27 January 2024: Clinical Research
Effectiveness of Corticosteroid Therapy in Enhancing Early Postoperative Recovery in Lumbar Spinal Stenosis Patients: A Retrospective Study
Haozhi Zhang1BCDEF, Changfa Huang1BDF, Daoyun Wang1BDF, Kuan Li2ABF, Xiao Han3ABF, Xin Chen4ABF, Zheng Li5ADEG*DOI: 10.12659/MSM.943233
Med Sci Monit 2024; 30:e943233
Abstract
BACKGROUND: The degree of postoperative symptom improvement in patients with lumbar spinal stenosis (LSS) is crucial to their postoperative rehabilitation process and functional exercise. Corticosteroids have certain anti-inflammatory effects. This study aimed to explore whether small doses of corticosteroids would improve postoperative neurological symptoms in patients with lumbar spinal stenosis.
MATERIAL AND METHODS: Patients with lumbar spinal stenosis who underwent open surgery were divided into a corticosteroid therapy group (CTG) and a non-corticosteroid therapy group (NCTG). They were followed up for 24 months after surgery. The numeric rating scale (NRS) for leg pain (NRS-LP) and leg numbness (NRS-LN), Oswestry Disability Index (ODI) scores, and Short Form Health Survey (SF-36) scores of the 2 groups were compared at different time points to evaluate the therapeutic effect.
RESULTS: Of the 232 eligible patients enrolled, 128 received corticosteroids and 104 did not. At the 1-month postoperative follow-up, patients in the CTG had significantly lower NRS-LP and NRS-LN scores than those in the NCTG (P=0.017; P=0.043). At the 3-month follow-up, the NRS-LP and ODI scores of patients in the CTG were significantly lower than those of the NCTG (P=0.015; P=0.027), and SF-36 scores were significantly higher than that of the NCTG (P=0.012). At the 6-month follow-up, the SF-36 scores of patients in the CTG was significantly higher than that of the NCTG (P=0.008).
CONCLUSIONS: Small doses of corticosteroid therapy for postoperative lumbar spinal stenosis reduced symptoms and improved quality of life scores after surgery. However, it had little long-term impact on final patient outcomes.
Keywords: 11-Hydroxycorticosteroids, Decompression, Surgical, Lumbar Vertebrae, Rehabilitation
Background
With lifestyle changes and the extension of the human life span, the incidence of lumbar spine diseases is gradually increasing. Severe lumbar spinal stenosis (LSS) is a relatively common disease [1], which often causes patients to experience symptoms such as pain, numbness, and movement dysfunction in the lower limbs, affecting the patients’ quality of life [2]. For patients with LSS, in addition to conservative treatment, surgical treatment is often an important option, aiming to relieve nerve compression, improve symptoms, and restore function.
In recent years, adjuvant treatment after surgery has attracted increasing attention from the medical community. Several studies suggest that after lumbar spinal decompression, there is a potential for further nerve damage attributable to ischemia-reperfusion injury following the alleviation of nerve and arterial compression. This damage is primarily associated with the inflammatory response induced by ischemia-reperfusion [3,4]. Corticosteroids have anti-inflammatory, anti-congestive, and stabilizing cell membrane effects [5]. It is reported that corticosteroids can help reduce the inflammatory reaction after surgery, promote nerve recovery, and thereby improve postoperative symptoms, such as pain and numbness [6,7]. Many doctors administer epidural injections of corticosteroids, often in combination with other pharmacological agents, to alleviate lower extremity symptoms in patients with LSS. This practice aims to diminish the patients’ likelihood of requiring surgery and to minimize the reliance on opioid analgesics [8–10]. However, the impact of this intervention on ameliorating postoperative symptoms in patients with LSS is still a subject of debate [11]. To date, few studies have focused on the efficacy of using postoperative systemic corticosteroids to ease symptoms in patients who have undergone conventional surgery.
Based on the above background, this retrospective cohort study aimed to systematically evaluate the effect of short-acting corticosteroid therapy after surgery in patients with severe LSS, explore whether corticosteroids can really help improve patients’ postoperative symptoms, and further clarify the effect and safety of corticosteroids on patients with severe LSS.
Through this study, we hope to provide clearer guidance for the postoperative treatment of patients with LSS, elucidate the impact of postoperative corticosteroid application on patient symptoms, and provide a more robust basis for doctors and patients to make informed decisions regarding treatment options.
Material and Methods
DATA ACQUISITION:
This retrospective cohort study was designed to evaluate whether small doses of corticosteroids could reduce postoperative symptoms in patients with LSS. We selected patients with a main diagnosis of LSS who underwent their first surgical treatment at Peking Union Medical College Hospital from January 2019 to September 2021. All patients were consecutively enrolled. The inclusion criteria were as follows: (1) age ≥50 years; (2) radiologically confirmed severe lumbar spinal stenosis; (3) preoperative severe lower limb pain or numbness significantly affecting daily life; and (4) underwent posterior open decompression, internal fixation, and bone graft fusion surgery performed by the same chief physician. The exclusion criteria were as follows: (1) combined with obvious lumbar disc herniation and treated with discectomy; (2) patients with long-term corticosteroid therapy due to other systemic diseases; (3) combined with severe stenosis of lower limb arteries, deep vein thrombosis, or any other vascular diseases; and (4) patients with incomplete follow-up data for 2 years after surgery. Patients enrolled were randomly assigned to either the corticosteroid treatment group (CTG) or the non-corticosteroid treatment group (NCTG). However, the CTG excluded patients with (1) allergies to corticosteroids; (2) diabetes; (3) myasthenia gravis; and (4) severe immunosuppression, which is mainly attributed to concerns regarding the safety and potential adverse effects associated with the use of corticosteroids.
All patient basic information was collected from the medical record system of Peking Union Medical College Hospital, including sex, age, body mass index (BMI), Charlson Comorbidity Index [12], total disease duration, number of fused vertebrae, number of decompressed vertebrae, number of interbody cages used, operation time, blood loss, drainage tube indwelling time postoperatively, and preoperative and postoperative sagittal vertical axis and its variation (Table 1). The Charlson Comorbidity Index was calculated to evaluate the patient’s physical condition before surgery. The sagittal vertical axis was determined by calculating the horizontal distance from the plumb line passing through the seventh cervical vertebra to the posterior-superior corner of the first sacral vertebra [13]. The drainage tube was removed once the wound drainage volume decreased to less than 100 mL/day.
This study was conducted strictly with the principles outlined in the Declaration of Helsinki regarding research involving human participants. Ethical approval for this retrospective analysis was obtained from the Ethics Committee of Peking Union Medical College Hospital (approval number: K3123). Informed consent was obtained from all patients upon admission for treatment. All personal identifiers were removed or altered before analysis to ensure the confidentiality and privacy of the participants’ data.
SURGICAL PROCEDURE AND POSTOPERATIVE RECOVERY:
In this study, all patients underwent conventional open surgery, which was performed under general anesthesia. The primary analgesic drug used during intraoperative anesthesia was remifentanil, which was often used in conjunction with sedatives such as propofol and etomidate. Upon positioning, the spinous processes, bilateral articular processes, and roots of the transverse processes were exposed. Subsequently, titanium pedicle screws (Legacy, Medtronic, USA) were bilaterally inserted into the pedicles. Following this, 2 titanium rods of the appropriate length were bent and secured between the nuts. After rod placement, laminectomy and spinal decompression were conducted. The extent of laminectomy typically spans from the lower one-fifth of the first affected vertebra to the upper two-thirds of the vertebral body at the last level of the stenotic segment. In the next step, bilateral modified facet joint fusion, an innovative technology developed by the author’s team [14], was performed. To elaborate, the articular surfaces of the facet joints on both sides were meticulously ground away using a high-speed abrasive drill, creating a bone graft bed. Allogeneic cancellous bone particles combined with autologous cancellous bone were implanted into this bed. This procedure ensures intervertebral stability by fusing the facet joints, thus obviating the need for intervertebral fusion. In our previous studies, it was observed that a significant proportion (93.6%) of patients achieved favorable outcomes in terms of spinal fusion [14]. Postoperatively, patients were routinely administered non-steroidal anti-inflammatory drugs (NSAIDs) for 3 to 7 days to manage pain and inflammation. We generally avoided routine opioid use postoperatively. However, in cases in which a patient experienced unbearable pain that impeded their rest, we administered a temporary tramadol intramuscular injection. Patients typically required hospitalization for approximately 7 days following surgery. Prior to discharge, all patients were confirmed to be free from tramadol dependence. Beyond this standard protocol, a subset of patients underwent an adjunct treatment involving intravenous dexamethasone 5 mg every 12 h for at least 3 consecutive days postoperatively, extending up to the time the patient was discharged. This adjunct treatment aimed to mitigate the inflammatory reaction potentially affecting the nerve roots, thereby categorizing patients into the 2 groups, CTG and NCTG. Generally, on the third day after surgery, the surgeon helped the patient get up next to the bed and try to stand next to the bed. By the end of the fifth day after surgery, the patient transitioned to autonomous walking. To enhance recovery, the surgeon additionally instructed the patient in waist and back muscle strengthening exercises, including the glute bridge [15], jogging, other related exercises, and the 5-point support, which was conducted as follows. The patient assumed a supine position and bent the knees. Using the soles of the feet, elbows, and shoulders as fulcrums, the patient proceeded to lift the pelvis until the shoulders, abdomen, and knees aligned in a straight line. Then, the pelvis was gradually lowered back to the starting position; the exercise was repeated for 20 to 30 consecutive times. After CTG patients were discharged from the hospital, it was not mandatory for them to continue corticosteroid treatment. Patients were advised to take oral dexamethasone acetate tablets (0.75 mg every 12 h) only when they experienced severe neurological symptoms. They were instructed that the medication should not be taken continuously for more than 1 week.
CLINICAL EFFICACY EVALUATION:
Patient self-assessment rating scales were distributed either through online web questionnaires or paper questionnaires, with the instruction that patients answer according to their circumstances. To accommodate elderly patients who could not use the web or paper questionnaires, we conducted follow-up interviews via voice calls and asked the same questions. We assessed clinical outcomes using the numeric rating scale (NRS) for leg pain (NRS-LP) and leg numbness (NRS-LN), along with a validated simplified Chinese version of the Oswestry Disability Index (ODI) scale [16], and the validated simplified Chinese version of the Short Form Health Survey (SF-36) [17]. Data collection occurred at specific time points: before surgery, and then at 1, 3, 6, 12, and 24 months after surgery. The score range for both the NRS-LP and NRS-LN is 0–9. A score of 9 indicates extreme difficulty in walking due to severe pain or numbness, significantly impacting daily life activities. Conversely, a score of 0 indicates the absence of any pain or numbness symptoms. Patients were instructed to assign scores based on the most intense symptoms experienced in their daily lives.
CORTICOSTEROID-RELATED COMPLICATIONS EVALUATION:
Corticosteroids, while potentially improving postoperative symptoms through their anti-inflammatory and immunosuppressive effects, can also lead to a variety of complications [18–20]. These complications can adversely affect the patient’s recovery. To further evaluate the safety of postoperative corticosteroids, we performed a detailed assessment of the postoperative corticosteroid-related complications observed in the 2 patient groups. Complications were assessed based on poor wound healing, infection, gastrointestinal adverse effects, gastrointestinal bleeding, skin itching or erythema, blood sugar abnormalities, sleep disorders, psychiatric disorders, and other complications mentioned in past literature. If complications occurred during hospitalization, these events were meticulously documented by the attending physician. Additionally, in the follow-up questionnaire issued 1 month after surgery, we asked patients about whether they had experienced any of the aforementioned complications.
STATISTICAL ANALYSIS:
All statistical analyses were conducted using the R tools (version 4.2.1) [21]. The “tableone” package [22] was used to describe patient demographics, while the “ggplot2” package [23] was used to create statistical visualizations. Data conforming to the normal distribution are presented as mean (standard deviation), and intergroup comparisons were performed using the independent sample t test. Conversely, data not adhering to the normal distribution are presented as medians (interquartile ranges), with intergroup comparisons conducted through the Mann-Whitney U test. For categorical variables, comparisons were executed using the chi-square test. A P value less than 0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS:
A total of 295 patients were initially considered for inclusion in the study. Among them, 19 patients were excluded because they were complicated with severe lumbar disc herniation and underwent discectomy, 3 patients were excluded because they were complicated with other underlying diseases and required long-term corticosteroid treatment, 7 patients were excluded because they were complicated with lower limb vascular diseases such as lower limb arterial stenosis and deep vein thrombosis, and 34 patients were excluded because they did not receive a complete 2-year postoperative follow-up. Consequently, 232 patients were deemed eligible and were enrolled in the study. Of these, 128 patients were administered dexamethasone 5 mg every 12 h for 3 consecutive days after surgery in addition to regular NSAID treatment for 3 to 7 days, while the remaining 104 patients received only the standard NSAID regimen for the same duration (Figure 1). All patients had complete follow-up data for the 24 months after surgery.
SYMPTOM RECOVERY OUTCOMES:
Follow-up for patients in both groups was maintained through to 24 months after surgery. The values of NRS-LP, NRS-LN, ODI, and SF-36 were collected at different postoperative time points (Table 2). Figure 2 illustrates the mitigating trend of NRS-LP, while Figure 3 similarly illustrates the mitigation in NRS-LN. In contrast, Figure 4 shows the recovery trajectory of the ODI, while Figure 5 shows the recuperation process of the SF-36.
At the 1-month postoperative follow-up, patients in the CTG had significantly lower NRS-LP and NRS-LN scores than did those in the NCTG [NRS-LP: CTG 2(2,3) vs NCTG 3(2,3) (
COMPARATIVE ANALYSIS OF SCORE VARIATIONS:
We also conducted a comparative analysis of score variations at each follow-up time point for each patient in the 2 groups. This involved calculating the absolute value of the difference between each patient’s preoperative scores (NRS-LP, NRS-LN, ODI, and SF-36) and their corresponding scores at each postoperative follow-up time point. These variations are denoted as ΔNRS-LP, ΔNRS-LN, ΔODI, and ΔSF-36, respectively. Furthermore, a statistical analysis was performed to evaluate the significance of the differences in these score changes between the 2 groups of patients. The results of this analysis are presented in Table 3. At the 1-month postoperative follow-up, patients in the CTG had significantly higher ΔNRS-LP, ΔNRS-LN, ΔODI, and ΔSF-36 than those in the NCTG [ΔNRS-LP: CTG 5(4,5) vs NCTG 4(4,5) (P=0.017); ΔNRS-LN: CTG 2(1,3) vs NCTG 1(0,2) (P<0.001); ΔODI: CTG 10(5.5,14) vs NCTG 6(2,12) (P=0.002); ΔSF-36: CTG 10(5,13) vs NCTG 7(3.75,12) (P=0.004)]. At the 3-month postoperative follow-up, patients in the CTG had significantly higher ΔNRS-LP, ΔNRS-LN, ΔODI, and ΔSF-36 than those in the NCTG [ΔNRS-LP: CTG 5(5,5) vs NCTG 5(4.75,5) (P=0.015); ΔNRS-LN: CTG 3(1,4) vs NCTG 2(1,3) (P=0.012); ΔODI: CTG 22(16,26) vs NCTG 19(14,24) (P=0.003); ΔSF-36: CTG 16(11.75,22) vs NCTG 13(9,19) (P=0.002)]. At the 6-month postoperative follow-up, patients in the CTG had significantly higher ΔODI and ΔSF-36 than those in the NCTG [ΔODI: CTG 28(22,34) vs NCTG 24(20,32) (P=0.011); ΔSF-36: CTG 17.5(13,23.25) vs NCTG 14.5(10,20.25) (P=0.001)], and there was no significant difference in ΔNRS-LP and ΔNRS-LN. In the follow-up after 6 months, there were no statistically significant differences in ΔNRS-LP, ΔNRS-LN, ΔODI, and ΔSF-36 between the 2 groups of patients.
CORTICOSTEROID-RELATED COMPLICATIONS ANALYSIS:
The data collection of postoperative corticosteroid-related complications of all enrolled patients was completed at the 1-month postoperative follow-up (Table 4). Eleven patients experienced poor wound healing (5 in CTG and 6 in NCTG, P=0.507). Four patients experienced infection (2 in CTG and 2 in NCTG, P=1.000). Thirty-eight patients experienced gastrointestinal adverse effects (19 in CTG and 11 in NCTG, P=0.335). No patients experienced gastrointestinal bleeding in either group. Four patients experienced skin itching or erythema (3 in CTG and 1 in NCTG, P=0.766). Five patients experienced blood sugar abnormalities (0 in CTG and 5 in NCTG, P=0.017). Six patients experienced sleep disorders (2 in CTG and 4 in NCTG, P=0.500). Three patients experienced psychiatric disorders (2 in CTG and 1 in NCTG, P=1.000). For other complications, 1 patient developed a foot drop (in CTG), and 1 patient developed a cerebrospinal fluid fistula (in NCTG).
Discussion
After lumbar spine surgery, patients frequently experience a constellation of symptoms, including low back pain, lower limb pain, lower limb numbness, and other neurological symptoms [24]. Unfortunately, for a subset of individuals, postoperative symptoms are not merely disruptive to daily activities but can also precipitate psychological distress and depression, thereby compounding the complexity of rehabilitation and detrimentally influencing postoperative recovery quality [25]. Consequently, it is of paramount importance to identify an effective and safe therapeutic approach to mitigate pain and numbness after surgery, thus enhancing patient quality of life and facilitating recovery.
Traditional modalities for managing postoperative pain, such as the administration of analgesic medications, including opioids and NSAIDs, are commonplace [26]. While these agents are adept at controlling pain, their attendant adverse effects are nontrivial and warrant cautious consideration. Specifically, in the early postoperative phase, the concomitant use of NSAIDs can exacerbate gastrointestinal adverse effects, a spectrum ranging from indigestion and stomach pain to the development of ulcers [27], especially when the patient has just completed surgery and is in poor eating condition. Similarly, although opioids are efficacious for pain relief, they are encumbered by serious potential risks, such as dependency and respiratory depression.
Considering these concerns, corticosteroid therapy has emerged as a compelling alternative for postoperative pain management. Corticosteroids, with their potent anti-inflammatory properties, can theoretically attenuate pain and augment neurological function by diminishing the inflammatory response secondary to surgery. Our study has demonstrated that short-acting corticosteroid therapy administered postoperatively significantly mitigated pain and numbness in the lower extremities during the early postoperative period.
Nevertheless, corticosteroid use is not devoid of risk and can lead to other complications. Some literature suggests that corticosteroids might precipitate hyperglycemia in patients with diabetes [28], induce immune suppression, with a subsequent heightened risk of perioperative infection [29], and exacerbate osteoporosis [30], potentially culminating in fusion failure. Hence, it is incumbent upon physicians to judiciously assess patient-specific variables when considering postoperative corticosteroid use and to implement stringent monitoring and risk mitigation strategies. In our cohort, corticosteroid therapy was not administered to patients with diabetes, myasthenia gravis, immunodeficiency disorders, or other patients for whom corticosteroid use might pose adverse effects. Still, there is currently no statistical evidence to indicate that patients in the CTG had a higher incidence of corticosteroid-related complications than did patients in the NCTG, suggesting that with appropriate management, the risks associated with corticosteroids can be judiciously managed.
In the early postoperative period, pain and numbness symptom scores in the CTG were significantly lower than those in the control group. This outcome aligns with the anti-inflammatory, anti-congestive, and cell membrane-stabilizing effects of corticosteroids. By mitigating the inflammatory response, corticosteroids may facilitate a reduction in local tissue reactions and nerve ischemia-reperfusion injury after surgical trauma, leading to a faster return to normal neurological function.
However, it is worth noting that the differences between the 2 groups gradually narrowed over time. At 6 months after surgery, the symptom scores of the 2 groups of patients were comparable to the preoperative levels, and there was no significant difference. This finding suggests that although corticosteroid therapy can provide short-term efficacy advantages, its impact on long-term efficacy appears to be limited.
In administering corticosteroids, it is crucial to thoroughly assess the potential for adverse events. Our evaluation involved quantifying 8 frequent corticosteroid-related complications in 2 patient cohorts. We observed a statistically significant difference in hyperglycemia. Notably, all 5 patients exhibiting abnormal blood sugar levels had pre-existing diabetes, underscoring a predisposed sensitivity. This specific outcome emerged as patients with diabetes were deliberately exempt from receiving corticosteroids at the onset of treatment, to mitigate the risk of hyperglycemia. Consequently, the data on postoperative complications do not support a statistically significant correlation between corticosteroid therapy and an elevated risk of complications in the broader patient population. This reinforces the premise that short-term, low-dose corticosteroid use postoperatively is safe.
Based on our study’s findings, the use of corticosteroids does not significantly alter patient long-term prognosis. However, their early administration after surgery effectively mitigates pain and numbness in the lower limbs during the immediate postoperative phase. This facilitates earlier ambulation and enables the initiation of rehabilitation exercises sooner.
Concurrently, it was observed that patients undergoing short-term postoperative corticosteroid therapy did not exhibit a significantly higher incidence of corticosteroid-related complications than the control group. This observation implies a potentially favorable benefit-risk ratio associated with the use of corticosteroids in this specific clinical setting.
This study is subject to several limitations: First, the retrospective nature of this study precludes random assignment, introducing an inherent degree of chance and potential bias in patient corticosteroid usage. Second, our exclusion of patients with less than 2 years of follow-up, which was a necessary criterion for our study design, introduced potential follow-up bias. Third, the assessment of postoperative pain can be compromised by individual variability in patient sensitivity to NSAIDs. Fourth, the use of the NRS for evaluation is susceptible to patient perception, which can introduce subjectivity into the measurements. Lastly, this study did not explored the ramifications of varying doses and treatment durations of corticosteroids. However, with ongoing research, there is potential for more refined tailoring of corticosteroid dosage and duration for diverse patient groups. Such advancements could offer enhanced direction for clinical treatment strategies.
Conclusions
For patients with LSS undergoing posterior open lumbar decompression surgery, the administration of short-term corticosteroid therapy in the immediate postoperative period was associated with a reduction in pain and numbness in the lower limbs. This therapy was been observed to decrease the pain and numbness experienced by patients at 1 month and 3 months after surgery. Furthermore, it led to a reduction in the ODI scores and an increase in the SF-36 scores within 6 months after surgery. This has certain benefits for patients’ postoperative symptom improvement. However, during prolonged follow-up, the use of corticosteroids appears to have minimal influence on the patients’ ultimate outcomes. From a safety perspective, our data indicate that the use of corticosteroids is not associated with an increased risk of postoperative complications.
Figures
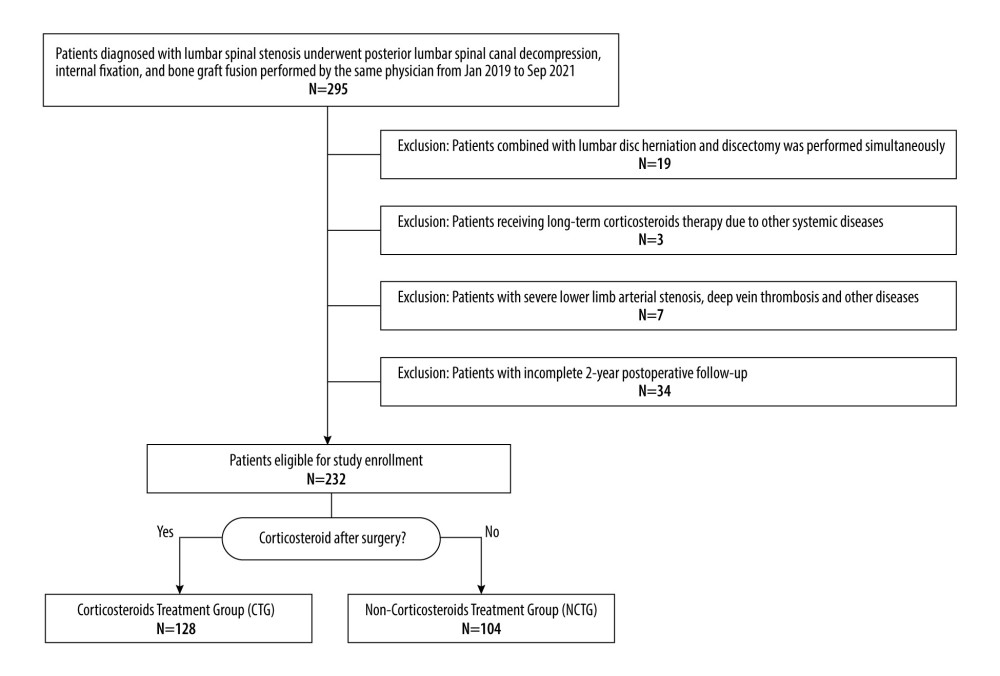 Figure 1. Inclusion flowchart.
Figure 1. Inclusion flowchart. 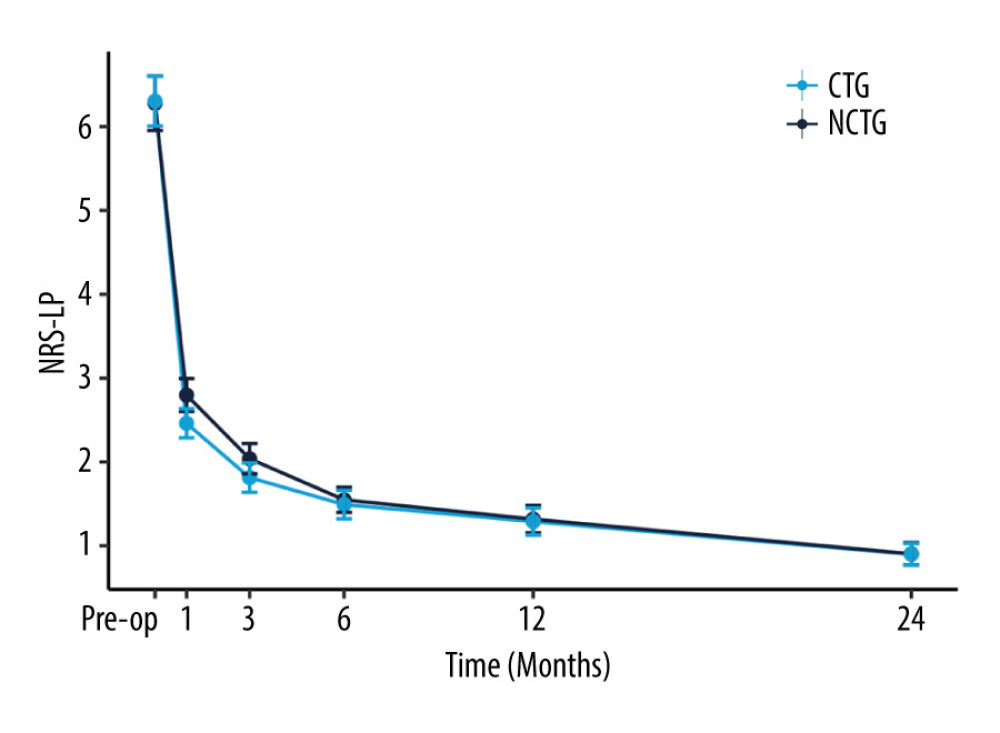 Figure 2. The recovery outcome of leg pain using the numeric rating scale (NRS-LP).
Figure 2. The recovery outcome of leg pain using the numeric rating scale (NRS-LP). 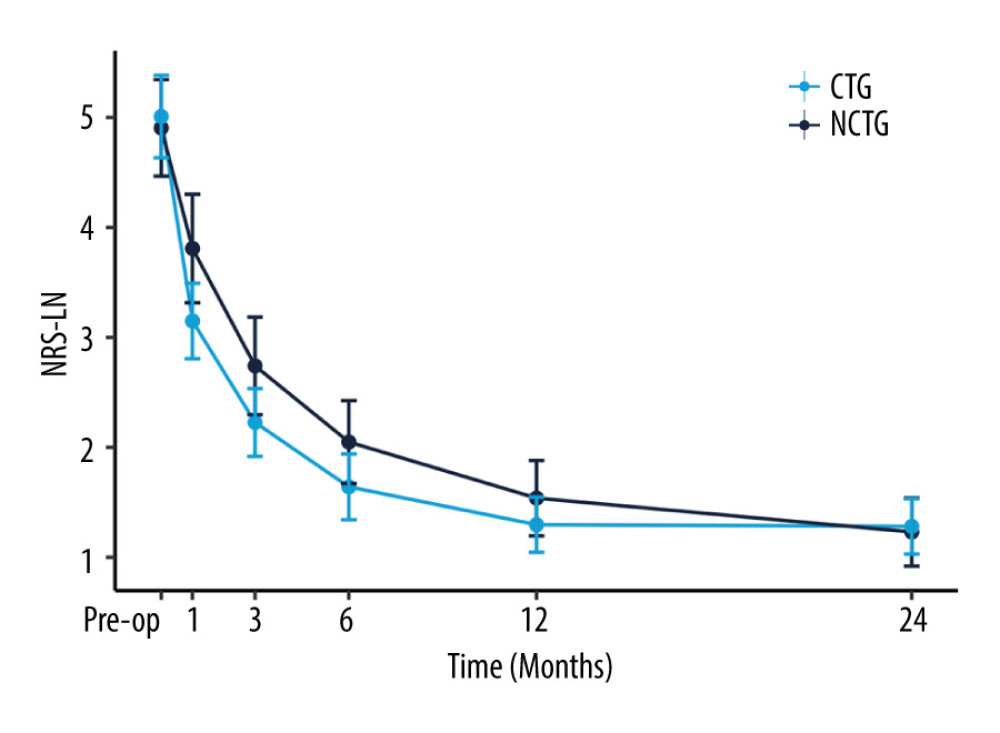 Figure 3. The recovery outcome of leg numbness leg pain using the numeric rating scale.
Figure 3. The recovery outcome of leg numbness leg pain using the numeric rating scale. 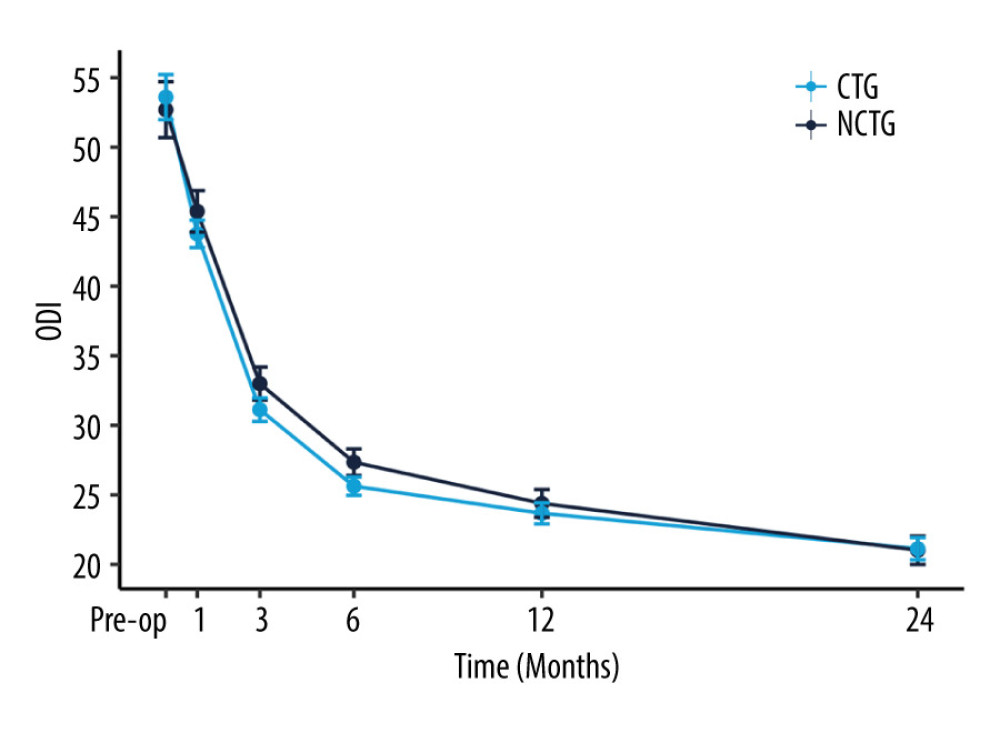 Figure 4. The recovery outcome of Oswestry Disability Index (ODI).
Figure 4. The recovery outcome of Oswestry Disability Index (ODI). 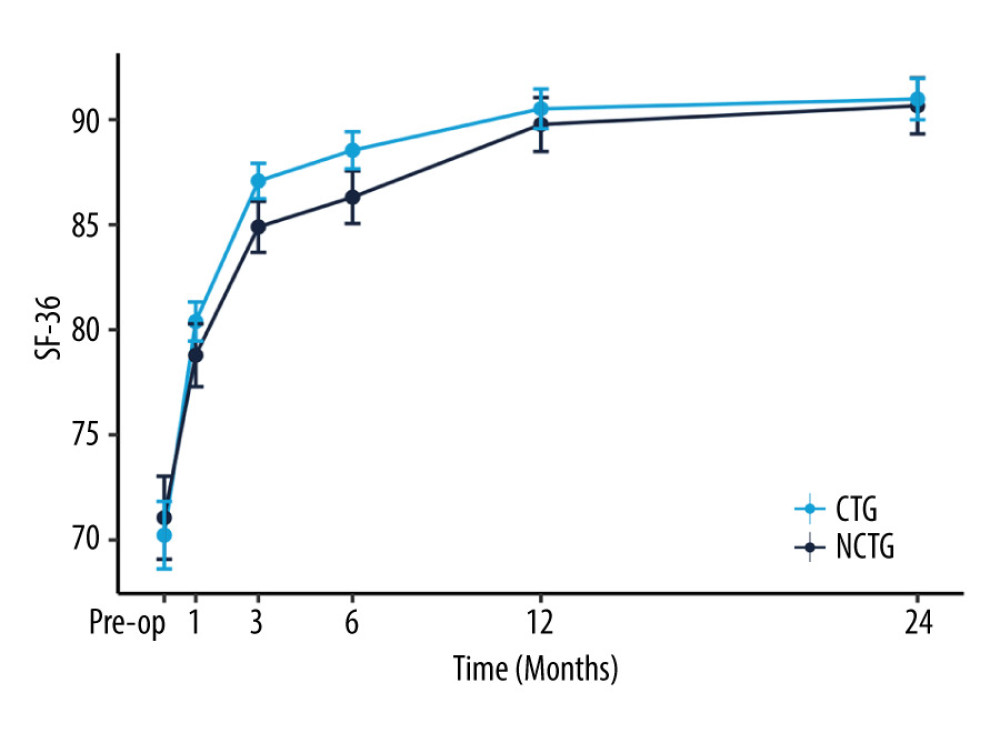 Figure 5. The recovery outcome of Short Form Health Survey (SF-36).
Figure 5. The recovery outcome of Short Form Health Survey (SF-36). References
1. Katz JN, Zimmerman ZE, Mass H, Makhni MC, Diagnosis and management of lumbar spinal stenosis: A review: JAMA, 2022; 327(17); 1688-99
2. Siebert E, Prüss H, Klingebiel R, Failli V, Lumbar spinal stenosis: Syndrome, diagnostics and treatment: Nat Rev Neurol, 2009; 5(7); 392-403
3. Smith PD, Puskas F, Meng X, The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury: Circulation, 2012; 126(11 Suppl 1); S110-17
4. Sun Z, Wang Y, Pang X, Mechanisms of polydatin against spinal cord ischemia-reperfusion injury based on network pharmacology, molecular docking and molecular dynamics simulation: Bioorg Chem, 2023; 140; 106840
5. Manchikanti L, Role of neuraxial steroids in interventional pain management: Pain Physician, 2002; 5(2); 182-99
6. Turan A, Sessler Daniel I, Steroids to ameliorate postoperative pain: Anesthesiology, 2011; 115(3); 457-59
7. Lee MS, Moon HS, Safety of epidural steroids: A review: Anesth Pain Med (Seoul), 2021; 16(1); 16-27
8. Friedly JL, Comstock BA, Turner JA, A randomized trial of epidural glucocorticoid injections for spinal stenosis: N Engl J Med, 2014; 371(1); 11-21
9. Manchikanti L, Cash KA, McManus CD, Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis: Pain Physician, 2012; 15(5); 371-84
10. Tafazal S, Ng L, Chaudhary N, Sell P, Corticosteroids in peri-radicular infiltration for radicular pain: A randomised double blind controlled trial. One year results and subgroup analysis: Eur Spine J, 2009; 18(8); 1220-25
11. Chou R, Pinto RZ, Fu R, Systemic corticosteroids for radicular and non-radicular low back pain: Cochrane Database Syst Rev, 2022; 10(10); CD012450
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation: J Chronic Dis, 1987; 40(5); 373-83
13. Jackson RP, McManus AC, Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size. A prospective controlled clinical study: Spine (Phila Pa 1976), 1994; 19(14); 1611-18
14. Ren Z, Li Z, Li S, Modified facet joint fusion for lumbar degenerative disease: Case series of a fusion technique, clinical outcomes, and fusion rate in 491 patients: Oper Neurosurg, 2020; 19(3); 255-63
15. Gasibat Q, Alexe CI, Raveica G, Decoding hip muscle activation: A comparative electromyographic analysis of turn-out bent knee pulse and single-leg banded glute bridge exercises in healthy female subjects: Eur J Investig Health Psychol Educ, 2023; 13(9); 1612-23
16. Liu H, Tao H, Luo Z, Validation of the simplified Chinese version of the Oswestry Disability Index: Spine (Phila Pa 1976), 2009; 34(11); 1211-16 discussion 1217
17. Li L, Wang HM, Shen Y, Chinese SF-36 Health Survey: Translation, cultural adaptation, validation, and normalisation: J Epidemiol Community Health, 2003; 57(4); 259-63
18. Koshi EJ, Young K, Mostales JC, Vo KB, Burgess LP, Complications of corticosteroid therapy: a comprehensive literature review: J Pharm Technol, 2022; 38(6); 360-67
19. Timm V, Timo E, Christoph T, Roland B, Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature: Eur Respir J, 2018; 52(4); 1800703
20. Li J-X, Cummins CL, Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions: Nat Rev Endocrinol, 2022; 18(9); 540-57
21. R Core Team: R: A language and environment for statistical computing, 2022, R Foundation for Statistical Computing Available from: https://www.R-project.org/
22. Yoshida K, Bartel A: tableone: Create ‘Table 1’ to describe baseline characteristics with or without propensity score weights, 2022 Available from: https://rdrr.io/cran/tableone/
23. Wickham H: ggplot2: Elegant graphics for data analysis, 2016, New York, Springer-Verlag
24. Hara N, Oka H, Yamazaki T, Predictors of residual symptoms in lower extremities after decompression surgery on lumbar spinal stenosis: Eur Spine J, 2010; 19(11); 1849-54
25. Jackson KL, Rumley J, Griffith M, Correlating psychological comorbidities and outcomes after spine surgery: Global Spine J, 2020; 10(7); 929-39
26. Bajwa SJ, Haldar R, Pain management following spinal surgeries: An appraisal of the available options: J Craniovertebr Junction Spine, 2015; 6(3); 105-10
27. Sivaganesan A, Chotai S, White-Dzuro G, The effect of NSAIDs on spinal fusion: A cross-disciplinary review of biochemical, animal, and human studies: Eur Spine J, 2017; 26(11); 2719-28
28. Deutsch AJ, Schroeder PH, Mandla R, Type 2 diabetes polygenic score predicts the risk of glucocorticoid-induced hyperglycemia in patients without diabetes: Diabetes Care, 2023; 46(8); 1541-45
29. Muffly B, Ayeni A, Jones C, Periprosthetic joint infection risk after primary total knee arthroplasty: Are all preoperative corticosteroid injections the same?: J Arthroplasty, 2023 [Online ahead of print]
30. Sørensen NN, Andreasen CM, Jensen PR, Disturbed bone marrow adiposity in patients with Cushing’s syndrome and glucocorticoid- and postmenopausal-induced osteoporosis: Front Endocrinol (Lausanne), 2023; 14; 1232574
Figures
 Figure 1. Inclusion flowchart.
Figure 1. Inclusion flowchart. Figure 2. The recovery outcome of leg pain using the numeric rating scale (NRS-LP).
Figure 2. The recovery outcome of leg pain using the numeric rating scale (NRS-LP). Figure 3. The recovery outcome of leg numbness leg pain using the numeric rating scale.
Figure 3. The recovery outcome of leg numbness leg pain using the numeric rating scale. Figure 4. The recovery outcome of Oswestry Disability Index (ODI).
Figure 4. The recovery outcome of Oswestry Disability Index (ODI). Figure 5. The recovery outcome of Short Form Health Survey (SF-36).
Figure 5. The recovery outcome of Short Form Health Survey (SF-36). Tables
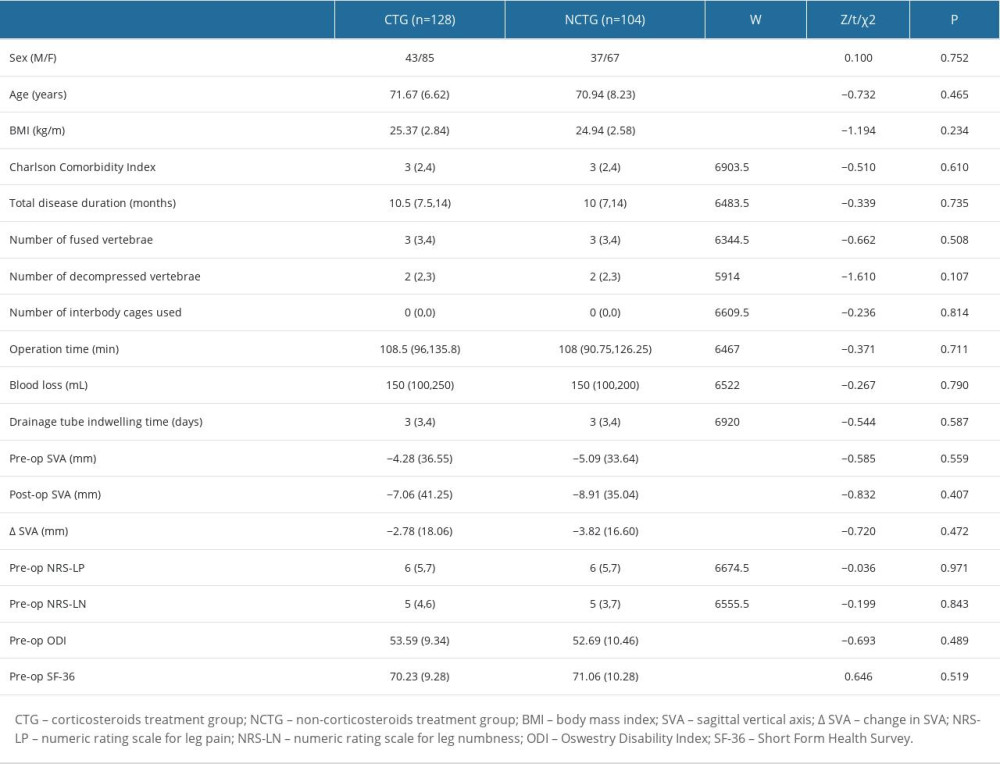 Table 1. Patient baseline characteristics.
Table 1. Patient baseline characteristics.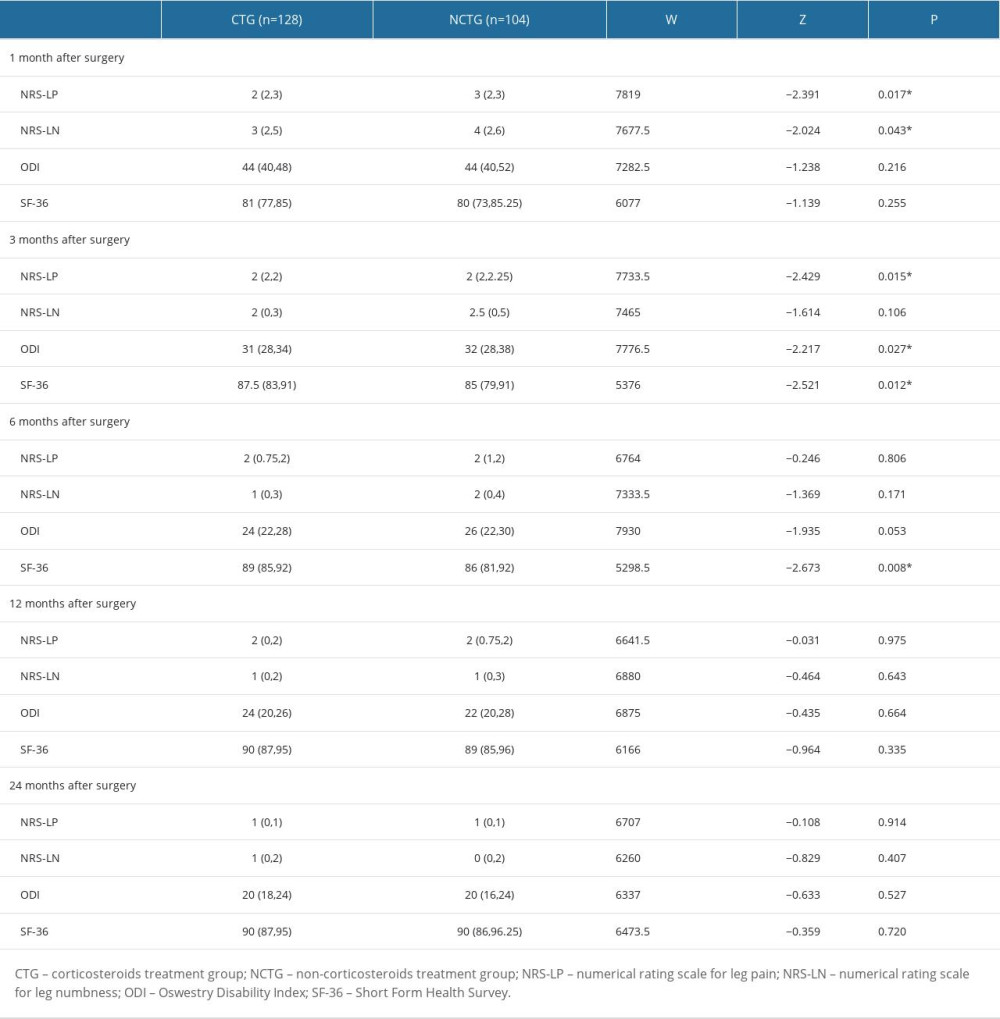 Table 2. Symptom recovery outcomes within 24 months.
Table 2. Symptom recovery outcomes within 24 months.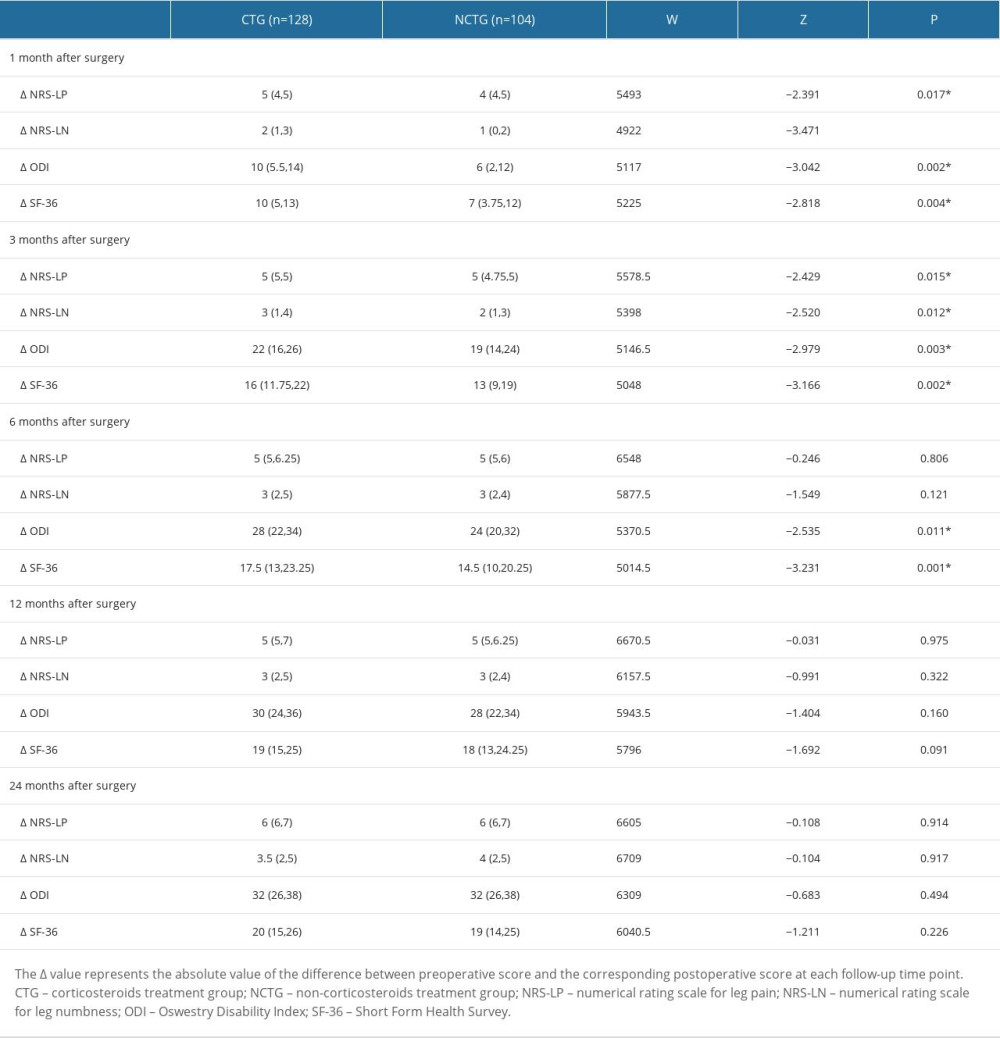 Table 3. Postoperative scores compared with preoperative scores (score variation [Δ]).
Table 3. Postoperative scores compared with preoperative scores (score variation [Δ]).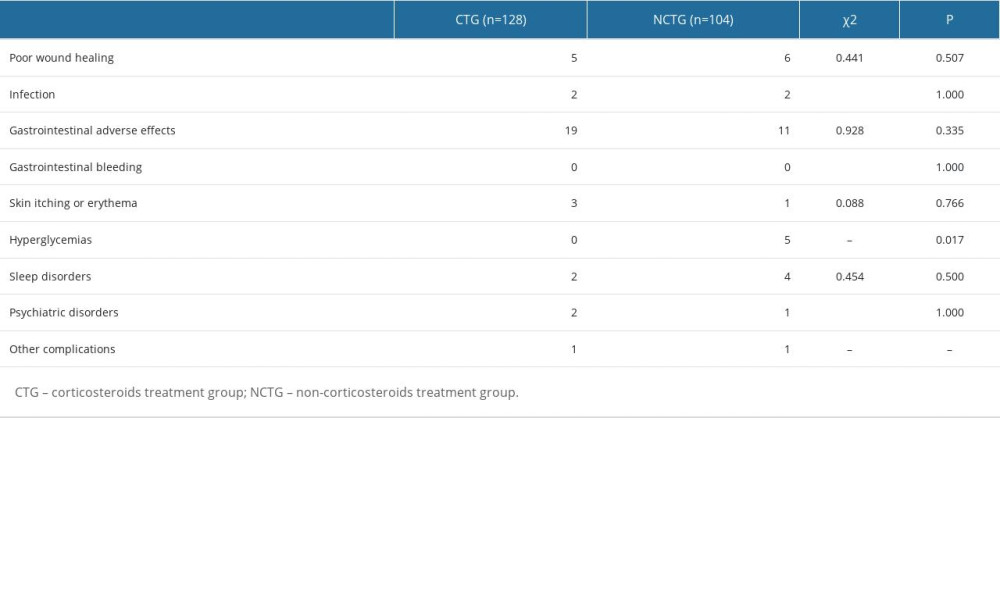 Table 4. Postoperative complications.
Table 4. Postoperative complications. Table 1. Patient baseline characteristics.
Table 1. Patient baseline characteristics. Table 2. Symptom recovery outcomes within 24 months.
Table 2. Symptom recovery outcomes within 24 months. Table 3. Postoperative scores compared with preoperative scores (score variation [Δ]).
Table 3. Postoperative scores compared with preoperative scores (score variation [Δ]). Table 4. Postoperative complications.
Table 4. Postoperative complications. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








