13 January 2024: Clinical Research
Impact of Smoking on Salivary Lipid Profile and Oxidative Stress in Young Adults: A Comparative Analysis between Traditional Cigarettes, E-Cigarettes, and Heat-Not-Burn Products
Sara ZiębaDOI: 10.12659/MSM.942507
Med Sci Monit 2024; 30:e942507
Abstract
BACKGROUND: Smoking nicotine is considered to be one of the most harmful addictions, leading to the development of a number of health complications, including many pathologies in the oral cavity. The aim of this study was to examine the effect of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on profiles of salivary lipids and lipid peroxidation products in the unstimulated and stimulated saliva of healthy young adults with a smoking habit of up to 3 years.
MATERIAL AND METHODS: We enrolled 3 groups of 25 smoking patients each and a control group matched for age, gender, and oral status. In saliva collected from patients from the study groups and participants from the control group, the concentrations of sphingolipids: sphingosine, sphinganine, sphingosine-1-phosphate, ceramides, and salivary lipid peroxidation products – malondialdehyde (MDA) and 4-hydroxynonenal (HNE) – were measured. The normality of distribution was assessed using the Shapiro-Wilk test. For comparison of the results, one-way analysis of variance (ANOVA) followed by post hoc Tukey test was used.
RESULTS: We demonstrated that each type of smoking causes a decrease in the concentration of salivary lipids, and there was an increased concentration of salivary MDA and 4-HNE.
CONCLUSIONS: Smoking in the initial period of addiction leads to an increase in the concentration of lipid peroxidation products through increased oxidative stress, leading to disturbance of the lipid balance of the oral cavity (eg, due to damage to cell membranes).
Keywords: cigarette smoking, Electronic Nicotine Delivery Systems, Lipid Peroxidation, Lipids, Oxidative Stress, Salivary Glands
Background
The negative effects of smoking tobacco and e-cigarette vaping are primarily evaluated in terms of causes of development of cardiovascular diseases, lung cancer, respiratory chronic inflammatory diseases, or disorders of the gastrointestinal microbiota [1–3]. However, other tissues also succumb to toxic effects of nicotine contained in commonly available nicotine carriers, including the oral cavity and upper respiratory tract [4,5].
The oral cavity is the place of first contact with cigarette smoke in the human body. Evidence has shown that smoking is a risk factor in the development and progression of periodontal diseases, cancer, and precancerous conditions of the oral cavity area, as well as salivary gland dysfunction and disorders of saliva composition [6–9].
It is well known that smoking traditional cigarettes leads to redox imbalance. We can observe increased production of free radicals, which can cause damage to cell membranes or DNA. It has been demonstrated that long-term smoking leads to a decrease in the activity of endogenous salivary enzymatic antioxidants such as SOD, CAT, and Px, and significantly reduces the efficiency of non-enzymatic endo- and exo-antioxidant systems: GSH, UA, and vitamin C [6,10,11]. Similarly, e-cigarettes can induce oxidative stress and increase the expression of advanced glycation end products (AGEs) and their cellular receptors (RAGEs) in gingival and periodontal tissues within just 1 year of starting smoking [12–14]. Furthermore, in an in vitro study, Ganapathy et al [13] showed that a 14-day exposure of cells to e-cigarette aerosol extracts increases DNA damage in oral epithelial cells, which is expressed by increased concentrations of 8-oxo-dG levels. Long-term smoking of traditional cigarettes and e-cigarettes reduces the content of salivary components of specific and non-specific immunity, such as sIgA, peroxidase, lactoferrin, and lysozyme [6,15,16].
Moreover, saliva contains a wide variety of lipids, including cholesterol and its esters, fatty acids, triglycerides, wax esters, and polar lipids such as phosphatidylcholine, phosphatidylethanolamine, sulfides, and glycolipids, including ceramides [15,16]. Ceramides are composed of sphingosine linked by an amide bond to any fatty acid. The most common ceramides are C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer, C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, and C24: 0-Cer. These lipids form cell membranes and are also precursors of more complex sphingolipids, such as sphingomyelin, ceramide-1 phosphate, and glycerosphingolipids. In addition to their structural function, ceramides determine the process of cell differentiation, proliferation, and apoptosis, and regulate the process of protein phosphorylation, which is essential in signal transduction [16,17]. Sphingolipids, on the other hand, show antimicrobial and antiviral activity in a dose-dependent manner, and induce cellular damage. Pretreatment of cells with sphingosine prevents the viral spike protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) from interacting with host cell receptors and inhibits the propagation of herpes simplex virus type 1 (HSV-1) in macrophages [18]. Cigarette smoke strongly activates inflammatory pathways in lungs and in myocardial and skeletal muscle cells, which increases biosynthesis of ceramide and its derivatives in these tissues [3,19,20]. High concentration of this group of lipids in response to exposure to cigarette smoke has been linked to endothelial barrier dysfunction, emphysema, inflammation, and altered myocardial mitochondrial function [21,22]. Lipidomic profiling of sputum samples showed increased levels of 28 ceramides in long-term smokers with COPD (chronic obstructive pulmonary disease) compared to long-term smokers without COPD. Differences between smokers without COPD and people who have never smoked cigarettes revealed significant changes only in the level of salivary glycosphingolipids. Interestingly, disorders in plasma sphingolipid composition were observed only in smokers of traditional cigarettes, while subjects using e-cigarettes only showed dysregulation of tricarboxylic acid cycle-related metabolites [22].
Lipids perform many important functions in the oral cavity, from structural to functional. In addition to their key role in maintaining the integrity and function of cells, they affect the processes of digestion, protection, and communication, as well as maintaining the internal balance in the oral cavity [17]. Lipids contained in saliva help to moisturize and protect the mucous membranes, facilitating eating, speaking, and other functions of the oral cavity. In the oral cavity, lipids can form a thin protective layer on the surface of the teeth and mucous membranes, which helps protect against the effects of irritants and infectious substances and prevents excessive evaporation of water from tissue surfaces [17,23].
Considering the role of saliva and its lipids in maintaining oral homeostasis, we decided to evaluate the effect of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of selected sphingolipids (eg, sphingosine, sphinganine, and sphingosine-1-phosphate), ceramides, and the lipid peroxidation products 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) in unstimulated and stimulated saliva from healthy young adults who had been smoking for 1–3 years.
Material and Methods
SUBJECTS:
A group of 75 smokers was enrolled in the study group. Smokers were divided into 4 subgroups according to the type of the smoking: Group 1 was traditional cigarette smokers (n=25), Group 2 was e-cigarette smokers (n=25), and Group 3 was heated tobacco device smokers (n=25). Each patient in the study group had been smoking for 1–3 years and used only 1 of the 3 methods of delivering nicotine to the body. Participants smoked on average about 10 cigarettes a day. The control group consisted of non-smokers (n=25) matched by age and gender to the subjects from the study group. The study participants were under continuous care of the Department of Restorative Dentistry at the Medical University of Białystok, reporting regularly for follow-up visits. The number of subjects was determined according to our previous study, assuming power of the test=0.8 (α=0.05) using Fisher’s formula [24]. All study subjects were young adults, under the age of 30 years, in generally good health (no chronic diseases of any kind), without any oral inflammatory lesions, with a normal BMI (within the range of 18.5–25), drinking alcohol only occasionally, and not taking psychoactive drugs. At that time, participants in the study were not using fixed orthodontic appliances or retainers, Invisalign splints, did not have removable dentures, fixed restorations, implants, or titanium implants. The subjects had not taken medicines, vitamins, or other dietary supplements within 6 months before the study. Their diet was typical, consisting of 70% carbohydrates, 20% proteins, and 10% fats.
SALIVA COLLECTION AND DENTAL EXAMINATION:
Saliva was collected by an experienced person (S. Z.) at a prior dental examination, including assessment of DMFT (decayed, missing, and filled teeth), GI (gingival index), and PPD (periodontal pocket depth). The examination was performed under artificial lighting, using a mirror, an explorer, and a periodontal probe (WHO, 621). The examiner was previously calibrated, and 20 patients were randomly examined by another dentist (A. Z.). Interrater agreement for DMFT was r=1.0, for GI: r=0.96, for PPD: r=0.9. The tested material consisted of unstimulated and stimulated saliva, collected via the spitting method between 8 and 10 a.m. Before collection of the diagnostic material, patients were instructed not to smoke or consume food or beverages other than water and not to perform any oral hygiene procedures at least 2 hours before the visit. To avoid patients’ embarrassment, saliva was collected in a separate room, in a sitting position, with the head slightly inclined downwards, with minimal movement of the face and lips. Before spitting unstimulated saliva into a plastic centrifuge tube, patients rinsed their mouths 3 times with room-temperature water. Saliva collected within the first minute was discarded. Unstimulated saliva was then collected for 15 minutes into a calibrated tube. Stimulated saliva was gathered in a similar manner for 5 minutes, during which 20 μl of citric acid was spotted on the dorsal surface of the patient’s tongue every 30 seconds. Prior to centrifugation, the volume of the spat secretion was measured (with a calibrated pipette) and the rate of saliva secretion was determined by dividing the volume of saliva in the tube by the time required to obtain it. The saliva was centrifuged for 20 minutes at 4°C, 10000×g, then the fluid was collected from above the sediment, frozen at −84°C, and stored until assays were performed, but no longer than 4 months.
LIPIDS ANALYSIS:
The concentration of sphingolipids (sphingosine (Sph), sphinganine (SPA), sphingosine-1-phosphate (S1P) and ceramides (C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer, C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, C24: 0-Cer) in saliva was measured according to the method described by Blachnio-Zabielska et al via ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC/MS/MS), with minor modification [21]. Briefly, an internal standard mixture (Sph-d7, SPA-d7, S1P-d7, C15: 0-d7-Cer, C16: 0-d7-Cer, C18: 1-d7-Cer, C18: 0-d7-Cer, 17C20: 0-Cer, C24: 1-d7-Cer and C24-d7-Cer) (Avanti Polar Lipids, Alabaster, Al, USA) and an extraction mixture (isopropanol: ethyl acetate, 15: 85; v/v) (Merck, Saint Louis, MO, USA) were added to each sample (100 μL of saliva). Samples were then vortexed, sonicated, and centrifuged (5 minutes at 3000 g, 4°C). The supernatants were transferred to new vials and the pellets were re-extracted. Both supernatants were combined and evaporated under a nitrogen stream and reconstituted in solvent B (2 mM ammonium formate (Sigma-Aldrich, Saint Louis, MO, USA), 0.1% formic acid (Honeywell Fluka, Morris Township, NJ, USA) in methanol (Merck, Saint Louis, MO, USA)). Sphingolipids were analyzed with a Sciex QTRAP 6500 + triple quadrupole mass spectrometer (AB Sciex Germany GmbH, Darmstadt, Germany) using a positive ion electrospray ionization (ESI) source (except for S1P, which was analyzed in the negative mode) with multiple reaction monitoring (MRM) against standard curves constructed for each compound. The chromatographic separation was performed on a reverse-phase Zorbax SB-C8 column 2.1×150 mm, 1.8 μm (Agilent Technologies, Santa Clara, CA, USA) in binary gradient using 1 mM ammonium formate (Sigma-Aldrich, Saint Louis, MO, USA), 0.1% formic acid (Honeywell Fluka, Morris Township, NJ, USA) in water (Merck, Saint Louis, MO, USA) as solvent A, 2 mM ammonium formate (Sigma-Aldrich, Saint Louis, MO, USA) and 0.1% formic acid (Honeywell Fluka, Morris Township, NJ, USA) in methanol (Merck, Saint Louis, MO, USA) as solvent B at the flow rate of 0.4 mL/min. To acquire and process the data, we used Analyst (Software version 1.7., AB Sciex Germany GmbH, Darmstadt, Germany) and Sciex OS-Q (AB Sciex Germany GmbH, Darmstadt, Germany).
OXIDATIVE DAMAGE ASSAYS:
MDA concentration was assayed colorimetrically using the thiobarbituric acid reactive substances (TBARS) method with 1,3,3,3-tetraethoxypropane (Sigma-Aldrich, Saint Louis, MO, USA) as a standard [25]. The absorbance was measured at 535 nm with microplate reader ELx800 and Gen5 2.01 software (BioTek Instruments, Winooski, VT, USA).
4-HNE concentrations was measured using a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Cell Biolabs, Inc., San Diego, CA, USA, and USCN Life Science). The absorbance was measured at 405 nm with microplate reader ELx800 and Gen5 2.01 software (BioTek Instruments, Winooski, VT, USA).
STATISTICAL ANALYSES:
GraphPad Prism 8.3.0 for MacOS (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Normality of distribution was assessed using the Shapiro-Wilk test. For comparison of the quantitative variables, one-way analysis of variance (ANOVA) followed by the Tukey post hoc test was used. The statistical significance level was established at
Results
CLINICAL AND STOMATOLOGICAL FINDINGS:
There were no significant differences in age, BMI, duration of addiction, unstimulated and stimulated saliva flow rate, DMFT, API, PBI, and PPD among the 3 study groups and among the study groups vs the control group. Clinical and stomatological characteristics of participants are presented in Table 1.
UNSTIMULATED (US) AND STIMULATED (S) SALIVA:
SpH concentration was significantly lower in US and S in all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (US:
SPA concentration was significantly lower in US and S of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (US:
The concentration of S1P was significantly lower in US of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (
Similarly, ceramide C14 content was considerably lower in unstimulated saliva of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (
The concentrations of ceramides C16 and C24 were significantly lower in US and S of all nicotine users (IQOS, e-cigarette users, CS) compared to the control group (C16, US:
The level of ceramide C18 was considerably lower in US and S of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (US:
Ceramide C18 concentration was clearly lower in US of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (
Ceramide C20 concentration was significantly lower in US and S of all nicotine users (IQOS, e-cigarette users, CS) compared to the control group (US:
Ceramide C22 concentration was significantly lower in US of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (
The concentration of ceramide C24 1 and total Cer was notedly lower in US and S of all nicotine users (IQOS, e-cigarette users, CS) compared to the controls (US, C 24.1:
The content of ceramide C24 1 in the S of the e-cig group was significantly elevated compared to the groups: IQOS (
The concentration of 4-HNE and MDA in US was significantly higher in the group of traditional cigarette smokers compared to the controls (4-HNE
Graphical presentation of the results is presented in Figures 1–4.
Discussion
LIMITATIONS:
Our study has several limitations. Due to the small group size, this should be regarded as a pilot study. The people qualified for the study and control groups were considered matched, with deficiencies in systemic diseases and other factors that may directly affect the increase in oxidative stress in the body. Therefore, fewer patients were included in the study.
Because of the young age of our participants and relatively short smoking histories (up to 3 years), our findings have limited generalizability to long-term or older smokers who may experience different oral health effects. The results may not capture the full spectrum of oral health effects associated with smoking, particularly in older individuals with longer smoking durations.
Moreover, only some salivary lipids were included in our study, so the results do not reflect the overall effect of smoking on the salivary lipid profile. This study also did not compare heavy vs light smokers.
Conclusions
The results of our research clearly show that:
Figures
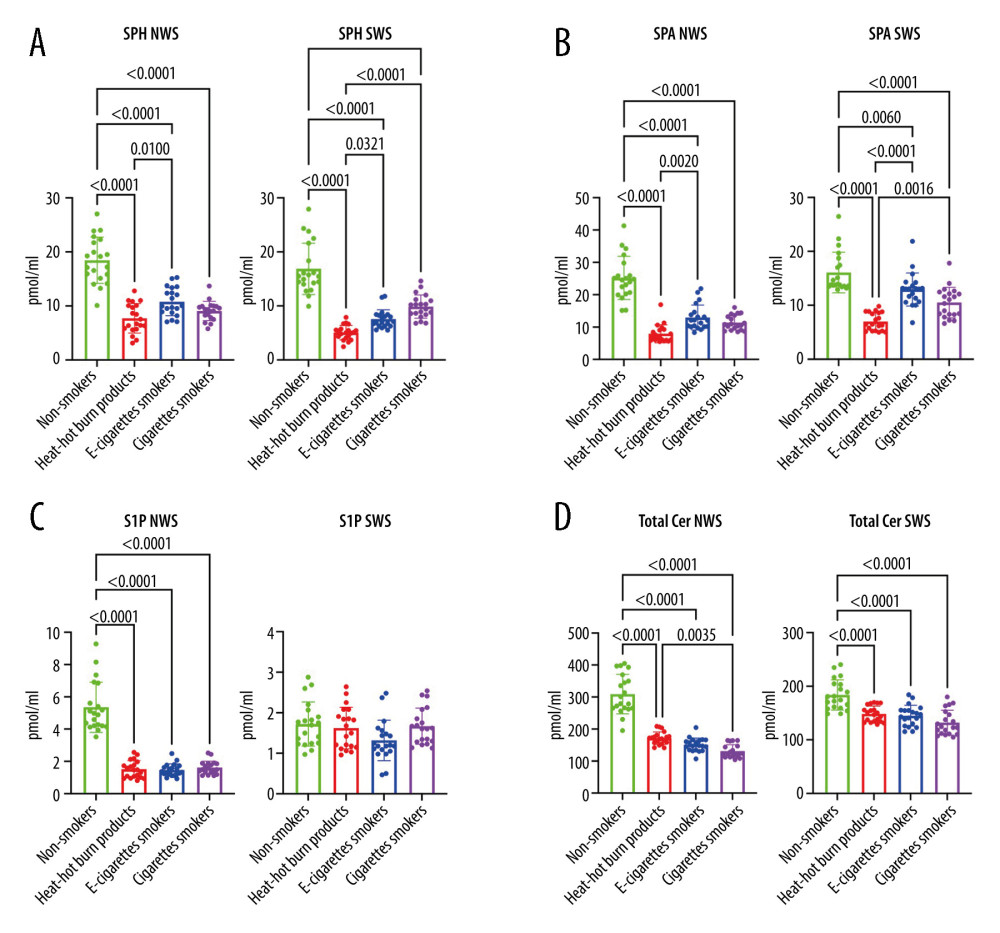 Figure 1. (A–D)Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. NWS – non-stimulated whole saliva; SPH – sphingosine; SPA – sphinganine; S1P – sphingosine-1-phosphate; SWS – stimulated whole saliva; Total Cer – total ceramide; p<0.0001. The figure was created in GraphPad Prism.
Figure 1. (A–D)Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. NWS – non-stimulated whole saliva; SPH – sphingosine; SPA – sphinganine; S1P – sphingosine-1-phosphate; SWS – stimulated whole saliva; Total Cer – total ceramide; p<0.0001. The figure was created in GraphPad Prism. 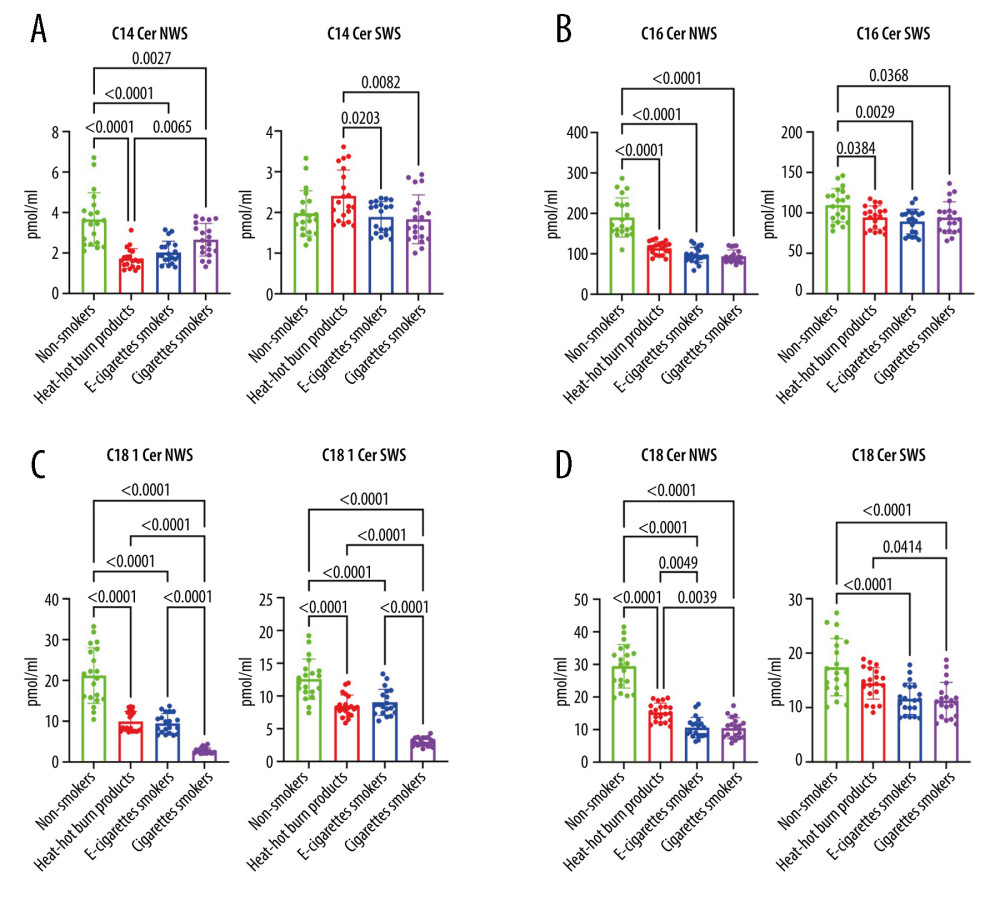 Figure 2. (A–D Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 2. (A–D Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. 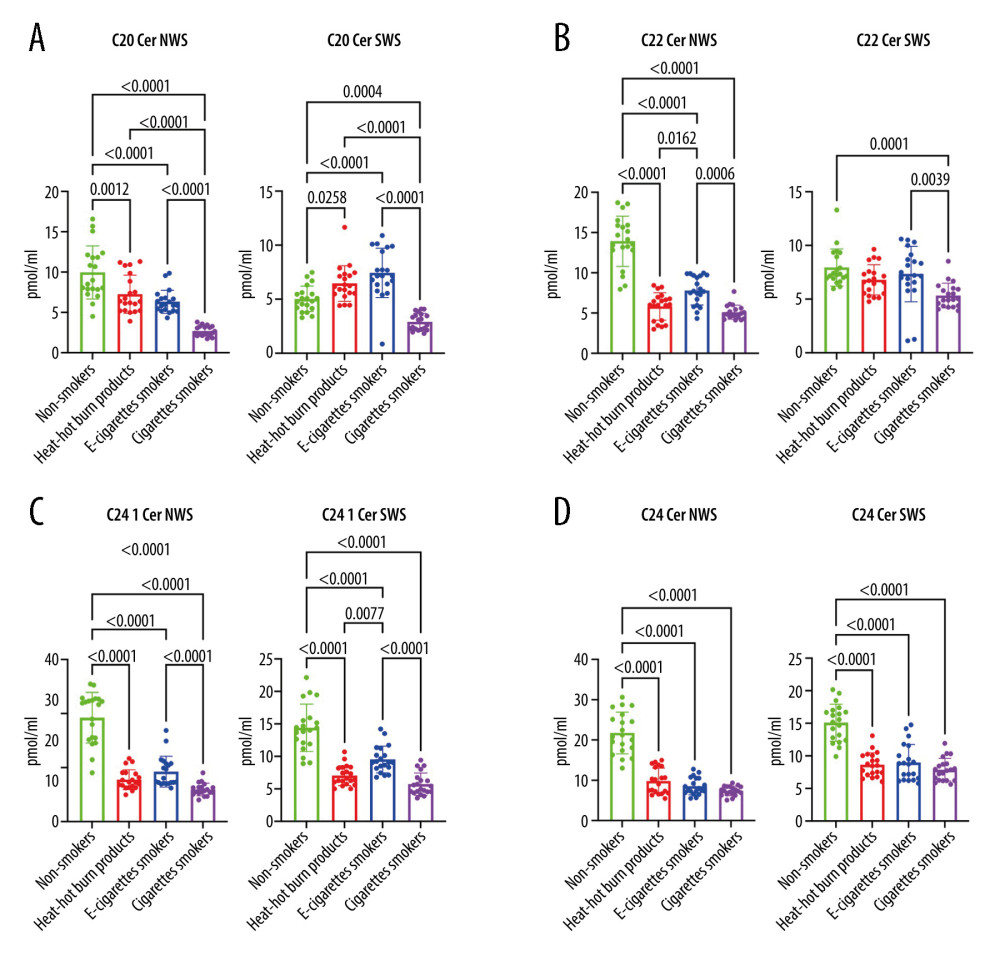 Figure 3. (A–D) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, C24: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 3. (A–D) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, C24: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. 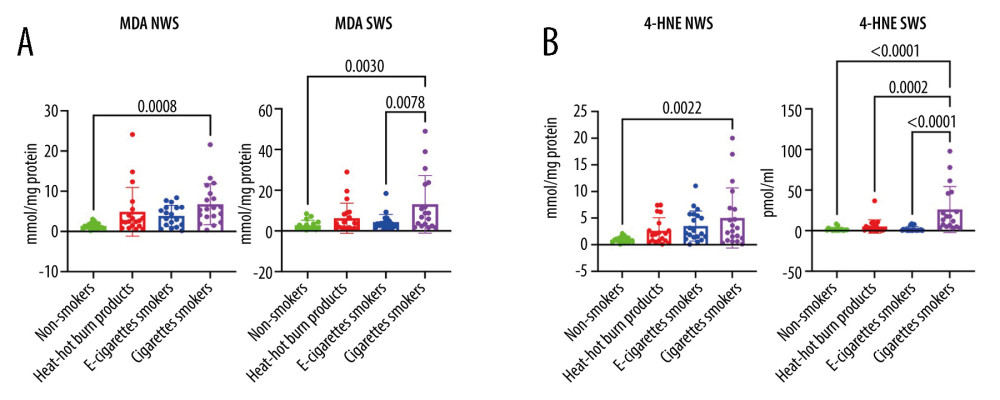 Figure 4. (A, B) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipid peroxidation products in unstimulated and stimulated saliva. 4-HNE – 4-hydrokxynonenal; MDA – malondialdehyde; NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 4. (A, B) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipid peroxidation products in unstimulated and stimulated saliva. 4-HNE – 4-hydrokxynonenal; MDA – malondialdehyde; NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. References
1. Babizhayev MA, Yegorov YE, Smoking and health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration: Fundam Clin Pharmacol, 2011; 25(4); 425-42
2. Hu X, Fan Y, Li H, Impacts of cigarette smoking status on metabolomic and gut microbiota profile in male patients with coronary artery disease: A multi-omics study: Front Cardiovasc Med, 2021; 8; 766739
3. Tong X, Chaudhry Z, Lee CC, Cigarette smoke exposure impairs β-cell function through activation of oxidative stress and ceramide accumulation: Mol Metab, 2020; 37; 100975
4. Ford PJ, Rich AM, Tobacco use and oral health: Addiction, 2021; 116(12); 3531-40
5. Szyfter K, Napierala M, Florek E, Molecular and health effects in the upper respiratory tract associated with tobacco smoking other than cigarettes: Int J Cancer, 2019; 144(11); 2635-43
6. Waszkiewicz N, Zalewska A, Szajda SD, The effect of chronic alcohol intoxication and smoking on the activity of oral peroxidase: Folia Histochem Cytobiol, 2012; 50(3); 450-55
7. Waszkiewicz N, Jelski W, Zalewska A, Salivary alcohol dehydrogenase in non-smoking and smoking alcohol-dependent persons: Alcohol, 2014; 48(6); 611-16
8. Waszkiewicz N, Chojnowska S, Zalewska A, Salivary hexosaminidase in smoking alcoholics with bad periodontal and dental states: Drug Alcohol Depend, 2013; 129(1–2); 33-40
9. Wu CN, Chang WS, Shih LC, Interaction of DNA repair gene XPC with smoking and betel quid chewing behaviors of oral cancer: Cancer Genomics Proteomics, 2021; 18(3 Suppl); 441-49
10. Nagler RM, Altered salivary profile in heavy smokers and its possible connection to oral cancer: Int J Biol Markers, 2007; 22(4); 274-80
11. Reznick AZ, Klein I, Eiserich JP, Cross CE, Nagler RM, Inhibition of oral peroxidase activity by cigarette smoke: In vivo and in vitro studies: Free Radic Biol Med, 2003; 34(3); 377-84
12. ArRejaie AS, Al-Aali KA, Alrabiah M, Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers: J Periodontol, 2019; 90(4); 367-74
13. Al-Aali KA, Alrabiah M, ArRejaie AS, Peri-implant parameters, tumor necrosis factor-alpha, and interleukin-1 beta levels in vaping individuals: Clin Implant Dent Relat Res, 2018; 20(3); 410-15
14. Sundar IK, Javed F, Romanos GE, Rahman I, E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts: Oncotarget, 2016; 7(47); 77196-204
15. Cichońska D, Kusiak A, Kochańska B, Influence of electronic cigarettes on selected antibacterial properties of saliva: Int J Environ Res Public Health, 2019; 16(22); 4433
16. Waszkiewicz N, Szajda SD, Jankowska A, The effect of acute ethanol intoxication on salivary proteins of innate and adaptive immunity: Alcohol Clin Exp Res, 2008; 32(4); 652-56
17. Matczuk J, Żendzian-Piotrowska M, Maciejczyk M, Kurek K, Salivary lipids: A review: Adv Clin Exp Med, 2017; 26(6); 1023-31
18. Wu Y, Liu Y, Gulbins E, Grassmé H, The anti-infectious role of sphingosine in microbial diseases: Cells, 2021; 10(5); 1105
19. Tippetts TS, Winden DR, Swensen AC, Cigarette smoke increases cardiomyocyte ceramide accumulation and inhibits mitochondrial respiration: BMC Cardiovasc Disord, 2014; 14(1); 165
20. Thatcher MO, Tippetts TS, Nelson MB, Ceramides mediate cigarette smoke-induced metabolic disruption in mice: Am J Physiol Endocrinol Metab, 2014; 307(10); E919-E27
21. Blachnio-Zabielska AU, Persson XMT, Koutsari C, A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans: Rapid Commun Mass Spectrom, 2012; 26(9); 1134-40
22. Wang Q, Ji X, Rahman I, Dysregulated metabolites serve as novel biomarkers for metabolic diseases caused by e-cigarette vaping and cigarette smoking: Metabolites, 2021; 11(6); 345
23. Zalewska A, Knaś M, Maciejczyk M, Antioxidant profile, carbonyl and lipid oxidation markers in the parotid and submandibular glands of rats in different periods of streptozotocin induced diabetes: Arch Oral Biol, 2015; 60(9); 1375-86
24. Jung SH, Stratified Fisher’s exact test and its sample size calculation: Biom J, 2014; 56(1); 129-40
25. Buege JA, Aust SD, Microsomal lipid peroxidation: Methods Enzymol, 1978; 52; 302-10
26. Humphrey SP, Williamson RT, A review of saliva: Normal composition, flow, and function: J Prosthet Dent, 2001; 85(2); 162-69
27. Gerreth P, Maciejczyk M, Zalewska A, Comprehensive evaluation of the oral health status, salivary gland function, and oxidative stress in the saliva of patients with subacute phase of stroke: A case-control study: J Clin Med, 2020; 9(7); 2252
28. Greabu M, Battino M, Mohora M, Saliva – a diagnostic window to the body, both in health and in disease: J Med Life, 2009; 2(2); 124-32
29. Soleimani F, Dobaradaran S, De-la-Torre GE, Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review: Sci Total Environ, 2022; 813; 152667
30. Fenoll-Palomares C, Muñoz-Montagud JV, Sanchiz V, Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers: Rev Esp Enferm Dig, 2004; 96(11); 773-83
31. Voelker MA, Simmer-Beck M, Cole M: J Dent Hyg, 2013; 87(1); 30-37
32. Marsh PD, Do T, Beighton D, Devine DA, Influence of saliva on the oral microbiota: Periodontol 2000, 2016; 70(1); 80-92
33. Zięba S, Maciejczyk M, Zalewska A, Ethanol- and cigarette smoke-related alternations in oral redox homeostasis: Front Physiol, 2022; 12; 793028
34. Nakonieczna-Rudnicka M, Bachanek T, Piekarczyk W, Kobyłecka E, Secretory immunoglobulin A concentration in non-stimulated and stimulated saliva in relation to the status of smoking: Przegl Lek, 2016; 73(10); 704-7
35. Tarbiah N, Todd I, Tighe PJ, Fairclough LC, Cigarette smoking differentially affects immunoglobulin class levels in serum and saliva: An investigation and review: Basic Clin Pharmacol Toxicol, 2019; 125(5); 474-83
36. Grana R, Benowitz N, Glantz SA, E-cigarettes: A scientific review: Circulation, 2014; 129(19); 1972-86
37. Rom O, Pecorelli A, Valacchi G, Reznick AZ, Are E-cigarettes a safe and good alternative to cigarette smoking?: Ann NY Acad Sci, 2015; 1340; 65-74
38. Belok SH, Parikh R, Bernardo J, Kathuria H, E-cigarette, or vaping, product use-associated lung injury: A review: Pneumonia, 2020; 12(1); 657-63
39. Dawson CT, Maziak W, Renormalization and eegulation of e-cigarettes: Am J Public Health, 2016; 106(3); 569
40. Ratajczak A, Jankowski P, Strus P, Feleszko W, Heat not burn tobacco product – a new global trend: Impact of heat-not-burn tobacco products on public health, a systematic review: Int J Environ Res Public Health, 2020; 17(2); 409
41. Clapp PW, Peden DB, Jaspers I, E-cigarettes, vaping-related pulmonary illnesses, and asthma: A perspective from inhalation toxicologists: J Allergy Clin Immunol, 2020; 145(1); 97-99
42. Gotts JE, Jordt SE, McConnell R, Tarran R, What are the respiratory effects of e-cigarettes?: BMJ, 2019; 366; I5275
43. Cichońska D, Kusiak A, Kochańska B, Influence of electronic cigarettes on selected antibacterial properties of saliva: Int J Environ Res Public Health, 2019; 16(22); 4433
44. Lestari DA, Tandelilin RTC, Rahman FA, Degree of acidity, salivary flow rate and caries index in electronic cigarette users in Sleman Regency, Indonesia: Journal of Indonesian Dental Association, 2020; 3(1); 449
45. Faridoun A: A comparison of the levels of salivary biomarkers between conventional smokers and electronic cigarette users (a pilot study) Available in: http://hdl.handle.net/10713/9615
46. Caterino M, Fedele R, Carnovale V, Lipidomic alterations in human saliva from cystic fibrosis patients: Sci Rep, 2023; 13(1); 600
47. Fineide F, Chen X, Bjellaas T, Characterization of lipids in saliva, tears and minor salivary glands of Sjögren’s syndrome patients using an HPLC/MS-based approach: Int J Mol Sci, 2021; 22(16); 8997
48. Gault CR, Obeid LM, Hannun YA, An overview of sphingolipid metabolism: from synthesis to breakdown: Adv Exp Med Biol, 2010; 688; 1-23
49. Taha TA, Mullen TD, Obeid LM, A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death: Biochim Biophys Acta, 2006; 1758(12); 2027-36
50. Mencarelli C, Martinez-Martinez P, Ceramide function in the brain: When a slight tilt is enough: Cell Mol Life Sci, 2013; 70(2); 181-203
51. Okada T, Kajimoto T, Jahangeer S, Nakamura S, Sphingosine kinase/sphingosine 1-phosphate signalling in central nervous system: Cell Signal, 2009; 21(1); 7-13
52. Attard R, Dingli P, Doggen CJM, The impact of passive and active smoking on inflammation, lipid profile and the risk of myocardial infarction: Open Heart, 2017; 4(2); e000620
53. Gossett LK, Johnson HM, Piper ME, Smoking intensity and lipoprotein abnormalities in active smokers: J Clin Lipidol, 2009; 3(6); 372-78
54. Herath P, Wimalasekera S, Amarasekara T, Effect of cigarette smoking on smoking biomarkers, blood pressure and blood lipid levels among Sri Lankan male smokers: Postgrad Med J, 2022; 98(1165); 848-54
55. Butera A, Pascadopoli M, Pellegrini M, Oral microbiota in patients with peri-implant disease: A narrative review: Applied Sciences, 2022; 12(7); 3250
56. Vale GC, Mayer MPA, Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis: Arch Oral Biol, 2021; 128; 105174
57. Butera A, Pascadopoli M, Pellegrini M, Domiciliary use of chlorhexidine vs. postbiotic gels in patients with peri-implant mucositis: A split-mouth randomized clinical trial: Applied Sciences, 2022; 12(6); 2800
58. Maciejczyk M, Zalewska A, Ładny JR, Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly: Oxid Med Cell Longev, 2019; 2019; 4393460
Figures
 Figure 1. (A–D)Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. NWS – non-stimulated whole saliva; SPH – sphingosine; SPA – sphinganine; S1P – sphingosine-1-phosphate; SWS – stimulated whole saliva; Total Cer – total ceramide; p<0.0001. The figure was created in GraphPad Prism.
Figure 1. (A–D)Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. NWS – non-stimulated whole saliva; SPH – sphingosine; SPA – sphinganine; S1P – sphingosine-1-phosphate; SWS – stimulated whole saliva; Total Cer – total ceramide; p<0.0001. The figure was created in GraphPad Prism. Figure 2. (A–D Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 2. (A–D Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C14: 0-Cer, C16: 0-Cer, C18: 1-Cer, C18: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. Figure 3. (A–D) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, C24: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 3. (A–D) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipids in unstimulated and stimulated saliva. Cer – ceramides (C20: 0-Cer, C22: 0-Cer, C24: 1-Cer, C24: 0-Cer); NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. Figure 4. (A, B) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipid peroxidation products in unstimulated and stimulated saliva. 4-HNE – 4-hydrokxynonenal; MDA – malondialdehyde; NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism.
Figure 4. (A, B) Influence of smoking traditional cigarettes, e-cigarettes, and heat-not-burn products on the concentration of lipid peroxidation products in unstimulated and stimulated saliva. 4-HNE – 4-hydrokxynonenal; MDA – malondialdehyde; NWS – non-stimulated whole; SWS – stimulated whole saliva; P<0.0001. The figure was created in GraphPad Prism. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952