21 November 2023: Review Articles
Circadian Rhythms in Cardiovascular Function: Implications for Cardiac Diseases and Therapeutic Opportunities
Jiayue LinDOI: 10.12659/MSM.942215
Med Sci Monit 2023; 29:e942215
Abstract
ABSTRACT: Circadian rhythms are internal 24-h intrinsic oscillations that are present in essentially all mammalian cells and can influence numerous biological processes. Cardiac function is known to exhibit a circadian rhythm and is strongly affected by the day/night cycle. Many cardiovascular variables, including heart rate, heart rate variability (HRV), electrocardiogram (ECG) waveforms, endothelial cell function, and blood pressure, demonstrate robust circadian rhythms. Many experiential and clinical studies have highlighted that disruptions in circadian rhythms can ultimately lead to maladaptive cardiac function. Factors that disrupt the circadian rhythm, including shift work, global travel, and sleep disorders, may consequently enhance the risk of cardiovascular diseases. Some cardiac diseases appear to occur at particular times of the day or night; therefore, targeting the disease at particular times of day may improve the clinical outcome. The objective of this review is to unravel the relationship between circadian rhythms and cardiovascular health. By understanding this intricate interplay, we aim to reveal the potential risks of circadian disruption and discuss the emerging therapeutic strategies, specifically those targeting circadian rhythms. In this review, we explore the important role of circadian rhythms in cardiovascular physiology and highlight the role they play in cardiac dysfunction such as ventricular hypertrophy, arrhythmia, diabetes, and myocardial infarction. Finally, we review potential translational treatments aimed at circadian rhythms. These treatments offer an innovative approach to enhancing the existing approaches for managing and treating heart-related conditions, while also opening new avenues for therapeutic development.
Keywords: Arrhythmias, Cardiac, Chronobiology Disorders, Circadian Rhythm, Heart Rate
Background
Circadian rhythms are a natural phenomenon that consists of approximately 24-h intrinsic oscillations that regulate the sleep–wake cycle and various other biological processes [1]. In mammalians, circadian rhythms are affected by 2 sets of circadian clocks: the central/primary clock and the periphery clock. The central clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, whereas peripheral clocks are present in tissues [2,3].
The central circadian clock system plays a pivotal role in the generation of circadian rhythms in mammals [4]. In this way, it regulates the biological clocks of peripheral organs (eg, heart, liver, lung, and brain) through the nervous system and endocrine hormones, thereby fine-tuning the biological clock to changes in the external environment [5]. Stimulation of SCN with external stimuli such as light signals triggers the production of biological rhythms which align internal body changes with the external environment [6]. The peripheral biological clocks exist in cells, tissues, and organs, where they function by directly modulating the expression of target genes. In so doing, they maintain homeostasis of the local internal environment [7].
Circadian processes can be viewed as an integrated system composed of genes that alter various aspects of organ, tissue, and cell function, including in the myocardium. Various studies have identified the presence of peripheral clocks in cardiovascular cells [8,9]. Numerous cardiovascular variables, including heart rate, cardiac contractility, stroke volume, ECG waveforms (RR, PR, QRS, and QT intervals), endothelial function, and blood pressure, exhibit robust circadian rhythms [10–14]. In humans, the circadian clock system is an evolved process synchronized with the alternating light-to-dark changes of the external environment – a phenomenon termed entrainment. Consequently, humans are awake during the daytime and sleep at night. Numerous biological processes and phenotypes, including blood hormone levels [15], body temperature [16], sleep [17], and locomotor activity display a circadian pattern [18]. In the cardiovascular system, circadian rhythms modulate cardiac contraction and metabolic activities and maintain other functions, such as heart rate and blood pressure, which are higher during the daytime and lower at night [15].
Circadian disruption broadly refers to multiple types of circadian clock disturbances, including circadian misalignment [19] and circadian desynchrony [20] or desynchronization [21]. These disturbances can manifest across various biological levels, from cellular and tissue scales to organismal and systemic scales. Circadian misalignment is a mismatch between an individual’s internal circadian clock and their external environment or social schedule. Circadian desynchrony or desynchronization both refer to a variance in the cycles of 2 or more rhythms. Both concepts can be quantified by measuring the phase angle differences and comparing the estimated durations of the rhythms.
Cardiac disorders are multifactorial, and circadian disruption has been shown to enhance their risk by 40–63% [22–24], particularly in diseases such as arrhythmia and acute myocardial infarction [25]. This paper reviews basic studies investigating the mechanisms of circadian clock disorders, as well as clinical studies exploring the impact of circadian clock disorders on the cardiovascular system. In this review, we provide a detailed discussion of the significance and impact of circadian rhythms on cardiovascular function. Moreover, we dissect the correlations between circadian rhythms and cardiovascular diseases. Lastly, a summary of potential translational therapies targeting circadian rhythms are reviewed to offer insights into the use of current management and treatment strategies of cardiac diseases and guide future development of new therapies.
Circadian Genes
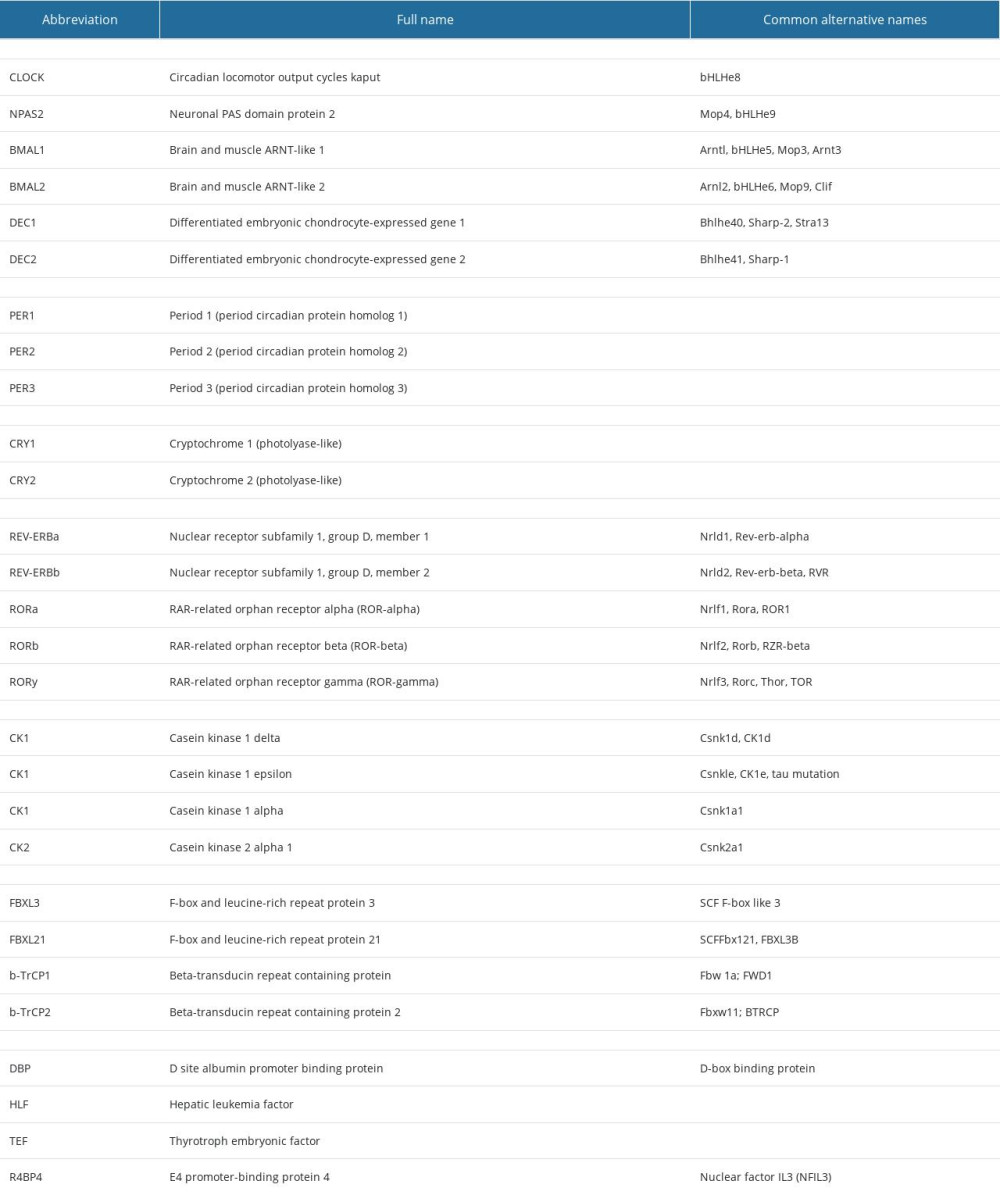
The circadian clock in most mammalian cells is driven by an autoregulatory transcriptional–translation feedback network. This circadian clock is regulated by a set of molecules that form self-sustained transcriptional feedback loops with a 24-h cycle, ultimately giving rise to fluctuations in the proteome and cellular activity [9]. The core circadian “clock gene” products are shown in Table 1.
In mammals, the circadian clock machinery includes the core circadian molecules BMAL1 and CLOCK (and its paralog, NPAS2). Upon heterodimerization, the BMAL1: CLOCK complex binds to enhancer-box (E-box) domains in the promoters of various target genes. A key function of this complex is the regulation of 2 cryptochrome genes (
In addition to the feedback loops, circadian rhythms are governed by a range of alternative processes. For instance, circadian rhythms are modulated by polyadenylation, histone modification, methylation, and non-coding RNAs. The precision and robustness of circadian rhythms rely heavily on the indispensability of translational and epigenetic processes [28]. By modulating the expression of different components of the circadian clock process, the clock can regulate a plethora of cellular processes, such as signal transduction, transcription, translation, metabolism, and ion homeostasis. Consequently, it is estimated that circadian clocks regulate a 3–16% of the transcriptome [29].
Circadian Clock-Related Cardiac Function
LIGHT:
The endogenous diurnal rhythm of the SCN is synchronized to the diurnal cycle most effectively by light. Herein, light is the most potent external cue for SCN, which can affect neuronal firing frequency, alter circadian gene expression [48], and affect other peripheral clock functions (eg, in the heart). Because light influences daily routine, cardiovascular risk also shows a day–night difference. Most cardiovascular incidents occur in the morning from 6 a.m. to noon), and there is another peak from 6 p.m. to midnight, which indicates a bimodal pattern of risk [49]. The “morning shift” in cardiac sympatho-vagal balance is probably involved in the risk of cardiovascular disease at that time. Therefore, light-caused circadian clock disruption may be a risk factor in cardiovascular disease, contributing to increased HR and HRV during high-risk periods. Because the endogenous circadian rhythm is best synchronized to the diurnal change by light [50], it is reasonable to infer that exposure to light regulates cardiovascular function [51]. Thus, understanding the interaction between light exposure, circadian rhythms, and cardiovascular health may suggest potential avenues for formulating preventive and therapeutic strategies.
PHYSICAL ACTIVITY:
Physical activity is also an important factor in circadian rhythm orchestration and cardiovascular function. It is well known that the circadian rhythm of blood pressure is regulated by physical activity. Cardiovascular events have demonstrated a positive correlation with physical activity during the early morning hours in individuals with hypertension. However, the surge in cardiovascular incidents during this morning peak cannot be exclusively attributed to daily variations in external factors such as activity [52]. Rather, it is more likely to be associated with the interplay of activity-induced circadian alterations in blood pressure, vascular tone, catecholamines, platelet aggregation, elevation in plasminogen activator inhibitor-1, heart rate (HR), and fluctuations in the beat-to-beat interval. Conversely, the cardiometabolic requirements exhibit significant fluctuations throughout the day and night, aligning with physical activity patterns. A study indicated that physical and mental activities can incite ischemic episodes during the morning hours in individuals with stable coronary artery disease [53]. Additionally, an activity-independent circadian influence maintains a heightened level of ischemic incidents during the awake period. Consequently, the temporal fluctuations in cardiovascular indicators stem from a combination of external activity and intrinsic circadian influences [54].
FEEDING:
Mammals exhibit daily anticipatory activity to cycles of food availability. Early studies have demonstrated an SCN-independent food-entrainable oscillator (FEO), which is separate from the light-entrained oscillator (LEO) located in the SCN. The FEO is characterized by increased locomotor activity in the hours preceding food delivery. This anticipatory behavior is also referred to as food anticipatory activity (FAA) [55]. It has previously been well-documented that the circadian rhythm is generated by circadian genes at the molecular level, and this may be the molecular basis of generation of food-entrained rhythms. Numerous studies in GM mice have revealed the role of clock gene and FAA [56–59]. It was reported that Clock-mutant mice still have FAA, which demonstrated that the Clock gene is not necessary for expression of FEO, but suggests that FEO is mediated by a molecular mechanism distinct from that of the SCN [56]. Dudley et al found that NPAS2 knockout mice also exhibit similar results regarding FEO [57]. Studies have also reported that food entrainment decreases in BMAL1-deficient mice [58] and Cry1/Cry2-deficient and Per2-mutant (but not Per1-deficient) mice [59]. Together, these studies provide strong evidence for a functional food-entrainable oscillator. In addition to this, the occurrence of FAA in animals with complete SCN lesions reflects the presence of an SCN-independent oscillator acting as a pacemaker that regulates locomotor activity rhythms. The FEO misalignment may lead to some metabolic changes; for example, Clock-mutant mice showed an altered feeding rhythm, hyperphagia, and obesity, and a metabolic syndrome of hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia. The alternation of glucose homeostasis is associated with severe comorbidities of cardiovascular disease [60].
Effects of Circadian Disruption on Cardiovascular Function
CARDIAC HYPERTROPHY:
Various genetically engineered mice with circadian gene modifications show signs of cardiac hypertrophy and ventricular dysfunction. Cardiomyocyte-specific CLOCK-mutant (CCM) mice, which express a dominant negative CLOCK-mutant protein lacking the transactivation domain, subjected to circadian disruption exhibited lower heart rates, attenuated diurnal variations, lower cardiac efficiency, and a higher mortality rate relative to control animals [77]. Mice displayed altered fractional shortening (FS), left ventricular ejection fraction, septal wall thickness, increased biventricular mass, and increased biventricular weight-to-body weight ratio compared to controls [78,79]. The cardiomyocyte cross-sectional area was also enhanced, indicating cellular hypertrophy in CCM hearts. SERCA2a, MHC-α, and MHC-β were significantly elevated by circadian disruption in both CCM and control mice, and hypertrophy is known to be time-of-day dependent. Upon administration of isoproterenol, a pro-hypertrophic agonist, during the awake-to-sleep phase transition (ZT 0) or during the sleep-to-awake phase transition (ZT 12) for 7 consecutive days, biventricular weight-to-body weight ratio was found to be highest at ZT 0 in CCM mice relative to controls. While isoproterenol-induced cardiac hypertrophy exhibited time-of-day variation in control mice, this oscillation was abolished in CCM mice. Consistently, ANF, a marker of hypertrophy, was significantly elevated in isoproterenol-treated CCM hearts at ZT 0 and ZT 12 in the absence of a diurnal variation [79]. Moreover, genetic deletion of BMAL1 predisposed the heart to hypertrophic growth. Cardiac-specific BMAL1 knockout (BMAL1cko) mice exhibited significantly enlarged hearts, with a 15% increase in left ventricular weight from 4 weeks of age. Progressive eccentric hypertrophy-induced ventricular dilation was observed in 32-week-old BMAL1cko mice. The fractional shortening also significantly decreased (24.3%) and enlarged (17.12%) the left ventricular internal diameter in 36-week-old BMAL1cko mice relative to control littermates [80]. Moreover, reduced BMAL1 expression in the heart was correlated with a marked reduction in amplitude of the circadian rhythms of circadian genes and their downstream targets [81]. MHC-α and MHC-β mRNA levels were significantly reduced [80], while the expression of the collagen isoforms Col3a1 and Col4a1 and the cardiac dysfunction markers ANF and MHC-β were elevated. Additionally, Serca2a expression [81] and DBP diurnal variations were suppressed, while expression of the hypertrophic marker MCIP1 was elevated [79]. Together, these observations suggest that reduced BMAL1 expression enhances collagen expression in the myocardium, impairing myocardial contractility and causing subsequent cardiac dysfunction.
Circadian disruption exhibits complex effects on cardiac hypertrophy in a transverse aortic constriction (TAC) animal model. A study of 7-week-old rats did not find any differences in the circadian expression of the clock genes Bmal1, Clock, Per1–3, and CRY1–2, in rats subjected to TAC relative to age-matched controls. However, rhythmic expression of the clock-controlled transcription factors, DBP, HLF, and TEF was blunted [82]. Indeed, DBP/TEF/HLF triple knockout mice exhibit cardiac hypertrophy, left ventricular dysfunction, increased morbidity, and shortened life span [83]. Thus, DBP, HLF, and TEF might have major roles in cardiac function and hypertrophy. Housing TAC mice in 20-h light/dark cycles exacerbated pathophysiological signs, such as enhanced LVEDD and LVESD, as well as decreased FS and contractility. However, these TAC mice exhibit reduced hypertrophy and myocyte size relative to TAC mice housed in 24-h light/dark cycles [84]. Indicating that reduction of circadian disruption by housing under a shorter light/dark cycle might minimize cardiomyocyte hypertrophy, but not structural remodelling in TAC mice. Interestingly, transferring TAC mice from 20-h to 24-h light/dark conditions for 8 weeks rescued the pathological cardiac remodelling, resulting in reduced signs of vascular smooth muscle layer hyperplasia, enhanced myocyte volume, normal Per2 and Bmal1 expression relative to controls, and expression of hypertrophy markers, including ANF, BNP, ACE, and COLLAGEN [84]. Together, these findings showed that circadian disruption may rescue the cardiac hypertrophy mediated by the clock output targets DBP, HLF, and TEF. These studies revealed that such reversal of cardiac hypertrophy is mediated by the inhibition of circadian clock genes, including Bmal1 and Per2. These findings suggest that deregulation of circadian gene dynamics predispose individuals to cardiac maladaptations, improving our understanding of the genes associated with human cardiac diseases, especially in people exposed to irregular circadian patterns or performing shift work. This review lays the foundation for developing potential therapeutic interventions based on circadian gene pathways to combat cardiac dysfunction.
ARRHYTHMIA:
In humans, circadian disruption can alter the function of both the atria and ventricles, as short-term circadian disruption causes atrial dysfunction, while prolonged disruption causes ventricular dysfunction. A study involving 11 participants observed a marked increase in atrial premature beats after working a 24-h shift [7]. Ventricular dysfunction induced by circadian disruption correlates with the duration of exposure. Although premature ventricular contractions were not observed after 1 night shift [7], a 24-h ECG monitoring study indicated that significant exacerbation in ventricular dysfunction occurs with increased duration of exposure to circadian disruption, which correlates with cardiac disease. In a study of physicians with 58–106 months of night-shift experience, the number of ventricular premature beats between midnight and 6 am on a 24-h on-call shift was significantly elevated [85]. Following a 1-year study, Van Amelsvort et al reported similar findings, demonstrating that the frequency of premature ventricular complexes was significantly higher in 49 night shift workers relative to 22 daytime workers, and was significantly correlated with the number of work nights [64]. Ventricular ectopic beats occurrence also was correlated with frequency and duration of exposure to night shifts [86]. Individuals routinely working night shifts may face increased cardiac risks; therefore, people working prolonged night shift schedules may benefit from regular cardiac monitoring, especially if they display or report any symptoms of cardiac distress. This would allow for early detection and intervention if arrhythmias or other cardiac issues are identified.
A 10-year study reported significantly prolonged QTc in 158 night shift workers relative to 75 daytime workers but did not find differences in systolic or diastolic blood pressure [87]. Similar observations have been reported by Meloni et al, who observed significant QTc prolongation in participants with abnormal repolarization phases. However, it did not correlate with other ECG abnormalities, including conduction, heart rhythm disorders, BMI, or age. Prolonged QTc interval may lengthen action potential and is a putative pro-arrhythmogenic factor, especially for long QT (LQT) syndrome. LQT 1, 2, and 3 are the most common forms of LQT syndrome and result from cardiac ion channel dysfunction. LQT 1 and 2 result in decreased outward potassium currents, while LQT 3 enhances inward sodium currents [88]. In LQT1 patients, only 3% of abnormal ECG events occur during the night-time rest/sleep phase. However, in LQT2 and 3, abnormal ECG events occurring at night have been found to be 29% and 39%, respectively. Moreover, lethal events during rest/sleep without arousal occur in 49% and 64% of LQT 2 and LQT3 cases, respectively [89]. A recent clinical study of 26 LQT patients found that LQT1 patients exhibit modest QTc shortening, LQT2 patients exhibit modest lengthening at night versus daytime, and LQT3 patients exhibit clear QTc lengthening at night. These changes are not explained by heart rate changes or by the use of beta-blockers [90] and are thought to be associated with sodium and potassium channels. Moreover, the voltage-gated Na+ channel, Nav1.5, exhibits a circadian expression pattern in the heart, and circadian rhythm is dampened by BMAL1 ablation [91]. Similarly, the potassium channels Kv1.5, Kv4.2 and ERG1 exhibit circadian variations in gene expression and electrophysiological current function [92,93]. Circadian disruption modifies key ion channel activities regulated by circadian genes, inducing prolonged QTc intervals. This may explain the higher cardiac dysfunction, including sudden cardiac death, observed in shift workers.
ISCHEMIC HEART DISEASE:
Circadian rhythms play an indispensable role in the manifestation and progression of ischemic heart disease. Intriguingly, some cardiac diseases preferentially occur at a particular time of the day or night. In humans, attacks from ventricular arrhythmias and sudden cardiac death are common in the morning (the start of the awake period) [41]. Moreover, single-nucleotide polymorphisms in genes encoding for circadian clock components have been linked to an increased risk of myocardial infarction [94]. Durgan et al revealed that the heart’s tolerance to ischemia/reperfusion (I/R) damage varies based on the time of day, with the most significant damage occurring at the sleep-to-wake transition. This time-of-day dependence in I/R tolerance is mediated by the cardiomyocyte circadian clock [95]. Gaining insight into and potentially influencing this biological clock could present innovative approaches for addressing cardiac dysfunctions caused by ischemia. This is particularly relevant given that the highest-risk time period coincides with the typical occurrence of heart attacks in humans. A recent study by Zhao et al found that shift workers had an increased susceptibility to myocardial infarction reperfusion injury [96]. Shift work was associated with increased infarct size and increased risk of major adverse cardiac events. Consistent with the clinical findings, shift work simulation in sheep and mice worsened reperfusion injury in acute myocardial ischemia. Mechanistically, it was identified that a novel nuclear receptor subfamily 1 group D member 1/cardiotrophin-like cytokine factor 1 axis in the heart played a key role in regulating the pathological effects of shift work on the myocardium [96]. Using a mouse model of short-term rhythm disruption, Faisal et al found that disruption of diurnal rhythm after MI impaired healing and exacerbated maladaptive cardiac remodelling, demonstrating that the short-term rhythm disruptions interfered with an early inflammatory phase of LV remodelling, adversely affecting the innate immune infiltration, and adversely affecting scar formation [97]. Rhythm disruption can also dramatically alter hepatic clock gene expression, bile acid metabolism, and lipid homeostasis, contributing to dyslipidemia [98].
DIABETES:
The relationship between circadian rhythms and diabetes is intricately connected, and understanding this association is pivotal for managing the disease effectively. Studies have demonstrated the link between disruptions to circadian rhythms, such as those experienced by shift workers or irregular sleep patterns, can increase the risk of developing diabetes. Irregular schedules can lead to impaired glucose tolerance, insulin resistance, and obesity [99]. Circadian rhythms have a substantial impact on glucose metabolism regulation. In humans, glucose tolerance and insulin sensitivity follow a natural daily cycle, peaking in the morning and reaching their lowest point at night. This diurnal fluctuation can influence blood glucose levels, as the level of insulin diminishes during night-time hours, potentially resulting in elevated fasting blood glucose levels among individuals with diabetes. Furthermore, eating meals at consistent times that align with the natural circadian rhythm may help stabilize blood glucose levels. Skipping meals or eating irregularly can lead to glucose spikes and contribute to diabetes mismanagement [74].
Circadian Rhythm Modulation for Cardiovascular Disease Treatment
The essential role of circadian rhythms in biological processes is fundamental in both health and disease. Recognizing and understanding the importance of circadian rhythms has given rise to the idea that circadian clocks could potentially be leveraged for treating and managing cardiovascular diseases. Circadian rhythms display osculating patterns; therefore, developing a treatment that can take advantage of circadian governance may have great therapeutic value. Hence, a crucial therapeutic approach involves mitigating these risks during the periods of highest vulnerability in the circadian cycle or reinstating the circadian phase and amplitude to their typical patterns.
A key consideration of therapeutically targeting the circadian rhythm is timing. Although patient gender and comorbidities are often considered during treatment, the timing of the treatment is often overlooked. Many clinical studies investigating cardiovascular disease treatment also fail to mention the timing of treatment administration [25]. For example, hypertension exhibits a higher occurrence in the early morning. Hence, it is noteworthy that most once-daily antihypertensive drugs are prescribed for use at around 8 am. This timing leads to peak drug plasma levels during the day, when adverse effects are most common. A study by Hermida et al revealed that administration of an angiotensin-converting enzyme inhibitor at bedtime resulted in lower nocturnal blood pressure compared to morning administration [100], and also found that administration of 5 mg/day of ramipril at bedtime effectively reduced BP for the entire 24-h period. In contrast, when the drug was taken in the morning, its efficacy was reduced, lasting only for 16 h and did not effectively lower nocturnal blood pressure [100]. Night-time BP has been determined to be a better predictor of cardiovascular mortality compared to daytime or 24-h BP. Therefore, controlling nocturnal BP is crucial. This is particularly important because increased nocturnal BP and a non-dipping pattern are linked to end-organ injury and cardiovascular events, and this decrease improved cardiovascular outcomes [100,101]. Furthermore, a recent study found that acutely targeting the circadian driver REV-ERB using SR9009 (non-selective REV-ERBα/β dual agonist) at the time of perfusion over-prolonged cardiac recovery and repair following myocardial ischemia-reperfusion injury [102]. The single-dose treatment resulted in an estimated 50% reduction in infarct size and decreased risk of heart failure development. Treatment resulted in the downregulation of the cardiac NLRP3 inflammasome, reducing the inflammatory response and allowing the healing process to become dysregulated.
An alternate treatment approach could involve shifting the phase of the circadian clock to a particular phase within physiological rhythms or a specific time-of-day configuration that would yield the most advantageous outcomes in a particular scenario. For instance, myocardial infarction typically occurs in the morning, but manipulating the functional and molecular components of the heart to mimic an environment similar to the afternoon or evening might hold great potential. Myocardial infarction patients typically present with increased levels of thrombocytes in the morning, when the risk of plaque rupture is significantly higher. However, Bonten et al observed that administration of aspirin at bedtime significantly reduced platelet activity in the morning in comparison to the morning administration of aspirin [103]. The use of time-specific therapy has also been implemented in stem-cell-mediated repair. Stem cells derived from both cardiac and non-cardiac patients (multipotent stem cells) have undergone evaluation for their potential regenerative and paracrine effects in the clinical setting [104]. Stem cells have been shown to have circadian clocks, but investigation into whether employing stem cells at a particular time of day enhances patient outcomes remains unclear.
Another primary approach to minimize disruptions in the circadian rhythm involves reducing desynchronization and aversion. This can be accomplished by exposing patients to regular 24-h signals, ensuring adequate light exposure, and maintaining a dark environment during the night. When circadian disruption is evitable, such as during shift work, several strategies have been developed to reduce the harmful effects, including managing shift schedules, taking short naps during shifts, and regular food intake, which together may limit the impact of shift work [105].
Conclusions
Circadian clocks orchestrate various critical biological processes in virtually all cardiovascular cell types and a diverse spectrum of cardiovascular physiologies undergo circadian oscillations, including blood pressure, ECG pattern, heart rate, and metabolism. The development, progression, and outcome of various cardiovascular diseases are closely linked to aberrant circadian rhythms. Disruption of this circadian regulation has been proven to lead to malfunction in cellular or organ processes, ultimately triggering pathological conditions. A more comprehensive understanding of the molecular mechanisms that underlie cardiovascular diseases holds the potential to yield novel treatment strategies or improve current strategies. Therapeutically, there is increasing recognition of the potential benefits of treatments for modulating the circadian rhythm in cardiovascular disease. Timing of treatment, often overlooked in clinical studies, can have profound effects on efficacy. For instance, night-time administration of some drugs has been shown to improve cardiovascular outcomes compared to morning doses. This circadian–cardiovascular connection underscores the need for clinicians and researchers to optimize the timing of interventions and to develop strategies to mitigate circadian disruptions. Such an understanding could unlock novel therapeutic avenues and improve clinical outcomes of patients with cardiovascular diseases.
Figures
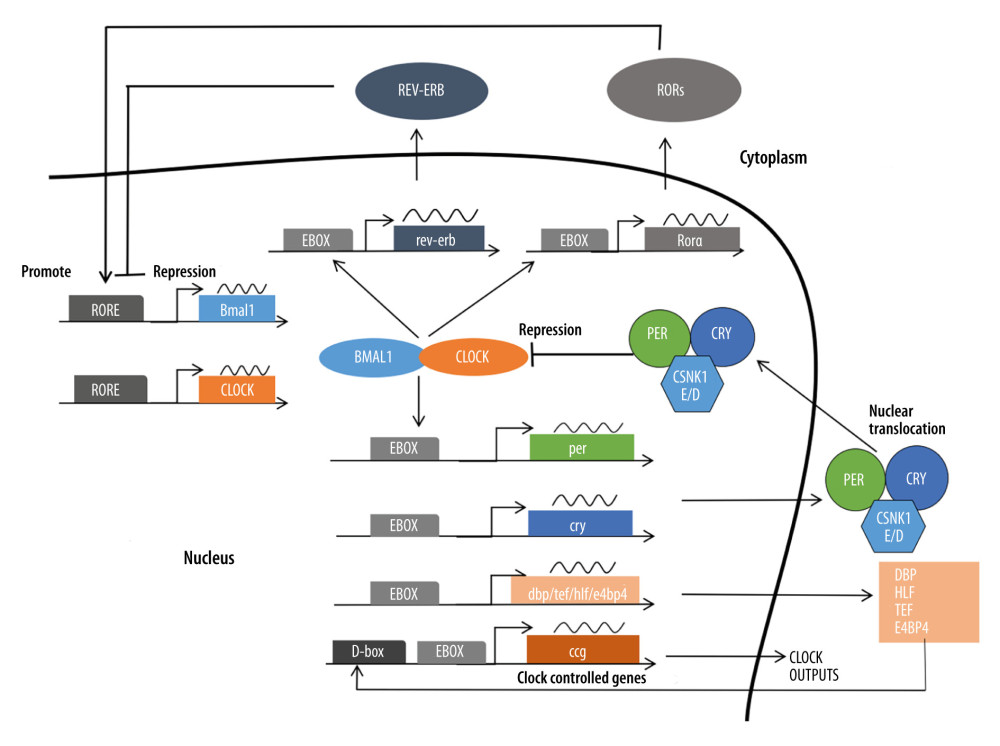 Figure 1. The molecular mechanism of the circadian clock in mammals. Constituting the core circadian clock is an autoregulatory transcriptional feedback loop involving the activators CLOCK and BMAL1 and their target genes Per1, Per2, Cry1, and Cry2, whose gene products form a negative-feedback repressor complex.
Figure 1. The molecular mechanism of the circadian clock in mammals. Constituting the core circadian clock is an autoregulatory transcriptional feedback loop involving the activators CLOCK and BMAL1 and their target genes Per1, Per2, Cry1, and Cry2, whose gene products form a negative-feedback repressor complex. 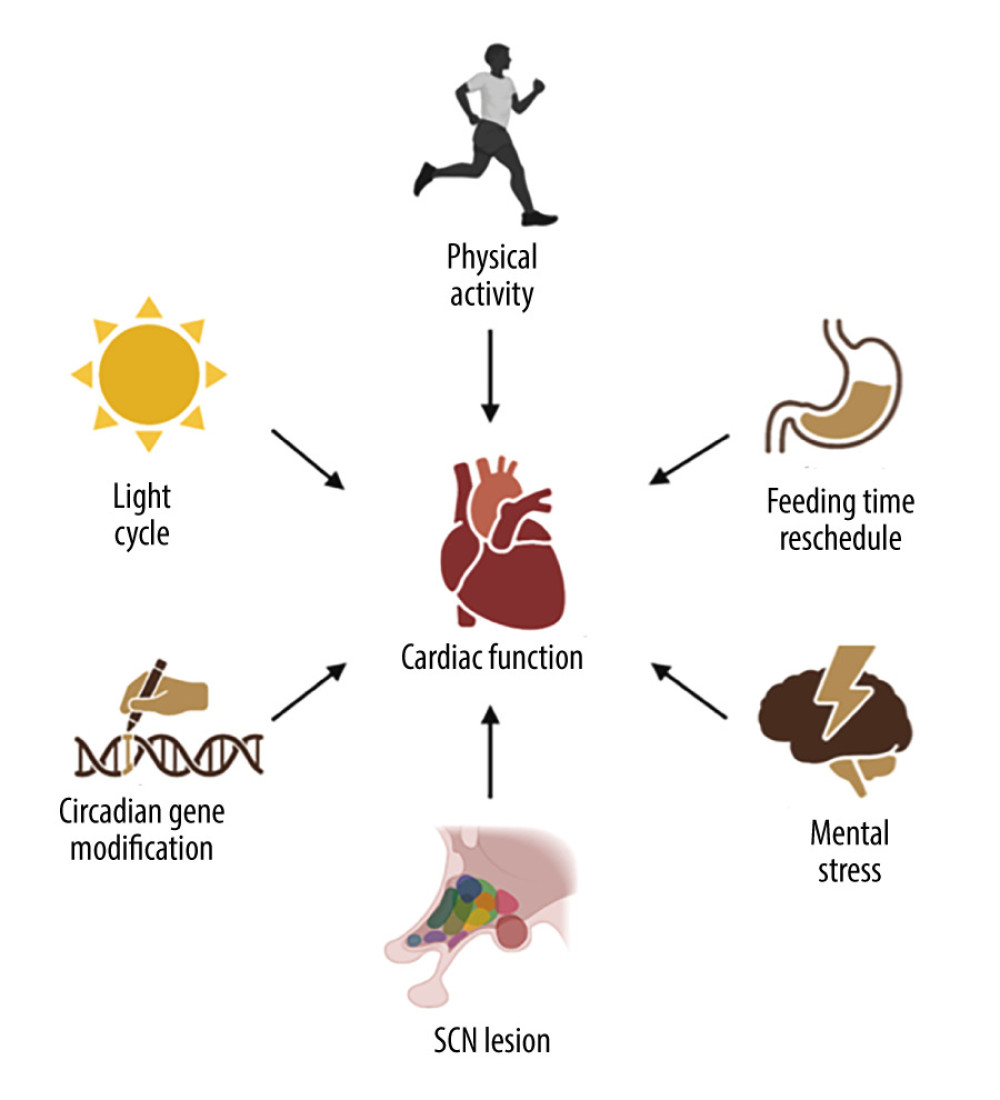 Figure 2. Environmental and behavioral cues to trigger circadian disruption in animals. Circadian disruption can be triggered by environmental cues, mainly light/dark cycle manipulations, behavioral cues such as feeding time rescheduling, physical activities, and mental stress, suprachiasmatic nuclei lesions, and gene modification.
Figure 2. Environmental and behavioral cues to trigger circadian disruption in animals. Circadian disruption can be triggered by environmental cues, mainly light/dark cycle manipulations, behavioral cues such as feeding time rescheduling, physical activities, and mental stress, suprachiasmatic nuclei lesions, and gene modification. References
1. Hastings MH, Reddy AB, Maywood ES, A clockwork web: Circadian timing in brain and periphery, in health and disease: Nat Rev Neurosci, 2003; 4(8); 649-61
2. Ralph M, Foster R, Davis F, Menaker M, Transplanted suprachiasmatic nucleus determines circadian period: Science, 1990; 247(4945); 975-78
3. King DP, Takahashi JS, Molecular genetics of circadian rhythms in mammals: Annu Rev Neurosci, 2000; 23(1); 713-42
4. Sanchez REA, Kalume F, de la Iglesia HO, Sleep timing and the circadian clock in mammals: Past, present and the road ahead: Semin Cell Dev Biol, 2022; 126; 3-14
5. Emens JS, Burgess HJ, Effect of light and melatonin and other melatonin receptor agonists on human circadian physiology: Sleep Med Clin, 2015; 10(4); 435-53
6. Hastings MH, Maywood ES, Brancaccio M, Generation of circadian rhythms in the suprachiasmatic nucleus: Nat Rev Neurosci, 2018; 19(8); 453-69
7. Adams SL, Roxe DM, Weiss J, Ambulatory blood pressure and holter monitoring of emergency physicians before, during, and after a night shift: Acad Emerg Med, 1998; 5(9); 871-77
8. Do MT, Yau KW, Intrinsically photosensitive retinal ganglion cells: Physiol Rev, 2010; 90(4); 1547-81
9. Mohawk JA, Green CB, Takahashi JS, Central and peripheral circadian clocks in mammals: Annu Rev Neurosci, 2012; 35; 445-62
10. Durgan DJ, Young ME, The cardiomyocyte circadian clock: Emerging roles in health and disease: Circ Res, 2010; 106(4); 647-58
11. Collins HE, Rodrigo GC, Inotropic response of cardiac ventricular myocytes to β-adrenergic stimulation with isoproterenol exhibits diurnal variation: Involvement of nitric oxide: Circ Res, 2010; 106(7); 1244-52
12. Young ME, The circadian clock within the heart: Potential influence on myocardial gene expression, metabolism, and function: Am J Physiol Heart Circ Physiol, 2005; 290(1); H1-H16
13. Portaluppi F, Hermida RC, Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy: Adv Drug Deliv Rev, 2007; 59(9); 940-51
14. Martino TA, Young ME, Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology: J Biol Rhythms, 2015; 30(3); 183-205
15. Serin Y, Acar Tek N, Effect of circadian rhythm on metabolic processes and the regulation of energy balance: Ann Nutr Metab, 2019; 74(4); 322-30
16. Refinetti R, Circadian rhythmicity of body temperature and metabolism: Temperature (Austin), 2020; 7(4); 321-62
17. Potter GD, Skene DJ, Arendt J, Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures: Endocr Rev, 2016; 37(6); 584-608
18. Cascallares G, Riva S, Franco DL, Role of the circadian clock in the statistics of locomotor activity in Drosophila: PLoS One, 2018; 13(8); e0202505
19. Baron KG, Reid KJ, Circadian misalignment and health: Int Rev Psychiatry, 2014; 26(2); 139-54
20. Lewy AJ, Sack RL, Exogenous melatonin’s phase-shifting effects on the endogenous melatonin profile in sighted humans: A brief review and critique of the literature: J Biol Rhythms, 1997; 12(6); 588-94
21. Aschoff J, Circadian rhythms in man: Science, 1965; 148(3676); 1427-32
22. Morris CJ, Purvis TE, Hu K, Scheer FAJL, Circadian misalignment increases cardiovascular disease risk factors in humans: Proc Natl Acad Sci USA, 2016; 113(10); E1402-E11
23. Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN study: Sleep, 2011; 34(11); 1487-92
24. Bøggild H, Knutsson A, Shift work, risk factors and cardiovascular disease: Scand J Work Environ Health, 1999; 25(2); 85-99
25. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW, Circadian rhythms and the molecular clock in cardiovascular biology and disease: Nat Rev Cardiol, 2019; 16(7); 437-47
26. Kwapis JL, Alaghband Y, Kramar EA: Nat Commun, 2018; 9(1); 3323
27. Yoo SH, Mohawk JA, Siepka SM, Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm: Cell, 2013; 152(5); 1091-105
28. Mendoza-Viveros L, Bouchard-Cannon P, Hegazi S, Molecular modulators of the circadian clock: Lessons from flies and mice: Cell Mol Life Sci, 2017; 74(6); 1035-59
29. Zhang R, Lahens NF, Ballance HI, A circadian gene expression atlas in mammals: Implications for biology and medicine: Proc Natl Acad Sci USA, 2014; 111(45); 16219-24
30. Solocinski K, Gumz ML, The circadian clock in the regulation of renal rhythms: J Biol Rhythms, 2015; 30(6); 470-86
31. Lee Y, Field JM, Sehgal A, Circadian rhythms, disease and chronotherapy: J Biol Rhythms, 2021; 36(6); 503-31
32. Oishi K, Fukui H, Ishida N, Rhythmic expression of BMAL1 mRNA is altered in Clock mutant mice: Differential regulation in the suprachiasmatic nucleus and peripheral tissues: Biochem Biophys Res Commun, 2000; 268(1); 164-71
33. Durgan DJ, Hotze MA, Tomlin TM, The intrinsic circadian clock within the cardiomyocyte: Am J Physiol Heart Circ Physiol, 2005; 289(4); H1530-41
34. Dierickx P, Vermunt MW, Muraro MJ, Circadian networks in human embryonic stem cell-derived cardiomyocytes: EMBO Rep, 2017; 18(7); 1199-212
35. Leibetseder V, Humpeler S, Svoboda M, Clock genes display rhythmic expression in human hearts: Chronobiol Int, 2009; 26(4); 621-36
36. Young ME, Temporal partitioning of cardiac metabolism by the cardiomyocyte circadian clock: Exp Physiol, 2016; 101(8); 1035-39
37. Bray MS, Shaw CA, Moore MW, Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression: Am J Physiol Heart Circ Physiol, 2008; 294(2); H1036-47
38. Tsai JY, Kienesberger PC, Pulinilkunnil T, Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock: J Biol Chem, 2010; 285(5); 2918-29
39. McGinnis GR, Tang Y, Brewer RA, Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart: J Mol Cell Cardiol, 2017; 110; 80-95
40. Durgan DJ, Pat BM, Laczy B, O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock: J Biol Chem, 2011; 286(52); 44606-19
41. Black N, D’Souza A, Wang Y, Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms: Heart Rhythm, 2019; 16(2); 298-307
42. Schroder EA, Burgess DE, Zhang X, The cardiomyocyte molecular clock regulates the circadian expression of Kcnh2 and contributes to ventricular repolarization: Heart Rhythm, 2015; 12(6); 1306-14
43. Chen Y, Zhu D, Yuan J, CLOCK-BMAL1 regulate the cardiac L-type calcium channel subunit CACNA1C through PI3K-Akt signaling pathway: Can J Physiol Pharmacol, 2016; 94(9); 1023-32
44. Tong M, Watanabe E, Yamamoto N, Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart: Biol Rhythm Res, 2013; 44(4); 519-30
45. Yamashita T, Sekiguchi A, Iwasaki YK, Circadian variation of cardiac K+ channel gene expression: Circulation, 2003; 107(14); 1917-22
46. D’Souza A, Wang Y, Anderson C, A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate: Heart Rhythm, 2021; 18(5); 801-10
47. Millar-Craig MW, Bishop CN, Raftery EB, Circadian variation of blood-pressure: Lancet, 1978; 1(8068); 795-97
48. Kaneko M, Cahill GM, Light-dependent development of circadian gene expression in transgenic zebrafish: PLoS Biol, 2005; 3(2); e34
49. Fournier S, Taffe P, Radovanovic D, Myocardial infarct size and mortality depend on the time of day-a large multicenter study: PLoS One, 2015; 10(3); e0119157
50. Czeisler CA, Gooley JJ, Sleep and circadian rhythms in humans: Cold Spring Harb Symp Quant Biol, 2007; 72; 579-97
51. Chellappa SL, Lasauskaite R, Cajochen C, In a heartbeat: Light and cardiovascular physiology: Front Neurol, 2017; 8; 541
52. Scheer FA, Hu K, Evoniuk H, Impact of the human circadian system, exercise, and their interaction on cardiovascular function: Proc Natl Acad Sci USA, 2010; 107(47); 20541-46
53. Parker JD, Testa MA, Jimenez AH, Morning increase in ambulatory ischemia in patients with stable coronary artery disease. Importance of physical activity and increased cardiac demand: Circulation, 1994; 89(2); 604-14
54. Sato M, Matsuo T, Atmore H, Akashi M, Possible contribution of chronobiology to cardiovascular health: Front Physiol, 2013; 4; 409
55. Carneiro BT, Araujo JF, Food entrainment: major and recent findings: Front Behav Neurosci, 2012; 6; 83
56. Pitts S, Perone E, Silver R, Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice: Am J Physiol Regul Integr Comp Physiol, 2003; 285(1); R57-67
57. Dudley CA, Erbel-Sieler C, Estill SJ, Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice: Science, 2003; 301(5631); 379-83
58. Mieda M, Sakurai T, Bmal1 in the nervous system is essential for normal adaptation of circadian locomotor activity and food intake to periodic feeding: J Neurosci, 2011; 31(43); 15391-96
59. Takasu NN, Kurosawa G, Tokuda IT, Circadian regulation of food-anticipatory activity in molecular clock-deficient mice: PLoS One, 2012; 7(11); e48892
60. Turek FW, Joshu C, Kohsaka A, Obesity and metabolic syndrome in circadian Clock mutant mice: Science, 2005; 308(5724); 1043-45
61. Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA, Adverse metabolic and cardiovascular consequences of circadian misalignment: Proc Natl Acad Sci USA, 2009; 106(11); 4453-58
62. Scheer FAJL, Hu K, Evoniuk H, Impact of the human circadian system, exercise, and their interaction on cardiovascular function: Proc Natl Acad Sci USA, 2010; 107(47); 20541-46
63. Grimaldi D, Carter JR, Van Cauter E, Leproult R, Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults: Hypertension, 2016; 68(1); 243-50
64. van Amelsvoort LG, Schouten EG, Maan AC, Changes in frequency of premature complexes and heart rate variability related to shift work: Occup Environ Med, 2001; 58(10); 678-81
65. Chung MH, Kuo TB, Hsu N, Sleep and autonomic nervous system changes – enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses: Scand J Work Environ Health, 2009; 35(3); 180-87
66. Viola AU, Archer SN, James LM, PER3 polymorphism predicts sleep structure and waking performance: Curr Biol, 2007; 17(7); 613-18
67. Herrero L, Valcarcel L, da Silva CA, Altered circadian rhythm and metabolic gene profile in rats subjected to advanced light phase shifts: PLoS One, 2015; 10(4); e0122570
68. Boudreau P, Dumont GA, Boivin DB, Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart: PLoS One, 2013; 8(7); e70813
69. Esquirol Y, Perret B, Ruidavets JB, Shift work and cardiovascular risk factors: New knowledge from the past decade: Arch Cardiovasc Dis, 2011; 104(12); 636-68
70. Boivin DB, Boudreau P, James FO, Kin NM, Photic resetting in night-shift work: Impact on nurses’ sleep: Chronobiol Int, 2012; 29(5); 619-28
71. James FO, Cermakian N, Boivin DB, Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work: Sleep, 2007; 30(11); 1427-36
72. Wang XS, Armstrong ME, Cairns BJ, Shift work and chronic disease: The epidemiological evidence: Occup Med (Lond), 2011; 61(2); 78-89
73. Woller A, Gonze D, Circadian misalignment and metabolic disorders: A story of twisted clocks: Biology (Basel), 2021; 10(3); 207
74. Mason IC, Qian J, Adler GK, Scheer F, Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes: Diabetologia, 2020; 63(3); 462-72
75. Leso V, Vetrani I, Sicignano A, The impact of shift-work and night shift-work on thyroid: A systematic review: Int J Environ Res Public Health, 2020; 17(5); 1527
76. Brown M, Tucker P, Rapport F, The impact of shift patterns on junior doctors’ perceptions of fatigue, training, work/life balance and the role of social support: Qual Saf Health Care, 2010; 19(6); e36
77. Young ME, Razeghi P, Cedars AM, Intrinsic diurnal variations in cardiac metabolism and contractile function: Circ Res, 2001; 89(12); 1199-208
78. Bray MS, Shaw CA, Moore MWS, Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression: Am J Physiol Heart Circu Physiol, 2008; 294(2); H1036-H47
79. Durgan DJ, Tsai J-Y, Grenett MH, Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice: Chronobiol Int, 2011; 28(3); 187-203
80. Lefta M, Campbell KS, Feng H-Z, Development of dilated cardiomyopathy in Bmal1-deficient mice: Am J Physiol Heart Circ Physiol, 2012; 303(4); H475-H85
81. Young ME, Brewer RA, Peliciari-Garcia RA, Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart: J Biol Rhythms, 2014; 29(4); 257-76
82. Young ME, Razeghi P, Taegtmeyer H, Clock genes in the heart: Circ Res, 2001; 88(11); 1142-50
83. Wang Q, Maillard M, Schibler U, Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF: Am J Physiol Regul Integr Comp Physiol, 2010; 299(4); R1013-R19
84. Martino TA, Tata N, Belsham DD, Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization: Hypertension, 2007; 49(5); 1104-13
85. Rauchenzauner M, Ernst F, Hintringer F, Arrhythmias and increased neuro-endocrine stress response during physicians’ night shifts: A randomized cross-over trial: Eur Heart J, 2009; 30(21); 2606-13
86. Härenstam A, Theorell T, Orth-Gomèr K, Shift work, decision latitude and ventricular ectopic activity: A study of 24-hour electrocardiograms in Swedish prison personnel: Work & Stress, 1987; 1(4); 341-50
87. Murata K, Yano E, Shinozaki T, Cardiovascular dysfunction due to shift work: J Occup Environ Med, 1999; 41(9); 748-53
88. Schwartz PJ, Crotti L, Insolia R, Long-QT syndrome: From genetics to management: Circ Arrhythm Electrophysiol, 2012; 5(4); 868-77
89. Schwartz PJ, Priori SG, Spazzolini C, Genotype-phenotype correlation in the long-QT syndrome: Circulation, 2001; 103(1); 89-95
90. Stramba-Badiale M, Priori SG, Napolitano C, Gene-specific differences in the circadian variation of ventricular repolarization in the long QT syndrome: A key to sudden death during sleep?: Ital Heart J, 2000; 1(5); 323-28
91. Schroder EA, Lefta M, Zhang X, The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility: Am J Physiol Cell Physiol, 2013; 304(10); C954-C65
92. Yamashita T, Sekiguchi A, Iwasaki Y-k, Circadian variation of cardiac K+ channel gene expression: Circulation, 2003; 107(14); 1917-22
93. Schroder EA, Burgess DE, Zhang X, The cardiomyocyte molecular clock regulates the circadian expression of Kcnh2 and contributes to ventricular repolarization: Heart Rhythm, 2015; 12(6); 1306-14
94. Skrlec I, Milic J, Heffer M, Genetic variations in circadian rhythm genes and susceptibility for myocardial infarction: Genet Mol Biol, 2018; 41(2); 403-9
95. Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock: Circ Res, 2010; 106(3); 546-50
96. Zhao Y, Lu X, Wan F, Disruption of circadian rhythms by shift work exacerbates reperfusion injury in myocardial infarction: J Am Coll Cardiol, 2022; 79(21); 2097-115
97. Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function: Circ Res, 2014; 114(11); 1713-22
98. Ferrell JM, Chiang JY, Short-term circadian disruption impairs bile acid and lipid homeostasis in mice: Cell Mol Gastroenterol Hepatol, 2015; 1(6); 664-77
99. Wang L, Ma Q, Fang B, Shift work is associated with an increased risk of type 2 diabetes and elevated RBP4 level: Cross sectional analysis from the OHSPIW cohort study: BMC Public Health, 2023; 23(1); 1139
100. Hermida RC, Ayala DE, Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: Improved blood pressure control with bedtime dosing: Hypertension, 2009; 54(1); 40-46
101. Hermida RC, Ayala DE, Mojon A, Fernandez JR, Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk: J Am Coll Cardiol, 2011; 58(11); 1165-73
102. Reitz CJ, Alibhai FJ, Khatua TN, SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome: Commun Biol, 2019; 2; 353
103. Bonten TN, Snoep JD, Assendelft WJ, Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: A randomized cross-over trial: Hypertension, 2015; 65(4); 743-50
104. Madonna R, Van Laake LW, Davidson SM, Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure: Eur Heart J, 2016; 37(23); 1789-98
105. Neil-Sztramko SE, Pahwa M, Demers PA, Gotay CC, Health-related interventions among night shift workers: A critical review of the literature: Scand J Work Environ Health, 2014; 40(6); 543-56
Figures
 Figure 1. The molecular mechanism of the circadian clock in mammals. Constituting the core circadian clock is an autoregulatory transcriptional feedback loop involving the activators CLOCK and BMAL1 and their target genes Per1, Per2, Cry1, and Cry2, whose gene products form a negative-feedback repressor complex.
Figure 1. The molecular mechanism of the circadian clock in mammals. Constituting the core circadian clock is an autoregulatory transcriptional feedback loop involving the activators CLOCK and BMAL1 and their target genes Per1, Per2, Cry1, and Cry2, whose gene products form a negative-feedback repressor complex. Figure 2. Environmental and behavioral cues to trigger circadian disruption in animals. Circadian disruption can be triggered by environmental cues, mainly light/dark cycle manipulations, behavioral cues such as feeding time rescheduling, physical activities, and mental stress, suprachiasmatic nuclei lesions, and gene modification.
Figure 2. Environmental and behavioral cues to trigger circadian disruption in animals. Circadian disruption can be triggered by environmental cues, mainly light/dark cycle manipulations, behavioral cues such as feeding time rescheduling, physical activities, and mental stress, suprachiasmatic nuclei lesions, and gene modification. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952