04 August 2020: Lab/In Vitro Research
Role and Mechanism of Maresin-1 in Acute Lung Injury Induced by Trauma-Hemorrhagic Shock
Huayi Ma1ABCDEFG, Song Mo1ABCDEFG, Qushen Yi1BCD, Junhua Lai1BF, Huan Liu1BEFG*, Zhanying Shi2BEFGDOI: 10.12659/MSM.923518
Med Sci Monit 2020; 26:e923518
Abstract
BACKGROUND: It is reported that trauma hemorrhagic shock (THS) could resulted in organ injury and is related to a high mortality rate. Maresin-1 (MaR1), a derived medium through biosynthesis, is involved in inflammatory responses. However, the mechanism of MaR1 against acute lung injury needs to be further understood. This report aimed to explore whether MaR1 had a protective effect on lung injury.
MATERIAL AND METHODS: We constructed a THS-induced acute lung damage rat model and then treated the rats with MaR1. We determined Evan’s blue dye (EBD) lung permeability, lung permeability index, wet/dry (W/D) weight ratio, nitric oxide (NO) concentration and inducible nitric oxide synthase (iNOS) expression in lung tissue samples. The inflammation-related cytokines levels in the bronchoalveolar lavage fluid (BALF) and serum of rats were determined by enzyme-linked immunosorbent assay (ELISA). Finally, the TLR4/p38MAPK/NF-κB pathway was analyzed by quantitative real-time polymerase chain reaction and western blot assay.
RESULTS: The increased EBD ratio, lung permeability index and W/D weight ratio, NO concentration and iNOS levels were suppressed by MaR1 treatment. THS-induced over-production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in BALF and serum was suppressed by MaR1. Besides, the TLR4/p38MAPK/NF-κB pathway activation in THS-induced rats were inhibited by MaR1 treatment.
CONCLUSIONS: Our study showed that MaR1 could effectively alleviated THS-induced lung injury via inhibiting the excitation of the TLR4/p38MAPK/NF-κB pathway in THS-induced rats, suggesting that MaR1 might be a novel agent for lung damage treatment.
Keywords: acute lung injury, Toll-Like Receptor 4, Bronchoalveolar Lavage Fluid, Cytokines, Docosahexaenoic Acids, Nitric Oxide, Nitric Oxide Synthase Type II, Protective Agents, Shock, Hemorrhagic
Background
Acute lung injury, a primary cause of trauma hemorrhagic shock (THS), could develop into acute respiratory distress syndrome (ARDS) [1,2]. Acute lung injury can also cause dyspnea, endothelium damage, and gas exchange deterioration [3,4]. Previous studies have reported that severe THS may result in inflammatory response in multiple organ and high mortality [5]. Despite improvement in understanding the pathogenesis of THS, there is still no effective therapeutic strategies to treat THS.
Inflammatory response is one of the main causes in acute lung injury, and excessive inflammatory responses may result in a number of diseases, such as tissue destruction, fibrosis, and organ failure [6,7]. Thus, exploitation of effective drugs against inflammation may be conducive to treatment of THS. Studies have revealed that various regulators, such as lipoxins [8], resolvins [9], and protectins [10], could mediate inflammatory response. MaR1, an acid-derived regulator, is bio-synthesized by docosahexaenoic acid and conversion by oxidation. MaR1 reportedly blocks the bronchial epithelial cells immunoreaction and induces tissue regeneration [11,12]. Moreover, MaR1 exhibits various pharmacological and biological functions in the lung. Chatterjee et al. revealed that MaR1 could relieve inflammatory response in vascular smooth muscle [13]. However, whether MaR1 has a protective effect on lung injury by THS is unknown.
Based on previous investigations, in this study, we aimed to explore the role of MaR1 in THS-induced lung injury models and the related mechanisms.
Material and Methods
ANIMALS:
Male Sprague-Dawley rats (10 to 14 weeks old; 360 to 400 g) were purchased from Vital River Company (Beijing, China) and were kept under a temperature-controlled condition with 12 hours light/dark cycle, 60% to 65% humidity and allowed to feed freely. All protocols of animal experiments were conducted in accordance with Animal Care and Use Committee of the Fourth Affiliated Hospital of Guangxi Medical University.
MODEL OF THS AND TREATMENT:
Rats were assigned at random to 1 of 5 groups (n=20 for each group): sham, sham+MaR1, TSH, TSH+saline, TSH+MaR1. The rats in the sham group were injected by physiological saline (0.1 mL/100 g, intraperitoneal injection) and rats in the sham+MaR1 group were treated with 10 ng/mL MaR1 (Cayman, Michigan, USA, intravenously) [14]. The THS model was conducted according to previous research methods [15]. Twelve rats were randomly selected from each group: 4 rats were used for lung permeability evaluation, and the other 8 rats were used for collection of bronchoalveolar lavage fluid (BALF), orbital blood, serum, and lung tissue samples. And 8 rats in each group were used for survival analysis. At 24 hours after TSH induction, rats were anesthetized with pentobarbital (40 mg/kg) by intraperitoneal injection and sacrificed by cervical dislocation (death defined as the lack of heartbeat and breathing).
EVANS BLUE DYE (EBD):
Evans blue dye (EBD) levels were used to assess the lung permeability as described previously [16]. In brief, all rats were stimulated with 1% EBD reagent by jugular vein. Then 10 minutes later, blood samples (1 mL) were collected from the femoral artery catheter. After 20 minutes, the separated lungs were washed with normal saline 3 times, then BALF was obtained and centrifuged. The percentage of EBD in plasma and BALF samples were analyzed by measuring the OD620 using a micro-plate reader (BioTek, USA).
LUNG PERMEABILITY INDEX:
The ratio of BALF protein concentration to serum protein concentration were evaluated to measure lung permeability as described previously [15]. In short, supernatant was blended with saline or Coomassie brilliant blue, then the absorbance at 595 nm were determined on a micro-plate reader (BioTek, USA).
LUNG WET/DRY (W/D) WEIGHT RATIO MEASUREMENT:
To evaluate the severity of pulmonary edema, we quantified the lung wet/dry (W/D) weight ratio. In brief, the fresh lung samples were collected and wiped-dry with filter paper, then these samples were weighed before being dried. After 48 hours of being dried, we weigh these lung samples again.
ELISA ASSAY:
Lung injury was assessed by detecting the levels of pro-inflammatory factors as described previously. In short, tracheas were exposed, and lung tissue specimens were obtained, lavaged, and centrifuged. Then we measured interleukin-6 (IL-6) (Cat no. PI328) and tumor necrosis factor-α (TNF-α) (Cat no. PT516) levels in BALF or serum respectively using enzyme-linked immunosorbent assay (ELISA) kits (Beyotime, Shanghai, China) following the manufacturer’s protocols.
NITRIC OXIDE (NO) DETECTION:
To evaluate the nitric oxide (NO) levels in samples, we determined the sum of the concentration of stable NO metabolites, the nitrite concentration as described previously [17].
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION (QRT-PCR):
TRIzol reagent (Thermo Fisher Scientific, MA, USA) was adopted to separate total RNA from serum and tissues following the manufacturer’s protocol. The cDNA Synthesis Kit (Takara Bio Inc., Otsu, Japan) was adopted to reverse transcription of total RNA to cDNA and gene expression was measured by Real-time PCR system with SYBR Green Master mix (Invitrogen; Thermo Fisher Scientific, Inc., USA), following the manufacturer’s protocol. Primer sequences were as follows:
After 5 minutes of denaturation at 95°C, 35 cycles were performed: 94°C for 1 minute, 60°C for 1 minutes, 55°C for 60 seconds and final extension at 72°C for 10 minutes. The target genes levels were calculated using 2−ΔΔCq method [18].
WESTERN BLOT ASSAY:
Radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, China) was adopted to isolate protein from lung tissues. Then, protein fractions were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transferring to polyvinylidene difluoride (PVDF) membranes. The membranes were closed with 5% milk and incubated with TLR4 (1: 1000; Cell Signaling Technology, Danvers, MA, USA), MyD88 (1: 1000; Cell Signaling Technology), p-p65 (1: 1000; Cell Signaling Technology), p65 (1: 1000; Cell Signaling Technology), p-p38 (1: 1000; Cell Signaling Technology), p-p38 (1: 1000; Cell Signaling Technology), and GAPDH (1: 1000; Cell Signaling Technology) antibodies overnight at 4°C. Then the membranes were coupled with second antibody (1: 2000; Cell Signaling Technology) for 1 hour at room temperature. At last, the proteins were detected using enhanced chemiluminescence (Beyotime, Shanghai, China) with Canon imaging system and quantified by using Gel-Pro-Analyzer software version 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
STATISTICAL ANALYSIS:
Data were presented as the mean±standard deviation (SD). SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used to conduct statistical analyses. Differences among the groups were evaluated with Student’s
Results
MAR1 REDUCED THS-STIMULATED LUNG TISSUE BALF/PLASMA EBD RATIO, LUNG PERMEABILITY INDEX, AND W/D WEIGHT RATIO:
To explore the role of MaR1 in acute lung injury induced by THS, we firstly explored the effects of MaR1 on THS-induced rats lung tissues. Figure 1 exhibited that the BALF/plasma EBD ratio (Figure 1A), lung permeability index (Figure 1B) and W/D weight ratio (Figure 1C) in THS group were obviously higher than those in sham group. However, there was no statistic difference between the sham group and the sham+MaR1 group. Compared to the TSH+saline group, these 3 indicators in THS+MaR1 group were markedly decreased.
MAR1 REDUCED THE SECRETION OF INFLAMMATORY CYTOKINE IN THS-INDUCED RATS:
Then we investigate whether MaR1 had anti-inflammatory impacts in THS-induced rats, as it has been reported that inflammatory factors participated in the propagation of immunoreaction. In this study, we determined the particular factors by ELISA. As presented in Figure 2A and 2C, the TNF-α level in the BALF and serum of THS-treated rats were significantly enhanced compared to the sham treated rats. However, there was no obvious difference between the sham group and the sham+MaR1 group. In contrast, the levels of TNF-α were downregulated in the THS+MaR1 group compared with the TSH+saline group. In addition, the same trend of IL-6 levels in in serum and BALF of rats were observed (Figure 2B, 2D).
MAR1 REDUCED NO CONCENTRATIONS AND INDUCIBLE NITRIC OXIDE SYNTHASE (INOS) LEVELS ASSOCIATED WITH LUNG THS INJURY:
Inducible nitric oxide synthase (iNOS) has been identified as a regulator in the progression of lung injury during trauma-hemorrhagic shock, thus we investigated the roles of MaR1 in NO concentrations and iNOS levels during lung THS injury. We found that NO concentrations were obviously higher in the THS group relative to the sham group. A reduction was found in the THS+MaR1 group compared with the TSH+saline group (Figure 3A). Furthermore, results from qRT-PCR and western blot analysis showed that iNOS mRNA levels (Figure 3B) and protein expression (Figure 3C) were remarkably augmented in the THS group compared to the sham group. Compared with the TSH+saline group, iNOS was downregulated in the THS+MaR1 group. Meanwhile, both NO and iNOS levels had no outstanding difference between the sham group and the MaR1 group. These results indicated an association between NO and iNOS response in lung THS injury and MaR1 treatment.
MAR1 BLOCKED THE ACTIVATION OF THE TLR4/P38MAPK/NF-κB SIGNALING PATHWAY IN THS-STIMULATED ACUTE LUNG INJURY:
Finally, we investigated the mechanism of MaR1 in THS-induced acute lung injury. As shown in Figure 4A, increased protein levels of TLR4 and MyD88 were observed in lung tissues caused by THS and these levels were suppressed by MaR1 treatment. In addition, upregulation of TLR4 and MyD88 mRNA levels in lung tissues of THS rats were found, and these levels were suppressed by MaR1 (Figure 4B, 4C). However, the TLR4 and MyD88 protein expression and mRNA levels did not change in the sham group or the sham+MaR1 group.
Various reports have indicated that the p38MAPK/NF-κB pathway is a main regulator in inflammatory response. Thus, we detected the related protein expression in THS-induced lung injury. Our findings suggested that p-p65 and p-p38 levels were promoted in the THS group compared to the sham group, while the expression of p-p65 and p-p38 were inhibited by MaR1 treatment (Figure 5A). We also found that the ratio of p-p65/p65 (Figure 5B) and p-p38/p38 (Figure 5C) were enhanced in the THS group compare to the sham group. However, these increases were inhibited in the THS+MaR1 group compared with the TSH+saline group. Meanwhile, the ratio of p-p65/p65 and p-p38/p38 was not obviously change in the sham group and the sham+MaR1 group. Next, we measured the mRNA of p65 and p38 in different groups using qRT-PCR. Our data demonstrated that p65 (Figure 5D) and p38 (Figure 5E) mRNA levels had no statistic difference between all groups. These results revealed that MaR1 blocked the excitation of the TLR4/p38MAPK/NF-κB signaling pathway in THS-induced acute lung injury.
Discussion
In this report, our evidence showed that MaR1 had a protective effect in THS-induced lung damage. This effect probably depends on the regulation of MaR1 in immune response because MaR1 obviously reduced the release of inflammatory factors and restored damage tissues. Besides, we also found that the protective effects of MaR1 were exhibited by the reduction of EBD, lung permeability index, W/D weight ratio, NO concentration, and iNOS levels in BALF, serum, and lung tissues. Moreover, the related protein expression and mRNA levels in the TLR4/p38MAPK/NF-κB signaling pathway were altered after MaR1 treatment. These results revealed that MaR1 had protective effects in THS-induced lung damage partly via regulating the TLR4/p38MAPK/NF-κB signaling pathway.
Acute lung injury can be induced by THS, which is a complex disease in the clinical setting [17]. Imbalanced inflammation, uncontrolled accumulation of leukocytes and platelets, and the change in tissues barriers are the main pathophysiological characteristics of acute lung injury [19]. MaR1 is known to participate in various pharmacological functions, such as cells immunoreaction [20], tissue regeneration, and pain [21]. However, whether MaR1 has a protective effect in THS-induced lung injury and the underlying mechanisms in disease progress are still not well understood. EBD, lung permeability index, and W/D weight ratio have been widely used to evaluate the severity of pulmonary epithelial and vascular permeability. Therefore, we firstly used THS to establish lung injury model and determined the EBD ratio, lung permeability index, and W/D weight ratio in THS groups compared to sham groups. We found that the EBD ratio, lung permeability index, and W/D weight ratio were enhanced in THS groups compared to sham groups. Besides, these indicators were suppressed in THS+MaR1 group and there was no statistical difference between the sham groups and MaR1 groups. However, in the present study, we evaluated the successful establishment of the THS model only by lung permeability, lung permeability index, and pulmonary edema evaluation. Physiological indices and hypoxic parameters, which we did not analyzed, are also very important factors to evaluate to determine whether the THS model is successfully established. This was a limitation of our study.
Recent reports have shown that MaR1 regulates immunoreaction in lipopolysaccharide (LPS)-stimulated acute lung injury by reducing neutrophil accumulation and pro-inflammatory factors release [22]. Also, Chatterjee et al. demonstrated that MaR1 inhibited TNF, IL-1β, and IL-8 production in vascular smooth muscle and endothelial cells [23]. TNF-α and IL-6 are commonly used inflammatory markers, which exhibited vital roles in lung damage. In our study, we detected IL-6 and TNF-α levels in BALF or serum in the different rat groups. Results from ELISA analysis demonstrated that these indicators were upregulated in the THS groups and suppressed after treatment of MaR1, suggesting that MaR1 likely blocked inflammatory response and relieved lung damage in THS-induced rat models. In addition, iNOS has been identified as a regulator in lung injury development, and NO concentration has been used to evaluate the degree of lung injury during THS [24,25]. Harkin et al. found that iNOS inhibition could alleviate acute lung injury [26]. We measured the NO concentrations and iNOS levels in our lung injury rat models, our results in accordance with previous studies, indicating that NO concentrations and iNOS levels were obviously lower in the sham group relative to that in THS group. A reduction was observed in the THS+MaR1 group as compared with the TSH+saline group. Meanwhile, both NO and iNOS levels were no obvious difference between the sham group and the MaR1 group. These aforementioned data were at least partly indicated that MaR1 are participated in the progression of THS-induced acute lung injury.
Many reports have evidenced that various signaling pathways often been activated in inflammatory response, such as Nrf2/HO-1, NF-κB signaling pathway [27], and MAPK pathway [28]. TLR4 has been reported to be enhanced in THSinduced acute lung injury [29,30]. Studies have also revealed the activation of p38MAPK/NF-κB pathway in THS-induced acute lung injury and lung injury [29,31,32]. Furthermore, we measured the related protein expression in the TLR4/p38MAPK/NF-κB pathway in THS-induced lung injury models. Our findings suggested that levels of TLR4 and MyD88 were increased in lung tissues caused by THS and suppressed as MaR1 treatment. However, these levels did not change in the sham group and the MaR1 group. Besides, p-p65 and p-p38 levels were enhanced in the THS group and inhibited as MaR1 treatment. Also, the ratio of p-p65/p65 and p-p38/p38 were rise in the THS group compare to the sham group. However, these increases were inhibited in the THS+MaR1 group. Meanwhile, p65 and p38 mRNA levels had no statistic difference between all groups. However, only MaR1 of 10 ng/mL was used in this study and the effect of various dose of MaR1 on THS induced acute lung injury rats should be investigated. This was a limitation of this study which needs further investigation.
Conclusions
In conclusion, we provided the first evidence that MaR1 had a protective effect on THS induced lung injury. This protection possibly depends on inhibiting the activation of the TLR4/p38MAPK/NF-κB pathway in THS-induced rats. These data reveled that MaR1 may be a potential therapeutic approach for acute lung injury treatment.
Figures
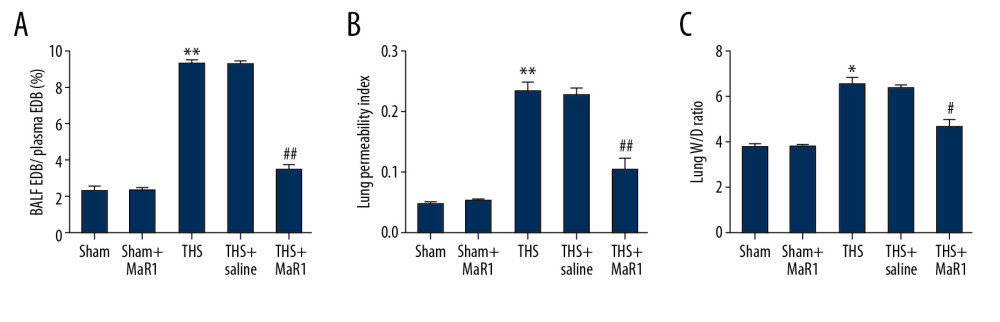 Figure 1. MaR1 reduced BALF/plasma EDB ratio, lung permeability index and wet/dry weight ratio in acute lung injury induced by THS. A lung injury model was induced by THS before intravenously injection of 10 ng/mL MaR1 or 0.1 mL/100 g physiological saline. (A) lung permeability to EBD was conducted. (B) lung permeability activity. (C) Wet/Dry weight ratio. *, ** P<0.05, 0.01 versus Sham; #, ## P<0.05, 0.01 versus THS+saline group. MaR1 – Maresin-1; BALF – bronchoalveolar lavage fluid; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 1. MaR1 reduced BALF/plasma EDB ratio, lung permeability index and wet/dry weight ratio in acute lung injury induced by THS. A lung injury model was induced by THS before intravenously injection of 10 ng/mL MaR1 or 0.1 mL/100 g physiological saline. (A) lung permeability to EBD was conducted. (B) lung permeability activity. (C) Wet/Dry weight ratio. *, ** P<0.05, 0.01 versus Sham; #, ## P<0.05, 0.01 versus THS+saline group. MaR1 – Maresin-1; BALF – bronchoalveolar lavage fluid; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. 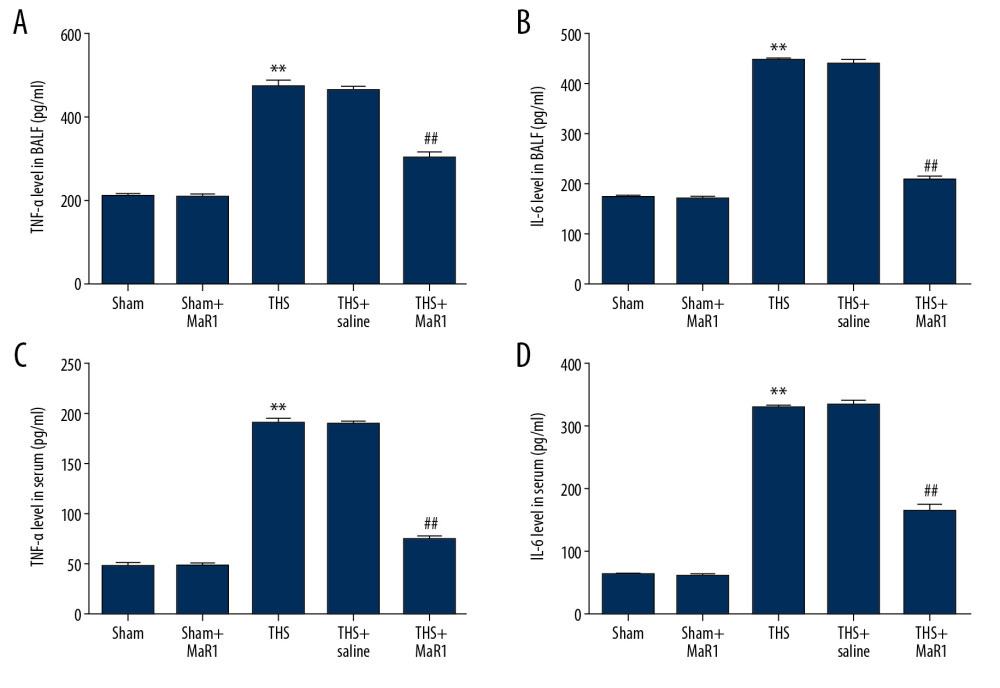 Figure 2. MaR1 ameliorated the release of inflammatory cytokine in THS-stimulated rats. After treatment, inflammatory cytokines in different groups were measured using ELISA. (A) The levels of TNF-α in BALF, (B) the levels of IL-6 in BALF, (C) the levels of TNF-α in serum (D) the levels of IL-6 in serum. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; ELISA – enzyme-linked immunosorbent assay; TNF – tumor necrosis factor; BALF – bronchoalveolar lavage fluid; IL – interleukin.
Figure 2. MaR1 ameliorated the release of inflammatory cytokine in THS-stimulated rats. After treatment, inflammatory cytokines in different groups were measured using ELISA. (A) The levels of TNF-α in BALF, (B) the levels of IL-6 in BALF, (C) the levels of TNF-α in serum (D) the levels of IL-6 in serum. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; ELISA – enzyme-linked immunosorbent assay; TNF – tumor necrosis factor; BALF – bronchoalveolar lavage fluid; IL – interleukin. 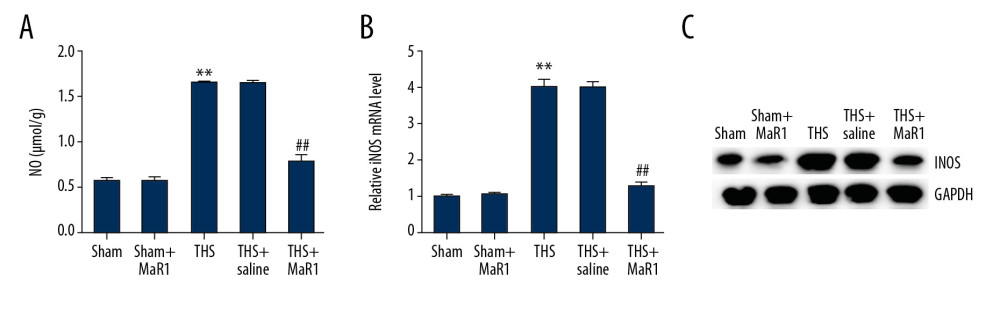 Figure 3. MaR1 increased NO concentrations and iNOS levels in THS-induced lung tissues. MaR1 or physiological saline were injected into THS-induced lung injury models. (A) The levels of NO were assessed. (B) qRT-PCR was used to measure the iNOS mRNA levels. (C) Western blot assay was adopted to assess the iNOS protein expression. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; NO – nitrogen oxide; iNOS – inducible nitric oxide synthase; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 3. MaR1 increased NO concentrations and iNOS levels in THS-induced lung tissues. MaR1 or physiological saline were injected into THS-induced lung injury models. (A) The levels of NO were assessed. (B) qRT-PCR was used to measure the iNOS mRNA levels. (C) Western blot assay was adopted to assess the iNOS protein expression. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; NO – nitrogen oxide; iNOS – inducible nitric oxide synthase; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. 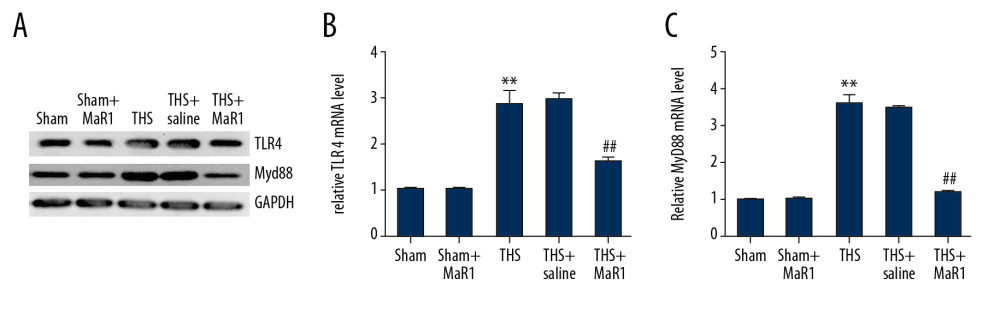 Figure 4. MaR1 suppressed the activation of TLR4 and MyD88 expression in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the relative genes expressions in different groups. (A) TLR4 and MyD88 protein expressions in lung tissues were assessed by western blot assay. TLR4 (B) and MyD88 (C) mRNA levels were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 4. MaR1 suppressed the activation of TLR4 and MyD88 expression in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the relative genes expressions in different groups. (A) TLR4 and MyD88 protein expressions in lung tissues were assessed by western blot assay. TLR4 (B) and MyD88 (C) mRNA levels were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. 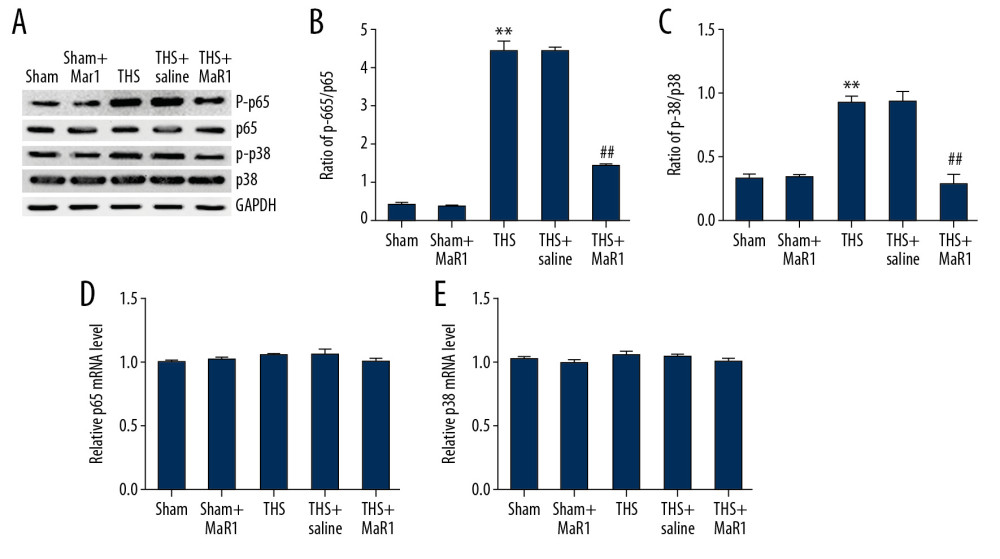 Figure 5. MaR1 suppressed the activation of p38MAPK/NF-κB pathway in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the proteins and relative genes expressions of p38MAPK/NF-κB pathway in different groups. (A) protein expression of p-p65 and p-p38 in lung tissues. The ratio of p-p65/p65 (B) and p-p38/p38 (C) were quantified. The mRNA levels of p65 (D) and p38 (E) in different groups were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 5. MaR1 suppressed the activation of p38MAPK/NF-κB pathway in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the proteins and relative genes expressions of p38MAPK/NF-κB pathway in different groups. (A) protein expression of p-p65 and p-p38 in lung tissues. The ratio of p-p65/p65 (B) and p-p38/p38 (C) were quantified. The mRNA levels of p65 (D) and p38 (E) in different groups were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. References
1. D’Alessio FR, Mouse models of acute lung injury and ARDS: Methods Mol Biol, 2018; 1809; 341-50
2. Traylor ZP, Aeffner F, Davis IC, Influenza A H1N1 induces declines in alveolar gas exchange in mice consistent with rapid post-infection progression from acute lung injury to ARDS: Influenza Other Respir Viruses, 2013; 7; 472-79
3. Farzipour S, Amiri FT, Mihandoust E, Radioprotective effect of diethylcarbamazine on radiation-induced acute lung injury and oxidative stress in mice: J Bioenerg Biomembr, 2020; 52(1); 39-46
4. Qin L, Li M, Tan HL, Mechanistic target of rapamycin-mediated autophagy is involved in the alleviation of lipopolysaccharide-induced acute lung injury in rats: Int Immunopharmacol, 2019; 78; 105790
5. Teng Y, Feng C, Liu Y, Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats: Exp Anim, 2018; 67; 313-20
6. Rocha J, Eduardo-Figueira M, Barateiro A, Erythropoietin reduces acute lung injury and multiple organ failure/dysfunction associated to a scald-burn inflammatory injury in the rat: Inflammation, 2015; 38; 312-26
7. Hukkanen RR, Liggitt HD, Murnane RD, Frevert CW, Systemic inflammatory response syndrome in nonhuman primates culminating in multiple organ failure, acute lung injury, and disseminated intravascular coagulation: Toxicol Pathol, 2009; 37; 799-804
8. Marginean A, Sharma-Walia N, Lipoxins exert antiangiogenic and anti-inflammatory effects on Kaposi’s sarcoma cells: Transl Res, 2015; 166; 111-33
9. Yaribeygi H, Atkin SL, Simental-Mendia LE, Anti-inflammatory effects of resolvins in diabetic nephropathy: Mechanistic pathways: J Cell Physiol; 2019 [Epub ahead of print]
10. Bosviel R, Joumard-Cubizolles L, Chinetti-Gbaguidi G, DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation: Free Radic Biol Med, 2017; 103; 146-54
11. Lv C, Jin Q, Maresin-1 inhibits oxidative stress and inflammation and promotes apoptosis in a mouse model of Caerulein-induced acute pancreatitis: Med Sci Monit, 2019; 25; 8181-89
12. Munir F, Jamshed MB, Shahid N, Protective effects of Maresin 1 against inflammation in experimentally induced acute pancreatitis and related lung injury: Am J Physiol Gastrointest Liver Physiol, 2019; 317; G333-41
13. Chatterjee A, Sharma A, Chen M, The pro-resolving lipid mediator Maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells: PLoS One, 2014; 9; e113480
14. Gong J, Wu ZY, Qi H, Maresin 1 mitigates LPS-induced acute lung injury in mice: Br J Pharmacol, 2014; 171; 3539-50
15. Li M, Li G, Yu B, Activation of hypoxia-inducible factor-1alpha via succinate dehydrogenase pathway during acute lung injury induced by trauma/hemorrhagic shock: Shock, 2020; 53(2); 208-16
16. Shi HP, Deitch EA, Da Xu Z, Hypertonic saline improves intestinal mucosa barrier function and lung injury after trauma-hemorrhagic shock: Shock, 2002; 17; 496-501
17. Teng Y, Feng C, Liu Y, Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats: Exp Anim, 2018; 67; 313-20
18. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method: Methods, 2001; 25; 402-8
19. Fan HJ, Liu SY, Zhang JP, Liu YNAssessment on pathophysiological indexes of acute lung injury induced by lipopolysaccharide in rats: Zhongguo Wei Zhong Bing Ji Jiu Yi Xue, 2006; 18; 485-87 [in Chinese]
20. Lu Z, Chang W, Meng S, Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury: Stem Cell Res Ther, 2019; 10; 372
21. Shen Y, Song J, Wang Y, M2 macrophages promote pulmonary endothelial cells regeneration in sepsis-induced acute lung injury: Ann Transl Med, 2019; 7; 142
22. Gong J, Wu ZY, Qi H, Maresin 1 mitigates LPS-induced acute lung injury in mice: Br J Pharmacol, 2014; 171; 3539-50
23. Chatterjee A, Sharma A, Chen M, The pro-resolving lipid mediator Maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells: PLoS One, 2014; 9; e113480
24. Kosutova P, Mikolka P, Kolomaznik M, Effects of S-nitroso-N-acetyl-penicillamine (SNAP) on inflammation, lung tissue apoptosis and iNOS activity in a rabbit model of acute lung injury: Adv Exp Med Biol, 2016; 935; 13-23
25. Zhang S, Li J, Li Y, Nitric oxide synthase activity correlates with OGG1 in ozone-induced lung injury animal models: Front Physiol, 2017; 8; 249
26. Harkin DW, Rubin BB, Romaschin A, Lindsay TF, Selective inducible nitric oxide synthase (iNOS) inhibition attenuates remote acute lung injury in a model of ruptured abdominal aortic aneurysm: J Surg Res, 2004; 120; 230-41
27. Wu Y, Lin Z, Yan Z, Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-kappaB signaling pathways: Int Immunopharmacol, 2019; 75; 105715
28. Li X, Guo Y, Huang S, Coenzyme q10 prevents the interleukin-1 beta induced inflammatory response via inhibition of MAPK signaling pathways in rat articular chondrocytes: Drug Dev Res, 2017; 78; 403-10
29. Wu XJ, Liu HM, Song XM, Penehyclidine hydrochloride inhibits TLR4 signaling and inflammation, and attenuates blunt chest trauma and hemorrhagic shock-induced acute lung injury in rats: Mol Med Rep, 2018; 17; 6327-36
30. Reino DC, Pisarenko V, Palange D, Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice: PLoS One, 2011; 6; e14829
31. Li T, Wu YN, Wang H, Dapk 1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-κB signaling pathway: Mol Immunol, 2020; 120; 13-22
32. do Nascimento Xavier BM, Ferreira LAMP, Ferreira LKDP, MHTP, a synthetic tetra tetrahydroisoquinoline alkaloid, attenuates lipopolysaccharide-induced acute lung injury via p38MAPK/p65NF-κB signaling pathway-TLR4 dependent: Inflamm Res, 2019; 68; 1061-70
Figures
 Figure 1. MaR1 reduced BALF/plasma EDB ratio, lung permeability index and wet/dry weight ratio in acute lung injury induced by THS. A lung injury model was induced by THS before intravenously injection of 10 ng/mL MaR1 or 0.1 mL/100 g physiological saline. (A) lung permeability to EBD was conducted. (B) lung permeability activity. (C) Wet/Dry weight ratio. *, ** P<0.05, 0.01 versus Sham; #, ## P<0.05, 0.01 versus THS+saline group. MaR1 – Maresin-1; BALF – bronchoalveolar lavage fluid; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 1. MaR1 reduced BALF/plasma EDB ratio, lung permeability index and wet/dry weight ratio in acute lung injury induced by THS. A lung injury model was induced by THS before intravenously injection of 10 ng/mL MaR1 or 0.1 mL/100 g physiological saline. (A) lung permeability to EBD was conducted. (B) lung permeability activity. (C) Wet/Dry weight ratio. *, ** P<0.05, 0.01 versus Sham; #, ## P<0.05, 0.01 versus THS+saline group. MaR1 – Maresin-1; BALF – bronchoalveolar lavage fluid; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. Figure 2. MaR1 ameliorated the release of inflammatory cytokine in THS-stimulated rats. After treatment, inflammatory cytokines in different groups were measured using ELISA. (A) The levels of TNF-α in BALF, (B) the levels of IL-6 in BALF, (C) the levels of TNF-α in serum (D) the levels of IL-6 in serum. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; ELISA – enzyme-linked immunosorbent assay; TNF – tumor necrosis factor; BALF – bronchoalveolar lavage fluid; IL – interleukin.
Figure 2. MaR1 ameliorated the release of inflammatory cytokine in THS-stimulated rats. After treatment, inflammatory cytokines in different groups were measured using ELISA. (A) The levels of TNF-α in BALF, (B) the levels of IL-6 in BALF, (C) the levels of TNF-α in serum (D) the levels of IL-6 in serum. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; ELISA – enzyme-linked immunosorbent assay; TNF – tumor necrosis factor; BALF – bronchoalveolar lavage fluid; IL – interleukin. Figure 3. MaR1 increased NO concentrations and iNOS levels in THS-induced lung tissues. MaR1 or physiological saline were injected into THS-induced lung injury models. (A) The levels of NO were assessed. (B) qRT-PCR was used to measure the iNOS mRNA levels. (C) Western blot assay was adopted to assess the iNOS protein expression. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; NO – nitrogen oxide; iNOS – inducible nitric oxide synthase; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 3. MaR1 increased NO concentrations and iNOS levels in THS-induced lung tissues. MaR1 or physiological saline were injected into THS-induced lung injury models. (A) The levels of NO were assessed. (B) qRT-PCR was used to measure the iNOS mRNA levels. (C) Western blot assay was adopted to assess the iNOS protein expression. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; NO – nitrogen oxide; iNOS – inducible nitric oxide synthase; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. Figure 4. MaR1 suppressed the activation of TLR4 and MyD88 expression in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the relative genes expressions in different groups. (A) TLR4 and MyD88 protein expressions in lung tissues were assessed by western blot assay. TLR4 (B) and MyD88 (C) mRNA levels were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 4. MaR1 suppressed the activation of TLR4 and MyD88 expression in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the relative genes expressions in different groups. (A) TLR4 and MyD88 protein expressions in lung tissues were assessed by western blot assay. TLR4 (B) and MyD88 (C) mRNA levels were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. Figure 5. MaR1 suppressed the activation of p38MAPK/NF-κB pathway in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the proteins and relative genes expressions of p38MAPK/NF-κB pathway in different groups. (A) protein expression of p-p65 and p-p38 in lung tissues. The ratio of p-p65/p65 (B) and p-p38/p38 (C) were quantified. The mRNA levels of p65 (D) and p38 (E) in different groups were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA.
Figure 5. MaR1 suppressed the activation of p38MAPK/NF-κB pathway in acute lung injury stimulated by THS. Western blot assay and qRT-PCR analyses were adopted to evaluate the proteins and relative genes expressions of p38MAPK/NF-κB pathway in different groups. (A) protein expression of p-p65 and p-p38 in lung tissues. The ratio of p-p65/p65 (B) and p-p38/p38 (C) were quantified. The mRNA levels of p65 (D) and p38 (E) in different groups were determined using qRT-PCR. ** P<0.01 versus Sham; #, ## P<0.01 versus THS+saline group. MaR1 – Maresin-1; THS – trauma hemorrhagic shock; qRT-PCR – quantitative real-time polymerase chain reaction; mRNA – messenger RNA. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








