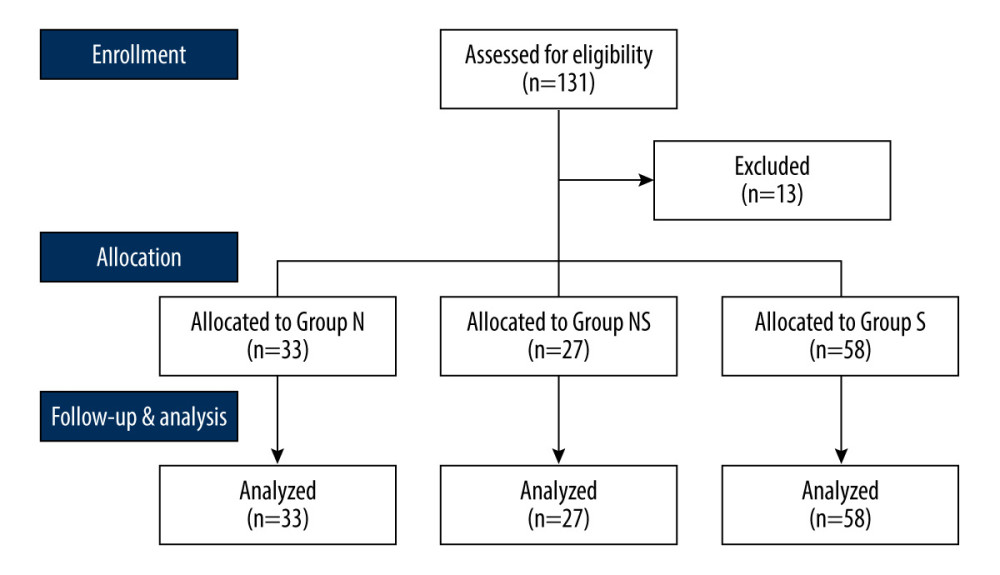 Figure 1. Study flow diagram. Design of the study to compare the impact of different types of α−1 adrenergic blockers on the sedative effects of the α−2 adrenergic agonist dexmedetomidine in 118 patients undergoing urologic surgery. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. This Figure was created by MS PowerPoint, version 2016, Microsoft.
Figure 1. Study flow diagram. Design of the study to compare the impact of different types of α−1 adrenergic blockers on the sedative effects of the α−2 adrenergic agonist dexmedetomidine in 118 patients undergoing urologic surgery. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. This Figure was created by MS PowerPoint, version 2016, Microsoft. 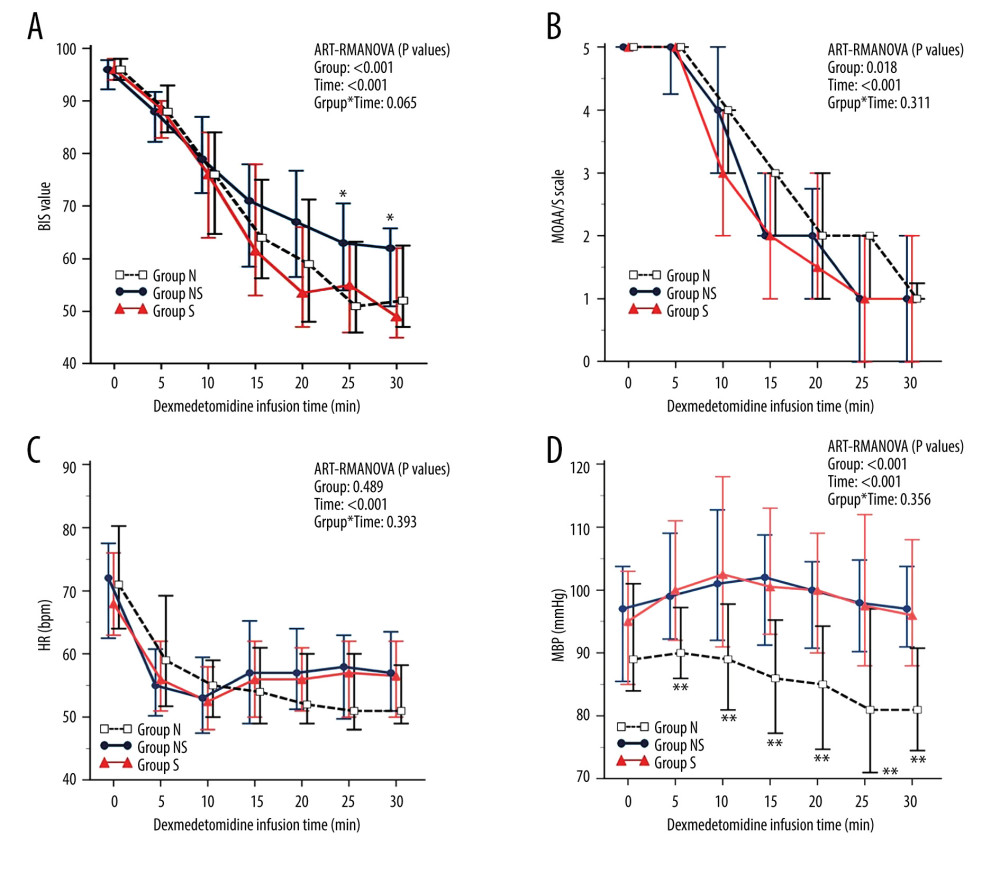 Figure 2. The changes in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), heart rate (HR) (C), and mean blood pressure (MBP) (D) after dexmedetomidine administration. After dexmedetomidine administration, group NS showed less sedation than groups N and S, as evidenced by higher BIS scores at 25 and 30 minutes. Group N consistently had lower MBP than groups NS and S. However, the MOAA/S scale and HR were similar across all groups. Data are presented as the median and interquartile range (IQR). * P<0.05 (group NS vs groups N and S) and ** P<0.01 (group N vs groups NS and S). ART-RMANOVA, aligned rank transformed ANOVA. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. BIS – bispectral index; MOAA/S scale – Modified Observer’s Assessment of Alertness/Sedation scale; HR – heart rate; MBP – mean blood pressure. This figure was created by MedCalc, version 20, MedCalc Software, Ltd.
Figure 2. The changes in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), heart rate (HR) (C), and mean blood pressure (MBP) (D) after dexmedetomidine administration. After dexmedetomidine administration, group NS showed less sedation than groups N and S, as evidenced by higher BIS scores at 25 and 30 minutes. Group N consistently had lower MBP than groups NS and S. However, the MOAA/S scale and HR were similar across all groups. Data are presented as the median and interquartile range (IQR). * P<0.05 (group NS vs groups N and S) and ** P<0.01 (group N vs groups NS and S). ART-RMANOVA, aligned rank transformed ANOVA. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. BIS – bispectral index; MOAA/S scale – Modified Observer’s Assessment of Alertness/Sedation scale; HR – heart rate; MBP – mean blood pressure. This figure was created by MedCalc, version 20, MedCalc Software, Ltd. Tables
Table 1. Bispectral index (BIS) scores (A) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores (B).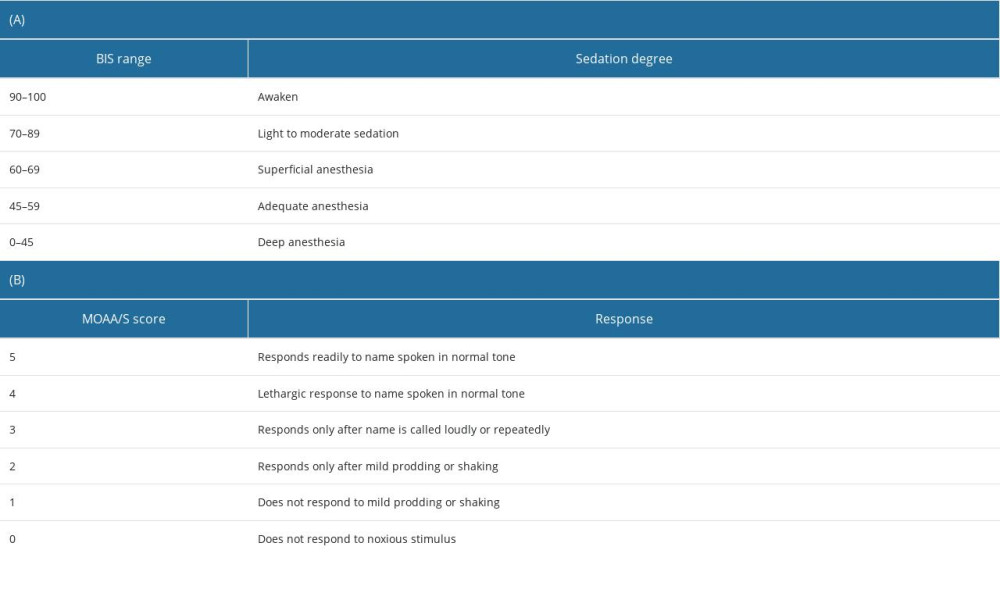 Table 2. Demographic data, medication, and patient characteristics.
Table 2. Demographic data, medication, and patient characteristics.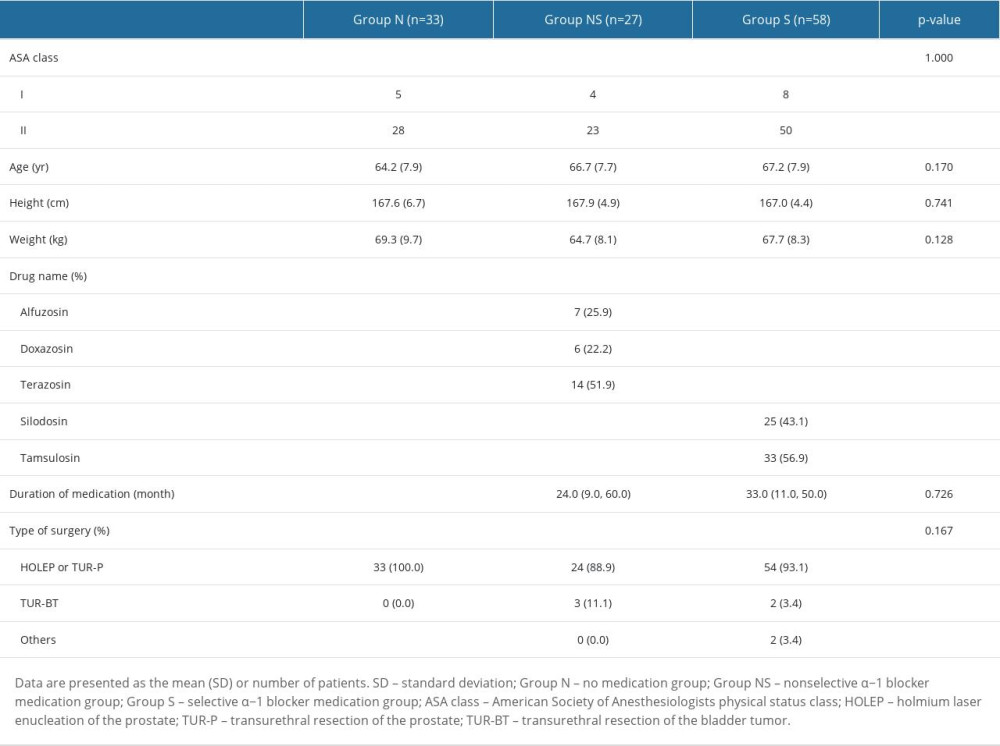 Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E).
Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E).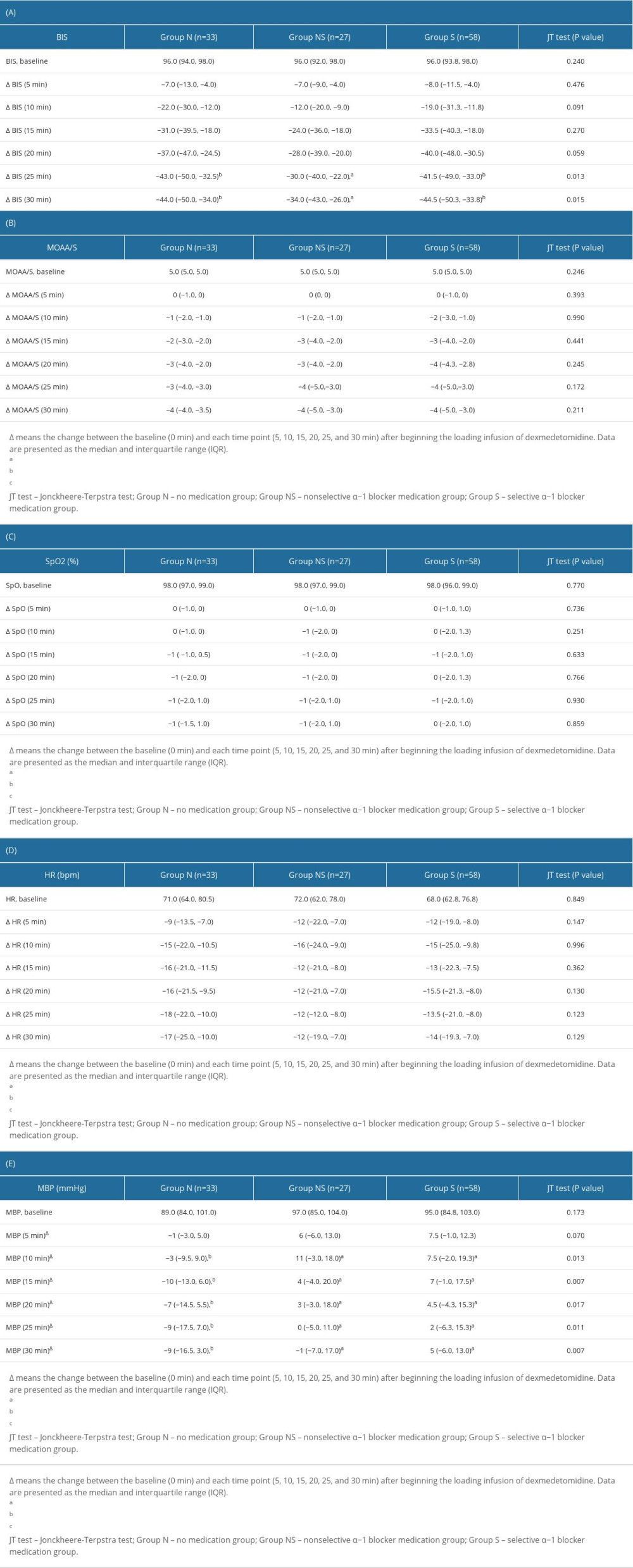
References
1. Chughtai B, Forde JC, Thomas DDM, Benign prostatic hyperplasia: Nat Rev Dis Primers, 2016; 2(1); 16031
2. Parsons JK, Benign prostatic hyperplasia and male lower urinary tract symptoms: Epidemiology and risk factors: Curr Bladder Dysfunct Rep, 2010; 5(4); 212-18
3. Lepor H, Alpha-blockers for the treatment of benign prostatic hyperplasia: Urol Clin North Am, 2016; 43(3); 311-23
4. Lokeshwar SD, Harper BT, Webb E, Epidemiology and treatment modalities for the management of benign prostatic hyperplasia: Transl Androl Urol, 2019; 8(5); 529-39
5. Handzel DM, Briesen S, Rausch S, Kälble T, Cataract surgery in patients taking alpha-1 antagonists: Know the risks, avoid the complications: Dtsch Arztebl Int, 2012; 109(21); 379-84
6. Zaman F, Bach C, Junaid I, The floppy iris syndrome – what urologists and ophthalmologists need to know: Curr Urol, 2012; 6(1); 1-7
7. Akinaga J, García-Sáinz JA, Pupo AS, Updates in the function and regulation of α(1) -adrenoceptors: Br J Pharmacol, 2019; 176(14); 2343-57
8. Andersson KE, Lepor H, Wyllie MG, Prostatic alpha 1-adrenoceptors and uroselectivity: Prostate, 1997; 30(3); 202-15
9. McConnell JD, Barry MJ, Bruskewitz RC, Benign prostatic hyperplasia: Diagnosis and treatment. Agency for Health Care Policy and Research: Clin Pract Guidel Quick Ref Guide Clin, 1994(8); 1-1
10. McVary KT, BPH: epidemiology and comorbidities: Am J Manag Care, 2006; 12(5 Suppl); S122-28
11. Koo C-H, Ryu J-H, Anesthetic considerations for urologic surgeries: Korean J Anesthesiol, 2020; 73(2); 92-102
12. Di Cianni S, Rossi M, Casati A, Spinal anesthesia: An evergreen technique: Acta Biomed, 2008; 79(1); 9-17
13. Liu SS, McDonald SB, Current issues in spinal anesthesia: Anesthesiology, 2001; 94(5); 888-906
14. Gertler R, Brown HC, Mitchell DH, Silvius EN, Dexmedetomidine: A novel sedative-analgesic agent: Proc (Bayl Univ Med Cent), 2001; 14(1); 13-21
15. Hong JY, Kim WO, Yoon Y, Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anaesthesia in elderly patients: Acta Anaesthesiol Scand, 2012; 56(3); 382-87
16. Ramsay MA, Luterman DL, Dexmedetomidine as a total intravenous anesthetic agent: Anesthesiology, 2004; 101(3); 787-90
17. Mantz J, Josserand J, Hamada S, Dexmedetomidine: New insights: Eur J Anaesthesiol, 2011; 28(1); 3-6
18. Jakob SM, Ruokonen E, Grounds RM, Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials: JAMA, 2012; 307(11); 1151-60
19. Riker RR, Shehabi Y, Bokesch PM, Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial: JAMA, 2009; 301(5); 489-99
20. Virtanen R, Savola JM, Saano V, Nyman L, Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist: Eur J Pharmacol, 1988; 150(1–2); 9-14
21. Doze VA, Chen BX, Maze M, Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors: Anesthesiology, 1989; 71(1); 75-79
22. Guo TZ, Tinklenberg J, Oliker R, Maze M, Central alpha 1-adrenoceptor stimulation functionally antagonizes the hypnotic response to dexmedetomidine, an alpha 2-adrenoceptor agonist: Anesthesiology, 1991; 75(2); 252-56
23. Liu J, Singh H, White PF, Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation: Anesth Analg, 1997; 84(1); 185-89
24. Nunes RR, Chaves IM, de Alencar JC, Bispectral index and other processed parameters of electroencephalogram: An update: Rev Bras Anestesiol, 2012; 62(1); 105-17
25. Wobbrock JO, Findlater L, Gergle D, Higgins JJ, The aligned rank transform for nonparametric factorial analyses using only anova procedures
26. Stone EA, Lin Y, Sarfraz Y, Quartermain D, Marked behavioral activation from inhibitory stimulation of locus coeruleus alpha1-adrenoceptors by a full agonist: Brain Res, 2009; 1291; 21-31
27. Chiu TH, Chen MJ, Yang YR, Action of dexmedetomidine on rat locus coeruleus neurones: Intracellular recording in vitro: Eur J Pharmacol, 1995; 285(3); 261-68
28. Docherty JR, The pharmacology of α(1)-adrenoceptor subtypes: Eur J Pharmacol, 2019; 855; 305-20
29. de Wit M, Epstein SK, Administration of sedatives and level of sedation: Comparative evaluation via the Sedation-Agitation Scale and the Bispectral Index: Am J Crit Care, 2003; 12(4); 343-48
30. LeBlanc JM, Dasta JF, Pruchnicki MC, Bispectral index values, sedation-agitation scores, and plasma Lorazepam concentrations in critically ill surgical patients: Am J Crit Care, 2012; 21(2); 99-105
31. Mathur RP, Nayak S, Sivaramakrishnan R, Jain V, Role of alpha blockers in hypertension with benign prostatic hyperplasia: J Assoc Physicians India, 2014; 62(9 Suppl); 40-44
Figures
 Figure 1. Study flow diagram. Design of the study to compare the impact of different types of α−1 adrenergic blockers on the sedative effects of the α−2 adrenergic agonist dexmedetomidine in 118 patients undergoing urologic surgery. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. This Figure was created by MS PowerPoint, version 2016, Microsoft.
Figure 1. Study flow diagram. Design of the study to compare the impact of different types of α−1 adrenergic blockers on the sedative effects of the α−2 adrenergic agonist dexmedetomidine in 118 patients undergoing urologic surgery. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. This Figure was created by MS PowerPoint, version 2016, Microsoft. Figure 2. The changes in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), heart rate (HR) (C), and mean blood pressure (MBP) (D) after dexmedetomidine administration. After dexmedetomidine administration, group NS showed less sedation than groups N and S, as evidenced by higher BIS scores at 25 and 30 minutes. Group N consistently had lower MBP than groups NS and S. However, the MOAA/S scale and HR were similar across all groups. Data are presented as the median and interquartile range (IQR). * P<0.05 (group NS vs groups N and S) and ** P<0.01 (group N vs groups NS and S). ART-RMANOVA, aligned rank transformed ANOVA. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. BIS – bispectral index; MOAA/S scale – Modified Observer’s Assessment of Alertness/Sedation scale; HR – heart rate; MBP – mean blood pressure. This figure was created by MedCalc, version 20, MedCalc Software, Ltd.
Figure 2. The changes in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), heart rate (HR) (C), and mean blood pressure (MBP) (D) after dexmedetomidine administration. After dexmedetomidine administration, group NS showed less sedation than groups N and S, as evidenced by higher BIS scores at 25 and 30 minutes. Group N consistently had lower MBP than groups NS and S. However, the MOAA/S scale and HR were similar across all groups. Data are presented as the median and interquartile range (IQR). * P<0.05 (group NS vs groups N and S) and ** P<0.01 (group N vs groups NS and S). ART-RMANOVA, aligned rank transformed ANOVA. Group N – no medication group; Group NS – nonselective α−1 blocker medication group; Group S – selective α−1 blocker medication group. BIS – bispectral index; MOAA/S scale – Modified Observer’s Assessment of Alertness/Sedation scale; HR – heart rate; MBP – mean blood pressure. This figure was created by MedCalc, version 20, MedCalc Software, Ltd. Tables
 Table 1. Bispectral index (BIS) scores (A) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores (B).
Table 1. Bispectral index (BIS) scores (A) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores (B). Table 2. Demographic data, medication, and patient characteristics.
Table 2. Demographic data, medication, and patient characteristics. Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E).
Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E). Table 1. Bispectral index (BIS) scores (A) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores (B).
Table 1. Bispectral index (BIS) scores (A) and Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale scores (B). Table 2. Demographic data, medication, and patient characteristics.
Table 2. Demographic data, medication, and patient characteristics. Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E).
Table 3. The change (Δ) in the bispectral index (BIS) (A), Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (B), oxygen saturation (SpO2) (C), heart rate (HR) (D), and mean blood pressure (MBP) (E). In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








