10 October 2021: Animal Study
Pretreatment with Methylene Blue Protects Against Acute Seizure and Oxidative Stress in a Kainic Acid-Induced Status Epilepticus Model
Yong-feng Wang1E, Yan Luo2C, Gao-lei Hou1B, Rui-jing He3C, Hao-yun Zhang3F, Yan-Li Yi3D, Ying Zhang3B, Zhi-qiang Cui3AG*DOI: 10.12659/MSM.933469
Med Sci Monit 2021; 27:e933469
Abstract
BACKGROUND: The aim of the present study was to investigate the potential anticonvulsant effect of methylene blue (MB) in a kainic acid (KA)-induced status epilepticus (SE) model. The effects of MB on levels of oxidative stress and glutamate (Glu) also were explored.
MATERIAL AND METHODS: Sixty C57BL/6 mice were randomly divided into 5 equal-sized groups: (1) controls; (2) KA; (3) MB 0.5 mg/kg+KA; (4) MB 1 mg/kg+KA; and (5) vehicle+KA. The SE model was established by intra-amygdala microinjection of KA. Behavioral observations and simultaneous electroencephalographic records of the seizures in different groups were analyzed to determine the potential anticonvulsant effect of MB. The influences of MB on oxidative stress markers and glutamate were also detected to explore the possible mechanism.
RESULTS: MB afforded clear protection against KA-induced acute seizure, as measured by the delayed latency of onset of generalized seizures and SE, decreased percentage of SE, and increased survival rate in mice with acute epilepsy. MB markedly increased the latency to first onset of epileptiform activity and decreased the average duration of epileptiform events, as well as the percentage of time during which the epileptiform activity occurred. Administration of MB prevented KA-induced deterioration of oxidative stress markers and Glu.
CONCLUSIONS: MB is protective against acute seizure in SE. This beneficial effect may be at least partially related to its potent antioxidant ability and influence on Glu level.
Keywords: Kainic Acid, Status epilepticus, Amygdala, Animals, Electrodes, Implanted, Electroencephalography, Glutamic Acid, Hippocampus, Humans, Methylene Blue, Mice, Neuroprotective Agents
Background
Epilepsy is one of the most prevalent neurological disorders. Temporal lobe epilepsy (TLE), the most common form of epilepsy in adults, is characterized by recurrent seizures that arise in the medial structures of the temporal lobe, including the hippocampus and amygdala [1]. The International League Against Epilepsy defines status epilepticus (SE) as seizure activity that is clinical and/or seen on an electroencephalogram (EEG), or recurrent seizure activity without recovery to baseline that lasts ≥5 min [2]. SE is a common neurological emergency with a high mortality rate, and it can trigger epileptogenesis, the process of developing epilepsy. Although existing medications can suppress seizures associated with TLE or SE to a certain degree, in about one-third of patients, they lose effectiveness over time or result in adverse effects [3,4]. Thus, it is imperative to develop alternative therapies to control refractory epilepsy and to prevent epileptogenesis.
Accumulating evidence suggests that disordered excitatory and inhibitory neurotransmitter balance plays a vital role in the pathology of epilepsy. The development of SE is the combined result of excessive release of an excitatory neurotransmitter and/or insufficient release of an inhibitory neurotransmitter [5]. In the nervous system, glutamate (Glu) is the primary excitatory neurotransmitter and gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter. Kainic acid (KA) is an excitatory Glu analog which has been widely used to induce SE and TLE in rodents [6,7]. High levels of extracellular Glu during SE can overstimulate Glu receptors, leading to increased influx of calcium, and ultimately, to neuronal cell death, which is referred to as neuro-excitotoxicity. Glu-mediated excitotoxicity can induce severe oxidative stress, result in excessive accumulation of free radicals, and give rise to an imbalance in endogenous free radicals and antioxidant enzymes, which further promotes epileptic seizures [8]. Therefore, alleviating injury associated with oxidative stress will be protective against epilepsy [9]. Malondialdehyde (MDA) is the final product of lipid peroxidation and its level reflects the state of free radicals. Free radical scavengers, such as superoxide dismutase (SOD) and reduced glutathione (GSH), could protect against oxidative stress injury [10]. Previous clinical research has demonstrated that patients with SE had significantly lower concentrations of SOD and GSH and higher concentrations of MDA compared to controls [11]. KA triggered a decrease in SOD activity and increase in MDA concentration [12]. Therefore, we speculate that a large amount of MDA is produced and the activities of SOD and GSH are weakened during SE induced by KA, resulting in a serious oxidative stress response, a process that may be alleviated or even reversed by antioxidants.
Methylene blue (MB) is a water-soluble thiophenazine compound that functions as a powerful antioxidant. Figure 1 shows the chemical structure and redox balance of MB [13]. It can reduce oxidative damage to the body by removing reactive oxygen free radicals from tissue in the brain [14–17]. MB already has been shown to exert a nerve-protective effect in ischemic brain damage, Leber optic neuropathy, Alzheimer disease, Parkinson disease, and other neurodegenerative diseases [18–20]. Therefore, in recent years, the role of MB in treatment of various central nervous system (CNS) diseases has generated widespread concern. MB has been confirmed to prevent methylmalonate-induced seizures on EEG and oxidative damage in rat striatum [17]. However, to the best of our knowledge, there has been no definitive evidence of the anticonvulsive effects of MB in a KA-induced acute seizure model until now. Based on favorable outcomes in other CNS diseases, we hypothesized that MB would protect against acute behavioral seizure and oxidative stress during SE. Hence, the aim of the present study was to investigate the efficacy of MB in alleviating deleterious effects of KA seen in animal behavior and on EEG. Its effects on the levels of oxidative stress and Glu were also explored.
Material and Methods
ANIMALS AND REAGENTS:
Clean-grade adult male C57BL/6 mice that weighed 25 to 30 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license No. 11400700208619). They were housed in a group (maximum of 5 mice per cage) at a constant temperature (25+2°C), provided a fixed 12-h alternating cycle of light and dark (light on at 08: 00), and allowed free access to food and water. All the animal experiment procedures complied with the guidelines for laboratory animal management issued by the Ministry of Science and Technology of the People’s Republic of China [1988] No. 134, which were in accordance with internationally recognized guidelines from the National Institutes of Health. Great efforts were devoted to minimizing the number of animals and decreasing their pain.
MB and KA were purchased from Qichuan Pharmaceutical Group Co., Ltd. (Jiangsu, China) and Sigma (St. Louis, Missouri, United States), respectively. The detection kits for GSH, SOD, and MDA were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). MB and KA were both dissolved in 100 mM of buffered phosphate (PBS, pH=7.4).
EXPERIMENTAL PROCEDURE:
Mice were randomly divided into 5 groups of 12 mice each. The controls received saline intra-amygdala microinjection. The KA group received a KA intra-amygdala microinjection. The 0.5 mg/kg+KA group received 0.5 mg/kg of MB intraperitoneally (i.p.) 30 min before KA injection. The 1 mg/kg+KA group received 1 mg/kg of MB 30 min before KA injection. The vehicle+KA group received an i.p. injection of saline 30 min before KA injection.
Figure 2 is a schematic overview of the configuration of the guider cannula for KA injection and EEG recording. Briefly, the mice were deeply anesthetized with pentobarbital (45 mg/kg, i.p.) and mounted on a stereotaxic apparatus (RWD Life Science, China). Following the Mouse Brain in Stereotaxic Coordinates of Paxinos and Franklin (2nd edition), a guider cannula (RWD Life Science, China) was implanted in the right amygdala. Three stainless-steel screws that touched the cortex and were used for EEG recording also were implanted. Two other screws were used to affix the dental cement. All the electrodes were connected to a miniature receptacle and attached to the skull of the mice with the guider cannula and screws using dental acrylic cement. After surgery, the animals were housed individually and were allowed to recover for at least 10 days. On the day of the experiment, the guider stylet was removed and a stainless-steel injection cannula was placed 1 mm below the tip of the guider cannula. KA, at a dose of 0.2 μL (1.5 μg/μL), was injected into the amygdala through the cannula. The injection, which was done using a 5-μL Hamilton microsyringe, continued for 1 min and the needle was kept in for another minute. The mice in the control group received an injection with an equal amount of saline following the same procedure.
BEHAVIORAL EVALUATION OF SEIZURE PROGRESSION:
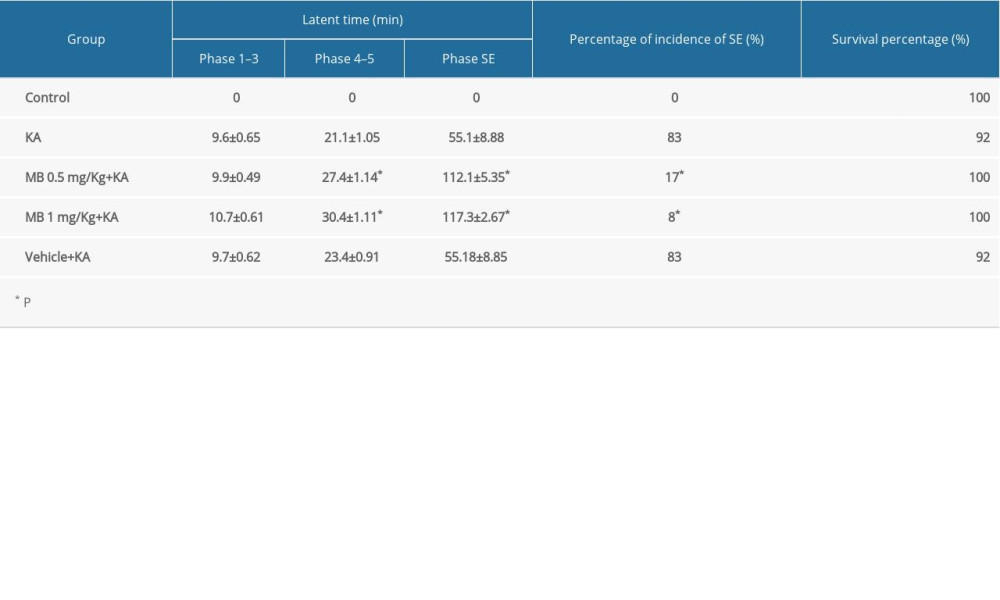
After administration of KA, mouse behavior was observed for 3 h. Seizure activity was assessed according to Racine’s classification [21]: phase 1, facial clonus; phase 2, nodding; phase 3, forelimb clonus; phase 4, forelimb clonus with rearing; phase 5, rearing, jumping, and falling. Phases 1 to 3 and 4 to 5 were considered focal seizures and generalized seizures, respectively [22]. Mice that reached phases 4 to 5 and maintained that level for at least 30 min were considered to have experienced SE. The latent time to reach each seizure phase including SE, the proportion of occurrence of SE, and the survival rates of the mice were recorded and used to quantitatively analyze seizure progression.
EEG RECORDING AND DATA ANALYSIS:
The animals were continuously monitored for 2 h following administration of KA with a kg KA PowerLab video EEG system (AD instruments, Australia). EEG recordings were sampled at 1 kHz using a 1000× gain preamplifier (bandpass-filtered 1–500 Hz) and analyzed by 2 individuals who were blinded to the experimental conditions. As described in the literature [23], the beginning of a seizure was defined as the time at which the amplitude of paroxysmal activity exceeded at least twice the standard error of mean (SEM) baseline amplitude. Seizure latency was defined as the time elapsed from administration of KA to the beginning of the first seizure recorded on EEG. The average duration of epileptiform activity was the average of the duration of each episode of epileptiform activity, including both rhythmic spiking activity and seizures observed during the full recording period. The total time showing epileptiform activity (% epileptiform activity) was calculated as the cumulative time spent in seizures during a 120-min recording period divided by 120 min. The durations of separate EEG events were determined from the start of the repetitive EEG pattern until the return to baseline. The average duration of the EEG events observed over 2 h was defined as the average seizure duration.
SAMPLE PROCESSING FOR ASSESSMENT OF MDA/SOD/GSH AND GLU/GABA:
Immediately after the behavioral evaluation, the animals were anesthetized and sacrificed. The left hippocampus was quickly collected and frozen in liquid nitrogen. Then, the tissues were stored at −80°C for subsequent detection. For all of the experimental procedures, the hippocampus tissue was prepared as a 10% tissue homogenate in an ice-cold 0.9% saline solution. After centrifugation (3500 rpm, 4°C, 15 min), the supernatant was collected to detect the content of MDA, SOD, and GSH using corresponding detection kits and following the manufacturer’s instructions. As previously reported [24,25], spectrophotometric measurement was performed at 532 nm, 550 nm, and 412 nm, respectively.
The same supernatant also was used to detect levels of Glu and GABA with liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS consisted of an Agilent 1200 liquid chromatography system (United States) equipped with a quaternary solvent delivery system, an autosampler, and a column compartment and a 3200 QTRAPTM system (Applied Biosystems, Foster City, California, United States) with a hybrid triple quadrupole linear ion trap mass spectrometer equipped with Turbo V sources and a Turbolon Spray interface [26].
STATISTICAL ANALYSIS:
Results from the experiment were presented as the mean±SEM. All statistical analyses were conducted using SPSS 22.0 Statistics Software for Windows (SPSS, Inc., Chicago, Illinois, United States). Means among multiple groups were compared with 1-way analysis of variance followed by Student-Newman-Keuls post hoc tests for different pair-wise comparisons. The number of animals that experienced SE and survived were calculated as percentages (percentage SE and percentage survival, respectively), and compared using a chi-square test.
Results
MB PRETREATMENT RELIEVES BEHAVIOR-RELATED EVENTS OF SE:
Ten minutes before saline or MB administration, normal behavior was observed in every group. After saline solution/MB administration, observation continued for another 30 min and there was no significant change in behavior. As shown in Table 1, animals in the KA group had progressive convulsive behavior and in 83% of them, it progressed to the SE phase; 92% survived 3 h after KA administration. Although there was a nonsignificant delay in the onset of focal seizure, MB pretreatment significantly delayed the onset of generalized seizures and SE (P<0.05, compared with KA/vehicle+KA group). MB pretreatment also reduced the incidence of SE and improved the survival rate of the animals. The inhibition effect of 0.5 mg/kg MB on acute seizure progression was equal to the effect of 1 mg/kg. None of the mice in the control group had seizure activity and there was no difference in seizure onset between the KA and vehicle+KA groups.
MB PRETREATMENT INCREASES LATENCY TO SE IN EEG AND DECREASES EPILEPTIFORM ACTIVITY OF SE:
Representative EEG patterns during SE are shown in Figure 3. Pattern A EEG activity fist emerged after KA administration (Figure 3A). As seizure severity increased, several other EEG patterns occurred (Figure 3B–3D), although patterns B to D were not temporally correlated with specific epileptic behavior. Complete representative traces from EEG recording results are shown in Figure 4. In vehicle-pretreated controls, KA administration resulted in uninterrupted seizures on EEG that persisted throughout the recording period. However, i.p. injection of both 0.5 and 1 mg/kg MB (Figure 4A) was effective in reducing epileptiform activity. Statistical analyses revealed that MB pretreatment augmented the latency to the first onset of epileptiform activity compared with that in the vehicle-pretreated mice (Figure 4B, P<0.05). During the 2 h after KA administration, MB pretreatment also reduced the average duration of epileptiform events (Figure 4C, P<0.05) and the percentage of time exhibiting epileptiform activity (Figure 4D, P<0.05). These effects were dose-dependent and the prophylactic effect of 1 mg/kg MB was much better than the effect of 0.5 mg/kg (Figure 4C, 4D, P<0.05).
MB PRETREATMENT ALLEVIATES KA-INDUCED DETERIORATION TRENDS IN OXIDATIVE STRESS MARKERS:
As shown in Figure 5, the level of MDA in the KA group increased significantly 3 h after KA administration compared with the control group (Figure 5A, P<0.05), whereas pretreatment with MB significantly weakened the increase in MDA in acute epileptic mice (P<0.05). Unlike MDA, SOD activity decreased significantly 3 h after KA administration compared with the control group (Figure 5B, P<0.05), and pretreatment with MB inhibited the declining trend in SOD activity (P<0.05). The variation in the GSH trend was the same pattern as that for SOD, which decreased after KA administration, and pretreatment with MB prevented the decreasing trend (Figure 5C, P<0.05). In addition, the inhibitory effects of MB on changes in nonoxidative stress markers were dose-dependent. In acute epileptic mice, the effect was markedly greater with 1 mg/kg than with 0.5 mg/kg of MB (Figure 5A–5C). Regarding the levels of MDA, SOD, and GSH, there was no significant difference between the vehicle+KA and KA groups.
MB PRETREATMENT ATTENUATES EXCESSIVE RELEASE OF GLU AND UPWARD TRENDING OF GLU/GABA DURING SE:
As shown in Figure 6A, compared with the control group, the levels of Glu (μg/g wet tissue) were increased significantly in the other 4 groups 3 h after treatment with KA (P<0.05). There was no difference in Glu between the KA and vehicle+KA groups (P>0.05). Compared with the KA and vehicle+KA groups, the level of Glu in the KA+MB 0.5 mg/kg group showed a declining trend, but there were no significant differences between them (P>0.05). However, the level of Glu in the KA+MB 1 mg/kg group was much lower than that in the other 3 KA-treated groups (P<0.05). The results suggested that MB pretreatment significantly lowered the level of Glu 3 h after KA administration. As shown in Figure 6B, there was no significant difference in the levels of GABA (μg/g wet tissue) in the groups (P>0.05), although the level of GABA in the KA+MB 1 mg/kg group showed an upward trend compared with the other 4 groups. Of note is that the level of Glu/GABA had a change in pattern similar to that for Glu (Figure 6A, 6C) and it was much lower in the KA+MB 1 mg/kg group than in the other 3 KA-treated groups (Figure 6C, P<0.05). These results indicate that MB pretreatment could attenuate excessive release of Glu and upward trending of Glu/GABA during an acute seizure.
Discussion
One of the major obstacles to management of SE is the unsatisfactory effect of the antiepileptic drugs currently being used. Therefore, there is an urgent need to identify alternative treatments that can efficiently control acute seizure and degeneration of neurons. In the present study, we investigated the possible anticonvulsant effects of MB on behavioral, EEG, and neurochemical alterations elicited by KA. We have confirmed for the first time that: (1) MB pretreatment affords clear protection against KA-induced acute seizure, which was measured by the delayed latency to onset of generalized seizures and SE, decreased percentage of SE, and increased the rate of in mice with acute epilepsy; (2) MB pretreatment markedly increased the latency to first onset of epileptiform activity and decreased the average duration of epileptiform events as well as the percentage of time exhibiting epileptiform activity; and (3) KA-induced trends in deterioration of oxidative stress markers and Glu can be prevented by pretreatment with MB.
Animal models of epilepsy have shown significant value in exploring basic mechanisms underlying epileptogenesis and the development of therapeutic substitution [27]. One of the most mature models is for KA. In the present study, we studied the anticonvulsant effect of KA in a KA-induced SE model. We used both behavioral observations and simultaneous EEG records of seizures because behavioral observations alone are not a reliable way to evaluate the efficacy of anticonvulsant treatments [28]. The combination of behavioral observations and simultaneous EEG records of seizures more accurately reflects the anticonvulsant effect of MB. Our previous study found that MB had a protective effect in a model of SE induced by prolonged stimulation of the basolateral amygdala [29]. These findings, combined with results from the present study, clearly show that MB pretreatment exerts anticonvulsant effects on acute seizure induced by prolonged stimulus or KA injection into the amygdala. It should be noted that the results do not mean that MB is effective for other types of epileptic seizures, such as those induced by 4-aminopyridine or bicuculine.
In clinical practice, MB has been used for many years to facilitate memory [30–33] and promote neuroprotection because of its potency in enhancing metabolism and as an antioxidant [34]. Because MS has strong lipophilic properties, it can rapidly cross the blood-brain barrier and easily accumulate in brain tissue to exert a direct neuroprotective effect [16,18,35]. The potential antioxidant effect of MB has been confirmed in several clinical and preclinical studies [17,36,37]. In a model of cerebral ischemia reperfusion injury, MB was shown to reduce reactive oxygen species (ROS) and oxidative stress injury by altering transmission of electrons in the respiratory chain [38]. The mechanism for electron transfer to oxygen is due to a pivotal chemical property of MB, that is, the capacity for autooxidation (Figure 1). MB can accept electrons from an electron transport donor and, in turn, transfer electrons to oxygen to form water. Evidence from several studies suggests that MB may be a scavenger of free radicals by acting as an alternative electron acceptor for tissue oxidases [39,40]. In KA-induced SE, Glu-mediated excitotoxicity causes overproduction of ROS and lipid peroxidation and decreases the activity of antioxidant enzymes [41]. Consistent with previous research, our results also showed that KA-treated mice had an increased level of MDA and a decreased level of SOD [10]. MB alters trends in deterioration of these biomarkers during SE, which may be related to its forceful antioxidative ability. Compared with low doses of MB, high doses result in better effects, possibly because targets more sensitive to oxidative damage require additional antioxidant protection [17]. Certainly, inflammatory processes and injuries are known to occur in KA-induced seizure [42,43], but whether MB is capable of reducing inflammation needs to be verified in our future research.
MB also may influence the glutamatergic system. In rat hippocampal slices, Glu-mediated synaptic transmission was inhibited by relatively high levels of MB [44]. In patients with Alzheimer disease, a favorable effect on cognitive functions was observed after treatment with MB, partly due to the influence of MB on the glutamatergic system [45]. In addition, MB has been suggested to effectively reduce S-nitroso-N-acetyl penicillamine-mediated Glu release. In our study, we found that MB pretreatment led to decreased levels of Glu and Glu/GABA, which were accompanied by a significant reduction in severity of KA-induced SE. Therefore, we believe that reduced production of Glu is involved in the anticonvulsant effect of MB, although it is difficult to determine whether that is a specific effect of MB or a non-specific effect of attenuated seizure activity. The potential mechanism accounting for it still needs to be explored.
One of the potential limitations of the present study may be the short period of EEG recording and relatively short period of behavioral observation after administration of KA. In future studies, telemetry could be used to extend EEG recording and explore longer-term outcomes related to the anticonvulsant and neuroprotective effects of MB. Another limitation is that previous studies demonstrated that a 4-mg/kg dose of MB was the most reliable for enhancing memory and it significantly improved both long-term behavioral habituation and object memory recognition [32]. However, we used 2 doses of MB in the present study, which were both lower than 4 mg/kg. Thus, it would be meaningful to perform a dose-response curve with different non-neurotoxic concentrations of MB to determine whether MB at higher concentrations would produce a better anticonvulsant effect in acute seizures.
Conclusions
Our results suggest that MB is able to protect against acute seizure induced by KA, but not all seizures. At least in part, this beneficial effect may be related to its potent antioxidant ability and influence on the Glu level. These results suggest that further studies are warranted to determine whether MB is a viable adjuvant therapy for preventing and/or treating epilepsy in humans.
Figures
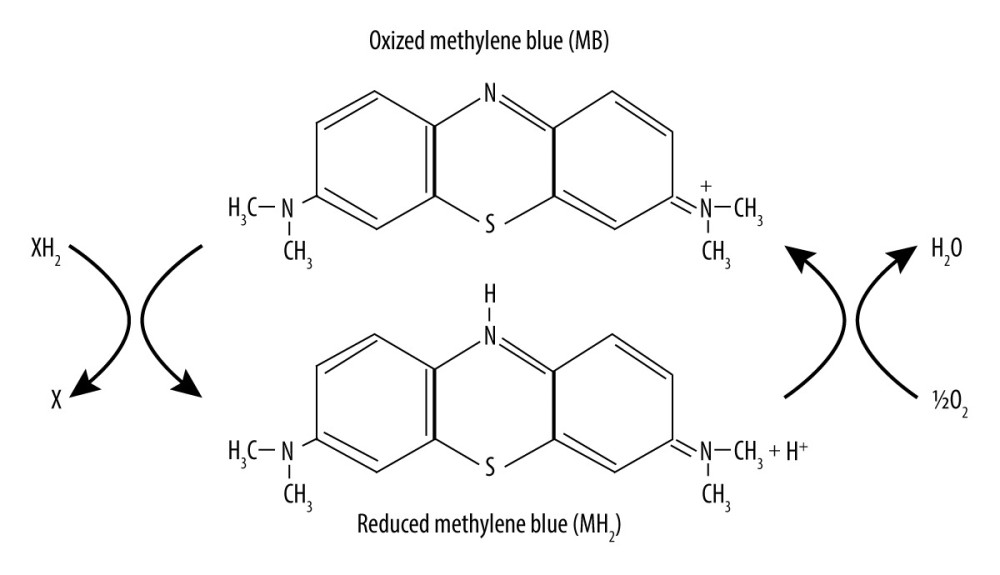 Figure 1. Chemical structure and redox balance of methylene blue (MB). MB has powerful autoxidizable properties. It can accept electrons from an electron donor in its oxidized form and acts as an electron donor in its reduced form. At low concentrations in vivo, the 2 forms of MB are at equilibrium, forming a reversible reduction-oxidation system.
Figure 1. Chemical structure and redox balance of methylene blue (MB). MB has powerful autoxidizable properties. It can accept electrons from an electron donor in its oxidized form and acts as an electron donor in its reduced form. At low concentrations in vivo, the 2 forms of MB are at equilibrium, forming a reversible reduction-oxidation system. 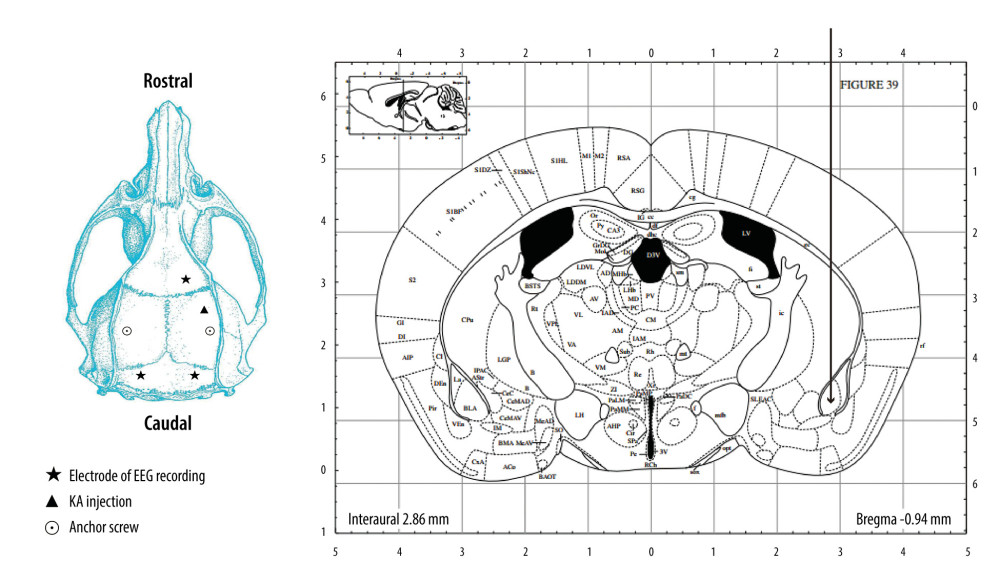 Figure 2. Schematic overview of the configuration of the guider cannula for kainic acid (KA) injection and electroencephalogram (EEG) recording. KA was injected into the right amygdala (anterior −0.94 mm, medial −2.85 mm, dorsal 4.75 mm) (indicated by the arrow) through a guider cannula (indicated by the vertical black line). For EEG recording, a single stainless-steel screw was implanted in the right frontal lobe to be used as a recording electrode. Two screws then were mounted over the left and right occipital lobes to act as an EEG reference and ground electrode, respectively. Two other screws were implanted to affix the dental cement.
Figure 2. Schematic overview of the configuration of the guider cannula for kainic acid (KA) injection and electroencephalogram (EEG) recording. KA was injected into the right amygdala (anterior −0.94 mm, medial −2.85 mm, dorsal 4.75 mm) (indicated by the arrow) through a guider cannula (indicated by the vertical black line). For EEG recording, a single stainless-steel screw was implanted in the right frontal lobe to be used as a recording electrode. Two screws then were mounted over the left and right occipital lobes to act as an EEG reference and ground electrode, respectively. Two other screws were implanted to affix the dental cement. 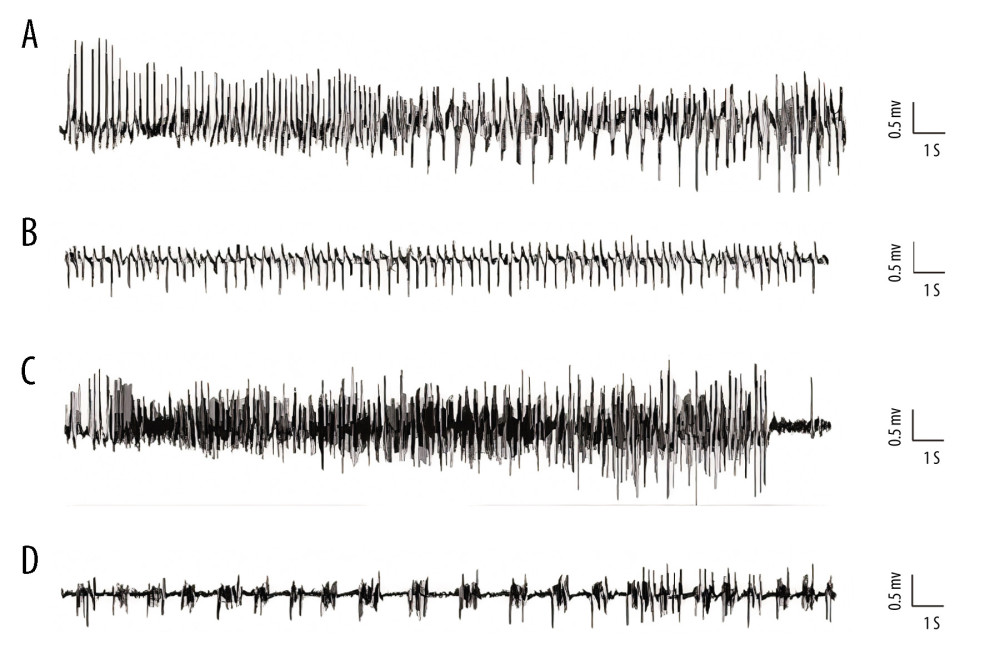 Figure 3. Representative kainic acid-induced seizure patterns on electroencephalogram in the present study. (A) Irregular high-amplitude spikes. (B) periodic epileptiform discharges. (C) High-frequency bursting. (D) A combination of periodic epileptiform discharges and high-frequency bursts of short duration. (Prism 8, version 8.3.0).
Figure 3. Representative kainic acid-induced seizure patterns on electroencephalogram in the present study. (A) Irregular high-amplitude spikes. (B) periodic epileptiform discharges. (C) High-frequency bursting. (D) A combination of periodic epileptiform discharges and high-frequency bursts of short duration. (Prism 8, version 8.3.0). 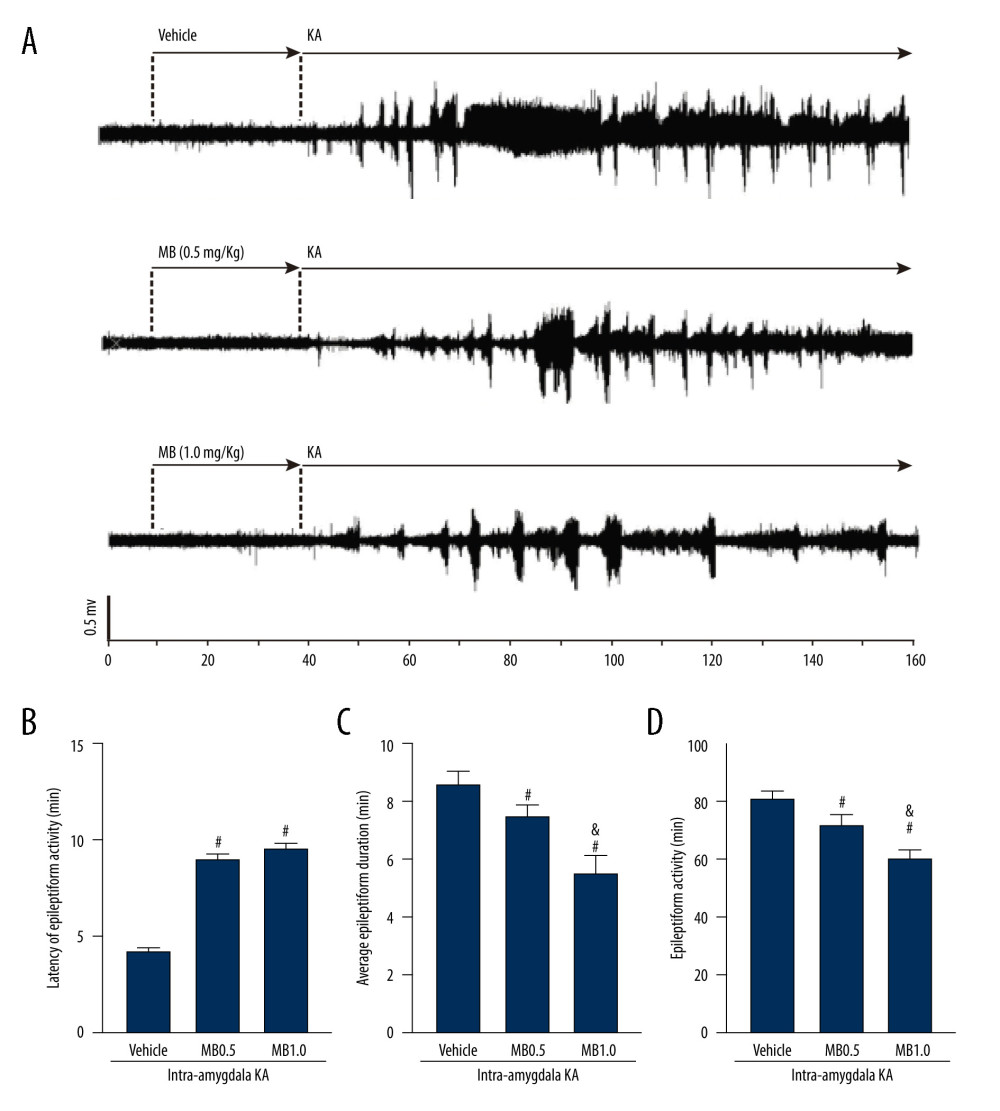 Figure 4. Effect of methylene blue (MB) on kainic acid (KA)-induced epileptiform activity in vivo. (A) Representative tracings showing KA-induced epileptiform activity for the full recording period in vivo in mice pretreated with vehicle, 0.5 mg/kg of MB, and 1 mg/kg of MB, respectively. Electroencephalogram tracings are labeled to indicate drug administration times (vehicle, MB, and KA) accordingly. Histograms showing the latency (minutes) (B), average epileptiform duration (minutes) (C), and epileptiform activity (percentage) (D) of epileptiform activity in KA-treated mice in different group. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group). # P<0.05 vs vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0).
Figure 4. Effect of methylene blue (MB) on kainic acid (KA)-induced epileptiform activity in vivo. (A) Representative tracings showing KA-induced epileptiform activity for the full recording period in vivo in mice pretreated with vehicle, 0.5 mg/kg of MB, and 1 mg/kg of MB, respectively. Electroencephalogram tracings are labeled to indicate drug administration times (vehicle, MB, and KA) accordingly. Histograms showing the latency (minutes) (B), average epileptiform duration (minutes) (C), and epileptiform activity (percentage) (D) of epileptiform activity in KA-treated mice in different group. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group). # P<0.05 vs vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0). 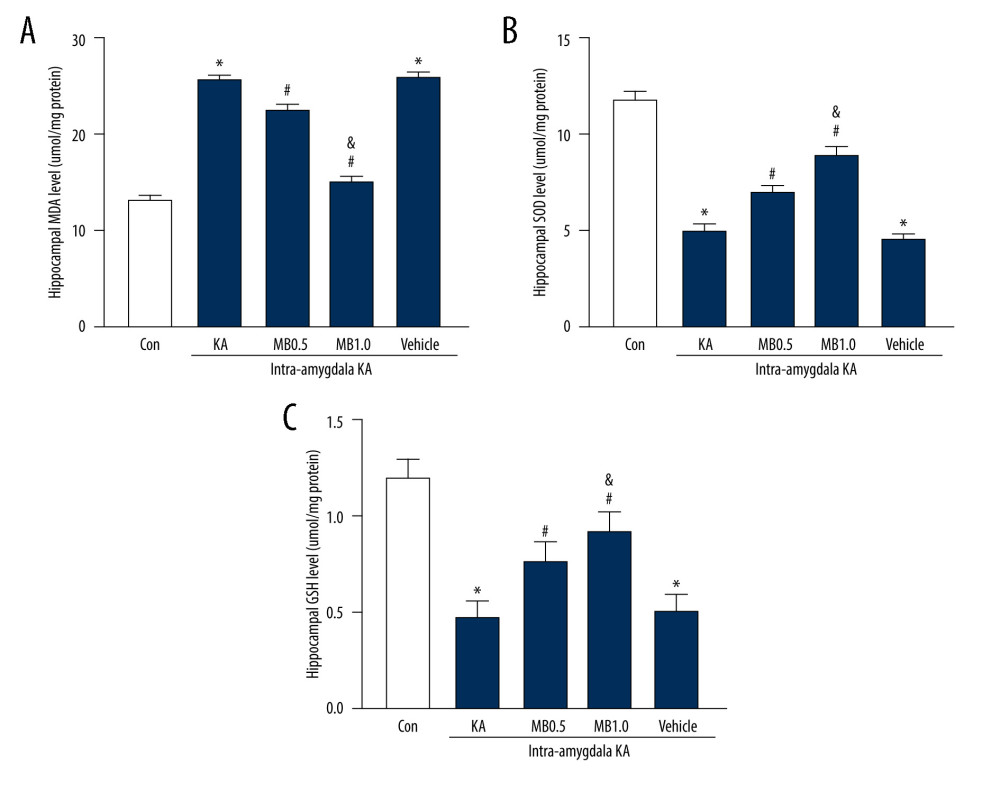 Figure 5. Effect of methylene blue (MB) on levels of hippocampus (A) malondialdehyde, (B) superoxide dismutase, and (C) glutathione 3 h after kainic acid (KA) administration. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA or vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0).
Figure 5. Effect of methylene blue (MB) on levels of hippocampus (A) malondialdehyde, (B) superoxide dismutase, and (C) glutathione 3 h after kainic acid (KA) administration. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA or vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0). 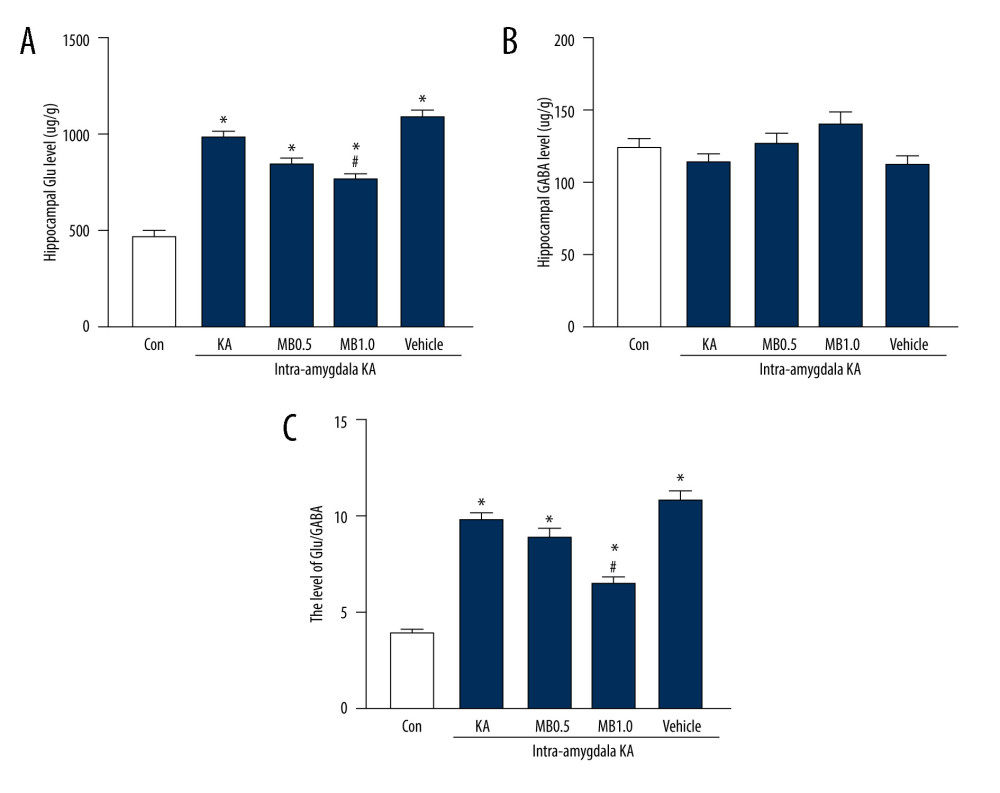 Figure 6. Effect of methylene blue (MB) on levels of (A) glutamate (Glu), (B) gamma-aminobutyric acid (GABA), and (C) Glu/GABA in kainic acid (KA)-treated mice. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA group or MB 0.5 mg/kg+KA group or vehicle+KA group. (Prism 8, version 8.3.0).
Figure 6. Effect of methylene blue (MB) on levels of (A) glutamate (Glu), (B) gamma-aminobutyric acid (GABA), and (C) Glu/GABA in kainic acid (KA)-treated mice. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA group or MB 0.5 mg/kg+KA group or vehicle+KA group. (Prism 8, version 8.3.0). References
1. Chakravarty K, Ray S, Kharbanda PS, Lal V, Baishya J, Temporal lobe epilepsy with amygdala enlargement: A systematic review: Acta Neurol Scand, 2021; 144(3); 236-50
2. Bhatia K, De Jesus O, New onset refractory status epilepticus: StatPearls, 2021, Treasure Island FL, © 2021, StatPearls Publishing LLC.
3. Rogawski MA, Loya CM, Reddy K, Neuroactive steroids for the treatment of status epilepticus: Epilepsia, 2013; 54(0 6); 93-98
4. Holtkamp M, Should barbiturates be used in refractory status epilepticus?: J Clin Neurophys, 2016; 33(1); 22-24
5. Eid T, Thomas MJ, Spencer DD, Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy: Lancet, 2004; 363(9402); 28-37
6. Dariani S, Baluchnejadmojarad T, Roghani M, Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy: J Molecular Neurosci, 2013; 51(3); 679-86
7. Rusina E, Bernard C, Williamson A, The kainic acid models of temporal lobe epilepsy: eNeuro, 2021; 8(2) ENEURO.0337-20.2021
8. Ueda Y, Yokoyama H, Nakajima A, Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus: Exp Brain Res, 2002; 147(2); 219-26
9. Shin EJ, Jeong JH, Chung YH, Role of oxidative stress in epileptic seizures: Neurochem Int, 2011; 59(2); 122-37
10. Si PP, Zhen JL, Cai YL, Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress: Neurosci Let, 2016; 618; 19-24
11. Kalita J, Misra UK, Singh LS, Tiwari A, Oxidative stress in status epilepticus: A clinical-radiological correlation: Brain Res, 2019; 1704; 85-93
12. Liu N, Lin MM, Huang SS, NADPH and mito-apocynin treatment protects against KA-induced excitotoxic injury through autophagy pathway: Front Cell Dev Biol, 2021; 9; 612554
13. Rojas JC, Bruchey AK, Gonzalez-Lima F, Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue: Progress Neurobiol, 2012; 96(1); 32-45
14. Talley Watts L, Long JA, Chemello J, Methylene blue is neuroprotective against mild traumatic brain injury: J Neurotrauma, 2014; 31(11); 1063-71
15. Zhao M, Liang F, Xu H, Methylene blue exerts a neuroprotective effect against traumatic brain injury by promoting autophagy and inhibiting microglial activation: Mol Med Rep, 2016; 13(1); 13-20
16. Oz M, Lorke DE, Hasan M, Petroianu GA, Cellular and molecular actions of methylene blue in the nervous system: Med Res Rev, 2011; 31(1); 93-117
17. Furian AF, Fighera MR, Oliveira MS, Methylene blue prevents methylmalonate-induced seizures and oxidative damage in rat striatum: Neurochem Int, 2007; 50(1); 164-71
18. Mori T, Koyama N, Segawa T, Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice: J Biol Chem, 2014; 289(44); 30303-17
19. Shen Q, Du F, Huang S, Neuroprotective efficacy of methylene blue in ischemic stroke: An MRI study: PLoS One, 2013; 8(11); e79833
20. Rojas JC, John JM, Lee J, Gonzalez-Lima F, Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy: Neurotox Res, 2009; 15(3); 260-73
21. Racine RJ, Gartner JG, Burnham WM, Epileptiform activity and neural plasticity in limbic structures: Brain Res, 1972; 47(1); 262-68
22. Ishikawa A, Mizuno Y, Sakai K, Kainic acid-induced seizures in the common marmoset: Biochem Biophys Res Commun, 2020; 525(3); 595-99
23. Maguire JL, Stell BM, Rafizadeh M, Mody I, Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety: Nature Neurosci, 2005; 8(6); 797-804
24. Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction: Anal Biochem, 1979; 95(2); 351-58
25. Baluchnejadmojarad T, Roghani M, Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress: Behav Brain Res, 2011; 224(2); 305-10
26. Kan MC, Wang WP, Yao GD, Anticonvulsant effect of dexmedetomidine in a rat model of self-sustaining status epilepticus with prolonged amygdala stimulation: Neurosci Lett, 2013; 543; 17-21
27. Vezzani A, Pilocarpine-induced seizures revisited: What does the model mimic?: Epilepsy Curr, 2009; 9(5); 146-48
28. Vermoesen K, Smolders I, Massie A, The control of kainic acid-induced status epilepticus: Epilepsy Res, 2010; 90(1–2); 164-66
29. Cui ZQ, Li WL, Luo Y, Methylene Blue exerts anticonvulsant and neuroprotective effects on self-sustaining status epilepticus (SSSE) induced by prolonged basolateral amygdala stimulation in Wistar rats: Med Sci Monit, 2018; 24; 161-69
30. Callaway NL, Riha PD, Bruchey AK, Methylene blue improves brain oxidative metabolism and memory retention in rats: Pharmacol Biochem Behav, 2004; 77(1); 175-81
31. Callaway NL, Riha PD, Wrubel KM, Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats: Neurosci Lett, 2002; 332(2); 83-86
32. Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F, Memory facilitation by methylene blue: Dose-dependent effect on behavior and brain oxygen consumption: Euro J Pharmacol, 2005; 511(2–3); 151-58
33. Gonzalez-Lima F, Bruchey AK, Extinction memory improvement by the metabolic enhancer methylene blue: Learn Mem, 2004; 11(5); 633-40
34. Zhang X, Rojas JC, Gonzalez-Lima F, Methylene blue prevents neurodegeneration caused by rotenone in the retina: Neurotox Res, 2006; 9(1); 47-57
35. O’Leary JC, Li Q, Marinec P, Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden: Mol Neurodegen, 2010; 5; 45
36. Oz M, Lorke DE, Petroianu GA, Methylene blue and Alzheimer’s disease: Biochem Pharmacol, 2009; 78(8); 927-32
37. Volke V, Wegener G, Vasar E, Rosenberg R, Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo: Brain Res, 1999; 826(2); 303-5
38. Wen Y, Li W, Poteet EC, Alternative mitochondrial electron transfer as a novel strategy for neuroprotection: J Biol Chem, 2011; 286(18); 16504-15
39. Salaris SC, Babbs CF, Voorhees WD, Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury: Biochem Pharmacol, 1991; 42(3); 499-506
40. Kelner MJ, Bagnell R, Hale B, Alexander NM, Methylene blue competes with paraquat for reduction by flavo-enzymes resulting in decreased superoxide production in the presence of heme proteins: Arch Biochem Biophys, 1988; 262(2); 422-26
41. Yue L, Zhao L, Liu H, Adiponectin protects against glutamate-induced excitotoxicity via activating SIRT1-dependent PGC-1α expression in HT22 hippocampal neurons: Oxid Med Cell Longev, 2016; 2016 2957354
42. Oprica M, Eriksson C, Schultzberg M, Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system: J Cell Mol Med, 2003; 7(2); 127-40
43. Järvelä JT, Ruohonen S, Kukko-Lukjanov TK, Kainic acid-induced neurodegeneration and activation of inflammatory processes in organotypic hippocampal slice cultures: Treatment with cyclooxygenase-2 inhibitor does not prevent neuronal death: Neuropharmacol, 2011; 60(7–8); 1116-25
44. Vutskits L, Briner A, Klauser P, Adverse effects of methylene blue on the central nervous system: Anesthesiology, 2008; 108(4); 684-92
45. Wischik CM, Staff RT, Wischik DJ, Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease: J Alzheimers Dis, 2015; 44(2); 705-20
Figures
 Figure 1. Chemical structure and redox balance of methylene blue (MB). MB has powerful autoxidizable properties. It can accept electrons from an electron donor in its oxidized form and acts as an electron donor in its reduced form. At low concentrations in vivo, the 2 forms of MB are at equilibrium, forming a reversible reduction-oxidation system.
Figure 1. Chemical structure and redox balance of methylene blue (MB). MB has powerful autoxidizable properties. It can accept electrons from an electron donor in its oxidized form and acts as an electron donor in its reduced form. At low concentrations in vivo, the 2 forms of MB are at equilibrium, forming a reversible reduction-oxidation system. Figure 2. Schematic overview of the configuration of the guider cannula for kainic acid (KA) injection and electroencephalogram (EEG) recording. KA was injected into the right amygdala (anterior −0.94 mm, medial −2.85 mm, dorsal 4.75 mm) (indicated by the arrow) through a guider cannula (indicated by the vertical black line). For EEG recording, a single stainless-steel screw was implanted in the right frontal lobe to be used as a recording electrode. Two screws then were mounted over the left and right occipital lobes to act as an EEG reference and ground electrode, respectively. Two other screws were implanted to affix the dental cement.
Figure 2. Schematic overview of the configuration of the guider cannula for kainic acid (KA) injection and electroencephalogram (EEG) recording. KA was injected into the right amygdala (anterior −0.94 mm, medial −2.85 mm, dorsal 4.75 mm) (indicated by the arrow) through a guider cannula (indicated by the vertical black line). For EEG recording, a single stainless-steel screw was implanted in the right frontal lobe to be used as a recording electrode. Two screws then were mounted over the left and right occipital lobes to act as an EEG reference and ground electrode, respectively. Two other screws were implanted to affix the dental cement. Figure 3. Representative kainic acid-induced seizure patterns on electroencephalogram in the present study. (A) Irregular high-amplitude spikes. (B) periodic epileptiform discharges. (C) High-frequency bursting. (D) A combination of periodic epileptiform discharges and high-frequency bursts of short duration. (Prism 8, version 8.3.0).
Figure 3. Representative kainic acid-induced seizure patterns on electroencephalogram in the present study. (A) Irregular high-amplitude spikes. (B) periodic epileptiform discharges. (C) High-frequency bursting. (D) A combination of periodic epileptiform discharges and high-frequency bursts of short duration. (Prism 8, version 8.3.0). Figure 4. Effect of methylene blue (MB) on kainic acid (KA)-induced epileptiform activity in vivo. (A) Representative tracings showing KA-induced epileptiform activity for the full recording period in vivo in mice pretreated with vehicle, 0.5 mg/kg of MB, and 1 mg/kg of MB, respectively. Electroencephalogram tracings are labeled to indicate drug administration times (vehicle, MB, and KA) accordingly. Histograms showing the latency (minutes) (B), average epileptiform duration (minutes) (C), and epileptiform activity (percentage) (D) of epileptiform activity in KA-treated mice in different group. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group). # P<0.05 vs vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0).
Figure 4. Effect of methylene blue (MB) on kainic acid (KA)-induced epileptiform activity in vivo. (A) Representative tracings showing KA-induced epileptiform activity for the full recording period in vivo in mice pretreated with vehicle, 0.5 mg/kg of MB, and 1 mg/kg of MB, respectively. Electroencephalogram tracings are labeled to indicate drug administration times (vehicle, MB, and KA) accordingly. Histograms showing the latency (minutes) (B), average epileptiform duration (minutes) (C), and epileptiform activity (percentage) (D) of epileptiform activity in KA-treated mice in different group. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group). # P<0.05 vs vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0). Figure 5. Effect of methylene blue (MB) on levels of hippocampus (A) malondialdehyde, (B) superoxide dismutase, and (C) glutathione 3 h after kainic acid (KA) administration. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA or vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0).
Figure 5. Effect of methylene blue (MB) on levels of hippocampus (A) malondialdehyde, (B) superoxide dismutase, and (C) glutathione 3 h after kainic acid (KA) administration. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 mg/kg or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA or vehicle+KA group; & P<0.05 vs MB 0.5 mg/kg+KA group. (Prism 8, version 8.3.0). Figure 6. Effect of methylene blue (MB) on levels of (A) glutamate (Glu), (B) gamma-aminobutyric acid (GABA), and (C) Glu/GABA in kainic acid (KA)-treated mice. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA group or MB 0.5 mg/kg+KA group or vehicle+KA group. (Prism 8, version 8.3.0).
Figure 6. Effect of methylene blue (MB) on levels of (A) glutamate (Glu), (B) gamma-aminobutyric acid (GABA), and (C) Glu/GABA in kainic acid (KA)-treated mice. Values are expressed as means±standard error of the mean. (n=11 vehicle+KA group, n=12 MB 0.5 or 1 mg/kg+KA group) * P<0.05 vs control group; # P<0.05 vs KA group or MB 0.5 mg/kg+KA group or vehicle+KA group. (Prism 8, version 8.3.0). In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952