14 December 2023: Clinical Research
Association of Ischemia-Modified Albumin (IMA) in Saliva, Serum, and Urine with Diagnosis of Chronic Kidney Disease (CKD) in Children: A Case-Control Study
Julita SzulimowskaDOI: 10.12659/MSM.942230
Med Sci Monit 2023; 29:e942230
Abstract
BACKGROUND: Ischemia-modified albumin (IMA) is a secreted biomarker for ischemic oxidative stress. This case-control study aimed to evaluate the association of ischemia-modified albumin (IMA) in saliva, serum, and urine with diagnosis of chronic kidney disease (CKD) in 24 children.
MATERIAL AND METHODS: The study involved 24 children with CKD. CKD was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) diagnostic criteria. The control group consisted of 24 healthy children who were matched for age and gender to the experimental group. The concentration of IMA was determined by the colorimetric method in non-stimulated whole saliva (NWS), stimulated whole saliva (SWS), serum, and urine of children with CKD. The Mann-Whitney U test was used for inter-group comparisons.
RESULTS: IMA levels were significantly higher in NWS (P=0.0082) and SWS (P=0.0014) of children with CKD than in the control group. The concentration of IMA in NWS was correlated with standard indicators of kidney function, including the estimated glomerular filtration rate (r=-0.798, P≤0.0001), stage of CKD (r=0.814, P≤0.0001), and serum creatinine (r=0.711, P≤0.0001) and urea levels (r=0.738, P≤0.0001).
CONCLUSIONS: Salivary IMA concentration depends on renal function in children. Salivary IMA discriminates children with end-stage kidney disease from children with mild and moderate CKD and healthy children with high sensitivity and specificity. Further research is required, including assessment of the diagnostic usefulness and validation of the biomarker in a clinical diagnostic study.
Keywords: Chronic Kidney Disease-Mineral and Bone Disorder, ischemia-modified albumin, Saliva
Background
Chronic kidney disease (CKD) is a multi-symptom disorder caused by progressive, irreversible, and prolonged damage to the renal parenchyma. The progression of CKD leads to water-electrolyte, acid-base, and calcium-phosphorus imbalance, and it disrupts the production of erythropoietin and renin [1,2]. Therefore, CKD is one of the key risk factors for cardiovascular disease, and renal complications are one of leading causes of death among pediatric patients [3,4]. Early diagnosis significantly improves CKD outcomes and often delays the decision to begin renal replacement therapy. Early diagnosis also reduces the risk of renal complications and premature death [5]. The basic screening test for assessing kidney function involves measurement of serum creatinine levels, and its results are used to estimate the glomerular filtration rate (eGFR) [6]. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, CKD is classified into 5 stages based on GFR values [7]. However, CKD may be asymptomatic for a long period of time, and the clinical symptoms of kidney failure are often non-specific, especially in children. The disease is particularly difficult to diagnose in stages 1 and 2 when GFR values reach 60–90 mL/min/1.73 m2 and are indicative only of impaired glomerular filtration [5]. It should also be noted that in children, GFR changes with age and is influenced by body mass and height [8]. In view of the rising prevalence and incidence of CKD and the high economic burden of the disease, new biomarkers supporting early CKD diagnosis in children are being searched for around the world. Chronic kidney disease has emerged as a significant challenge to 21st century pediatric nephrology [2,9].
Most laboratory tests for evaluating kidney function rely on blood and urine samples. Other biological fluids that are easier to obtain from pediatric patients have been suggested for diagnostic purposes [10]. These include saliva, which is the fluid secreted by salivary glands that accumulates in the mouth. Use of saliva has numerous advantages. This non-infectious biological fluid can be sampled in an easy, stress-free, and non-invasive manner. Saliva can be sampled at home (without the assistance of medical personnel) several times a day [11]. Saliva is a filtrate of blood plasma, and it poses an alternative to blood collection in patients for whom blood sampling is problematic (such as patients with clotting disorders and children). Saliva remains viable for a longer time than urine, and it is a more abundant source of biomarkers [12].
Albumin is the main plasma protein that plays an important role in many physiological processes, including the maintenance of osmotic pressure and blood pH, transport of endogenous substances (fatty acids, hormones) and xenobiotics (drugs, toxins), and scavenging of radical oxygen species (ROS) [13]. Therefore, albumin is a biomarker of diseases that disrupt albumin production and/or increase albumin loss from the body. In addition to quantitative changes, albumin can also undergo structural modifications during oxidative stress, which leads to the synthesis of ischemia-modified albumin (IMA) [14]. Research has shown that IMA is a reliable early biomarker of ischemia (without myocardial necrosis), and its concentration is determined by kidney function in patients undergoing dialysis [15,16]. The clinical usefulness of IMA has also been postulated in other conditions involving oxidative stress and ischemia, such as diabetes, neurological disorders, pregnancy complications, hypothyroidism, and hyperthyroidism [15]. However, the diagnostic potential of IMA in CKD (including in children) and the applicability of saliva as a biological material for determining IMA concentration has not been evaluated to date. This case-control study aimed to evaluate the association of ischemia-modified albumin (IMA) in saliva, serum, and urine with diagnosis of chronic kidney disease (CKD) in 24 children.
Material and Methods
STUDY POPULATION:
The study was approved by the Local Bioethics Committee of the Medical University of Białystok (permission number R-I-002/43/2018). All study participants and/or their legal guardians gave written consent to participate in the study.
The study involved 24 children with CKD who were patients of the Pediatrics and Nephrology Clinic of the Children’s Clinical Hospital of the Medical University of Białystok. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines were used to define the diagnostic criteria and classification of pediatric CKD [1,17,18]. The diagnosis of CKD was based on fulfilling one of the following criteria: glomerular filtration rate (GFR) <60 mL/min/1.73 m2 for more than 3 months, which has health implications, regardless of the presence of other CKD biomarkers, or GFR > 60 mL/min/1.73 m2 accompanied by signs of structural injury or other biomarkers of renal dysfunction, including albuminuria, proteinuria, renal tubular dysfunction or pathological defects identified by histology or inferred from imaging studies.
The estimated glomerular filtration rate (eGFR) was calculated using the updated Schwartz formula as eGFR (mL/min/1.73 m2)=0.413×[height in cm/sCr] [2,19]. Each patient was also asked to collect urine in the 24-h period immediately prior to their nephrology clinic appointment, and 24-h excretion of urinary albumin and protein were assessed. Urine was collected into containers without preservative and stored at 4°C prior to analysis. Urine albumin (albuminuria) and protein (proteinuria) losses in normal individuals are usually <30 mg/24 h, and lower than 150 mg/24 h, respectively. Since diagnosis, all patients had adhered to a renal diet that was low in sodium and/or phosphorus and/or protein, depending on the patients’ condition and CKD stage [4,20,21].
The control group was matched for age and gender to the experimental group, and it consisted of 24 healthy children who visited the Specialist Dental Clinic of the Medical University of Białystok for regular dental check-ups.
The study population consisted solely of children with normal body mass (body mass index [BMI] appropriate for age and height) who had not been administered nonsteroidal anti-inflammatory drugs (NSAID), antibiotics, hormones, or vitamin or dietary supplements during the 3 months before the study. In both groups, the exclusion criteria were also chronic autoimmune disorders (diabetes, rheumatoid arthritis, lupus, Sjogren’s syndrome, scleroderma, psoriasis), infectious diseases (viral and bacterial), and gastrointestinal, lung, thyroid, oral, or periodontal diseases.
SALIVA:
The concentration of IMA was determined in non-stimulated (NWS) and stimulated (SWS) whole saliva. Whole saliva is a complex body fluid derived from the major and minor salivary glands and various extra-secretory components such as epithelial cells, leukocytes, and serum-like filtrate. NWS is continuously produced in the mouth, while SWS is produced in response to stimulation (eg, with citric acid). NWS and SWS samples were collected by spitting between 7 and 9 AM in a child-friendly room to guarantee maximum patient comfort. The participants had been asked to refrain from eating, brushing their teeth, or taking medications for a minimum of 8 h before saliva sampling. The participants rinsed their mouths with distilled water at room temperature, and they spat the saliva that had accumulated at the bottom of the oral cavity into a collection tube. NWS was collected over a period of 15 min, and SWS was collected after a 5-min break. Saliva production was stimulated by applying several drops of 2% citric acid (10 μL of the solution every 30 s) on the tip of the tongue. SWS was collected over a period of 5 min. Saliva samples were centrifuged (5000×g, 20 min, +4°C), and none of them were contaminated with blood [22].
Directly after saliva sampling, an experienced pediatric dentist (JS) conducted a dental examination. Artificial lighting, a dental mirror, a periodontal probe, and an exploratory probe were used during the examination, according to WHO criteria [23]. The dental examination involved a determination of decayed, missing, and filled teeth (DMFT), the Sulcus Bleeding Index (SBI) according to Muhemann and Son [24], the Gingival Index (GI) according to Löe and Silness [25], and the approximal plaque index (API) according to Lange [26]. The dmft score was also calculated for milk teeth. The inter-rater agreement between the examiner (JS) and another experienced dentist (AZ) was assessed in 15 patients. The reliability for DMFT, SBI, GI, and API was >0.95.
BLOOD AND URINE:
The concentration of IMA was also determined in blood and urine samples. Venous blood (4.9 ml) was drawn after an overnight rest and a fasting period into S-Monovette® Serum (Sarstedt) collection tubes. Blood samples were centrifuged (1500×g, 10 min, +4°C), and the upper layer (serum) was collected for analysis. Urine samples were collected into sterile disposable containers from the first-morning portion of urine from the middle stream. The samples were centrifuged (1500×g, 10 min, +4°C), and the layer above the sediment was sampled for analysis.
SAMPLE STORAGE:
We added 0.5 M butylated hydroxytoluene (10 μl/mL) to NWS, SWS, serum, and urine samples directly after centrifugation to prevent oxidation during storage. The samples were stirred and freeze-stored at −80°C for further analyses.
COLORIMETRIC ASSAY:
The concentration of IMA was determined by the colorimetric method described by Bar-Or et al [27]. In this assay, IMA is measured based on the color reaction between dithiothreitol (DTT) and a known amount of exogenous cobalt that is not bound by albumin. IMA has lower affinity for transition metal ions, including cobalt ions. In brief, 50 μL of cobalt chloride solution (1 g/L) was added to 200 μL of NWS, SWS, serum, or urine; the contents were stirred vigorously and incubated for 10 min in the dark. Next, 50 μL of DTT solution (1.5 g/L) was added to the sample and the contents were stirred again and incubated for 2 min. After incubation, 1.0 mL of NaCl solution (9.0 g/L) was added to the samples to stop the reaction. Absorbance was measured at a wavelength of 470 nm with a 96-well Infinite M200 PRO Multimode microplate reader (Tecan). The content of IMA was expressed in albumin binding serum units (ABSU). All determinations were made in duplicate samples. The precision of these measurements, expressed as coefficients of variation (CV), was <5%.
STANDARDIZATION OF RESULTS:
The concentration of the analyzed biomarkers in saliva and urine is determined by salivary gland secretion or kidney function; therefore, the results were standardized for total protein content. Total protein content was determined in the bicinchoninic acid (BCA) assay with the use of the Thermo Scientific Pierce BCA Protein Assay Kit (Rockford, IL, USA).
STATISTICAL ANALYSIS:
The results were processed statistically in GraphPad Prism 8.4.3. for MacOS (GraphPad Software, La Jolla, USA). Data were checked for normal distribution with the Shapiro-Wilk test. Due to the non-normality of the distribution, the Mann-Whitney U test was used for comparisons between the study and control groups, and the results were visualized as median values and the interquartile range of each group on graphs with individual data points. The correlations between biomarkers and clinical parameters were evaluated with the use of Spearman’s rank correlation coefficient
The size of the experimental group and the control group was determined based on the results of a pilot study. The
Results
CLINICAL DATA:
The clinical characteristics of the study participants are presented in Table 1.
Total salivary protein (NWS: P<0.0001; SWS: P=0.0026) and salivary flow rates (NWS: P<0.0001; SWS: P<0.0001) were significantly lower in children with CKD than in the control group. However, no differences in oral hygiene or periodontal health were observed between the experimental group and the control group (Table 2).
ISCHEMIA-MODIFIED ALBUMIN (IMA) CONCENTRATION IN SALIVA, SERUM, AND URINE:
The concentration of IMA in NWS (P=0.0082) and SWS (P=0.0014) samples was significantly higher in children with CKD than in the control group. The content of IMA in serum and urine samples did not differ significantly between groups (Figure 1).
CORRELATIONS WITH CLINICAL PARAMETERS:
In children with CKD, the content of IMA in NWS was significantly correlated with standard indicators of kidney function, including eGFR (r=−0.798, P≤0.0001), stage of CKD (r=0.814, P≤0.0001), and serum creatinine (r=0.711, P≤0.0001) and urea levels (r=0.738, P≤0.0001) (Table 3).
In children with CKD, the serum and urine levels of IMA were negatively correlated with systolic (r=−0.448, P=0.041) and diastolic blood pressure (r=−0.501, P=0.021) (Table 3).
The IMA content of NWS was not correlated with IMA concentration in SWS, serum, or urine (Table 3).
ROC ANALYSIS:
In NWS, IMA concentration was significantly correlated with standard indicators of kidney function [15]; therefore, the potential diagnostic utility of this biomarker was evaluated in the ROC analysis. The results revealed that salivary IMA cannot be used as a potential biomarker of early-stage CKD in children. The content of IMA in NWS did not discriminate children with stage 1 and 2 CKD from the control group; however, it differentiated children with stage 3 CKD from healthy children with 100% sensitivity and specificity (AUC=1; P=0.0209) (Table 4). The concentration of IMA in NWS was significantly higher in end-stage CKD (stages 4 and 5) than in the control group (P<0.0001). The ROC analysis demonstrated that IMA content differentiated children with stages 4 and 5 CKD from healthy children (P<0.0001) with high sensitivity (83.33%) and specificity (87.5%) (Table 4, Figure 2).
The content of IMA in NWS was significantly higher in children with severe kidney damage and end-stage CKD (stages 4 and 5) than in children with mild and moderate CKD (stages 1–3) (P=0.0022). The ROC analysis revealed that IMA content differentiated between children with stages 4 and 5 CKD and children with stages 1–3 CKD (P=0.0022) with high sensitivity (83.33%) and specificity (83.33%) (Table 4, Figure 2).
Discussion
Chronic kidney disease is one of the most prevalent chronic diseases in children. According to epidemiological studies, CKD is the leading cause of death among pediatric patients, and its prevalence and incidence continue to increase [28]. In addition, CKD is generally diagnosed only in late stages of kidney failure. Therefore, there is an urgent need for new biomarkers for early diagnosis of CKD [29]. This is the first study to analyze the concentration of IMA in the saliva, serum, and urine of children with CKD. The content of IMA in NWS and SWS was significantly higher in children with CKD than in the control group. Salivary IMA levels differentiated children with end-stage CKD from children with mild and moderate CKD and healthy children with high sensitivity and specificity.
Human albumin is a protein with a molecular weight of 65–69 kDa, and it is composed of 585 amino acids. The N-terminal sequence of albumin (N-Asp-Ala-His-Lys) is a binding site for transition metal ions [30]. Under oxidative stress, this sequence undergoes conformational changes that decrease albumin’s ability to bind cobalt ions. The structural modifications of albumin give rise to IMA, which is synthesized not only during ROS overproduction, but also during sodium/calcium pump disorders, mitochondrial dysfunction, and ischemia-reperfusion injury [14,31]. This is the first study to assess the diagnostic utility of IMA as a biomarker of kidney function in children. Although kidney failure is rarely caused by ischemic nephropathy in pediatric patients, excessive ROS production, disruptions in enzymatic and non-enzymatic antioxidant systems, and increased protein, lipid, and DNA oxidation at both the local (kidneys) and systemic (blood, urine) level have been reported in children. Our previous study revealed a redox imbalance in the saliva of children with CKD (reduced antioxidant barrier and intensified glycoxidative/nitrative changes in saliva biomolecules) [32–34]. Salivary gland inflammation was also noted in the experimental group, which can explain the increase in IMA concentration in NWS and SWS in children with CKD relative to the control group. Szulimowska et al observed a statistically significant increase in all salivary Th2 cytokines. The multivariate regression analysis also showed that salivary cytokines, chemokines, and growth factors depend on the secretory function of the salivary glands [35]. Despite the above, IMA content in the serum and urine did not differ significantly between the experimental group and the control group. In addition, IMA concentration in NWS was not correlated with serum or urine IMA levels, which could be attributed to differences in the origin of salivary biomarkers. Biomolecules can be translocated from the blood to saliva via intracellular (diffusion, filtration, active transport) or extracellular (ultrafiltration, transport across damaged membranes) transport mechanisms. Biomolecules are also produced by the salivary glands [36]. Interestingly, the salivary glands are highly sensitive to oxidative damage [37]. Mitochondrial activity in the salivary glands is comparable (or higher) to mitochondrial activity in the liver, which is the most metabolically active organ in the body [38]. This is not surprising because the oral mucosa is the only bodily tissue that is continuously exposed to numerous pro-oxidants, including air pollutants, stimulants, xenobiotics, and food products [39]. These observations suggest that in CKD patients, mitochondrial dysfunction occurs not only in the renal parenchyma, but also in the salivary glands, but further research is needed to confirm this hypothesis [40]. In the present study, a reduced salivary flow rate (hyposalivation) and lower salivary protein secretion were noted in children with CKD, which suggests that kidney damage compromises salivary gland function [32]. These disorders are caused by dehydration, use of drugs and medications, and disruption of neurotransmission in the salivary glands [41,42]. IMA is an early biomarker of oxidative damage in the body. A significant increase in IMA concentration was found only in the saliva of children with CKD, which could be attributed to high oxygen consumption in the salivary glands and the resulting susceptibility of salivary gland tissue to damage caused by oxidative stress/ischemia [43]. However, further research is needed to confirm this hypothesis.
An ideal analytical biomarker should be correlated with a pathological process; it should be characterized by high diagnostic sensitivity and specificity, and biomarker measurements should be reproducible with the use of validated and widely available analytical methods [44]. In the present study, IMA concentration in NWS was correlated with standard biomarkers of kidney function, including eGFR (negative correlation), stage of CKD, and serum creatinine and urea levels (positive correlations) [45]. Although this is not a clinical diagnostic study, these observations may suggest the potential diagnostic value of salivary IMA. Therefore, in the following stage of the study, the potential diagnostic utility of salivary IMA was evaluated in the ROC analysis. The ROC curve is a graphic representation of the relationship between the sensitivity and specificity of a laboratory test. The ROC curve is plotted to assess a test’s discriminative power and to compare the accuracy of several tests [46]. The concentration of IMA in NWS was significantly higher in children with severe kidney damage and end-stage CKD (stages 4 and 5) than in the control group. The results of the ROC analysis also demonstrated that salivary IMA levels were significantly higher in children with stages 4 and 5 CKD than in children with mild and moderate CKD (stages 1–3). This parameter discriminated children with stages 4 and 5 CKD from children with stages 1–3 CKD with high sensitivity (83.33%) and specificity (83.33%). The IMA content of NWS did not differentiate between children with stages 1 and 2 CKD and the control group, but it discriminated patients with stage 3 CKD from healthy subjects with 100% sensitivity and specificity. Therefore, salivary IMA can have a potential for assessing kidney function in children. Further research is required, including assessment of the diagnostic usefulness and validation of the biomarker in a clinical diagnostic study. The collection of saliva samples is a non-invasive and pain-free procedure that can be performed at home without medical assistance, which is a very important consideration in small children (by minimizing stress) and patients with blood clotting disorders (by eliminating the need for blood sampling). Saliva samples can be collected several times a day. In contrast to blood, saliva is a non-infectious fluid [11]. Salivary IMA levels are determined colorimetrically by measuring the reduction in IMA’s affinity for cobalt ions. The colorimetric assay enables rapid and relatively accurate analyses of even small samples of biological fluids. This low-cost analytical method can be applied in routine biochemical tests [47]. Nevertheless, IMA levels may be affected by dialysis therapy. Kiyici et al assessed serum IMA in patients with end-stage renal disease (ESRD) undergoing hemodialysis [48]. They showed that post-dialysis IMA and albumin-corrected IMA were significantly increased compared to pre-dialysis level. The study by Turedi et al also confirms that serum IMA are significantly higher in ESRD patients both before and after dialysis compared to healthy subjects [49].
The present study has several strengths. The concentration of IMA was analyzed in both NWS and SWS, as well as in the serum and urine of children with CKD. Only children with a healthy body mass who had not been administered NSAIDs, antibiotics, hormones, vitamins, or dietary supplements were included in the study to eliminate potential variations in the quality and quantity of saliva samples. Other exclusion criteria were chronic autoimmune diseases (diabetes, rheumatoid arthritis, lupus, Sjogren’s syndrome, scleroderma, psoriasis), infectious diseases (viral and bacterial), and gastrointestinal, lung, thyroid, oral, and periodontal diseases [50]. Children with CKD and healthy children were matched for gender, age, oral hygiene, and periodontal health, and the control group and experimental group were homogeneous. The concentrations of salivary biomarkers can be influenced by the secretory activity of salivary glands, and IMA content was standardized to total protein content to objectively compare the quantitative concentrations of IMA in saliva samples. The size of the population sample was determined based on the results of a pilot study, but further research is needed to evaluate the clinical utility of salivary IMA in a larger population of children with CKD. In addition, IMA does not appear to be exclusively specific for kidney function. Oxidative stress/ischemia in other tissues and organs can also increase IMA concentration [51,52]. In addition, IMA concentration can be influenced by blood albumin levels. It was reported that the amount of free cobalt decreased with increasing albumin concentrations [53]. Therefore, further research is needed to assess the clinical utility of IMA in children with CKD and comorbidities.
Conclusions
Our study indicates that salivary IMA concentrations depend on renal function in children. Salivary IMA discriminates children with end-stage kidney disease from children with mild and moderate CKD and healthy children with high sensitivity and specificity. IMA levels in the serum and urine of patients do not have potential diagnostic value. Further research is required, including assessment of the diagnostic usefulness and validation of the biomarker in a clinical diagnostic study.
The research plan and the conclusions are presented graphically in Figure 3.
Figures
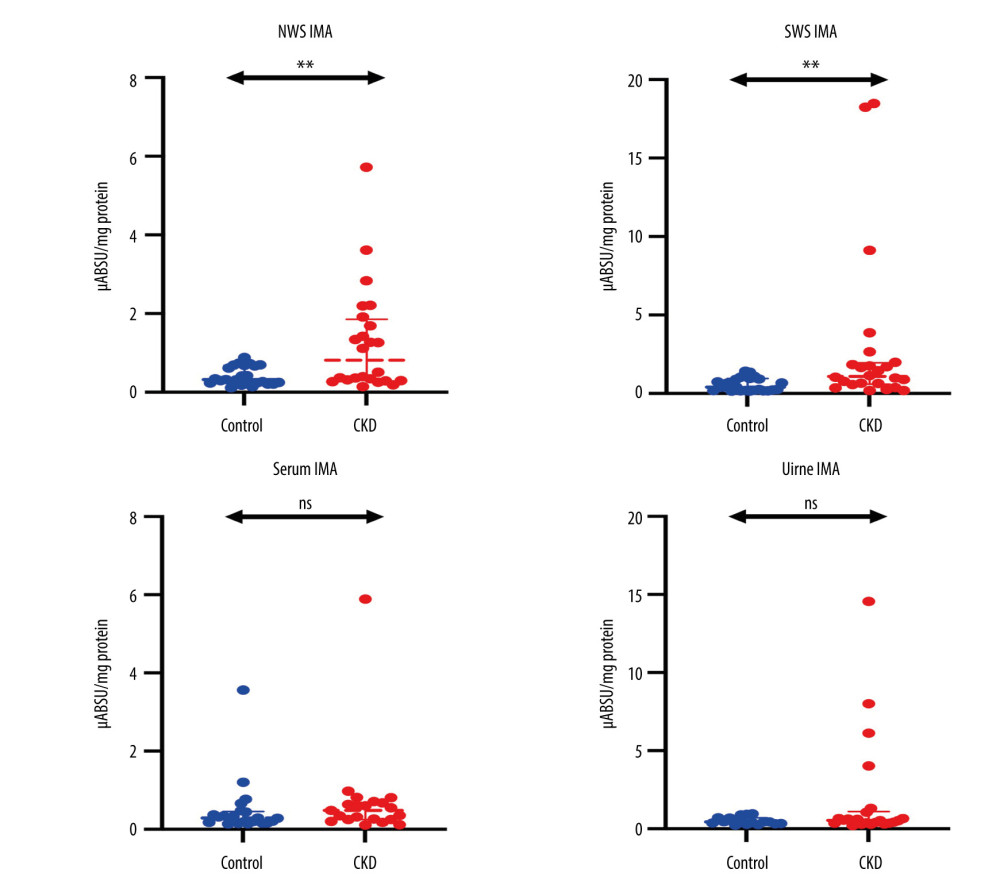 Figure 1. Concentration of ischemia-modified albumin (IMA) in non-stimulated (NWS) and stimulated whole saliva (SWS), serum, and urine of children with chronic kidney disease (CKD) relative to the control group. The Mann-Whitney U test was used for inter-group comparisons. NWS – non-stimulated whole saliva; SWS – stimulated whole saliva; ns – non-significant; ** P<0.01.
Figure 1. Concentration of ischemia-modified albumin (IMA) in non-stimulated (NWS) and stimulated whole saliva (SWS), serum, and urine of children with chronic kidney disease (CKD) relative to the control group. The Mann-Whitney U test was used for inter-group comparisons. NWS – non-stimulated whole saliva; SWS – stimulated whole saliva; ns – non-significant; ** P<0.01. 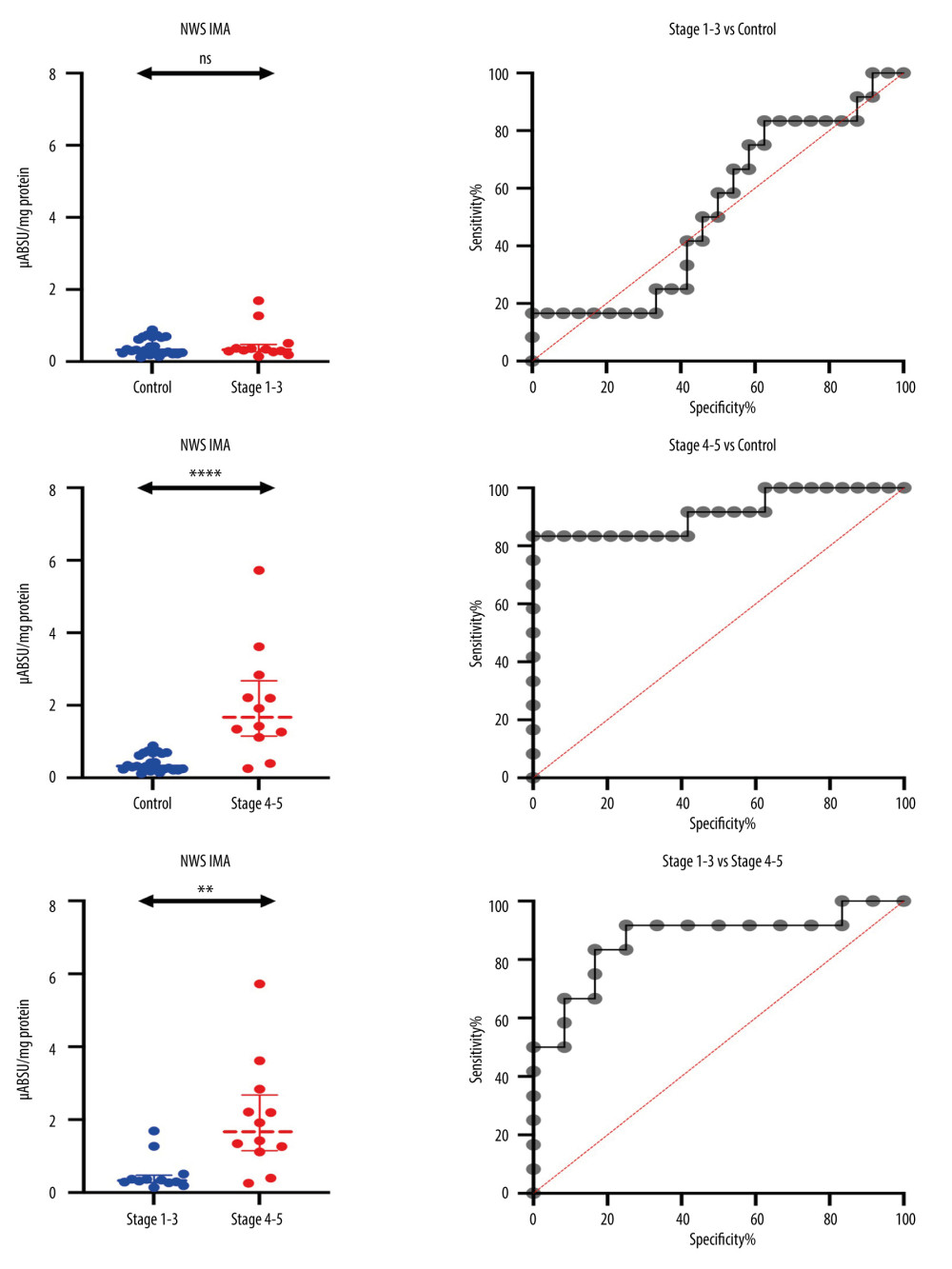 Figure 2. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva (NWS) of children with mild and moderate CKD, children with stages 4–5 and healthy children. ns: non-significant; ** P<0.01; **** P<0.0001.
Figure 2. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva (NWS) of children with mild and moderate CKD, children with stages 4–5 and healthy children. ns: non-significant; ** P<0.01; **** P<0.0001. 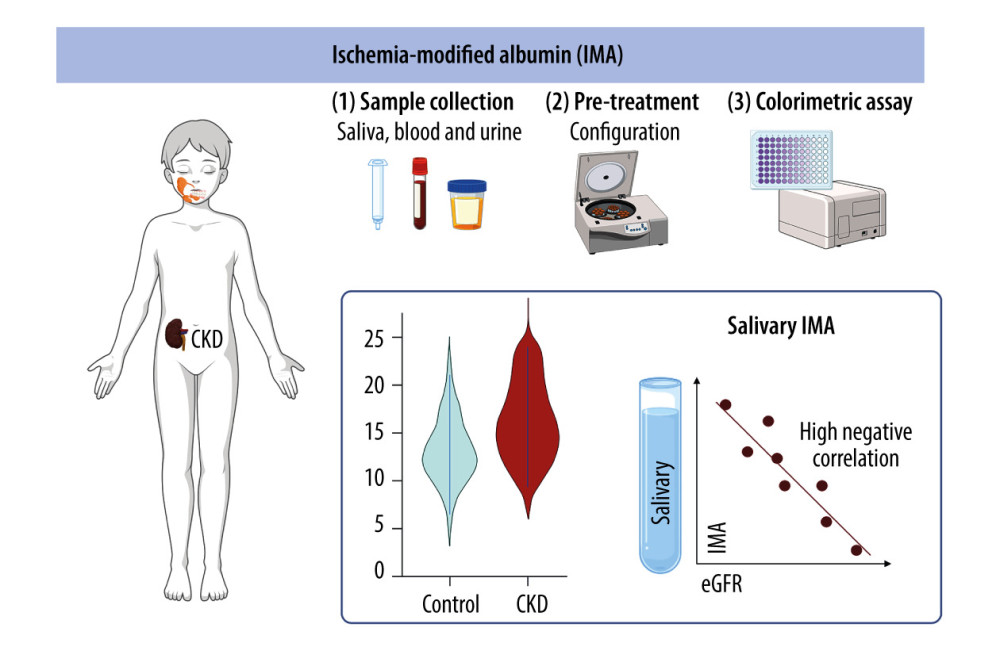 Figure 3. Research plan and conclusions (generated using biorender.com). The concentration of IMA in non-stimulated whole saliva is negatively correlated with the estimated glomerular filtration rate (eGFR). IMA levels in the serum and urine of patients do not have diagnostic value for assessing the progression of chronic kidney disease (CKD).
Figure 3. Research plan and conclusions (generated using biorender.com). The concentration of IMA in non-stimulated whole saliva is negatively correlated with the estimated glomerular filtration rate (eGFR). IMA levels in the serum and urine of patients do not have diagnostic value for assessing the progression of chronic kidney disease (CKD). Tables
Table 1. Clinical characteristics of children with chronic kidney disease (CKD) and healthy children.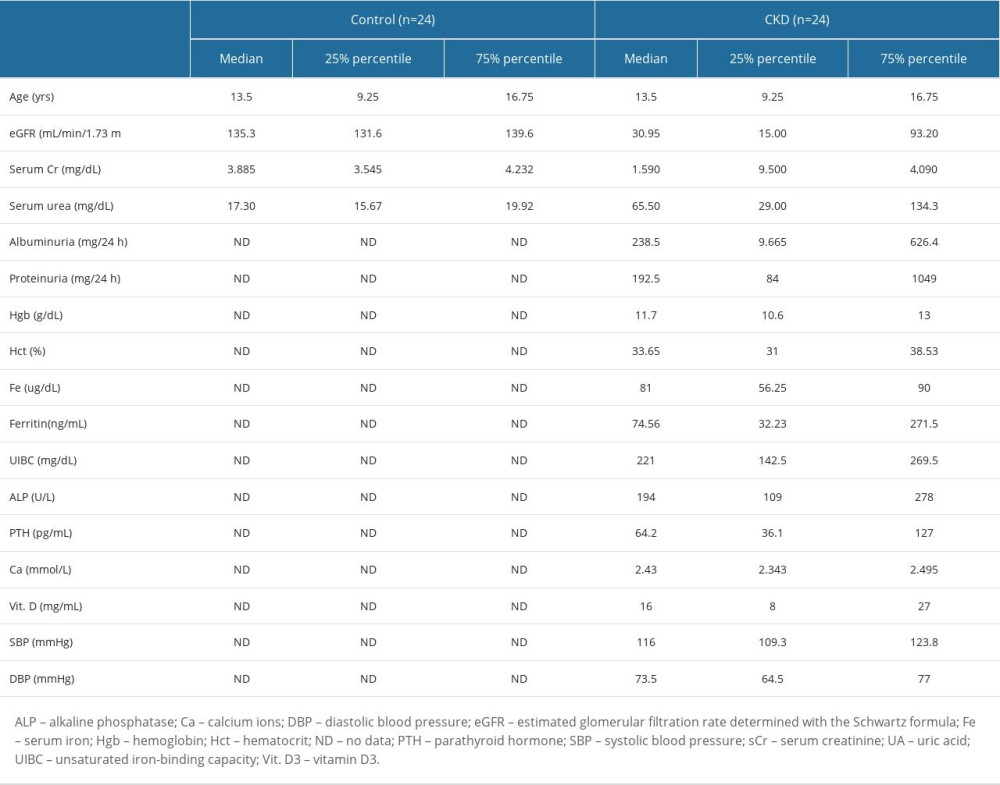 Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health.
Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health.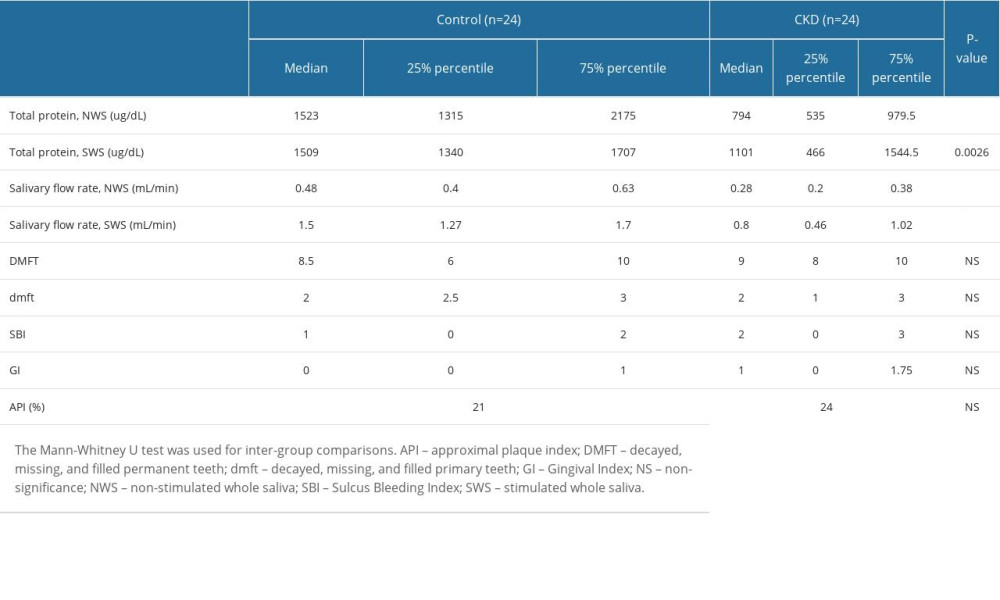 Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD).
Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD).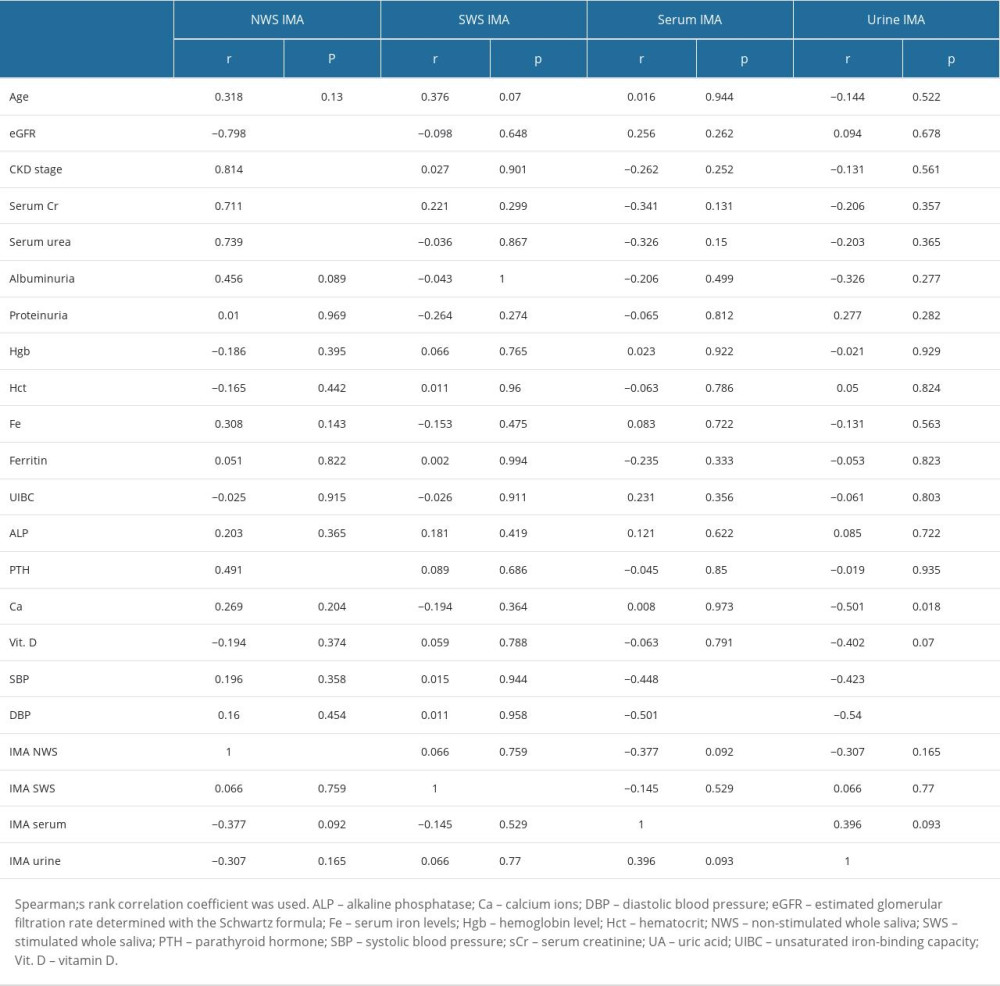 Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5).
Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5).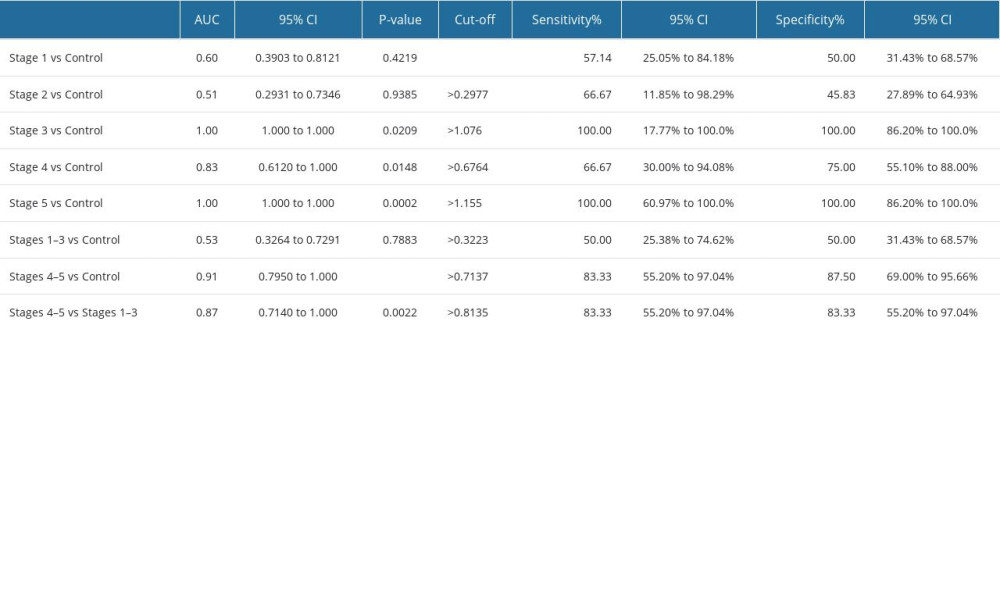
References
1. Kaspar CDW, Bholah R, Bunchman TE, A review of pediatric chronic kidney disease: Blood Purif, 2016; 41(1–3); 211-17
2. Kovesdy CP, Epidemiology of chronic kidney disease: An update 2022: Kidney Int Suppl, 2022; 12(1); 7-11
3. Ene-Iordache B, Perico N, Bikbov B, Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): A cross-sectional study: Lancet Glob Health, 2016; 4(5); e307-19
4. Darlington O, Dickerson C, Evans M, Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: Evidence from a systematic literature review: Adv Ther, 2021; 38(2); 994-1010
5. Evans M, Lewis RD, Morgan AR, A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives: Adv Ther, 2022; 39(1); 33-43
6. Levey AS, Stevens LA, Schmid CH, A new equation to estimate glomerular filtration rate: Ann Intern Med, 2009; 150(9); 604-12
7. Inker LA, Astor BC, Fox CH, KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD: Am J Kidney Dis, 2014; 63(5); 713-35
8. Pottel H, Delanaye P, Schaeffner E, Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C: Nephrol Dial Transplant, 2017; 32(3); 497-507
9. Mizdrak M, Kumrić M, Kurir TT, Božić J, Emerging biomarkers for early detection of chronic kidney disease: J Pers Med, 2022; 12(4); 548
10. Tvarijonaviciute A, Martinez-Subiela S, Lopez Jornet P, Lamy E: Saliva in health and disease the present and future of a unique sample for diagnosis, 2020
11. Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Saliva diagnostics – current views and directions: Exp Biol Med (Maywood), 2017; 242(5); 459-72
12. Spielmann N, Wong DT, Saliva: Diagnostics and therapeutic perspectives: Oral Dis, 2011; 17(4); 345-54
13. Quinlan GJ, Martin GS, Evans TW, Albumin: Biochemical properties and therapeutic potential: Hepatology, 2005; 41(6); 1211-19
14. Oettl K, Stauber RE, Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties: Br J Pharmacol, 2007; 151(5); 580-90
15. Shevtsova A, Gordiienko I, Tkachenko V, Ushakova G, Ischemia-modified albumin: Origins and clinical implications: Dis Markers, 2021; 2021; 9945424
16. Mangoni AA, Zinellu A, Serum concentrations of ischaemia-modified albumin in acute coronary syndrome: A systematic review and meta-analysis: J Clin Med, 2022; 11(14); 4205
17. Andrassy KM, Comments on “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.”: Kidney Int, 2013; 84(3); 622-23
18. Levey AS, Coresh J, Balk E, National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification: Ann Intern Med, 2003; 139(2); 137-47
19. Mian AN, Schwartz GJ, Measurement and estimation of glomerular filtration rate in children: Adv Chronic Kidney Dis, 2017; 24(6); 348-56
20. Paglialonga F, Schmitt CP, Sodium handling in pediatric patients on maintenance dialysis: Pediatr Nephrol, 2023; 38(12); 3909-21
21. Wang AYM, March DS, Burton JO, Physical activity and nutrition in chronic kidney disease: Curr Opin Clin Nutr Metab Care, 2023; 26(4); 385-92
22. Maciejczyk M, Nesterowicz M, Szulimowska J, Zalewska A, Oxidation, glycation, and carbamylation of salivary biomolecules in healthy children, adults, and the elderly: Can saliva be used in the assessment of aging?: J Inflamm Res, 2022; 15; 2051-73
23. : Oral health surveys: basic methods https://www.who.int/publications-detail-redirect/9789241548649
24. Mühlemann HR, Son S, Gingival sulcus bleeding – a leading symptom in initial gingivitis: Helv Odontol Acta, 1971; 15(2); 107-13
25. Silness J, Loe H, Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition: Acta Odontol Scand, 1964; 22; 121-35
26. Lange DE, Plagmann HC, Eenboom A, Promesberger AClinical methods for the objective evaluation of oral hygiene: Dtsch Zahnarztl Z, 1977; 32(1); 44-47 in German
27. Bar-Or D, Lau E, Winkler JV, A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – a preliminary report: J Emerg Med, 2000; 19(4); 311-15
28. Harada R, Hamasaki Y, Okuda Y, Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies: Pediatr Nephrol Berl Ger, 2022; 37(6); 1215-29
29. Saucedo AL, Perales-Quintana MM, Paniagua-Vega D, Chronic kidney disease and the search for new biomarkers for early diagnosis: Curr Med Chem, 2018; 25(31); 3719-47
30. Al-Harthi S, Lachowicz JI, Nowakowski ME, Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin: J Inorg Biochem, 2019; 198; 110716
31. Lee P, Wu X, Review: Modifications of human serum albumin and their binding effect: Curr Pharm Des, 2015; 21(14); 1862-65
32. Gaze DC: Biomarkers of cardiac ischemia, 2013
33. Sbarouni E, Georgiadou P, Voudris V, Ischemia modified albumin changes – review and clinical implications: Clin Chem Lab Med, 2011; 49(2); 177-84
34. Maciejczyk M, Szulimowska J, Taranta-Janusz K, Salivary gland dysfunction, protein glycooxidation and nitrosative stress in children with chronic kidney disease: J Clin Med, 2020; 9(5); 1285
35. Maciejczyk M, Szulimowska J, Skutnik A, Salivary biomarkers of oxidative stress in children with chronic kidney disease: J Clin Med, 2018; 7(8); 209
36. Szulimowska J, Zalewska A, Taranta-Janusz K, Association between salivary cytokines, chemokines and growth factors and salivary gland function in children with chronic kidney disease: J Inflamm Res, 2023; 16; 1103-20
37. Pfaffe T, Cooper-White J, Beyerlein P, Diagnostic potential of saliva: Current state and future applications: Clin Chem, 2011; 57(5); 675-87
38. Maciejczyk M, Zalewska A, Ładny JR, Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly: Oxid Med Cell Longev, 2019; 2019; 4393460
39. Chibly AM, Aure MH, Patel VN, Hoffman MP, Salivary gland function, development, and regeneration: Physiol Rev, 2022; 102(3); 1495-52
40. Żukowski P, Maciejczyk M, Waszkiel D, Sources of free radicals and oxidative stress in the oral cavity: Arch Oral Biol, 2018; 92; 8-17
41. Srivastava A, Tomar B, Sharma D, Rath SK, Mitochondrial dysfunction and oxidative stress: Role in chronic kidney disease: Life Sci, 2023; 319; 121432
42. Tanaka J, Mishima K, Application of regenerative medicine to salivary gland hypofunction: Jpn Dent Sci Rev, 2021; 57; 54-59
43. Guggenheimer J, Moore PA, Xerostomia: Etiology, recognition and treatment: J Am Dent Assoc, 2003; 134(1); 61-69
44. Avezov K, Reznick AZ, Aizenbud D, Oxidative stress in the oral cavity: Sources and pathological outcomes: Respir Physiol Neurobiol, 2015; 209; 91-94
45. Biomarkers Definitions Working Group, Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework: Clin Pharmacol Ther, 2001; 69(3); 89-95
46. Aklilu AM, Diagnosis of chronic kidney disease and assessing glomerular filtration rate: Med Clin North Am, 2023; 107(4); 641-58
47. Hajian-Tilaki K, Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation: Casp J Intern Med, 2013; 4(2); 627-35
48. Kiyici A, Mehmetoğlu I, Karaoğlan H, Ischemia-modified albumin levels in patients with end-stage renal disease patients on hemodialysis: does albumin analysis method affect albumin-adjusted ischemia-modified albumin levels?: J Clin Lab Anal, 2010; 24(4); 273-77
49. Turedi S, Cinar O, Yavuz I, Differences in ischemia-modified albumin levels between end stage renal disease patients and the normal population: J Nephrol, 2010; 23(3); 335-40
50. Garbett NC, Miller JJ, Jenson AB, Chaires JB, Calorimetric analysis of the plasma proteome: Semin Nephrol, 2007; 27(6); 621-26
51. Tampa M, Mitran CI, Mitran MI, Ischemia-modified albumin – a potential new marker of oxidative stress in dermatological diseases: Med Kaunas Lith, 2022; 58(5); 669
52. Mangoni AA, Zinellu A, A systematic review and meta-analysis of serum concentrations of ischaemia-modified albumin in acute ischaemic stroke, intracerebral haemorrhage, and subarachnoid haemorrhage: Biomolecules, 2022; 12(5); 653
53. Coverdale JPC, Katundu KGH, Sobczak AIS, Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding: Prostaglandins Leukot Essent Fatty Acids, 2018; 135; 147-57
Figures
 Figure 1. Concentration of ischemia-modified albumin (IMA) in non-stimulated (NWS) and stimulated whole saliva (SWS), serum, and urine of children with chronic kidney disease (CKD) relative to the control group. The Mann-Whitney U test was used for inter-group comparisons. NWS – non-stimulated whole saliva; SWS – stimulated whole saliva; ns – non-significant; ** P<0.01.
Figure 1. Concentration of ischemia-modified albumin (IMA) in non-stimulated (NWS) and stimulated whole saliva (SWS), serum, and urine of children with chronic kidney disease (CKD) relative to the control group. The Mann-Whitney U test was used for inter-group comparisons. NWS – non-stimulated whole saliva; SWS – stimulated whole saliva; ns – non-significant; ** P<0.01. Figure 2. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva (NWS) of children with mild and moderate CKD, children with stages 4–5 and healthy children. ns: non-significant; ** P<0.01; **** P<0.0001.
Figure 2. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva (NWS) of children with mild and moderate CKD, children with stages 4–5 and healthy children. ns: non-significant; ** P<0.01; **** P<0.0001. Figure 3. Research plan and conclusions (generated using biorender.com). The concentration of IMA in non-stimulated whole saliva is negatively correlated with the estimated glomerular filtration rate (eGFR). IMA levels in the serum and urine of patients do not have diagnostic value for assessing the progression of chronic kidney disease (CKD).
Figure 3. Research plan and conclusions (generated using biorender.com). The concentration of IMA in non-stimulated whole saliva is negatively correlated with the estimated glomerular filtration rate (eGFR). IMA levels in the serum and urine of patients do not have diagnostic value for assessing the progression of chronic kidney disease (CKD). Tables
 Table 1. Clinical characteristics of children with chronic kidney disease (CKD) and healthy children.
Table 1. Clinical characteristics of children with chronic kidney disease (CKD) and healthy children. Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health.
Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health. Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD).
Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD). Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5).
Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5). Table 1. Clinical characteristics of children with chronic kidney disease (CKD) and healthy children.
Table 1. Clinical characteristics of children with chronic kidney disease (CKD) and healthy children. Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health.
Table 2. Total salivary protein, salivary flow rate, and examinations of oral hygiene and periodontal health. Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD).
Table 3. Correlations with clinical parameters in children with chronic kidney disease (CKD). Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5).
Table 4. Receiver operating characteristic (ROC) analysis of the content of ischemia-modified albumin (IMA) in non-stimulated whole saliva of children with different stages of CKD (stages 1–5). In Press
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








