14 April 2021: Animal Study
Zi Qi Decoction Alleviates Liver Fibrosis by Inhibiting the Toll-Like Receptor 4 (TLR4)-Related Nuclear Factor kappa b (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways
Jingwen Zhou1ABCE, Xiaolong Zhang1A, Lingfeng Wan1AD, Jun Yu2BCD, Tianci Li2BCD, Ziyu Lu3BCD, Nanyuan Fang1E, Lixia Sun1AG, Fang Ye2AG*DOI: 10.12659/MSM.929438
Med Sci Monit 2021; 27:e929438
Abstract
BACKGROUND: Hepatic stellate cells (HSCs) play a vital role in hepatic fibrogenesis. Our recent clinical study indicated that the Zi Qi decoction, a Traditional Chinese Medicine formula, exhibited good efficacy in alleviating liver fibrosis, but the underlying mechanism remains elusive.
MATERIAL AND METHODS: Rats repeatedly injected with CCl₄ and cells stimulated with lipopolysaccharide were used as in vivo and in vitro models for liver fibrosis, respectively. The viability of LX-2 cells was evaluated with MTT assay. Relative messenger RNA (mRNA) expression of representative extracellular matrix (ECM) components was detected with real-time quantitative polymerase chain reaction (RT-qPCR). Moreover, total and phosphorylation levels of ECM proteins and pathway-related proteins were detected with western blotting. Immunofluorescent staining was used to show the nuclear translocation of nuclear factor kappa b (NF-κB) p65. Hematoxylin & eosin (H&E) and Masson trichrome staining and immunohistochemistry were performed to evaluate the extent of liver fibrosis. The levels of alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), Hyp, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were tested with an enzyme-linked immunosorbent assay. In addition, 7.0T micro-magnetic resonance imaging (micro-MRI) was used to evaluate the severity of hepatic damage.
RESULTS: The Zi Qi decoction inhibited lipopolysaccharide-mediated upregulation of mRNA and protein levels of representative ECM proteins both in vivo and in vitro. The Zi Qi decoction also suppressed activation of the Toll-like receptor 4 (TLR4)-related NF-κB signaling pathway and subsequently inhibited the nuclear translocation of activated NF-κB. Moreover, another TLR4 downstream pathway, mitogen-activated protein kinase (MAPK), was simultaneously restrained. The results of liver pathology and MRI in rat models also suggested the efficacy of the Zi Qi decoction in attenuating liver damage.
CONCLUSIONS: The Zi Qi decoction inhibited liver fibrosis by inhibiting the TLR4-related NF-kB and MAPK signaling pathways and preventing activation of HSCs.
Keywords: Fibrosis, Toll-Like Receptor 4, Alanine Transaminase, Aspartate Aminotransferases, Complex Mixtures, Cytokines, Drugs, Chinese Herbal, hepatic stellate cells, Inflammation Mediators, Liver, Liver Cirrhosis
Background
Liver fibrosis is a wound-healing response to numerous factors, such as alcohol abuse, hepatitis B or C virus infection, and non-alcoholic fatty liver disease [1]. Fibrotic liver is characterized by excessive deposition of extracellular matrix (ECM) [2]. Types I and III collagen and alpha-smooth muscle actin (α-SMA), which act as hallmarks for HSC activation [3], are the most abundant extracellular matrix components within the ECM [4]. Previous studies have shown that HSCs play a vital role in inflammatory modulation, liver immunology, and hepatic fibrogenesis [5]. Quiescent HSCs are inactive and localized within the space of Disse [6]. Once activated, HSCs lose intracellular lipid droplets, and turn a star-like phenotype into a fibroblast-like phenotype [1,7]. This process accelerates the progression of liver fibrosis. Based on this scenario, inhibition of activation of HSCs has been considered a potential therapeutic target for treatment of liver fibrosis [8].
Recently, overgrowth of intestinal bacteria has gained increasing attention in the pathophysiology of liver fibrosis [9]. Bacterial lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, acts as one of the strongest known inducers of inflammation and has been found to be associated with hepatic fibrogenesis through direct interaction with HSCs [10]. Because LPS is the specific ligand of Toll-like receptor 4 (TLR4) on the HSC membrane, it can trigger multiple signaling pathways, including nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK). These 2 pathways have been well studied and play crucial roles in inflammatory processes [11] that influence the survival of hepatocytes, inflammation in Kupffer cells, and activation of HSCs [12]. As a result, numerous pro-inflammatory cytokines are released [13], such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1, and IL-6 [14]. These important cytokines, in turn, can promote activation of HSCs and then start a vicious cycle of continuous aggravation of liver fibrosis.
All existing drugs for liver fibrosis have adverse effects. Therefore, there is an urgent need to develop an effective and safe way to treat liver fibrosis and prevent further development of cirrhosis. For hundreds of years, Traditional Chinese Medicine (TCM) has been well studied as a way of preventing and relieving hepatic fibrosis [15,16]. Zi Qi decoction is a TCM, prescribed by TCM master Professor Zhou Zhongying, which has shown a good effect on alleviating liver fibrosis [17]. However, the underlying mechanism of Zi Qi decoction needs further exploration.
The aim of the present study was to clarify the key functional target and specific molecular mechanism of Zi Qi decoction in alleviating liver fibrosis as a way of furthering its use in therapy to reverse liver fibrosis and prevent progression into cirrhosis.
Material and Methods
ANTIBODIES:
Antibodies against Type I and III collagen, α-SMA, TLR4, and Erk1/2 were produced by Proteintech Group (Rosemont, United States). Antibodies against MyD88, phospho-NF-κB p65, NF-κB p65, GAPDH, p38 MAPK, and c-Jun NH2-terminal kinase (JNK) were purchased from Cell Signaling Technology (Danvers, United States). The phospho-JNK antibody was acquired from Abcam (Cambridge, United Kingdom). Antibodies against phospho-Erk1/2 and phospho-p38 were obtained from Beijing Biosynthesis Biotechnology (Beijing, China). The TRAF6 antibody was obtained from Boster Bio (Pleasanton, United States).
ANIMALS:
Fifty specific-pathogen-free, 6- to 8-week-old male Sprague Dawley (SD) rats that weighed 220 to 250 g were purchased from Zhejiang Academy of Medical Sciences (SCXK [Zhe] 2019-0002). The current experimental protocols were approved by the Committee on Laboratory Animal Care of Nanjing University of Chinese Medicine and all the rats were given humane care according to the guidelines of the National Institutes of Health (United States).
The experiment was started after 1 week of adaptive feeding. The rats were kept in standard conditions (12-h light/dark cycle, humidity 55±5%, temperature 22±2°C) and provided with free access to food and water. Fifty rats were randomly divided into 5 groups (control, vehicle group, ZQ-low, ZQ-middle, and ZQ-high). According to the available reference [18], 15% CCl4 diluted with olive oil 200 μL/100 g was injected into the rats intraperitoneally twice a week for 3 months to successfully establish CCl4-induced rat models. Then, the rats in each group were administered a different liquid intragastrically once a day for 8 weeks: controls, normal saline; vehicle group, normal saline; ZQ-low, 0.5 mL/100 g Zi Qi decoction; ZQ-middle, 1 mL/100 g Zi Qi decoction; and ZQ-high, 1.5 mL/100 g Zi Qi decoction. All the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate 24 h after the last treatment and 1 mL of blood and the liver tissue was collected.
ZI QI DECOCTION PREPARATION:
The Zi Qi decoction contains 9 kinds of TCM materials: 10 g
PREPARATION OF DRUG-CONTAINING SERUM:
Twenty healthy SD male rats that weighed 220 to 250 g were reared under normal conditions (12 h light/dark cycle, humidity 55±5%, temperature 22±2°C). They were randomly divided into control, vehicle, ZQ-low, ZQ-middle, and ZQ-high groups (n=4 each) and kept fasting for 12 h before the experiment. For the drug-containing serum group, 11 g/mL, 5.5 g/mL, and 2.75 g/mL of Zi Qi decoction were intragastrically administrated to the rats in the ZQ-high, ZQ-middle, and ZQ-low groups, respectively (1 mL/100 g of body weight). The 0.9% normal saline was administered intragastrically to the rats in the control and vehicle groups (1 mL/100 g of body weight) once daily at 9: 00 am for 8 days and they were fed a normal diet after 30 min. Based on our previous preliminary experiments, anesthesia was performed 1 h after the eighth intragastric administration, because the blood concentration was highest at that point, and 10 mL of blood was collected from the cervical aorta under aseptic conditions. It was left standing for 4 h at room temperature and a supernatant was extracted after the blood was centrifuged at 3500 r/min for 20 min. The RPMI-1640 medium drug-containing serum then was diluted, respectively into control, vehicle, ZQ-low (0.55 g/mL), ZQ-middle (1.1 g/mL), and ZQ-high (2.2 g/mL) sera. Finally, all of the serum was inactivated at 56°C for 30 min and bacteria were filtered with 0.2-μm millipore filters and stored at −70°C.
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY ANALYSIS:
The drug-containing serum samples of Zi Qi decoction was dissolved in methanol and filtered through a 0.22-μm membrane before loading into a high-performance liquid chromatography (HPLC) system. The analysis was performed with a Water E2695 high-performance liquid chromatograph from Waters Corporation (Massachusetts, United States). Paeoniflorin, verbascoside, paeonol, and catalpol were purchased from Ruifinsi Biological Technology (Chengdu, China). Quercitin, matrine, atractylotin, berberine hydrochloride, and shikonin were produced by Meilun Biotechnology (Dalian, China). These standard compounds were prepared as a control solution for the high-performance liquid chromatography analyses after being precisely weighed and dissolved with methanol, as shown in Supplementary Figure 1. The mobile phase consisted of A (0.1% phosphoric acid water)-B (acetonitrile) binary gradient elution with a flow rate of 1.0 mL/min.
CELL CULTURE:
The LX-2 cell line was acquired from the BeNa Culture Collection (BNCC) (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium (Gibco, New York, United States), containing 10% fetal bovine serum (Gibco, New York, United States), 100 units/mL of penicillin, and 100 μg/mL of streptomycin (Gibco, New York, United States) under humidified air containing 5% CO2 at 37°C. The LX-2 cells were stimulated with LPS (100 ng/mL) (Sigma-Aldrich, St Louis, United States) for 6 h according to the procedures used in the previous reports [19].
CELL VIABILITY ASSAY:
Approximately 1×104 LX-2 cells per well were cultured in 96-well plates and then divided into 5 groups (control, vehicle, ZQ-low, ZQ-middle, and ZQ-high) which were treated for 24 h with LPS and different concentrations of the Zi Qi decoction. Then, 20 μL of MTT (Solarbio, China) was added to each well and the cells were incubated for 4 h. The cell culture medium was then discarded and dimethyl sulfoxide (DMSO) was added to dissolve crystal violet for 30 min. Finally, optical density values were measured at 490 nm. Infrared value=(1−A test/A control)×100%.
HISTOLOGICAL AND IMMUNOHISTOCHEMICAL ANALYSIS:
After being fixed in 10% formalin for 24 h, the excised livers were harvested and fixed in 4% paraformaldehyde (Sigma-Aldrich). The samples then were cut into 5-mm tissue sections and mounted onto slides (Sigma-Aldrich). The slides were prepared for hematoxylin & eosin (H&E) staining, Masson’s trichrome staining, and Type I collagen and α-SMA immunohistochemistry to assess the degree of liver fibrosis. All procedures were performed as previously described [20].
IMMUNOFLUORESCENCE:
Cells were grown on glass-bottomed cell culture dishes and all the samples were blocked in 4% bovine serum albumin after sufficient fixture. Then, the samples were incubated with rabbit-derived antibodies and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies. For DNA staining, 4,6-diamidino-2-phenylindole (DAPI) (SouthernBiotech, Birmingham, Alabama, United States) was used. The slices were observed with a confocal laser scanning microscope (Olympus, Tokyo, Japan) under 40×magnification.
REAL-TIME QUANTITATIVE POLYMERASE CHAIN REACTION:
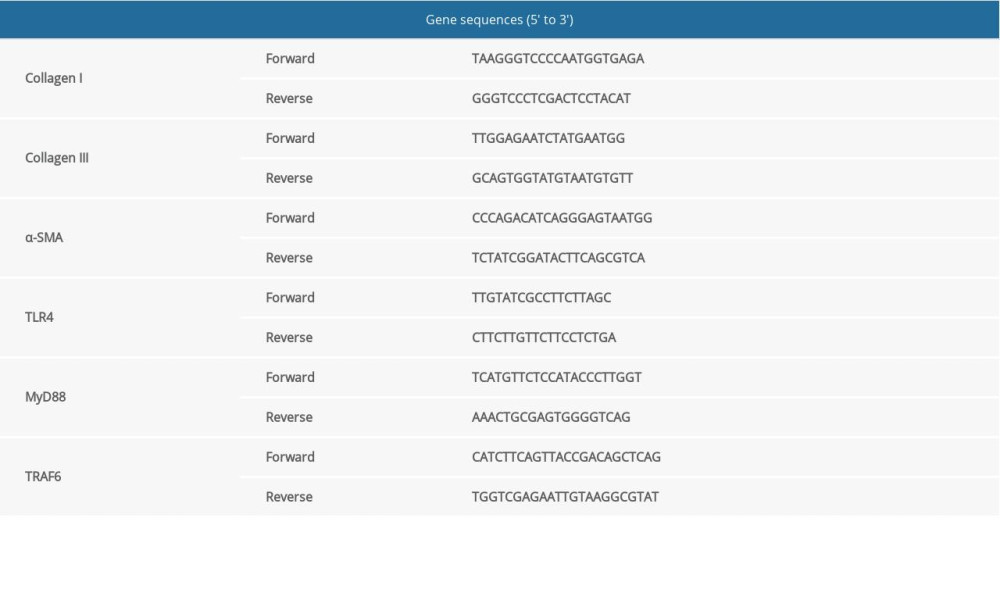
Total RNA was obtained from the cultured cells according to the manufacturer’s protocol. Real-time quantitative polymerase chain reaction was performed according to our previous protocol [21]. The expression level of GAPDH messenger RNA (mRNA) was set as an internal control. Primers for Types I and III collagen, α-SMA, TLR4, MyD88, TRAF6, and GAPDH were designed and used. Moreover, quantitative primers for amplification were used as described below (Table 1).
ENZYME-LINKED IMMUNOSORBENT ASSAY:
Serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and gamma-glutamyl transferase (GGT) in rats were measured using enzyme-lined immunosorbent assay kits (R&D systems, Minneapolis, United States) according to the manufacturer’s protocol and an appropriate amount of liver was homogenized in 0.9% saline. The suspension was used to detect the content of TNF-α, IL-6, and Hyp using standard commercial kits (R&D systems, Minneapolis, United States) according to the manufacturer’s instructions.
WESTERN BLOTTING:
As was done in our previous study [22], after treatment with Zi Qi decoction for 48 h, cell lysates were denatured and boiled in loading buffer. Proteins were transferred to nitrocellulose membranes (Bio-Rad, California, United States). The membranes were incubated with rabbit-derived primary antibodies overnight after being blocked in 5% skim milk for 1 h, and then incubated with rabbit-derived primary secondary antibodies. Finally, immunoreactive bands were revealed using enhanced chemiluminescence (Bio-Rad).
MICRO-MAGNETIC RESONANCE INSTRUMENTATION:
A Bruker Pharma Scan with 7.0T superconducting magnet resonance was used to perform micro-magnetic resonance imaging (MRI) of livers in rats from the Medical College of Southeast University (NanJing, China). Amira imaging PC-based software and Para vision 5 software were used to analyze and process the micro-MRI data.
STATISTICAL ANALYSIS:
The data were expressed as means ± standard deviations. All data were analyzed using 1-way analysis of variance in multiple comparisons to determinate significant differences. A least significant difference test was performed to assess for differences between groups.
Results
ZI QI DECOCTION INHIBITED ACTIVATION OF LX-2 CELLS:
To test the effect of Zi Qi decoction on activation of HSCs, LPS-stimulated LX-2 cells were used as an experimental cell model. According to the previous protocol [17], the MTT assay showed that at 48 h after administration, activation of LX-2 cells was inhibited in both the ZQ-middle and ZQ-high groups (Figure 1A). The RT-qPCR results also revealed that there was a dramatic decrease in the relative mRNA levels of Types I and III collagen and α-SMA in the ZQ-middle and ZQ-high groups compared with the LPS group (Figure 1B). The cellular protein levels of these 3 markers were downregulated after exposure to the Zi Qi decoction, especially in the ZQ-middle and ZQ-high groups (Figure 1C). These data suggest that Zi Qi decoction can suppress the activation of LX-2 cells and the deposition of ECM in a dose-dependent manner.
ZI QI DECOCTION INHIBITED TLR4-RELATED NF-κB AND MAPK SIGNALING PATHWAYS IN LX-2 CELLS:
To explore the pharmacological effect of Zi Qi decoction and better characterize the effect of the Zi Qi decoction on cellular signaling, we chose the high-dose Zi Qi decoction as a basic stimulating concentration for the next experiments. As is shown in Figure 2A, after stimulation with LPS, the LX-2 cells had increased mRNA levels of TLR4, MyD88, and TRAF6. However, the Zi Qi decoction inhibited these effects (Figure 2A). To identify the functional target of the Zi Qi decoction, TAK-242 and pyrrolidine dithiocarbamate (PDTC), which inhibit TLR4 and NF-κB, respectively, were applied. The results suggested that TAK-242 could suppress TLR4 and downstream NF-κB signaling but PDTC exhibited no significant effect on the recovery of TLR4/NF-κB signaling (Figure 2A).
Western blot results also indicated that protein levels of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 decreased markedly in both the ZQ and TAK-242 groups while the PDTC group showed no similar effects (Figure 2B). Furthermore, because activation of TLR4 on the cell surface can trigger translocation of NF-κB into the nucleus and result in the transcription of genes encoding inflammation-associated molecules and cytokines [23], an immunofluorescence assay was performed to evaluated the cellular location of NF-κB p65. The results suggested that after treatment with LPS, activated NF-κB p65 in the LX-2 cells was significantly translocated into the nucleus. However, when the cells were simultaneously treated with Zi Qi decoction, this effect apparently was restrained (Figure 2C).
Taken together with previous reports, these results show that TLR4 has another important downstream pathway, MAPK signaling, which is associated with inflammatory processes [13] and activation of HSCs [14]. Therefore, we further investigated whether Zi Qi decoction had a synergistic effect on the 2 TLR4-related signaling. The results indicated that compared with the LPS group, the relative phosphorylation levels of ERK, JNK, and p38 were significantly downregulated after Zi Qi decoction treatment (Figure 2D). These data indicated that the cellular MAPK pathway could also be suppressed by Zi Qi decoction. Therefore, Zi Qi decoction could inhibit the NF-κB and the MAPK signaling pathways simultaneously, both of which share the same upstream pathway for TLR4.
ZI QI DECOCTION AMELIORATED CCL4-INDUCED LIVER INJURY AND FIBROSIS IN VIVO:
Next, we performed some in vivo investigations to clarify the impact of Zi Qi decoction on liver fibrosis. As is shown in the results, long-term intraperitoneal injection of CCl4 obviously increased ALT, AST, and GGT, serum levels of which are widely used to evaluate the degree of liver damage. After 8 weeks of intragastric administration of different concentrations of the Zi Qi decoction, serum levels of all 3 enzymes greatly decreased regardless of the concentration of Zi Qi decoction that was used (Figure 3A–3C). The content of Hyp also was decreased after administration of the Zi Qi decoction (Figure 3D). In addition, because some pro-inflammatory cytokines, particularly TNF-α and IL-6, can be induced in response to hepatocyte injuries [24–26], we also tested the level of these 2 representative cytokines to evaluate the inflammatory state in fibrotic rats. The results showed that the levels of these 2 cytokines were obviously downregulated in the ZQ groups in a dose-dependent manner (Figure 3E, 3F). Therefore, it can be inferred that Zi Qi decoction can ameliorate liver injury and inflammation to some extent.
Furthermore, the 7.0T micro-MRI was used to evaluate the severity of hepatic damage in rats in each group. Liver tissue in the control group showed no changes and a tough appearance, disordered blood vessels, reduced liver volume, and dilated portal veins were obvious in the vehicle group. However, all the phenomena were improved across the different Zi Qi groups (Figure 3G). The apparent diffusion coefficient (ADC) values of the relative images were calculated using Para vision 5 software (Bruker) and ADC values were found to progressively decline with the severity of disease (Figure 3H).
The general appearance of rat liver was bright red and soft with a sharp edge in the control group, whereas in the vehicle group, the tissue was dark red, tough, and appeared to have granular tubercles with a dulled edge. In the ZQ-middle and ZQ-high groups, however, the liver was slightly softer than in the vehicle group. Moreover, granular tubercles on the surface in the ZQ-high group were not obvious compared with those in the vehicle group and the color of the liver tissue was brighter (Figure 3I, upper panel). H&E staining also showed that the control group had no changes in hepatic parenchyma and the liver tissue in the vehicle group showed liver parenchymal damage, inflammatory cell infiltration, steatosis, liver cell cord derangement, and hyperchromatic nucleus, consistent with typical liver sections in chronic CCl4-induced liver injury. However, fibrosis was alleviated by treatment with the Zi Qi decoction, especially in the ZQ-high group (Figure 3I, middle panel). Masson staining showed no alterations in the control group and large amounts of collagen deposition and obvious contorted liver parenchyma around the fibrotic nodules. Treatment with the Zi Qi decoction prevented collagen deposition and formation of regenerative nodules (Figure 3I, lower panel). Thus, these results showed that the Zi Qi decoction can reduce the severity of liver fibrosis in CCl4-induced rats.
ZI QI DECOCTION ALLEVIATED COLLAGEN DEPOSITION IN LIVER TISSUE IN A RAT MODEL OF CCL4-INDUCED FIBROSIS:
Because Zi Qi decoction had a significant effect on inhibition of intracellular fibrosis, it was necessary to investigate its underlying mechanism in vivo. RT-qPCR results suggested that all concentrations of the Zi Qi decoction induced an obvious decrease in the relative mRNA expression of Types I and III collagen and α-SMA (Figure 4A). Western blotting also showed that the Zi Qi decoction could significantly decrease protein expression of Types I and III collagen and α-SMA in liver tissue compared with the vehicle group (Figure 4B). Furthermore, immunohistochemistry showed that the positive staining of cells for α-SMA and Type I collagen appeared to increase significantly along the collagen septa bridging the portal areas and central vessels in the vehicle group. Moreover, expression of α-SMA and Type I collagen were significantly reduced compared with the vehicle group (Figure 4C). Taken together, Zi Qi decoction can suppress collagen deposition in a dose-dependent manner.
ZI QI DECOCTION REGULATED TLR4-RELATED NF-κB AND MAPK SIGNALING PATHWAYS IN LIVER TISSUE OF RATS WITH HEPATIC FIBROSIS:
To elucidate the inhibitory effects of the Zi Qi decoction on extracellular signaling, RT-qPCR and western blotting were performed. We found that the level of LPS increased in the animal models of hepatic fibrosis, in line with previous studies [26,27]. mRNA expression of TLR4, MyD88, and TRAF6 in the liver tissue decreased in the ZQ-middle and ZQ-high groups compared with the vehicle group (Figure 5A) and the protein expression of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in the liver tissue was markedly decreased in the ZQ-high group (Figure 5B). The relative phosphorylation levels of ERK, JNK, and p38 were obviously decreased in all of the Zi Qi groups, which indicated that the Zi Qi decoction can suppress either MAPK pathway (Figure 5C). All these data suggest that the results of the animal experiments were consistent with those from the cell experiments.
Discussion
Liver fibrosis is the early stage of cirrhosis and the annual incidence in the adult population worldwide is 0.3% to 0.6% [28,29]. How to prevent liver fibrosis and slow the process of cirrhosis has become an urgent problem for clinicians in modern society. In ancient times, doctors of TCM put forward the important principle of Superior Treatment Before Sickness, which includes prevention before disease and prevention after disease. For liver fibrosis, prevention of progression after the disease is diagnosed is the most important part of clinical practice. In the present study, the severity of hepatic fibrosis was alleviated or even reversed (Figure 3), which postponed the progression into liver cirrhosis or even hepatocellular carcinoma to some extent, embodying the theory of Superior Treatment Before Sickness of TCM. Great progress has been made in the prevention and treatment of liver fibrosis with TCM in recent years. Our previous studies demonstrated that the Zi Qi decoction could reduce the inflammatory response and relieve liver fibrosis, delaying its progression into cirrhosis [17].
In the present study, serological indicators reflecting liver injury (ALT, AST, and GGT) and liver inflammation (IL-6 and TNF-α) were significantly downregulated after Zi Qi decoction treatment in rats with hepatic fibrosis (Figure 3A–3I). We speculate that the Zi Qi decoction has the potential to ameliorate liver fibrosis by alleviating liver injury and inflammation. To better manifest early morphological changes in a rat liver model, advanced 7.0T micro-MRI was used in the present study. As was shown in our data, the ADC value was negatively correlated with the severity of hepatic fibrosis and the disordered blood vessels in the liver, small gridded structure, and dilated portal vein were alleviated in the liver sections after the intervention with the Zi Qi decoction (Figure 3G, 3H). Because liver MRI has been increasingly and widely used in the diagnosis of liver disease and quantitative MRI has the potential to characterize liver fibrosis [30], a PD-T2 image was selected to detect the degree of fibrosis and the ADC value was calculated based on a relative diffusion-weighted image. The pathological findings (Figure 3I) also were consistent with the previously described data from micro-MRI, which indicate that this innovative technology is better suited for identification of mild structural changes in rats.
Activation of HSCs is very important to the formation of liver fibrosis, and LPS, the specific ligand of TLR4, is also associated with hepatic fibrogenesis through direct interactions with HSCs [10]. Once identified, 2 representative downstream pathways of TLR4 that are closely related to inflammatory processes and immunoreactions – NF-κB and MAPK – were activated and subsequently influences the activation of HSCs [11]. In the present study, Zi Qi decoction suppressed collagen deposition (Figures 1, 4) and inhibited the TLR4/NF-κB pathway in vivo and in vitro. Moreover, TAK-242 suppressed TLR4 and downstream NF-κB signaling but PDTC had no significant effect on the recovery of TLR4/NF-κB signaling (Figures 2A–2C, 5A, 5B). TLR4 downstream MAPK signaling also was downregulated by treatment with the Zi Qi decoction, in vivo and in vitro (Figures 2D, 5C). These data suggest that the Zi Qi decoction has a TLR4-inhibitor-like effect on alleviating liver fibrosis. More importantly, this kind of effect was characterized with a multiple-target-selective feature, which reflects the unique advantages of TCM in the treatment of liver fibrosis.
Conclusions
Taken together, our results demonstrated that the Zi Qi decoction alleviated hepatic fibrosis by suppressing the activation of HSCs via inhibition of the TLR4-related NF-κB and MAPK signaling pathways. The present study suggests that the Zi Qi decoction may offer new insights into treatment of liver fibrosis, and in the near future, more detailed studies are needed to explore the relationship between the Zi Qi decoction, the intestinal tract, and liver fibrosis.
Figures
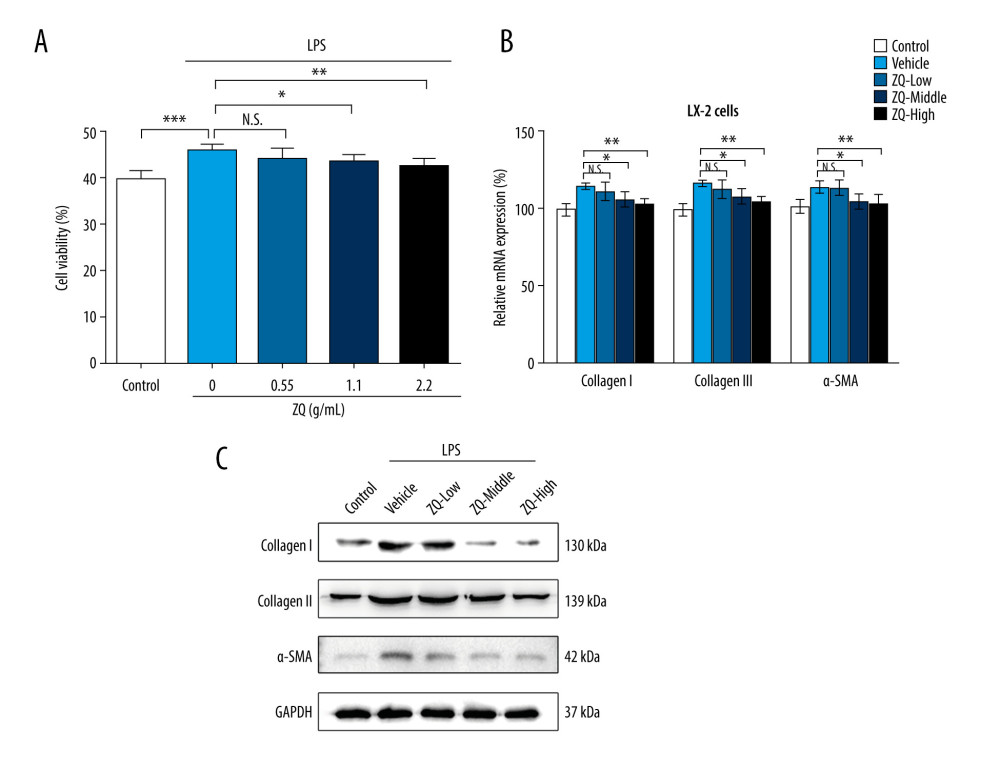 Figure 1. Zi Qi decoction plays a role in preventing activation of hepatic stellate cells. (A) The effects of Zi Qi decoction on cell viability at different concentrations. (B) The effects of Zi Qi decoction on the relative messenger RNA level of cellular Types I and III collagen and α-SMA at different concentrations. (C) The effects of Zi Qi decoction on the protein level of cellular Types I and III collagen and α-SMA at different concentrations. * P<0.05, ** P<0.01, *** P<0.001, compared with the LPS group. Data were presented as means±standard deviations.
Figure 1. Zi Qi decoction plays a role in preventing activation of hepatic stellate cells. (A) The effects of Zi Qi decoction on cell viability at different concentrations. (B) The effects of Zi Qi decoction on the relative messenger RNA level of cellular Types I and III collagen and α-SMA at different concentrations. (C) The effects of Zi Qi decoction on the protein level of cellular Types I and III collagen and α-SMA at different concentrations. * P<0.05, ** P<0.01, *** P<0.001, compared with the LPS group. Data were presented as means±standard deviations. 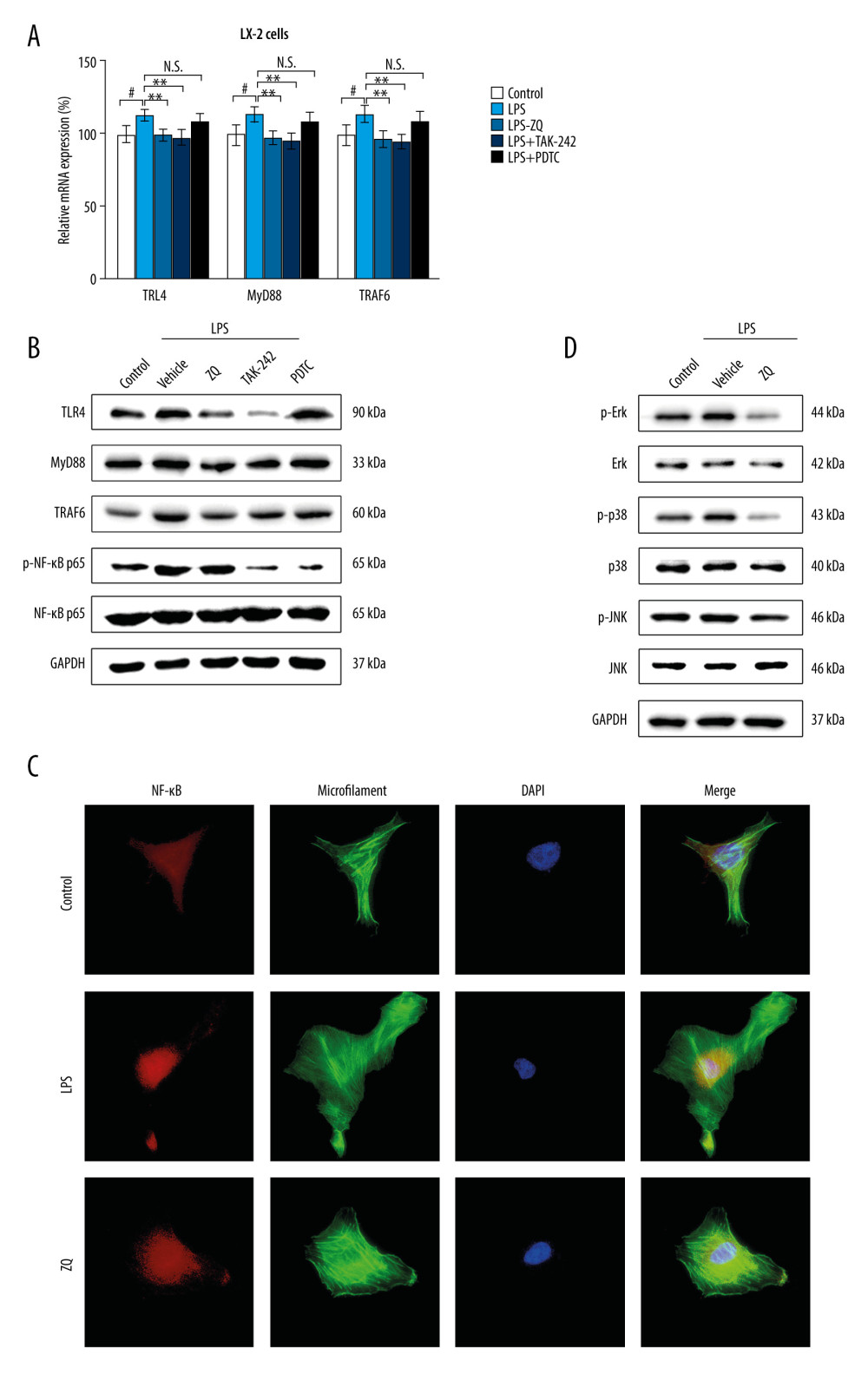 Figure 2. Lipopolysaccharide (LPS)-mediated Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vitro. (A) The effects of Zi Qi decoction on the relative messenger RNA level of cellular TLR4, MyD88, and TRAF6 in each group. (B) The effects of Zi Qi decoction on the protein level of cellular TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in each group. (C) Zi Qi decoction significantly suppressed the nuclear transportation of activated NF-κB p65 in LPS-induced LX-2 cells, as determined by immunofluorescence (n=3). Magnification ×40. (D) The protein expressions of ERK, c-Jun NH2-terminal kinase, and p38 and their relative phosphorylation levels in each group. # P<0.05, compared with the control group. * P<0.05, ** P<0.01, compared with the LPS group. Data were presented as means±standard deviations.
Figure 2. Lipopolysaccharide (LPS)-mediated Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vitro. (A) The effects of Zi Qi decoction on the relative messenger RNA level of cellular TLR4, MyD88, and TRAF6 in each group. (B) The effects of Zi Qi decoction on the protein level of cellular TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in each group. (C) Zi Qi decoction significantly suppressed the nuclear transportation of activated NF-κB p65 in LPS-induced LX-2 cells, as determined by immunofluorescence (n=3). Magnification ×40. (D) The protein expressions of ERK, c-Jun NH2-terminal kinase, and p38 and their relative phosphorylation levels in each group. # P<0.05, compared with the control group. * P<0.05, ** P<0.01, compared with the LPS group. Data were presented as means±standard deviations. 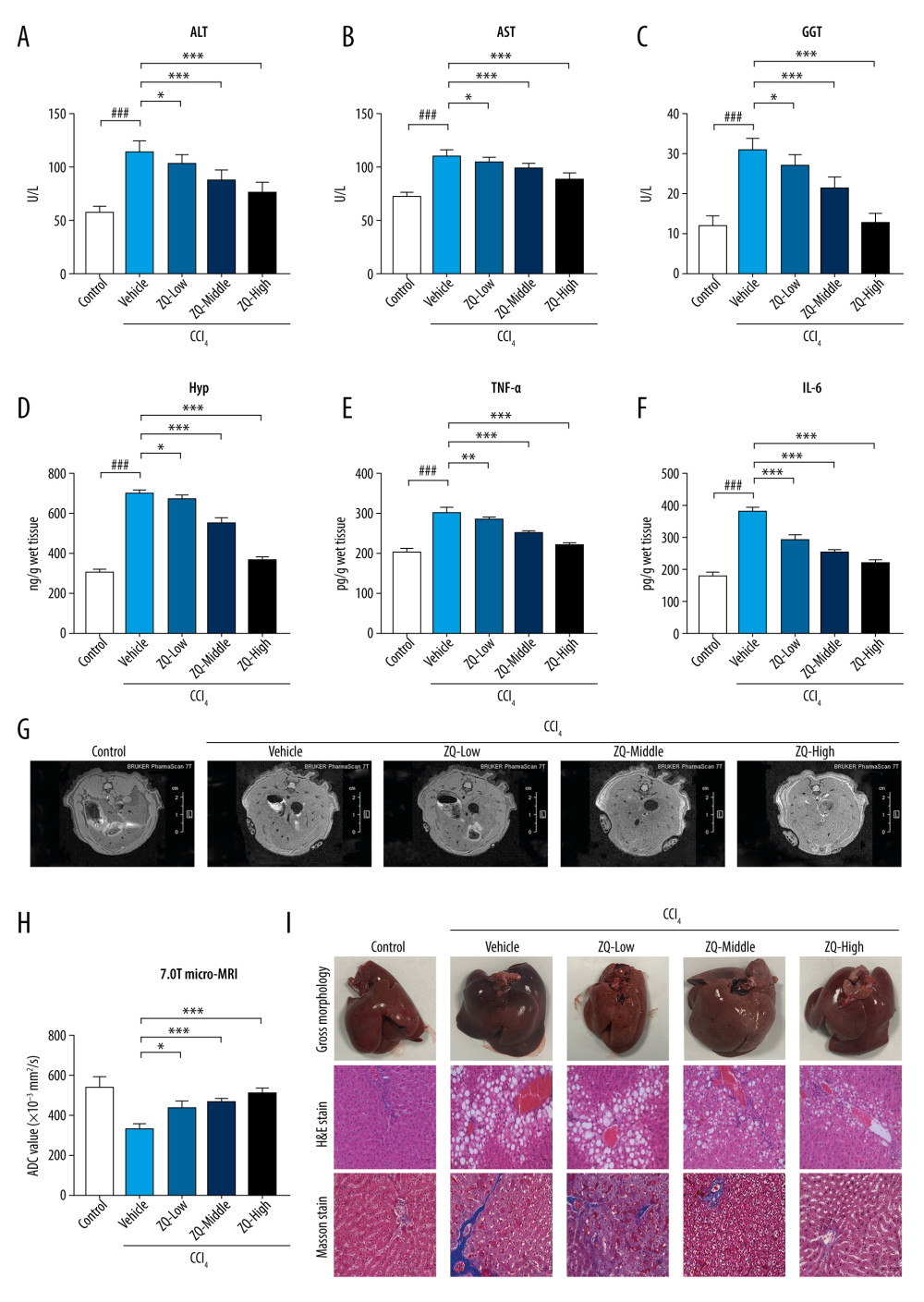 Figure 3. Representative indicators associated with liver fibrosis and Zi Qi decoction effects on macroscopic and microscopic hepatic architecture in CCl4-induced rats. The content of (A) alanine aminotransferase, (B) aspartate aminotransferase, (C) gamma-glutamyl transpeptidase, (D) Hyp, (E) tumor necrosis factor-α, and (F) interleukin-6 in each group were determined by enzyme-linked immunosorbent assay. (G) 7.0T micro-magnetic resonance imaging (micro-MRI) was used to evaluate the severity of hepatic damage of rats. (H) Apparent diffusion coefficient values of each slice of micro-MRI in each group when b value=50 s/mm2. (I) Representative images of macroscopic features (upper panel), hematoxylin & eosin staining (middle panel), and Masson staining (lower panel) of liver tissue from rats with hepatic fibrosis. Magnification ×200. ### P<0.001, * P<0.05, ** P<0.01, *** P<0.001, for comparison with the vehicle group. Data were presented as means±standard deviations.
Figure 3. Representative indicators associated with liver fibrosis and Zi Qi decoction effects on macroscopic and microscopic hepatic architecture in CCl4-induced rats. The content of (A) alanine aminotransferase, (B) aspartate aminotransferase, (C) gamma-glutamyl transpeptidase, (D) Hyp, (E) tumor necrosis factor-α, and (F) interleukin-6 in each group were determined by enzyme-linked immunosorbent assay. (G) 7.0T micro-magnetic resonance imaging (micro-MRI) was used to evaluate the severity of hepatic damage of rats. (H) Apparent diffusion coefficient values of each slice of micro-MRI in each group when b value=50 s/mm2. (I) Representative images of macroscopic features (upper panel), hematoxylin & eosin staining (middle panel), and Masson staining (lower panel) of liver tissue from rats with hepatic fibrosis. Magnification ×200. ### P<0.001, * P<0.05, ** P<0.01, *** P<0.001, for comparison with the vehicle group. Data were presented as means±standard deviations. 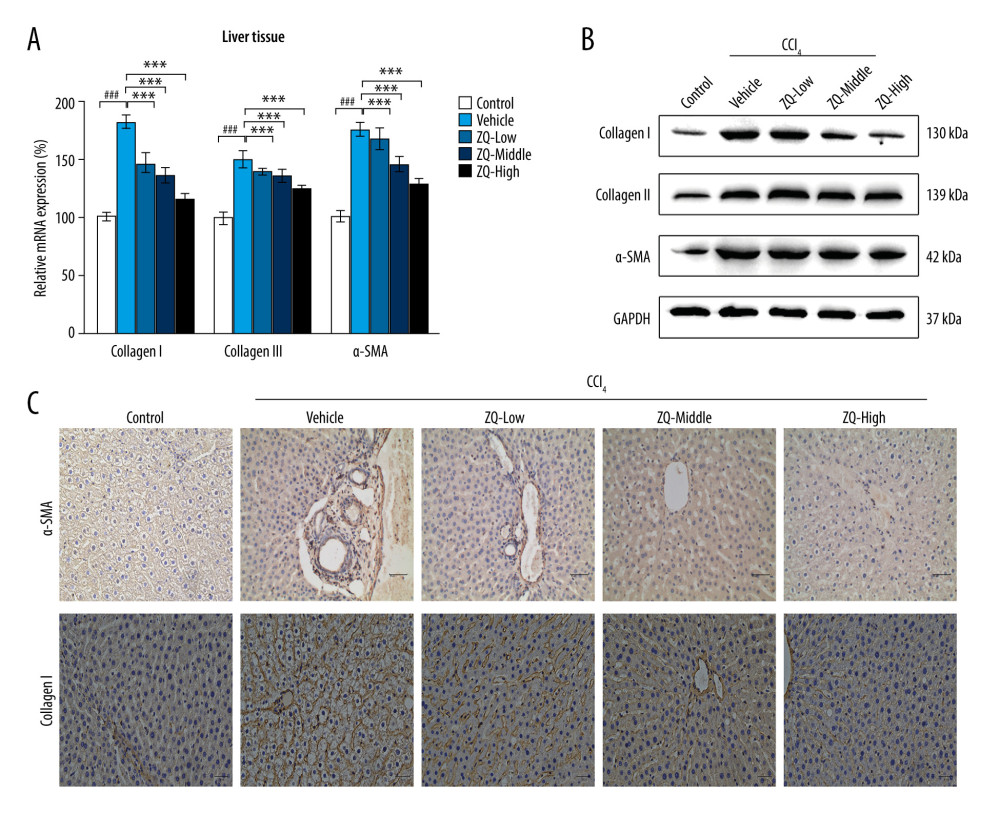 Figure 4. Effects of Zi Qi decoction on collagen deposition in liver tissue. (A) The effects of Zi Qi decoction on the relative messenger RNA level of Types I and III collagen and α-SMA in liver tissue of hepatic fibrosis rats. (B) The effects of Zi Qi decoction on the protein level of Types I and III collagen and α-SMA in liver tissue in each group. (C) Immunohistochemical staining assay of α-SMA and Type I collagen. Magnification ×200. ### P<0.001, compared with the control group. * P<0.05, ** P<0.01, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations.
Figure 4. Effects of Zi Qi decoction on collagen deposition in liver tissue. (A) The effects of Zi Qi decoction on the relative messenger RNA level of Types I and III collagen and α-SMA in liver tissue of hepatic fibrosis rats. (B) The effects of Zi Qi decoction on the protein level of Types I and III collagen and α-SMA in liver tissue in each group. (C) Immunohistochemical staining assay of α-SMA and Type I collagen. Magnification ×200. ### P<0.001, compared with the control group. * P<0.05, ** P<0.01, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations. 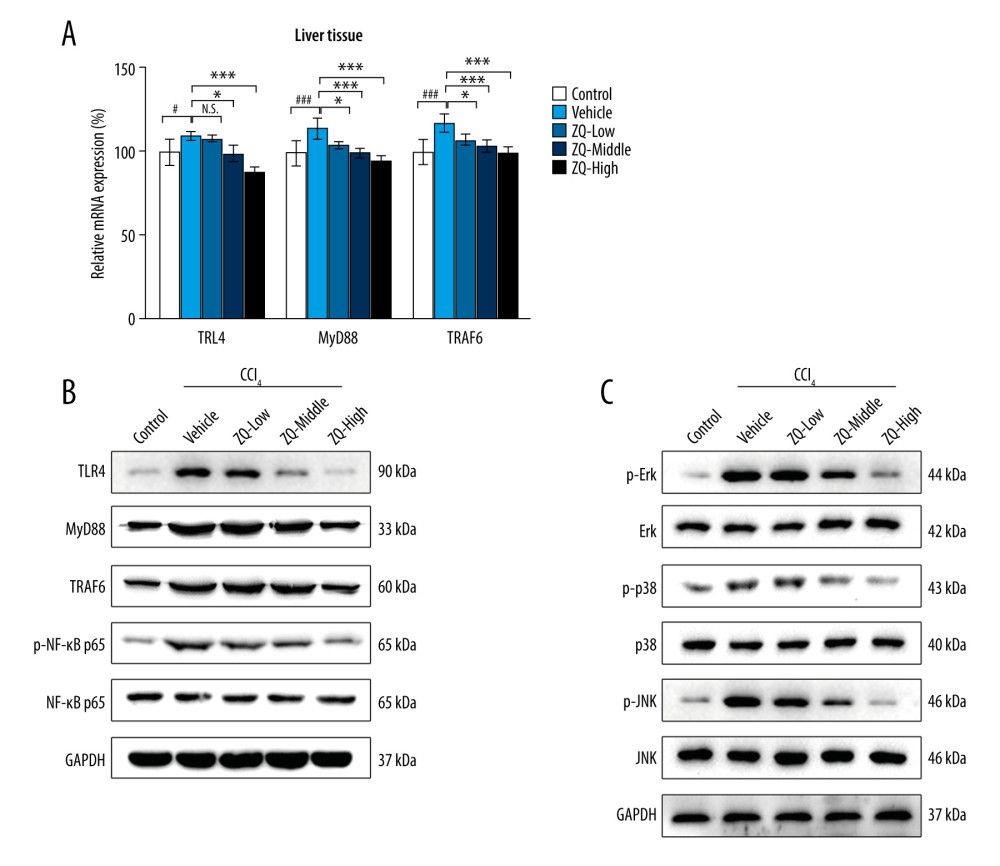 Figure 5. Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vivo. (A) Messenger RNA expression of TLR4, MyD88, and TRAF6 in liver tissue in each group. (B) Protein expression of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in liver tissue in each group. (C) Expression of MAPK pathway-related proteins and their relative phosphorylation levels in liver tissue in each group. # P<0.05, compared with the control group. * P<0.05, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations.
Figure 5. Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vivo. (A) Messenger RNA expression of TLR4, MyD88, and TRAF6 in liver tissue in each group. (B) Protein expression of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in liver tissue in each group. (C) Expression of MAPK pathway-related proteins and their relative phosphorylation levels in liver tissue in each group. # P<0.05, compared with the control group. * P<0.05, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations. References
1. Dewidar B, Meyer C, Dooley S, TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019: Cells, 2019; 8(11); 1419
2. Gan F, Liu Q, Liu Y, Lycium barbarum polysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-κB signaling pathway expression in wistar rats: Life Sci, 2018; 192; 205-12
3. Chiu YS, Wei CC, Lin YJ, IL-20 and IL-20R1 antibodies protect against liver fibrosis: Hepatology, 2014; 60(3); 1003-14
4. Schuppan D, Liver fibrosis: Common mechanisms and antifibrotic therapies: Clin Res Hepatol Gastroenterol, 2015; 39(Suppl 1); S51-59
5. Cummins CB, Wang X, Sommerhalder C, Natural compound oridonin inhibits endotoxin-induced inflammatory response of activated hepatic stellate cells: Biomed Res Int, 2018; 2018 6137420
6. Ding N, Hah N, Yu RT, BRD4 is a novel therapeutic target for liver fibrosis: Proc Natl Acad Sci USA, 2015; 112(51); 15713-18
7. Brenner DA, Reversibility of liver fibrosis: Gastroenterol Hepatol (NY), 2013; 9(11); 737-79
8. Wynn TA, Cellular and molecular mechanisms of fibrosis: J Pathol, 2008; 214(2); 199-210
9. Chen M, Liu J, Yang W, Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling: Autophagy, 2017; 13(11); 1813-27
10. Fouts DE, Torralba M, Nelson KE, Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease: J Hepatol, 2012; 56(6); 1283-92
11. Ikezoe T, Yang Y, Bandobashi K, Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-kappa B signal pathways: Mol Cancer Ther, 2005; 4(4); 578-86
12. Luedde T, Schwabe RF, NF-kappaB in the liver – linking injury, fibrosis and hepatocellular carcinoma: Nat Rev Gastroenterol Hepatol, 2011; 8(2); 108-18
13. de Oliveira RG, de Campos Castilho GR, da Cunha AL: J Ethnopharmacol, 2017; 202; 127-37
14. Zhang WB, Yang F, Wang Y, Inhibition of HDAC6 attenuates LPS-induced inflammation in macrophages by regulating oxidative stress and suppressing the TLR4-MAPK/NF-kappaB pathways: Biomed Pharmacother, 2019; 117; 109166
15. Cai FF, Bian YQ, Wu R, Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis: Biomed Pharmacother, 2019; 114; 108863
16. Li H, Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula: J Ethnopharmacol, 2020; 251; 112442
17. Xu JM, Xiao L, Huang JH, Zi Qi containing serum induced hepatic stellate cell proliferation after macrophage conditioned medium activation impact: Hepatol Digest Integrated Chinese Western Med, 2014; 24; 37-40
18. Yanguas SC, Cogliati B, Willebrords J, Experimental models of liver fibrosis: Arch Toxicol, 2016; 90(5); 1025-48
19. Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases: Hepatol Int, 2010; 4(4); 659-72
20. Zhao HW, Zhang ZF, Chai X, Oxymatrine attenuates CCl4-induced hepatic fibrosis via modulation of TLR4-dependent inflammatory and TGF-beta1 signaling pathways: Int Immunopharmacol, 2016; 36; 249-55
21. Wang Y, Zhang X, Tian J, Talin promotes integrin activation accompanied by generation of tension in talin and an increase in osmotic pressure in neurite outgrowth: FASEB J, 2019; 33(5); 6311-26
22. Zhang X, Li G, Guo Y, Regulation of ezrin tension by S-nitrosylation mediates non-small cell lung cancer invasion and metastasis: Theranostics, 2019; 9(9); 2555-71
23. Liu S, Khemlani LS, Shapiro RA, Expression of CD14 by hepatocytes: Upregulation by cytokines during endotoxemia: Infect Immun, 1998; 66(11); 5089-98
24. Tosello-Trampont AC, Landes SG, Nguyen V, Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production: J Biol Chem, 2012; 287(48); 40161-72
25. Sato T, Asanuma Y, Masaki Y, Changes in tumor necrosis factor-α and interleukin-1 beta production following liver surgery on cirrhotic patients: Hepatogastroenterol, 1996; 43(11); 1148-53
26. Seki E, De Minicis S, Osterreicher CH, TLR4 enhances TGF-beta signaling and hepatic fibrosis: Nat Med, 2007; 13(11); 1324-32
27. Grinko I, Geerts A, Wisse E, Experimental biliary fibrosis correlates with increased numbers of fat-storing and Kupffer cells, and portal endotoxemia: J Hepatol, 1995; 23(4); 449-58
28. Blachier M, Leleu H, Peck-Radosavljevic M, The burden of liver disease in Europe: A review of available epidemiological data: J Hepatol, 2013; 58(3); 593-608
29. Ganne-Carrie N, Epidemiology of liver cirrhosis: Rev Prat, 2017; 67(7); 726-30
30. Zhang H, Yang Q, Yu T, Comparison of T2, T1rho, and diffusion metrics in assessment of liver fibrosis in rats: J Magn Reson Imaging, 2017; 45(3); 741-50
Figures
 Figure 1. Zi Qi decoction plays a role in preventing activation of hepatic stellate cells. (A) The effects of Zi Qi decoction on cell viability at different concentrations. (B) The effects of Zi Qi decoction on the relative messenger RNA level of cellular Types I and III collagen and α-SMA at different concentrations. (C) The effects of Zi Qi decoction on the protein level of cellular Types I and III collagen and α-SMA at different concentrations. * P<0.05, ** P<0.01, *** P<0.001, compared with the LPS group. Data were presented as means±standard deviations.
Figure 1. Zi Qi decoction plays a role in preventing activation of hepatic stellate cells. (A) The effects of Zi Qi decoction on cell viability at different concentrations. (B) The effects of Zi Qi decoction on the relative messenger RNA level of cellular Types I and III collagen and α-SMA at different concentrations. (C) The effects of Zi Qi decoction on the protein level of cellular Types I and III collagen and α-SMA at different concentrations. * P<0.05, ** P<0.01, *** P<0.001, compared with the LPS group. Data were presented as means±standard deviations. Figure 2. Lipopolysaccharide (LPS)-mediated Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vitro. (A) The effects of Zi Qi decoction on the relative messenger RNA level of cellular TLR4, MyD88, and TRAF6 in each group. (B) The effects of Zi Qi decoction on the protein level of cellular TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in each group. (C) Zi Qi decoction significantly suppressed the nuclear transportation of activated NF-κB p65 in LPS-induced LX-2 cells, as determined by immunofluorescence (n=3). Magnification ×40. (D) The protein expressions of ERK, c-Jun NH2-terminal kinase, and p38 and their relative phosphorylation levels in each group. # P<0.05, compared with the control group. * P<0.05, ** P<0.01, compared with the LPS group. Data were presented as means±standard deviations.
Figure 2. Lipopolysaccharide (LPS)-mediated Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vitro. (A) The effects of Zi Qi decoction on the relative messenger RNA level of cellular TLR4, MyD88, and TRAF6 in each group. (B) The effects of Zi Qi decoction on the protein level of cellular TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in each group. (C) Zi Qi decoction significantly suppressed the nuclear transportation of activated NF-κB p65 in LPS-induced LX-2 cells, as determined by immunofluorescence (n=3). Magnification ×40. (D) The protein expressions of ERK, c-Jun NH2-terminal kinase, and p38 and their relative phosphorylation levels in each group. # P<0.05, compared with the control group. * P<0.05, ** P<0.01, compared with the LPS group. Data were presented as means±standard deviations. Figure 3. Representative indicators associated with liver fibrosis and Zi Qi decoction effects on macroscopic and microscopic hepatic architecture in CCl4-induced rats. The content of (A) alanine aminotransferase, (B) aspartate aminotransferase, (C) gamma-glutamyl transpeptidase, (D) Hyp, (E) tumor necrosis factor-α, and (F) interleukin-6 in each group were determined by enzyme-linked immunosorbent assay. (G) 7.0T micro-magnetic resonance imaging (micro-MRI) was used to evaluate the severity of hepatic damage of rats. (H) Apparent diffusion coefficient values of each slice of micro-MRI in each group when b value=50 s/mm2. (I) Representative images of macroscopic features (upper panel), hematoxylin & eosin staining (middle panel), and Masson staining (lower panel) of liver tissue from rats with hepatic fibrosis. Magnification ×200. ### P<0.001, * P<0.05, ** P<0.01, *** P<0.001, for comparison with the vehicle group. Data were presented as means±standard deviations.
Figure 3. Representative indicators associated with liver fibrosis and Zi Qi decoction effects on macroscopic and microscopic hepatic architecture in CCl4-induced rats. The content of (A) alanine aminotransferase, (B) aspartate aminotransferase, (C) gamma-glutamyl transpeptidase, (D) Hyp, (E) tumor necrosis factor-α, and (F) interleukin-6 in each group were determined by enzyme-linked immunosorbent assay. (G) 7.0T micro-magnetic resonance imaging (micro-MRI) was used to evaluate the severity of hepatic damage of rats. (H) Apparent diffusion coefficient values of each slice of micro-MRI in each group when b value=50 s/mm2. (I) Representative images of macroscopic features (upper panel), hematoxylin & eosin staining (middle panel), and Masson staining (lower panel) of liver tissue from rats with hepatic fibrosis. Magnification ×200. ### P<0.001, * P<0.05, ** P<0.01, *** P<0.001, for comparison with the vehicle group. Data were presented as means±standard deviations. Figure 4. Effects of Zi Qi decoction on collagen deposition in liver tissue. (A) The effects of Zi Qi decoction on the relative messenger RNA level of Types I and III collagen and α-SMA in liver tissue of hepatic fibrosis rats. (B) The effects of Zi Qi decoction on the protein level of Types I and III collagen and α-SMA in liver tissue in each group. (C) Immunohistochemical staining assay of α-SMA and Type I collagen. Magnification ×200. ### P<0.001, compared with the control group. * P<0.05, ** P<0.01, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations.
Figure 4. Effects of Zi Qi decoction on collagen deposition in liver tissue. (A) The effects of Zi Qi decoction on the relative messenger RNA level of Types I and III collagen and α-SMA in liver tissue of hepatic fibrosis rats. (B) The effects of Zi Qi decoction on the protein level of Types I and III collagen and α-SMA in liver tissue in each group. (C) Immunohistochemical staining assay of α-SMA and Type I collagen. Magnification ×200. ### P<0.001, compared with the control group. * P<0.05, ** P<0.01, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations. Figure 5. Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vivo. (A) Messenger RNA expression of TLR4, MyD88, and TRAF6 in liver tissue in each group. (B) Protein expression of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in liver tissue in each group. (C) Expression of MAPK pathway-related proteins and their relative phosphorylation levels in liver tissue in each group. # P<0.05, compared with the control group. * P<0.05, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations.
Figure 5. Toll-like receptor 4 (TLR4)-related nuclear factor kappa b (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways were inhibited by Zi Qi decoction in vivo. (A) Messenger RNA expression of TLR4, MyD88, and TRAF6 in liver tissue in each group. (B) Protein expression of TLR4, MyD88, TRAF6, and phospho-NF-κB p65 in liver tissue in each group. (C) Expression of MAPK pathway-related proteins and their relative phosphorylation levels in liver tissue in each group. # P<0.05, compared with the control group. * P<0.05, *** P<0.001, compared with the vehicle group. Data were presented as means±standard deviations. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952