27 July 2023: Clinical Research
Factors Associated with Severe Adenovirus Pneumonia in Children: A Retrospective Study from Guangzhou, China (2017–2019)
Yan Hong1ABCDEF, Qiang Wang1BCE, Yongling Song1BCE, Guangming Liu1BCDE, Jun Shen1ACDEG*DOI: 10.12659/MSM.939578
Med Sci Monit 2023; 29:e939578
Abstract
BACKGROUND: Adenovirus infections are prevalent in children, typically presenting with mild or asymptomatic symptoms. However, some children develop severe pneumonia necessitating hospitalization. This retrospective study aimed to identify risk factors associated with severe adenovirus pneumonia in children.
MATERIAL AND METHODS: We screened consecutive children admitted for community-acquired pneumonia at the Emergency Department of Guangzhou Women’s and Children’s Medical Center between 2017 and 2019. Adenovirus infection was confirmed through rapid respiratory virus assay, RT-PCR assay from respiratory secretions, or serum IgM antibodies. According to Chinese guidelines, patients with pneumonia were classified into severe and mild groups. We assessed risk factors for severe adenovirus pneumonia by comparing clinical features and laboratory indicators, then included differing factors between the 2 groups in a logistic regression analysis. Data analysis was performed using SPSS 26.0 software.
RESULTS: Our study included 173 children diagnosed with adenovirus pneumonia (117 severe, 56 mild). The median age was 40 months, with 64 male patients. Univariate analysis and binary logistic regression analysis revealed that pleural effusion (13.449 [1.226-147.510], p=0.033), electrolyte disturbances (15.149 [2.724-84.246], p=0.002), oxygen therapy (258.219 [20.684-3223.548], p<0.001), bronchoscopy (26.781 [6.088-117.805], p<0.001), and steroid administration (6.584 [1.497-28.953], p=0.013) were associated with the severity of adenovirus pneumonia.
CONCLUSIONS: This single-center retrospective study identified pleural effusion, the need for bronchoscopy, oxygen therapy, and steroid treatment, along with impaired serum electrolytes, as factors associated with severe adenovirus pneumonia in children.
Keywords: Adenovirus Infections, Human, Pneumonia, Risk Factors, Adult Children, Child, Child, Preschool, Female, Humans, Infant, Male, Adenoviridae Infections, China, Pneumonia, Viral, Retrospective Studies, Severity of Illness Index
Background
Pneumonia, one of the leading causes of mortality in children under 5 years of age, has been considered to be related to many microorganisms, including bacteria, fungi and viruses [1]. COVID-19, which has caused thousands of deaths in the last 3 years, has refocused people’s attention on the virus as a pathogen of pneumonia [2]. Viruses, including adenovirus, influenza virus, parainfluenza virus, respiratory syncytial virus, and bocavirus are among the known common pathogens which account for about 22% of the incidence of community-acquired pneumonia (CAP) [3]. Human adenovirus (HAdVs) is the fourth leading cause of viral pneumonia, but only 2.0% of CAP is caused by this agent [3]. However, 3.5% to 11.0% of pediatric cases of pneumonia are caused by adenovirus [4,5].
Adenovirus is a group of non-enveloped DNA viruses associated with a wide range of clinical manifestations, such as severe pneumonia, myocarditis, hepatitis, encephalitis, and disseminated disease [6]. Significantly, the clinical course of adenovirus infection in immunocompetent patients is usually benign and self-limited, but the morbidity and mortality rates in infants and young children or immunocompromised adults can be significantly higher [6,7]. Moreover, the severity of adenovirus pneumonia in children varies from mild bronchopneumonia to severe acute respiratory distress syndrome (ARDS) and can even be life-threatening due to adenovirus types, age of onset, immune status, and environment [8]. Previous reports showed that adenoviruses caused an increased number of immunocompetent children with severe adenovirus pneumonia in China [9]. Unfortunately, no effective vaccines or antivirals therapy were available for the treatment of adenovirus pneumonia in China [10]. The main approaches used in clinical practice include targeted support, immunomodulatory therapy, and complication management [11]. Therefore, severe pneumonia caused by adenovirus remains a serious challenge to clinical physicians.
HAdVs belong to the family Adenoviridae, genus Mastadenovirus, and are further subdivided into 7 species (A to G) and over 50 recognized types [12]. Virus species or types frequently determine clinical symptoms, disease severity, and infectious disease epidemiology patterns. HAdV acute respiratory illnesses (ARIs) are typically caused by viruses of the B, C, and E species. In pediatric populations, where infections are frequently subclinical or cause sporadic ARIs, Species C viruses are widespread and endemic. Species B (primarily types 3, 7, 11, 14, and 21) and E (type 4) viruses have been linked to epidemics of frequently severe ARIs affecting children and adults [13]. Diagnostic testing for HAdV in the lab includes viral isolation, antigen detection, polymerase chain reaction (PCR) detection, and serology test [12]. The diagnostic and management criteria for pediatric adenovirus infection in China are based on the Technical Guidelines for the Prevention and Control of Human Adenovirus Respiratory Infection (2019 Edition) [11] issued by the Chinese Center for Disease Control and Prevention. Cases with clinical manifestations of respiratory tract infection and any of the following test results can be considered as laboratory confirmed cases: (1) respiratory specimens tested positive for human adenovirus-specific nucleic acid; (2) respiratory specimens tested positive for human adenovirus-specific antigen; (3) human adenovirus was isolated and cultured from respiratory specimens; (4) double serum samples from acute and convalescent stages (sampling interval should be 2–4 weeks) with human adenovirus-specific IgG antibodies transitioning from negative to positive, or showing a 4-fold or higher increase. Cases with clinical manifestations of respiratory tract infection and a history of close contact with confirmed cases of human adenovirus infection within 8 days prior to onset can be diagnosed as clinically diagnosed cases of human adenovirus infection.
Efforts to improve childhood survival can be effective only if they are based on reasonably accurate information about the causes of mortality. In June 2019, the National Health Commission of China issued a guideline for the diagnosis and treatment of adenoviral pneumonia in children (2019), which recommended that the therapy of children with severe illness should be carefully evaluated [14]. In addition, several researchers have identified many possible risk factors for severe pneumonia caused by adenovirus, including adenovirus viremia [15], longer hospital stay [16], high serum lactate dehydrogenase (LDH) level, and low lymphocyte count [17]. However, the risk factors for severe adenovirus respiratory infection among children are controversial [18–20].
Therefore, this retrospective study of 173 children admitted to Guangzhou Women’s and Children’s Medical Center between 2017 and 2019 with adenovirus pneumonia aimed to identify factors associated with the severity of adenovirus pneumonia.
Material and Methods
ETHICS STATEMENT:
Human Ethics Committees of Guangzhou Women’s and Children’s Medical Center gave their approval to this study, ensuring that it was performed in accordance with the principles outlined in the Helsinki Declaration (Application ID: [2020]29301). Informed permission from patients was waived by the Ethics Committee because all data were acquired via the electronic medical record system, and there was no risk to participants and no violation of privacy or commercial interests.
STUDY POPULATION:
A total of 2357 consecutive children with community-acquired pneumonia who were hospitalized in the Emergency Department of Guangzhou Women’s and Children’s Medical Center between July 2017 and December 2019 were recruited retrospectively. Inclusion criteria were: (1) 29 days < Age <14 years; (2) those with confirmed pneumonia by chest X-ray; (3) those diagnosed with adenovirus infection using one of the criteria outlined in the Technical Guidelines for the Prevention and Control of Human Adenovirus Respiratory Infection (2019 Edition) published by the Chinese Center for Disease Control and Prevention. The patients who met the following exclusion criteria were excluded from this research: (1) those with hospital-acquired adenovirus pneumonia; (2) those with confirmed co-infection with other pathogens; (3) those with experience of other hospital therapy within the last 3 days before admission; (4) Re-admitted without a 7-day symptom-free period; (5) those with other severe diseases or organ dysfunction; (6) those with history of receiving corticosteroids before admission within 1 week; (7) those with abnormal measurement of immune function tests (including IgA, IgG, IgM, IgE, C3 and C4); (8) those with substantial missing data (Table 1). Finally, 173 cases were considered for this study.
ADENOVIRUS INFECTION: According to the diagnostic criteria for adenovirus infection in the Technical Guidelines for the Prevention and Control of Human Adenovirus Respiratory Infection (2019 Edition) [11] issued by the Chinese Center for Disease Control and Prevention, the etiological diagnosis for adenovirus infection was based on 1 of the following criteria: (1) positive adenovirus nucleic acid by rapid respiratory virus assay with nasopharyngeal swab; (2) positive adenovirus nucleic acid by RT-PCR test with the deeper respiratory tract specimens collected by bronchoscopy; (3) positive serum adenovirus-specific IgM antibody.
ADENOVIRUS PNEUMONIA: The diagnostic criteria for adenovirus pneumonia and classification of pneumonia severity were based on the diagnostic and treatment standards for adenovirus pneumonia in children (2019 Edition) issued by China’s National Health Commission and National Administration of Traditional Chinese Medicine [11].
CLASSIFICATION OF PNEUMONIA SEVERITY: According to the national guidelines for pediatric CAP issued by the National Health Commission of China, patients with adenovirus pneumonia were divided into mild and severe groups (Table 2) [14].
DATA COLLECTION AND MANAGEMENT:
According to the diagnostic and treatment standards for adenovirus pneumonia in children (2019 Edition) issued by China’s National Health Commission and National Administration of Traditional Chinese Medicine, the enrolled patients were classified into mild and severe groups (Table 2). The clinical course of each patient was obtained from the hospital’s electronic medical record database. Subsequently, the demographic characteristics (age, sex, and weight), clinical symptoms, and clinical signs (eg, temperature, blood pressure, pulse and respiratory rate, cough, and tachypnea), laboratory results (hematology, organ function, pathogen tests), chest imaging results, bronchoscopy results, diagnosis, treatment (eg, oxygen supplementation, immunoglobulin application and antibiotics administration), and outcome (improvement and cure) were recorded and analyzed. In addition, all these parameters were compared between mild and severe groups.
STATISTICAL ANALYSIS:
Patient’s characteristics and outcomes were summarized as median (interquartile range, IQR) for continuous variables and percentage for categorical variables, respectively. A univariate correlation analysis of demographic and laboratory variables was used to determine the statistical significance of the pairwise associations between the 2 groups. The Mann-Whitney test was used for quantitative variables with abnormally distributed data and chi-Square test or the Fisher’s exact test were performed for qualitative variables. In addition, binary logistic regression analysis was further performed to identify independent demographic and laboratory risk factors for severe pneumonia. Adjusted odd ratios (ORs) were estimated by multivariate logistic regression models. A
Results
BASELINE DEMOGRAPHIC CHARACTERISTICS:
From July 2017 to December 2019, 173 children (117 mild and 56 severe) were definitively diagnosed as having CAP caused by adenovirus infection in our hospital; there were 119 (68.79%) boys and 54 (31.21%) girls, with a male: female ratio of 2.20: 1.00. The patients’ age at onset ranged from 2 months to 108 months, and the median age was 27 months (range, 15–48). Among included patients, the mean total day of disease course was 17 (range, 12–22), and the median hospital stay was 9 days (range, 7–13), which were both significantly different in the patients with the mild and severe adenovirus pneumonia. The detailed results are shown in Table 3. No significant differences were found in the mean sex, age, and weight between the 2 groups. The fever course of patients hospitalized for adenovirus pneumonia was statistically significant, but not before admission.
CLINICAL MANIFESTATIONS:
Table 4 illustrates the detailed clinical symptoms of 173 children with pneumonia included in our study. All the patients were admitted to our hospital for the reason of clinical respiratory symptoms consistent with fever (100%), tachypnea (100%), and cough (100%). In addition, 9 of patients (5.2%) had acute respiratory failure requiring oxygenation support, which was significantly different between the mild and severe pneumonia groups. The clinical condition of 62 (35.84%) children was complicated by lobar consolidation (84.48%), small respiratory disease (4.84%), and pulmonary abscess (1.61%). Dry and wet rales could be heard in all 137 pediatric patients (79.19%) and 9 had bronchitis obliterans (5.2%). All the above respiratory symptoms were significantly different between the 2 groups. Extrapulmonary manifestations were also documented in this study at the same time. Gastrointestinal symptoms were reported in 81 of the patients (46.82%), including diarrhea in 27 patients (15.61%), peritoneal effusion in 17 patients (9.83%), and hepatosplenomegaly in 37 (21.39%). Neurological symptoms such as convulsions (6.94%) and poor consciousness (8.67%) were observed. Intriguingly, arrhythmia was significantly more common in patients with severe adenovirus pneumonia than in those with mild adenovirus pneumonia. In addition, severe pneumonia pediatric patients were also more vulnerable to electrolyte disturbances and coagulopathy, which may result in a poorer prognosis.
LABORATORY FINDINGS:
The patient’s blood and sputum were collected and cultured to identify whether there were microbial and viral infections. The positive blood cultures of 4 patients were due to the infection of streptococcus pneumoniae (S. pneumoniae), Corynebacterium aerogenes, and Staphylococcus hominis, respectively. The results of sputum cultures of patients with adenovirus pneumonia showed that a variety of bacteria were involved in the pathogenicity of infection, among which the most common bacteria in mild patients was S. pneumoniae (42.86%), but the most common infection in severe cases was Haemophilus influenzae (42.31%). In addition, one mild child with 3 consecutive positive sputum cultures for Haemophilus influenzae after standardized antibacterial treatment had documented that bacterial co-infection especially Haemophilus influenzae co-infection was very common during the process of adenovirus pneumonia, which is inconsistent with the results of professor Chen et al [8]. Possible concomitant other pathogen infection was observed in 56 patients, including respiratory syncytial virus (RSV) in 6 patients, influenza virus in 6 children (1 of parainfluenza virus, 2 of type A and 3 of type B1), and Mycoplasma pneumoniae (M. pneumoniae) in 43 patients (data not shown). All the above pathogen test results indicated that severe patients were accompanied by more pathogen infections (Table 5). A separate analysis was performed to compare the blood routine and the blood chemical indexes of mild patients with those of severe patients. Subsequently, a statistically significant difference was found in some of laboratory data between the groups, such as white blood cell (WBC), creatine kinase isoenzyme (CK-MB), and albumin (p<0.05). Furthermore, we found that the patients with the severe adenovirus pneumonia were accompanied by more consolidation and more infiltration via analyzing the computed tomography results of all involved children.
MANAGEMENT AND OUTCOMES:
As demonstrated in Table 6, the treatment options according to the guideline such as the management of steroid, antibiotics, immunoglobulins, vasoactive drugs, and acetylcysteine solutions, and the implementation of plasma transfusion were statistically different between the groups of mild adenovirus pneumonia and severe adenovirus pneumonia. The use rate of ventilators and fiberscopes in critically infectious patients was significantly higher than that in mild patients. None of the patients died of adenovirus infection or its complications. All patients were improved or cured before being discharged, but the prognosis of mild patients had more favorable prognosis than the severe ones.
MULTIVARIATE LOGISTIC REGRESSION ANALYSIS:
Multivariate logistic regression analysis was then further used to confirm the associations between the above-mentioned variables and adenovirus respiratory infection. As a result, the independently risk factors associated with severe adenovirus pneumonia were peritoneal effusion (13.449 [95% CI, 1.226–147.510], p=0.033) and electrolyte disturbances (15.149 [95% CI, 2.724–84.246], p=0.002) (Table 7). The crude odds ratios of sever adenovirus respiratory infection are also shown in Table 7. In addition, treatments such as oxygen inhalation (258.219 [95% CI, 20.684–3223.548], p=0.000), fiberscope usage (26.781 [95% CI, 6.088–117.805], p=0.000) and steroids administration (6.584 [95% CI, 1.497–28.953], p=0.013) may be associated with more severe adenovirus pneumonia.
Discussion
In this retrospective study, we revealed that seroperitoneum, electrolyte disturbances, oxygen therapy, bronchoscopy, and steroid delivery may be risk factors for severe adenovirus pneumonia in immunocompetent children. Seroperitoneum and electrolyte abnormalities were 2 clinical characteristics that may help predict the severity of pneumonia.
Since the beginning of the pandemic of adenovirus infection from May to August 2019 occurred in China, attentions on the adenovirus as one of the most common pathogens which can lead to a higher morbidity and mortality in children were increasingly focused [21,22]. Characterized by the specific seasonal epidemic trend, adenovirus pneumonia is a common childhood viral pneumonia appearing frequently in winter and spring in the southern regions of China, similar to other parts of the world [7,17]. Although the diseases caused by adenovirus were self-limiting in most immunocompetent people, it can bring serious consequences for the immunocompromised hosts [23]. Mounting evidence suggests that at least 5–10% of pediatric respiratory tract infections in children were caused by adenovirus [6]. However, no antiviral drug and vaccine thus far has been approved to treat adenovirus [24]. More importantly, retrospective analysis of the characteristics and clinical features of adenovirus pneumonia which can help clinicians make effective treatment decisions is still lacking [25]. Therefore, we analyzed 173 children who were hospitalized with adenovirus respiratory infection with respect to the epidemiology, clinical features, treatments, and risk factors of severe adenovirus pneumonia in this study.
Most of the children studied had adenovirus infection at under 3 years of age and the mean age of severe group was younger than that of the mild group, suggesting there may be a decline in the susceptibility to adenovirus infection with increasing age of children. Many published studies have confirmed that the immature immune function and low anti-infection ability of young children may contribute to a higher risk to the infection [21,26]. Previous studies have shown that young age, high viral load of adenovirus [15], duration of fever [20], longer hospital stay [16], coinfections [18], particularly with
Several previous studies have explored the risk factors of severe adenovirus pneumonia from different perspectives. Xu and colleagues divided children with severe adenovirus pneumonia into a survivor group and a non-survivor group and demonstrated that age less than 1 year, hypoxia, and thrombocytopenia were independent mortality risk factors in children with severe adenovirus pneumonia [18]. Zheng et al separated severe pneumonia in children under 5 years old into groups with and without adenovirus infection. They found that Adenovirus-infected children were more likely to be male, older than 1 year old, and less likely to have mixed viral infections and cardiovascular sickness, had more abnormal laboratory results, higher 30-day mortality, and longer hospital stays [19]. In addition, Huang et al classified children with severe adenovirus pneumonia into a severe group and a critical group, finding that severe disease and a poor prognosis were more common among children younger than 4 years old, those with continuous fever, those with significant lung lesions, and those with significantly elevated inflammatory cytokines such as IL6 [20].
Intravenous immunoglobulin or glucocorticoid therapy were demonstrated to accelerate the removal of adenovirus from the respiratory tract to a certain extent through mediating pediatric immune regulation [21]. In our study, these treatments were applied to varying degrees and achieved reliable efficacy based on the latest official guidance issued by the Health Commission of China [11]. Similarity to what has been found in Asia by other authors, patients with severe pneumonia were supported by more symptomatic and supportive treatment than those with mild pneumonia, such as oxygen inhalation, vasoactive drugs usage, steroid administration, and bronchoscopy due to the severity of infection [6,30]. Strikingly, most of the children in our series demonstrated a significant response to the treatment irrespective of the severity of adenovirus infection developed and none died.
Previous studies related to adenovirus pneumonia have identified many independent factors as predictors of the risk of pneumonia, including male sex, young age, leukocytosis, and elevated CRP levels [31]. In addition, liver dysfunction and nosocomial infection were also reported to be involved in the possibility of predicting the adenovirus pneumonia risk in PICU [28]. Wu et al [17] found high serum LDH level and low lymphocyte count may be independent risk factors for mortality in children with adenovirus. In our study, we identified other possible risk factors such as peritoneal effusion (13.449 [1.226–147.510], p=0.033) and electrolyte disturbances (15.149 [2.724–84.246], p=0.002) for severe adenovirus pneumonia. In addition, treatments such as oxygen inhalation (258.219 [20.684–3223.548], p=0.000), fiberscope usage (26.781 [6.088–117.805], p=0.000), and steroids administration (6.584 [1.497–28.953], p=0.013) can be used to predict the severity of adenovirus infection.
Unfortunately, the present study has several limitations. First, this study’s observational, retrospective approach made it easier to identify associations rather than causal correlations. Second, as a single-center study involving children of a single ethnicity, our conclusions may lack wide applicability and its evidence may not be transferable to other centers or ethnic groups. Third, there may be some bias in the results because the sample size was relatively small and not all confounding factors were collected and evaluated in this study. Methodologically, the respiratory tract samples during the break were not saved, making the viral load unclear and hard to be compared; the serotype of adenovirus was not detected due to the insufficiency of the remaining blood samples, which might affect the judgment of the outcomes; and due to the lack of dynamic changes in serum IgG levels of adenoviruses, it also affects the accuracy of diagnosis and prognosis judgment to some extent.
Conclusions
This retrospective study from a single center identified that pleural effusion, the requirement for bronchoscopy, the requirement for oxygen therapy and treatment with steroids, and impaired serum electrolytes were associated with severe adenovirus pneumonia.
Tables
Table 1. The inclusion and exclusion criteria for all cases.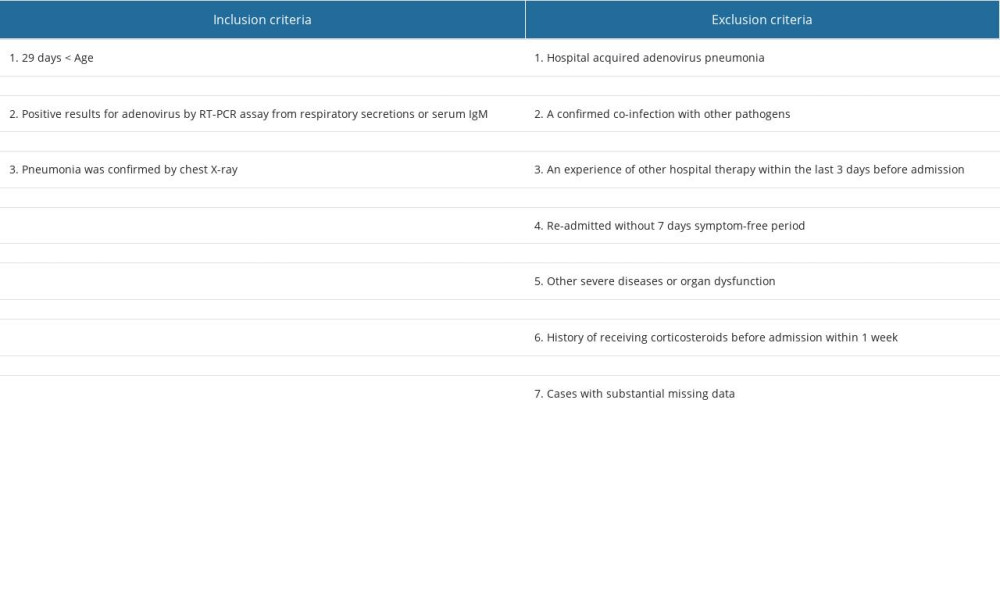 Table 2. The severity assessment of adenovirus pneumonia in children.
Table 2. The severity assessment of adenovirus pneumonia in children.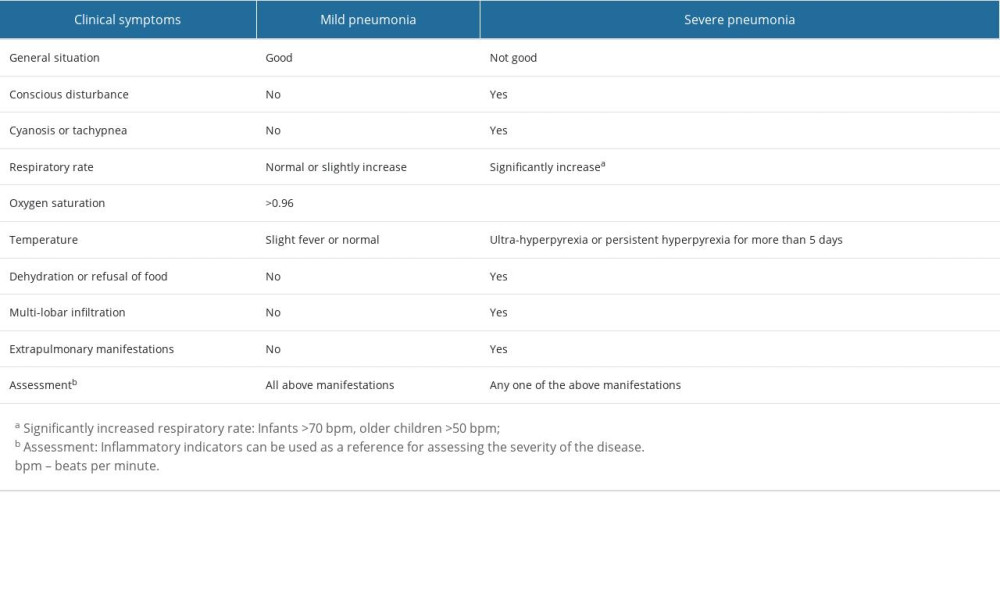 Table 3. Baseline characteristics of patients admitted to hospital between mild and severe group.
Table 3. Baseline characteristics of patients admitted to hospital between mild and severe group.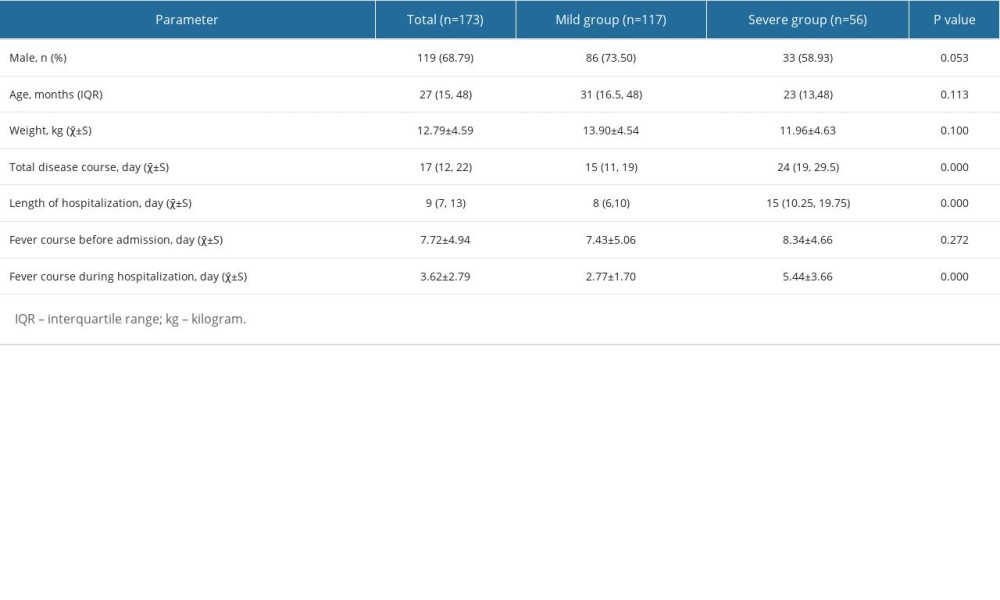 Table 4. Characteristics of children with adenovirus pneumonia according to disease severity.
Table 4. Characteristics of children with adenovirus pneumonia according to disease severity.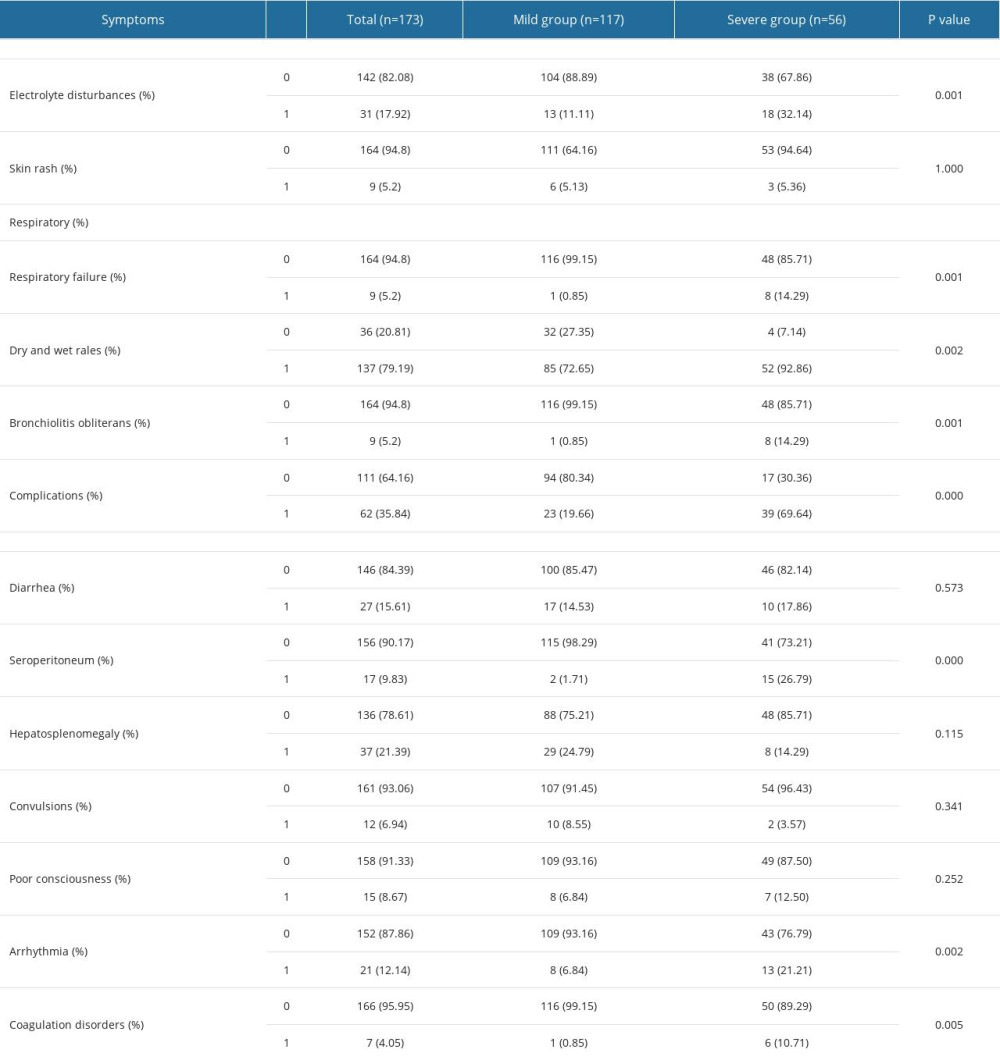 Table 5. Laboratory findings of hospitalized children with severe adenovirus pneumonia.
Table 5. Laboratory findings of hospitalized children with severe adenovirus pneumonia.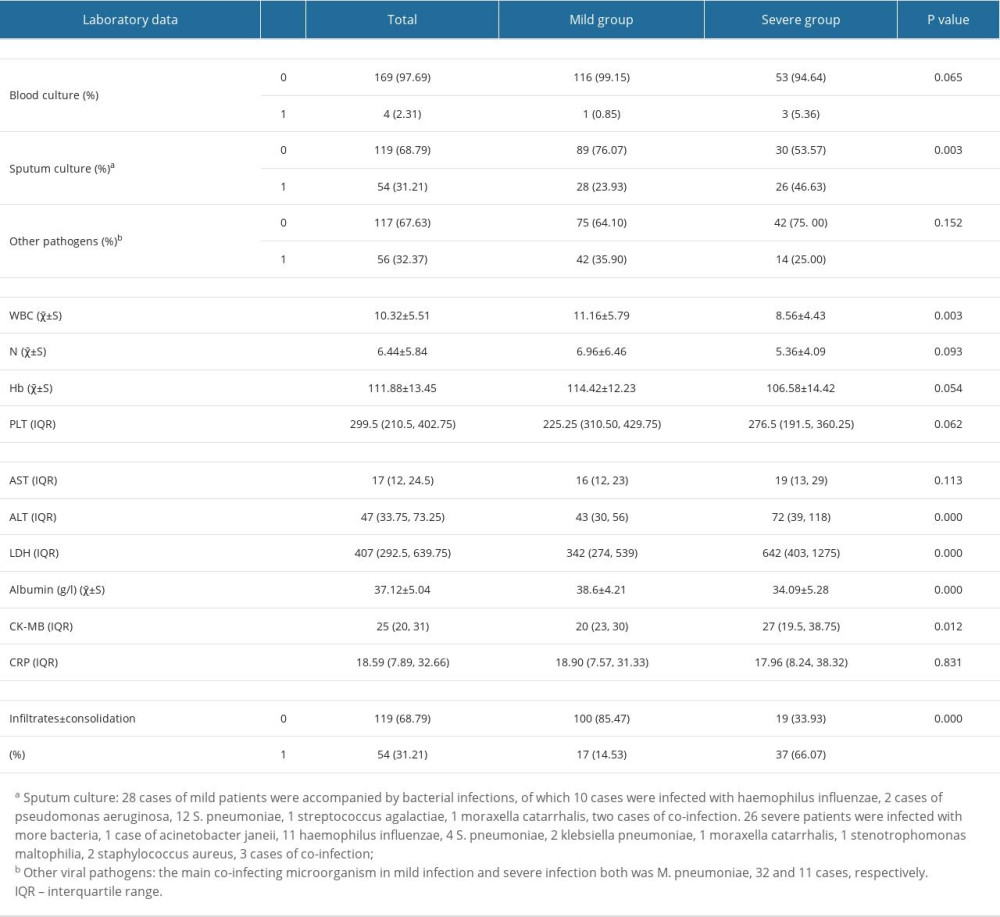 Table 6. The treatment and outcomes of patients between mild and severe group.
Table 6. The treatment and outcomes of patients between mild and severe group.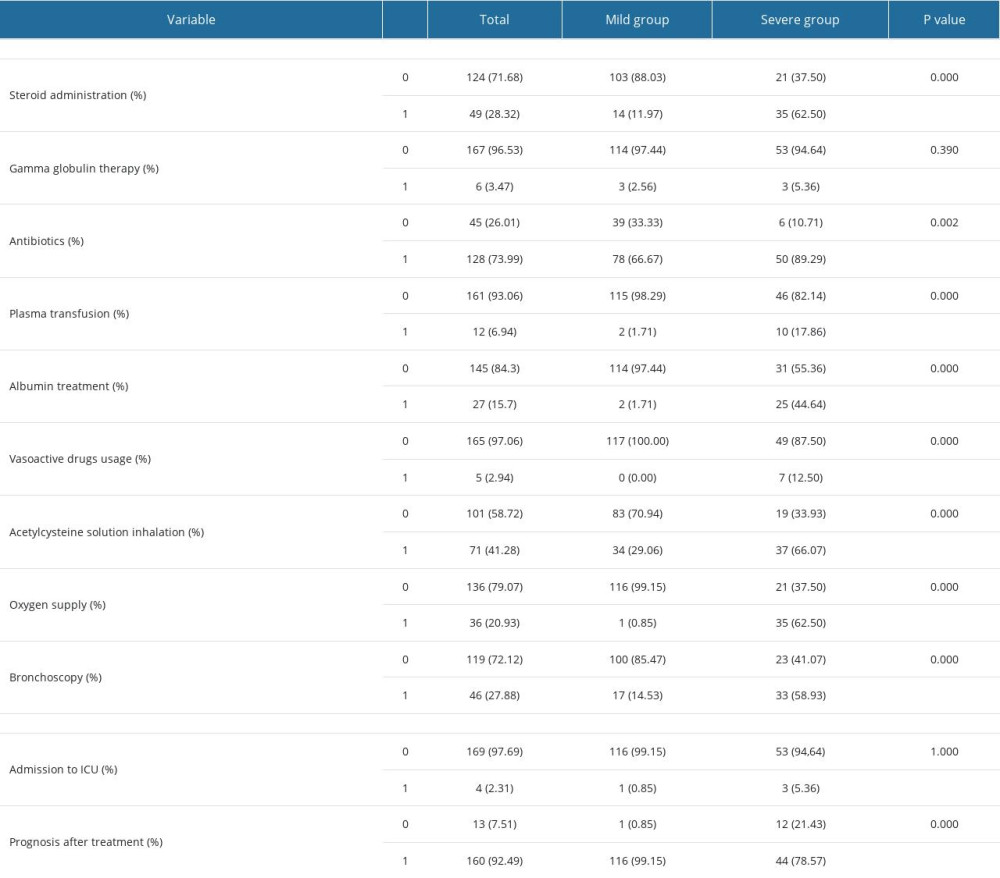 Table 7. Multivariate analysis of risk factors related to severe adenovirus pneumonia.
Table 7. Multivariate analysis of risk factors related to severe adenovirus pneumonia.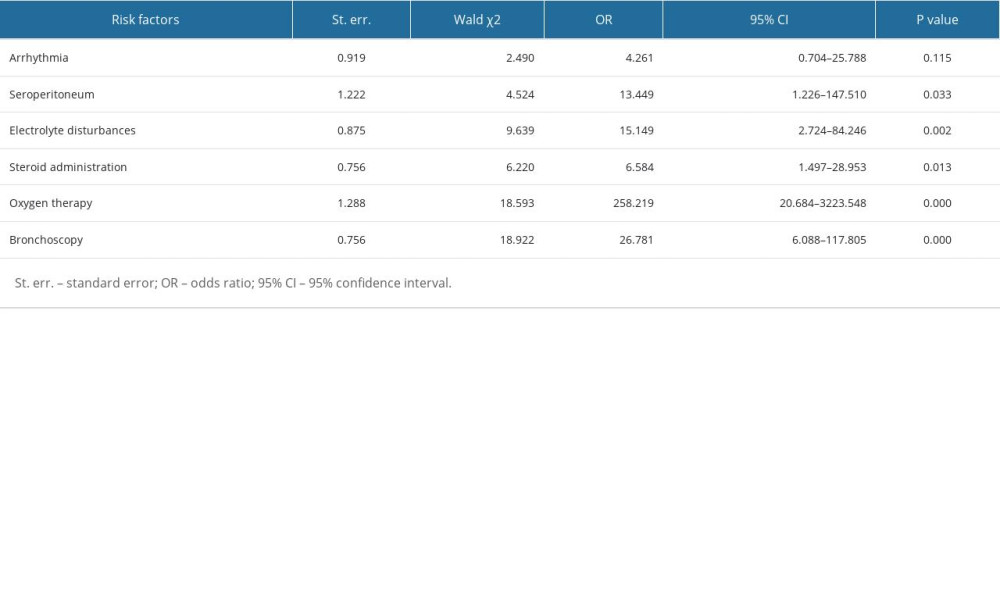
References
1. Bryce J, Boschi-Pinto C, Shibuya K, Black REGroup WHOCHER, WHO estimates of the causes of death in children: Lancet, 2005; 365(9465); 1147-52
2. Dagan R, van der Beek BA, Ben-Shimol S, The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: A prospective study: EBioMedicine, 2023; 90; 104493
3. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR, Viral pneumonia: Lancet, 2011; 377(9773); 1264-75
4. Jain S, Williams DJ, Arnold SR, Ampofo KCES Team, Community-acquired pneumonia requiring hospitalization among U.S. children: N Engl J Med, 2015; 372(9); 835-45
5. Liu C, Xiao Y, Zhang J, Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012: BMC Infect Dis, 2015; 15; 408
6. Lynch JP, Kajon AE, Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention: Semin Respir Criti Care Med, 2016; 37(4); 586-602
7. Lynch JP, Fishbein M, Echavarria M: Adenovirus Semin Respir Crit Care Med, 2011; 32(4); 494-511
8. Chen SP, Huang YC, Chiu CH, Clinical features of radiologically confirmed pneumonia due to adenovirus in children: J Clin Virol, 2013; 56(1); 7-12
9. Gu L, Liu Z, Li X, Severe community-acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing: J Clin Virol, 2012; 54(4); 295-301
10. Sun YP, Zheng XY, Zhang HX, Epidemiology of respiratory pathogens among children hospitalized for pneumonia in Xiamen: A Retrospective study: Infect Dis Ther, 2021; 10(3); 1567-78
11. Technical guidelines for prevention and control of human adenovirus respiratory infection (2019 edition): Zhonghua Yu Fang Yi Xue Za Zhi, 2019; 53(11); 1088-93 [in Chinese]
12. Shieh WJ, Human adenovirus infections in pediatric population – an update on clinico-pathologic correlation: Biomed J, 2022; 45(1); 38-49
13. Lu X, Trujillo-Lopez E, Lott L, Erdman DD, Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses: J Clin Microbiol, 2013; 51(4); 1089-93
14. Cao B, Huang Y, She DY, Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association: Clin Respir J, 2018; 12(4); 1320-60
15. Zhang R, Wang H, Tian S, Deng J, Adenovirus viremia may predict adenovirus pneumonia severity in immunocompetent children: BMC Infect Dis, 2021; 21(1); 213
16. Gray GC, McCarthy T, Lebeck MG, Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006: Clin Infect Dis, 2007; 45(9); 1120-31
17. Wu PQ, Zeng SQ, Yin GQ, Clinical manifestations and risk factors of adenovirus respiratory infection in hospitalized children in Guangzhou, China during the 2011–2014 period: Medicine (Baltimore), 2020; 99(4); e18584
18. Xu XH, Fan HF, Shi TT, Analysis of mortality risk factors in children with severe adenovirus pneumonia: A single-center retrospective study: Pediatr Neonatol, 2023; 64(3); 280-87
19. Zheng L, Liao W, Liang F, Clinical characteristics and outcomes of severe pneumonia in children under 5 years old with and without adenovirus infection in Guangzhou: Front Pediatr, 2021; 9; 599500
20. Huang H, Chen Y, Ma LYAnalysis of the clinical features and the risk factors of severe adenovirus pneumonia in children: Zhonghua Er Ke Za Zhi, 2021; 59(1); 14-19 [in Chinese]
21. Lou Q, Zhang SX, Yuan L, Clinical analysis of adenovirus pneumonia with pulmonary consolidation and atelectasis in children: J Int Med Res, 2021; 49(2); 300060521990244
22. Sun HQ, Zhang XX, Kuang XNEpidemiological analysis of 440 cases of respiratory adenovirus infections in children from the Suzhou area between 2006 and 2015: Zhongguo Dang Dai Er Ke Za Zhi, 2017; 19(1); 34-38 [in Chinese]
23. Ghanaiem H, Averbuch D, Koplewitz BZ, An outbreak of adenovirus type 7 in a residential facility for severely disabled children: Pediatr Infect Dis J, 2011; 30(11); 948-52
24. Echavarria M, Adenoviruses in immunocompromised hosts: Clin Microbiol Rev, 2008; 21(4); 704-15
25. Matthes-Martin S, Feuchtinger T, Shaw PJFourth European Conference on Infections in Leukemia, European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011): Transpl Infect Dis, 2012; 14(6); 555-63
26. Lion T, Adenovirus infections in immunocompetent and immunocompromised patients: Clin Microbiol Rev, 2014; 27(3); 441-62
27. Gao J, Xu L, Xu B: BMC Infect Dis, 2020; 20(1); 420
28. Shi J, Zhou Y, Wang F, A case series of children with adenovirus pneumonia: three-year experiences in a tertiary PICU: BMC Pediatr, 2020; 20(1); 375
29. He Y, Liu P, Xie L, Construction and verification of a predictive model for risk factors in children with severe adenoviral pneumonia: Front Pediatr, 2022; 10; 874822
30. Yao LH, Wang C, Wei TL, Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018: Virol J, 2019; 16(1); 78
31. Zhong L, Lin J, Dai J, Risk factors for the development of bronchiolitis obliterans in children with severe adenovirus pneumonia: A retrospective study with dose-response analysis: J Med Virol, 2020 [Online ahead of print]
Tables
 Table 1. The inclusion and exclusion criteria for all cases.
Table 1. The inclusion and exclusion criteria for all cases. Table 2. The severity assessment of adenovirus pneumonia in children.
Table 2. The severity assessment of adenovirus pneumonia in children. Table 3. Baseline characteristics of patients admitted to hospital between mild and severe group.
Table 3. Baseline characteristics of patients admitted to hospital between mild and severe group. Table 4. Characteristics of children with adenovirus pneumonia according to disease severity.
Table 4. Characteristics of children with adenovirus pneumonia according to disease severity. Table 5. Laboratory findings of hospitalized children with severe adenovirus pneumonia.
Table 5. Laboratory findings of hospitalized children with severe adenovirus pneumonia. Table 6. The treatment and outcomes of patients between mild and severe group.
Table 6. The treatment and outcomes of patients between mild and severe group. Table 7. Multivariate analysis of risk factors related to severe adenovirus pneumonia.
Table 7. Multivariate analysis of risk factors related to severe adenovirus pneumonia. In Press
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








