31 July 2023: Clinical Research
Early Lactate/Albumin and Procalcitonin/Albumin Ratios as Predictors of 28-Day Mortality in ICU-Admitted Sepsis Patients: A Retrospective Cohort Study
Fuxing Li1ABCDEF, Zhiqiang Ye2ABCE, Junqi Zhu1ABC, Shumin Gu1BC, Suqin Peng1BCE, Youling Fang1BCE, Longhua Hu1AEF*, Jianqiu Xiong3ACEFDOI: 10.12659/MSM.940654
Med Sci Monit 2023; 29:e940654
Abstract
BACKGROUND: Lactate/albumin (LA/ALB) and procalcitonin/albumin (PCT/ALB) ratios have been implicated in predicting mortality in sepsis patients. However, their prognostic value and relationship to sepsis severity require further investigation. This retrospective study aimed to assess the prognostic value of lactate/albumin (LA/ALB) and procalcitonin/albumin (PCT/ALB) ratios in septic patients admitted to the Intensive Care Unit (ICU).
MATERIAL AND METHODS: A total of 340 adult sepsis patients admitted to the ICU were included in the derivation cohort. LA/ALB and PCT/ALB ratios were calculated and analyzed in relation to sepsis severity and survival status. Additionally, a validation cohort of 75 sepsis patients from another medical center was selected.
RESULTS: In the derivation cohort, higher LA/ALB and PCT/ALB ratios and SOFA scores were significantly associated with increased mortality (P<0.001). The LA/ALB and PCT/ALB ratios positively correlated with SOFA score. Survival analysis revealed significantly higher 28-day mortality in sepsis patients with elevated PCT/ALB (≥0.256) and LA/ALB (≥0.079) ratios upon ICU admission. The constructed prediction model incorporating LA/ALB ratio, PCT/ALB ratio, and SOFA score yielded an AUC of 0.826, demonstrating good predictive ability. The associations between LA/ALB and PCT/ALB ratios and 28-day mortality in sepsis patients were validated in the validation cohort.
CONCLUSIONS: The LA/ALB and PCT/ALB ratios at ICU admission provide valuable prognostic information for predicting 28-day mortality in sepsis patients. Combining these ratios with SOFA score improves the assessment of prognosis in sepsis patients.
Keywords: Albumins, Lactates, Mortality, nomograms, procalcitonin, Sepsis, Adult, Humans, Retrospective Studies, Lactic Acid, ROC Curve, Organ Dysfunction Scores, Intensive Care Units, Prognosis
Background
Sepsis is described as an organ malfunction resulting from an uncontrolled host response to the infection, which is the leading cause of death among severely ill individuals and a significant public health concern worldwide [1,2]. A Lancet study reported there are nearly 50 million cases of sepsis worldwide each year, with 11 million sepsis-related deaths, and 1 person dying every 2.8 seconds from sepsis [3]. A study of 44 hospital ICUs in China found that the incidence of sepsis in ICUs was 20.6%, the case fatality rate was 35.5%, and the mortality rate was as high as 50% in severe sepsis [4]. For patients with sepsis, rapid and accurate assessment of prognosis on the first day of ICU admission is of great significance, but there is still a lack of effective clinical and laboratory-related indicators. Therefore, there is an immediate need for reliable predictive biomarkers to effectively evaluate the prognosis of sepsis patients and achieve precise diagnosis and treatment of sepsis patients.
As research has progressed, various biomarkers of sepsis have been revealed. At present, procalcitonin (PCT) [5] and C-reactive protein (CRP) [6] have been evaluated for use in the diagnosis and prognosis of sepsis, which have been included in the diagnostic criteria for sepsis by the Surviving Sepsis Campaign [7]. However, it should be noted that CRP and PCT both have some limitations, such as non-specificity and delayed response [8]. Moreover, some studies identified that PCT and CRP were unable to efficiently predict the risk of death in septic patients [9,10]; therefore, the value of PCT and CRP in the assessment of sepsis prognosis is still controversial. Meanwhile, other biomarkers, such as interleukin-37 (IL-37) [11], IL-26 [12] heparin-binding protein (HBP) [13], lactate (LA) [14], albumin (ALB) [15], macrophage/CD5L [16], and heat shock protein 90α (HSP90α) [17], have been found to be useful in the prognostic assessment of sepsis. Nevertheless, the specific role of most biomarkers in patients with sepsis is not well defined, and of the many biomarkers that have been studied, only a small fraction have been evaluated in large or replicated studies, while the sensitivity and specificity of single biomarkers were limited [18–20], which further limits the clinical application of the above indicators. Accordingly, to save medical resources, reduce costs, ensure the correct interventions, and further improve outcomes for septic patients, there is still a need to discover more new biomarkers applicable to clinical applications in an effort to optimize patient treatment.
Lactate/albumin (LA/ALB) ratio reflects the degree of cell damage and tissue hypoxia, and, combined with the nutritional status of body, is expected to be a biomarker for the prognosis of sepsis. It has been shown that the LA/ALB ratio can be used as an early prognostic marker in severely sick individuals, especially in those with heart failure or sepsis [21–23]. Moreover, procalcitonin/albumin (PCT/ALB) ratio is a new indicator that combines inflammation levels with nutritional status, which had been shown to contribute to the prognosis of sepsis in neonates [24]. Despite this, the correlation between the prognosis of sepsis and LA/ALB ratio, as well as PCT/ALB ratio, has rarely been studied. The present study evaluated the relationship between LA/ALB and PCT/ALB ratios on the first day of admittance to the ICU and the 28-day prognosis of patients with sepsis and attempted to develop a relevant predictive model.
Material and Methods
STUDY POPULATION:
We retrospectively collected data on septic patients admitted to the Intensive Care Unit (ICU) of the Second Affiliated Hospital of Nanchang University (China) from 1 January 2018 to 31 December 2022. There were 340 sepsis patients included in the derivation cohort. The validation cohort included 75 patients with sepsis admitted to the ICU at Dali University’s First Affiliated Hospital over the same period. Afterward, according to the septic patients’ 28-day mortality, the patients in the derivation cohort and validation cohort were assigned to either the survival group or the non-survival group. This research was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. Because of the retrospective and observational nature of the investigation and because the data were anonymous, the requirement for informed consent was waived by the Ethics Committee.
KEY INCLUSION AND EXCLUSION CRITERIA:
Inclusion criteria were: (1) All individuals fulfilled the diagnosis criteria of sepsis as recommended in the Third International Consensus Definitions for Sepsis and Septic Shock (sepsis 3.0) [25]; (2) Age 18–80 years; (3) All individuals had clinical data available.
Exclusion criteria were: (1) Women during pregnancy and lactation; (2) Severe liver and kidney dysfunction; (3) Major adverse cardiac and cerebrovascular events (myocardial infarction, cerebral hemorrhage, and cerebral infarction); (4) Patients with tumors, blood system diseases, immune system diseases, and other acute and life-threatening events; (5) Patients who received intravenous fluids containing albumin or lactate prior to blood collection; (6) Patients with missing key information.
CLINICAL AND LABORATORY DATA COLLECTION:
Data were obtained via manual and automated retrievals from the institutional electronic medical records regarding clinical characteristics, demographics, and laboratory results. Then, data were independently collected and entered into an Excel table (Microsoft Office 2020, USA) by 2 observers. The following clinical information was collected: Age, sex, vital signs (temperature, pulse rate, breath rate, and blood pressure), underlying disease (hypertension, diabetes mellitus, and pleural effusion), length of hospital stay, and the prognosis at 28 days following admission to the ICU. Within 24 h of admission to the ICU, the collected laboratory parameters included the results of blood culture, blood routine (white blood cells (×109/L), neutrophils (×109/L), lymphocytes (×109/L), hemoglobin (g/L), hematocrit (HCT,%), and platelet (PLT, ×109/L)), liver function (direct bilirubin (μmol/L), indirect bilirubin (μmol/L), total bilirubin (μmol/L), aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), and albumin (ALB, g/L)), renal function (creatinine (Crea, μmol/L), uric acid (UA, μmol/L)), blood urea nitrogen (BUN, mmol/L), triglycerides (mg/dL), total cholesterol (mg/dL), lactate (LA, mmol/L), procalcitonin (PCT, μg/L) and C-reactive protein (CRP, mg/L). PCT/ALB ratio was estimated according to the PCT serum levels and ALB, and LA/ALB ratio was calculated based on the levels of serum LA and ALB. Additionally, the acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scores were estimated on the day of admission to the ICU.
STATISTICS:
All statistics and plotting were conducted utilizing R version 4.2.3. Continuous variables were analyzed using the Mann-Whitney U test and expressed as the median and interquartile range (IQR). The enumeration data were adopted by chi-square test and reported as a percentage (%). Spearman correlation coefficient was employed to determine the relationship between the ratios of LA/ALB and PCT/ALB and the severity of sepsis (SOFA score). The receiver operating characteristic (ROC) curve was utilized to evaluate the abilities of LA/ALB, PCT/ALB, and SOFA scores on the prognosis of 28-day mortality in sepsis. Next, risk factors for 28-day mortality in sepsis were assessed using logistics regression analysis with best subsets variable reduction [26], and variables achieving univariate P<0.10 were included in multivariate logistic regression analysis. Furthermore, the optimum cutoff values from the ROC curve of LA/ALB (0.079) and PCT/ALB (0.256) in the derivation cohort were determined based on the Youden index, and the survival curves were plotted. The prediction model was developed using multivariate logistic regression analysis, and the nomogram was drawn. To assess the goodness of fit of the nomogram, the Hosmer-Lemeshow test was employed [27]. Then, utilizing the ROC curves and calibration plots, the internal validation of nomogram was accomplished, and decision curve analysis (DCA) was mapped to assess the clinical utility of predictive models. Finally, we validated the ability of LA/ALB and PCT/ALB to predict 28-day mortality in sepsis using data from 75 patients with sepsis at another medical institute, along with external validation of the prediction model. Differences with a P-value less than 0.05 were considered to be statistically significant (P<0.05).
Results
ELEVATED LA/ALB AND PCT/ALB RATIOS IN NON-SURVIVORS COMPARED TO THOSE IN SURVIVORS:
A total of 340 adult septic individuals meeting the clinical criteria for Sepsis-3.0, including 198 males and 142 females, having a median age of 56.0 years, were included in the derivation cohort. Then, these individuals were categorized into a survival group (n=238, 135 males and 103 females, median age 55.0 years) and a non-survival (n=102, 63 males and 39 females, median age 58.5 years) group based on the prognosis of septic individuals at 28 days. The clinical features and laboratory data of the survival and non-survival group are presented in Table 1. The median creatinine (Crea), lactate (LA), and procalcitonin (PCT) were significantly greater in non-survivors compared to those in survivors, and the median albumin (ALB) was lower in non-survivors in comparison with those in survivors (all P<0.05). According to the survival group, the ratio of serum LA to ALB (LA/ALB) (Figure 1A) and PCT to ALB (PCT/ALB) (Figure 1B) on ICU admission were also significantly elevated in the non-survival group (P<0.001). Meanwhile, non-survivors had significantly higher admission SOFA (Figure 1C) and APACHE II scores versus survivors (P<0.001).
THE RATIOS OF LA/ALB AND PCT/ALB WERE CONNECTED TO 28-DAY MORTALITY IN INDIVIDUALS SUFFERING FROM SEPSIS:
To evaluate the correlation between disease severity (SOFA score) and laboratory indexes, a correlation network analysis was performed (Figure 2). The spearman correlation analysis demonstrated low positive correlations between SOFA score and LA/ALB (r=0.297, P<0.001), LA (r=0.280, P<0.001), PCT/ALB (r=0.251, P<0.001), APACHE II (r=0.232, P<0.001), Crea (r=0.182, P<0.001), and a weak negative association between SOFA score and ALB (r=−0.208, P<0.001).
Furthermore, logistics regression analysis with best subsets variable reduction was performed to discover the independent risk factors of 28-day mortality in individuals with sepsis. Among the variables associated with 28-day mortality of sepsis patients in the univariate logistic regression analysis, PCT/ALB, LA/ALB, and SOFA score remained independent risks factors for 28-day mortality of sepsis patients in the multivariable analysis (Table 2).
THE ABILITY OF RATIOS OF LA/ALB AND PCT/ALB TO PREDICT 28-DAY MORTALITY IN SEPTIC PATIENTS:
Next, we used the ratios of LA/ALB and PCT/ALB to develop a receiver operating characteristic (ROC) curve analysis for predictive assessment of 28-day mortality in individuals with sepsis (Table 3). The area under the curve (AUC) of LA/ALB was 0.65 (95% CI 0.59–0.70), with 53.9% sensitivity and 74.4% specificity, which was greater than those in the AUC for PCT (AUC=0.62), ALB (AUC=0.62), lactate (AUC=0.64), creatinine (AUC=0.59), PCT/ALB (AUC=0.64) and APACHE II (AUC=0.64), but lower than SOFA score (AUC=0.83). The AUC of PCT/ALB combined with LA/ALB and SOFA score was 0.83, with sensitivity rising from 73.5% to 80.4% compared to the SOFA score alone.
Based on Youden’s index from the ROC curve in the derivation cohort, the result of optimal cutoff value of LA/ALB was 0.079 (Figure 3A), and the result of optimal cutoff value of PCT/ALB was 0.256 (Figure 3B), Kaplan-Meier survival analysis curves were constructed. Septic patients with higher ratios of LA/ALB and PCT/ALB had significantly higher 28-day mortality.
COLUMNAR PLOTS TO PREDICT THE 28-DAY MORTALITY IN PATIENTS WITH SEPSIS USING LA/ALB RATIO, PCT/ALB RATIO, AND SOFA SCORE:
According to the multivariate logistic regression model, the model having the highest score was chosen, and a predictive nomogram was constructed to assess the risk of 28-day mortality of sepsis patients (Figure 4). However, because LA/ALB ratio (LAR), PCT/ALB ratio (PAR), and SOFA score were nonnormal data, these continuous parameters were dichotomized by ROC curve-derived optimal cutoff values. The scores associated with the column line plots were 32 for LAR ≥0.079, 30 for PAR ≥0.256, and 100 for SOFA score ≥6.0, and the speculated probabilities were 0.1–0.8 for total integrals 22–174.
Then, the performance of the nomogram was estimated using the ROC curve and calibration plot. The model’s AUC value was 0.826 (0.723–0.843), indicating that the predictive model has a degree of discrimination (Figure 5A). Hosmer-Lemeshow test outcomes revealed no statistically significant variation between the model’s risk predicted values and the actual observed values (χ2=2.623, P=0.758). Furthermore, the calibration plot for the probability of 28-day mortality revealed good agreement between the prediction by nomogram and actual observation (Figure 5B).
CLINICAL UTILITY OF A MODEL FOR RISK OF 28-DAY MORTALITY IN SEPSIS PATIENTS:
The clinical decision curves for the risk of 28-day mortality in the sepsis model revealed that the model has clinical utility when the risk threshold varies from 0.07 to 0.74 (Figure 6).
VALIDATION OF PROGNOSTIC MODELS BASED ON EXTERNAL COHORT:
To confirm the predictive values of LAR and PAR, 75 sex- and age-matched adult septic patients from another medical institute were included in the external confirmation cohort (Table 4). In the validation cohort, non-surviving septic patients also had significantly higher LA/ALB ratio, PCT/ALB ratio, and SOFA score than survivors on ICU admission (Table 5). Notably, in the validation cohort the AUC of PCT/ALB combined with LA/ALB and SOFA score was 0.91 (0.82–0.96), with 94.6% of sensitivity and 71.1% specificity, rising from 0.86 for the SOFA score alone (Table 6).
Finally, to evaluate the performances of columnar plots, we used the independent cohort for external validation. The model performed well within both the derivation cohort and the confirmation cohort (Figure 7). Decision curve analysis (DCA) confirmed the predictive value of our prognostic model in the validation cohort, which has value for clinical use when the risk threshold extends from 0.04 to 0.84 in the validation cohort.
Discussion
LIMITATIONS:
Despite these findings, there are some restrictions associated with this investigation. Initially, as a retrospective analysis, although our study included medical centers in 2 different regions, selection bias could not be ruled out, while the sample size was limited, and a large prospective study of multiple centers is needed to confirm our findings. Second, the complex and unstable conditions of sepsis patients admitted to the ICU make it challenging to thoroughly assess the pre-admission background of these individuals, including the categorization of the source of sepsis and the days leading up to their ICU admission. Third, the investigation was performed only in adult septic individuals, but the increasing incidence of neonatal and pediatric sepsis requires further study to determine if it is useful to evaluate the prognosis of pediatric sepsis patients and neonatal sepsis patients. Fourth, we only evaluated the prognosis of sepsis by the above indicators on the day of admission to the ICU, and it is unclear whether changes in the patient’s condition cause changes in the above indicators after the clinical intervention, so dynamic monitoring of the above indicators can better assess the effect of treatment. Finally, although a predictive model was developed and validated, its predictive value is still limited, and as much clinical data as possible needs to be added to increase the model’s sensitivity and improve the prognostic prediction of septic patients.
Conclusions
In summary, our study found that LA/ALB and PCT/ALB ratios on the day of admission to the ICU were potential biomarkers to predict 28-day mortality in individuals with sepsis, while prediction models showed that LA/ALB and PCT/ALB ratios combined with SOFA score could better predict 28-day mortality in individuals with sepsis, but more prospective analyses are needed.
Figures
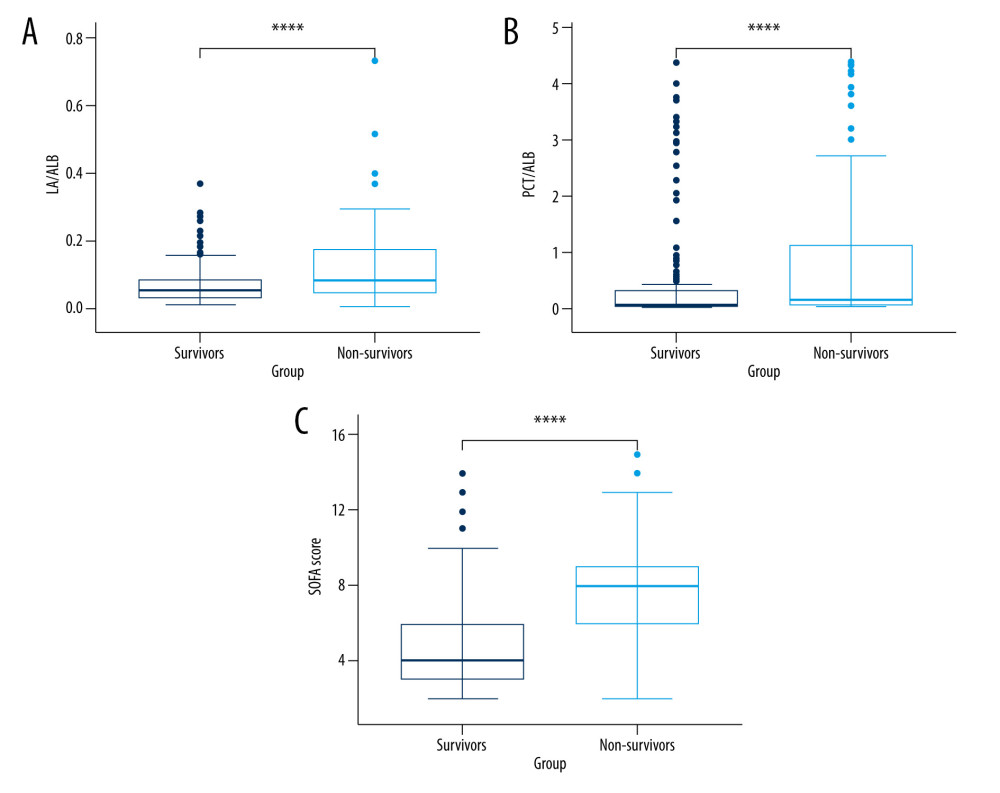 Figure 1. The levels of LA/ALB, PCT/ALB, and the SOFA score in survival and non-survival of septic patients. (A) The levels of LA/ALB across survival and non-survival groups. (B) The levels of PCT/ALB across survival and non-survival groups. (C) The levels of SOFA score across survival and non-survival groups. n=238 (survival group) and 102 (non-survival group), respectively. **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 1. The levels of LA/ALB, PCT/ALB, and the SOFA score in survival and non-survival of septic patients. (A) The levels of LA/ALB across survival and non-survival groups. (B) The levels of PCT/ALB across survival and non-survival groups. (C) The levels of SOFA score across survival and non-survival groups. n=238 (survival group) and 102 (non-survival group), respectively. **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria. 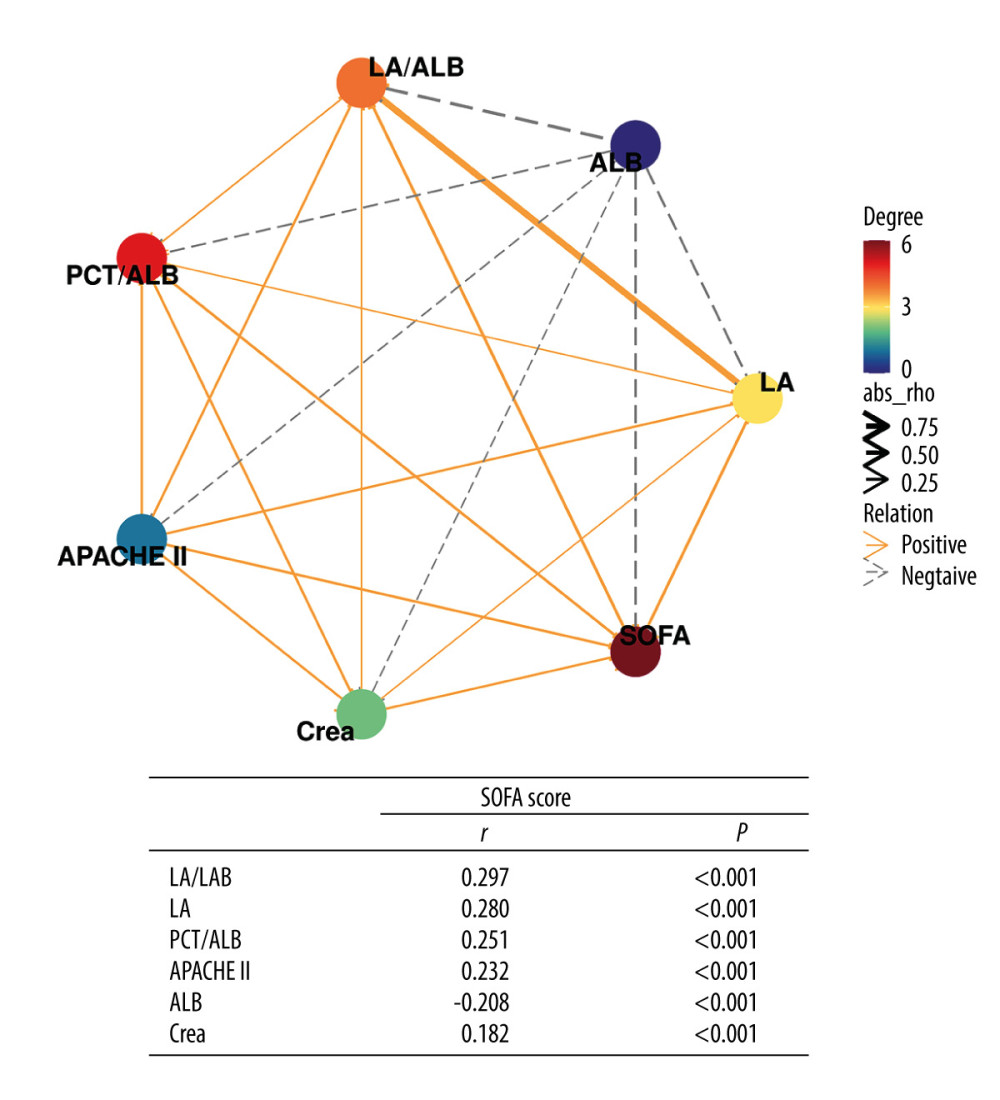 Figure 2. Correlation network of SOFA score with laboratory indexes (Spearman analysis). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 2. Correlation network of SOFA score with laboratory indexes (Spearman analysis). R version 4.2.3, The R Foundation, Vienna, Austria. 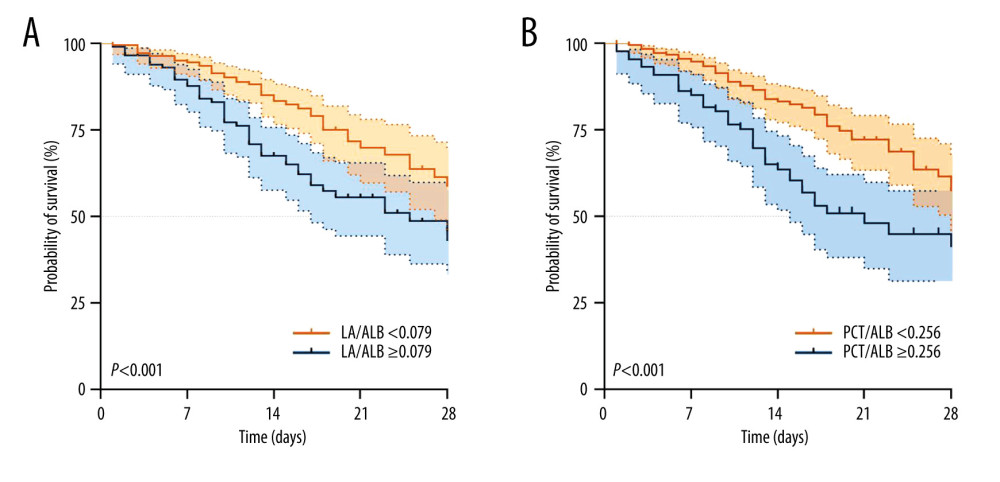 Figure 3. Kaplan-Meier survival curves of 340 adult septic patients based on the LAC/ALB (A) cutoff value (0.079) and PCT/ALB (B) cutoff value (0.256) on the day of ICU admission. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 3. Kaplan-Meier survival curves of 340 adult septic patients based on the LAC/ALB (A) cutoff value (0.079) and PCT/ALB (B) cutoff value (0.256) on the day of ICU admission. R version 4.2.3, The R Foundation, Vienna, Austria. 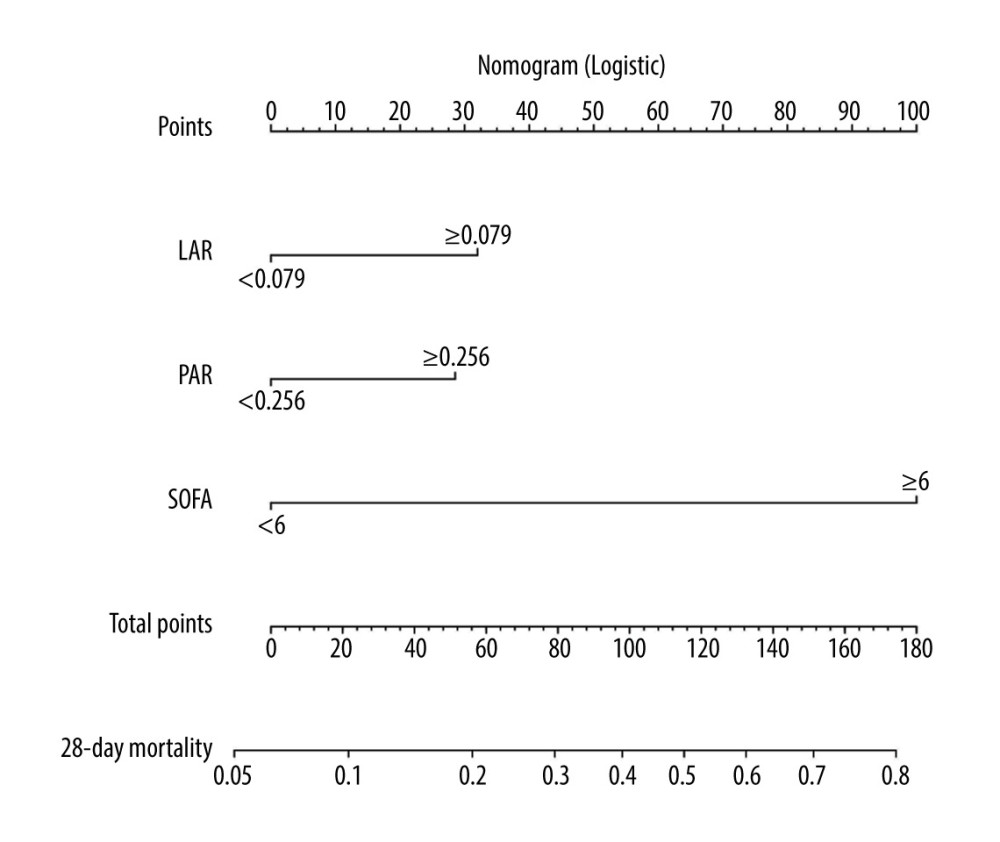 Figure 4. Nomogram for predicting the prognosis of septic patients. Nomogram, to draw an upward vertical line to the “Points” bar to calculate points. Based on the sum, draw a downward vertical line from the “Total Points” line to calculate the probability of 28-d mortality in sepsis for each patient. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 4. Nomogram for predicting the prognosis of septic patients. Nomogram, to draw an upward vertical line to the “Points” bar to calculate points. Based on the sum, draw a downward vertical line from the “Total Points” line to calculate the probability of 28-d mortality in sepsis for each patient. R version 4.2.3, The R Foundation, Vienna, Austria. 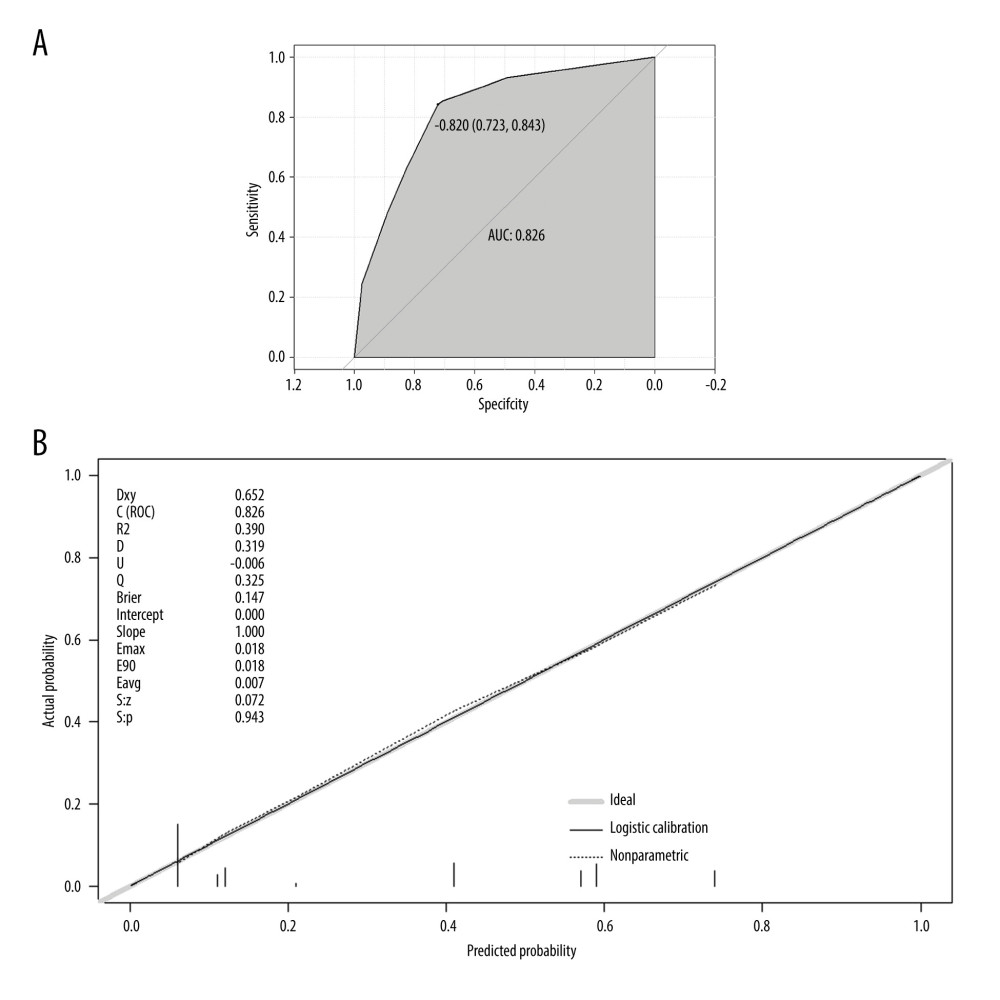 Figure 5. The validation of a predictive model for the prognosis of septic patients. (A) ROC curve of the prediction model for the prognosis of septic patients. (B) Calibration curve of the model for the prognosis of septic patients. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 5. The validation of a predictive model for the prognosis of septic patients. (A) ROC curve of the prediction model for the prognosis of septic patients. (B) Calibration curve of the model for the prognosis of septic patients. R version 4.2.3, The R Foundation, Vienna, Austria. 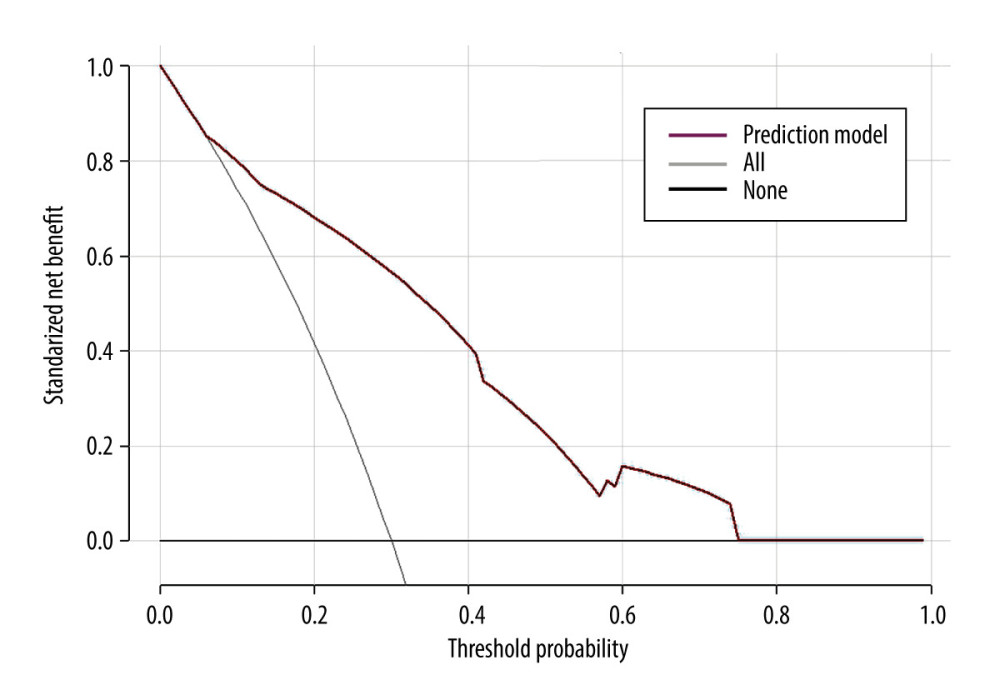 Figure 6. Clinical decision curve for the prognosis of septic patients. (X: probability with value, Y: net benefit, black line: hypothesis of no death in all septic patients, gray line: hypothesis of death in all septic patients). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 6. Clinical decision curve for the prognosis of septic patients. (X: probability with value, Y: net benefit, black line: hypothesis of no death in all septic patients, gray line: hypothesis of death in all septic patients). R version 4.2.3, The R Foundation, Vienna, Austria. 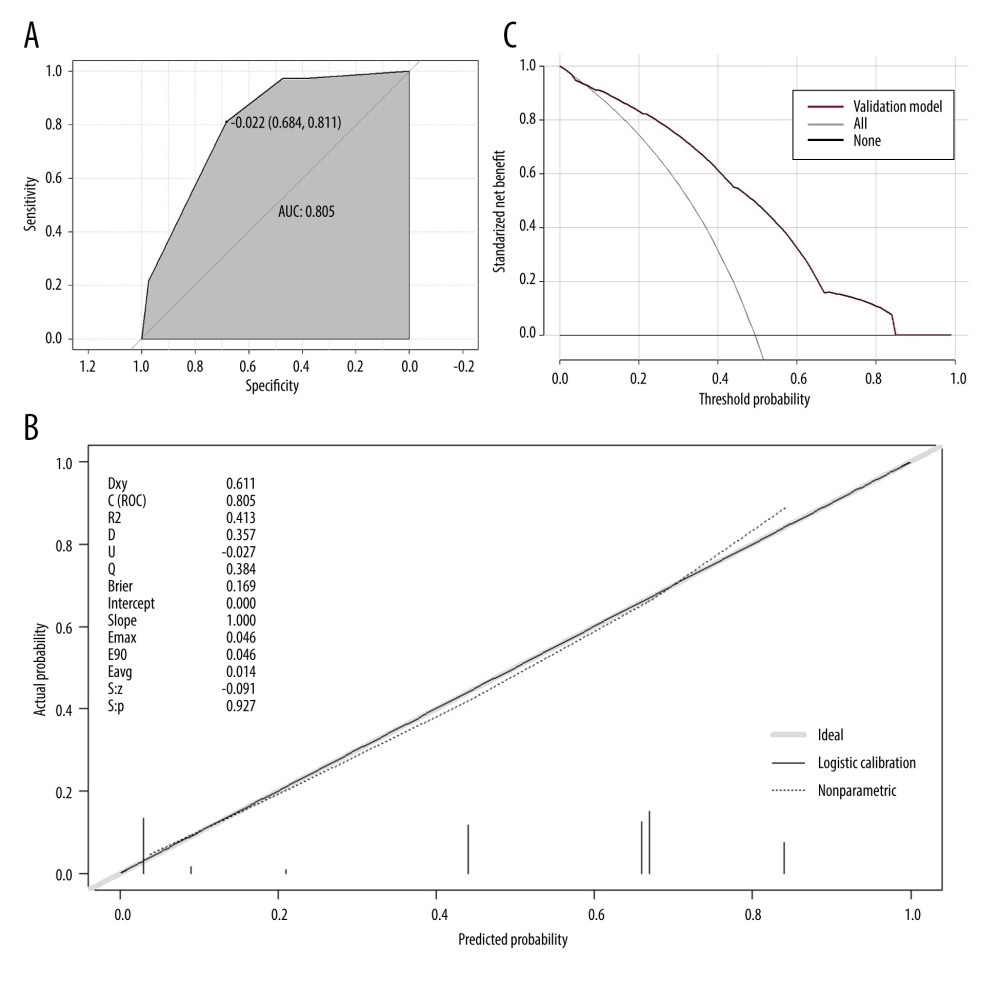 Figure 7. The validation of a predictive model for the prognosis of septic patients (validation cohort). (A) ROC curve of the prediction model for the prognosis of septic patients (validation cohort). (B) Calibration curve of the model for the prognosis of septic patients (validation cohort). (C) Clinical decision curve for the prognosis of septic patients (validation cohort). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 7. The validation of a predictive model for the prognosis of septic patients (validation cohort). (A) ROC curve of the prediction model for the prognosis of septic patients (validation cohort). (B) Calibration curve of the model for the prognosis of septic patients (validation cohort). (C) Clinical decision curve for the prognosis of septic patients (validation cohort). R version 4.2.3, The R Foundation, Vienna, Austria. Tables
Table 1. The comparisons of clinical features between the survival and non-survival groups of septic individuals.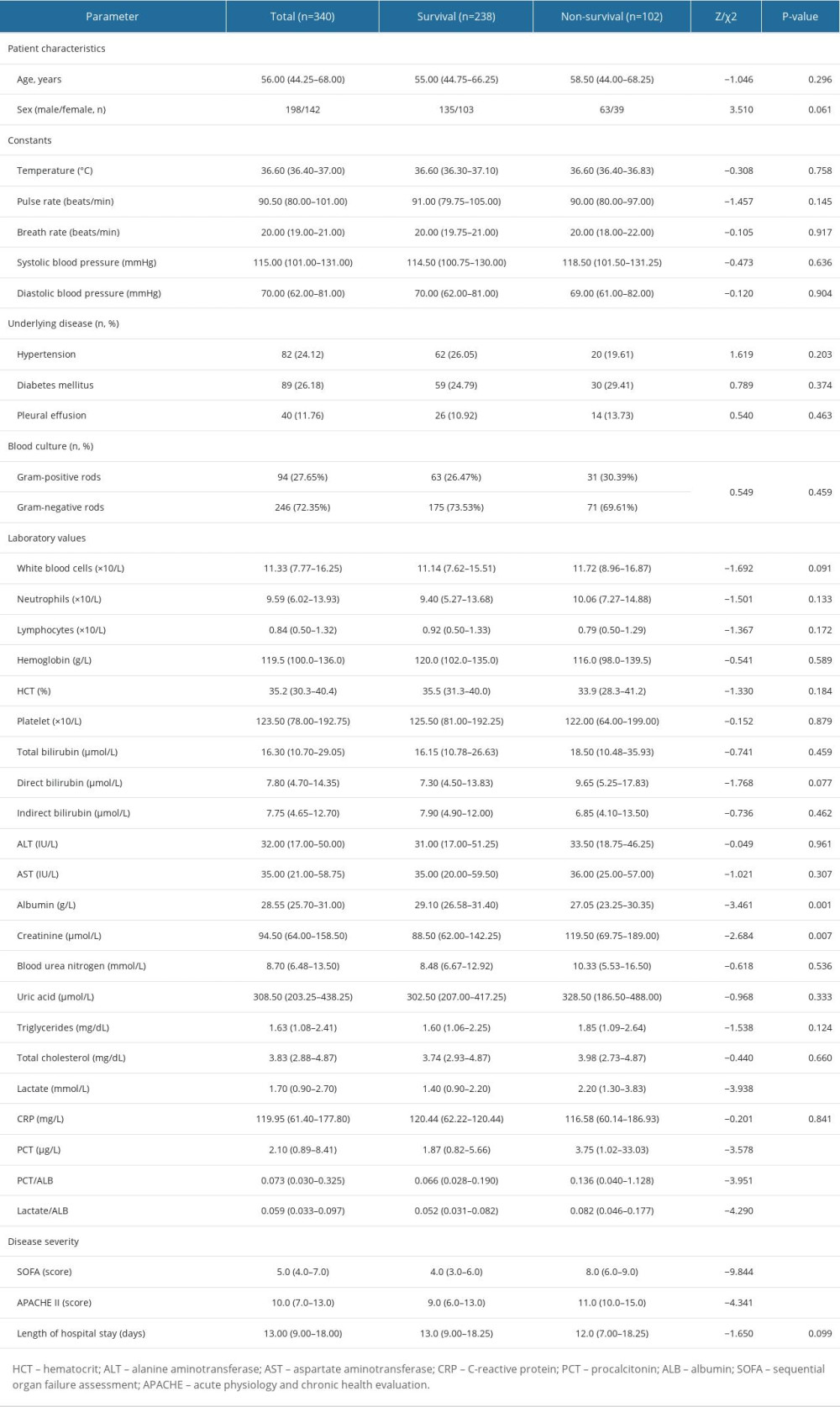 Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis.
Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis.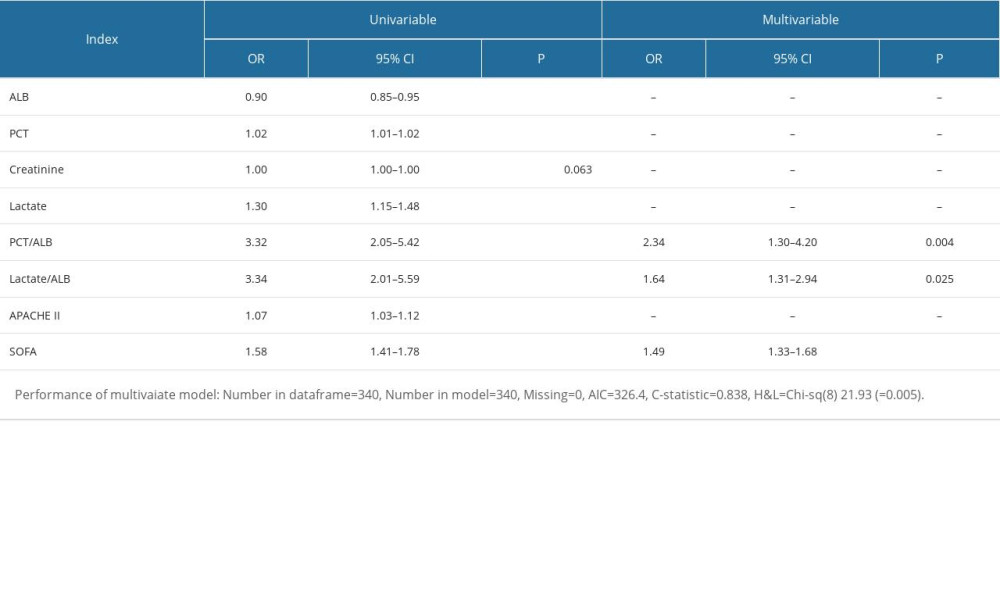 Table 3. Logistic regression with best subsets variable reduction.
Table 3. Logistic regression with best subsets variable reduction.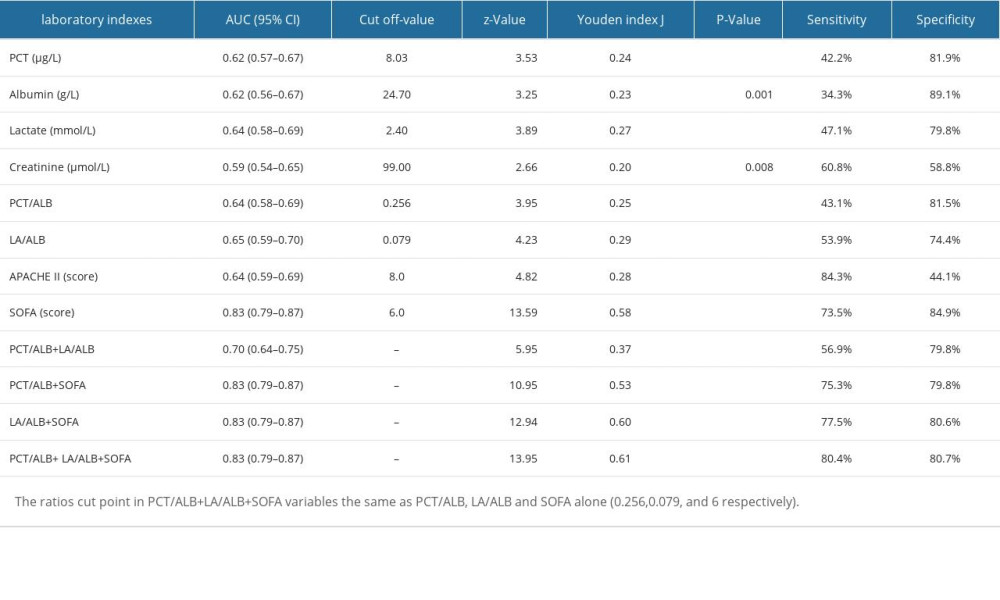 Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients.
Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients.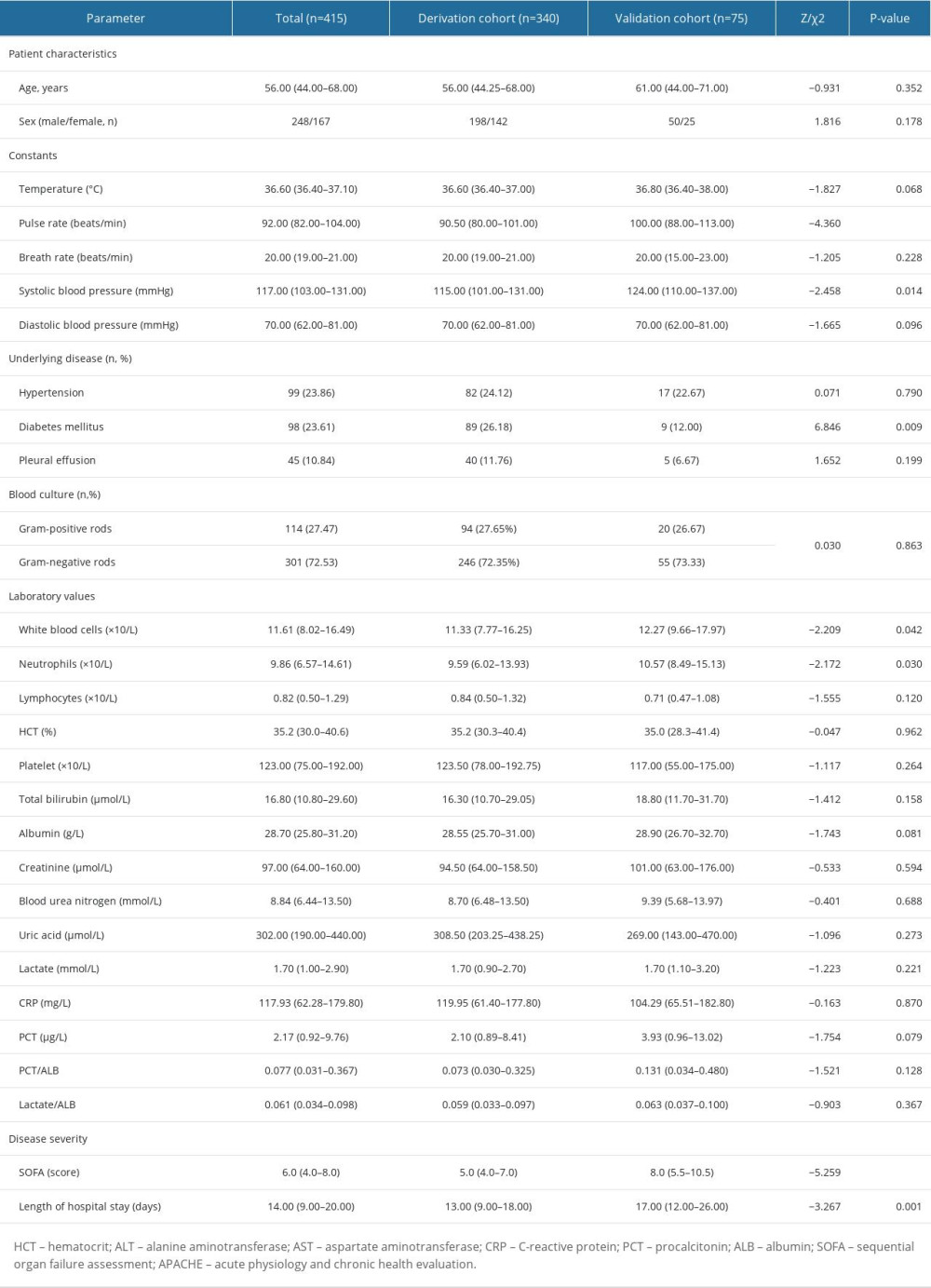 Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort).
Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort).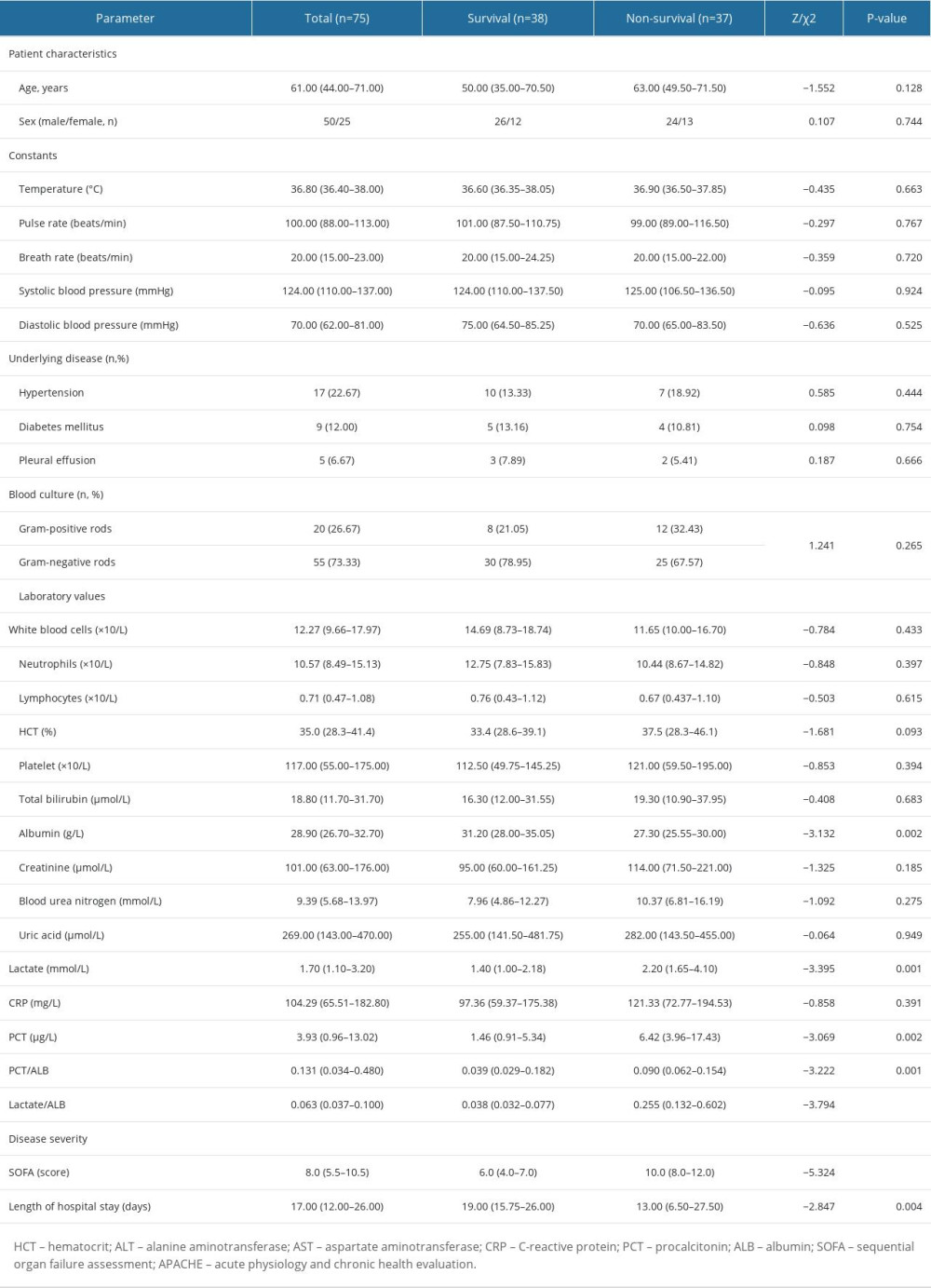 Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort).
Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort).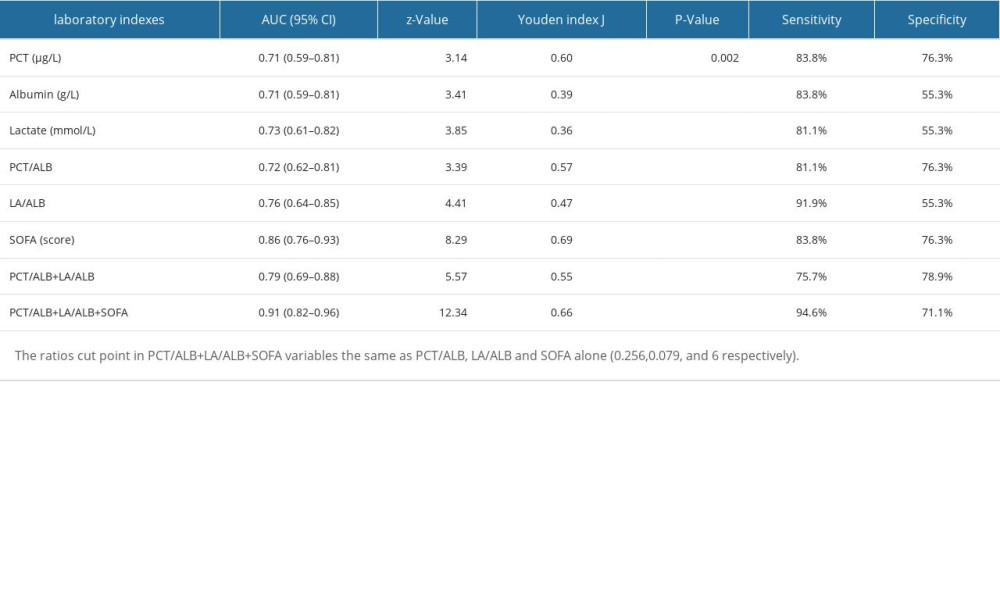
References
1. Salluh JIF, Soares M, Singer M, Spreading the knowledge on the epidemiology of sepsis: Lancet Infect Dis, 2017; 17(11); 1104-6
2. van den Berg M, van Beuningen FE, Ter Maaten JC, Bouma HR, Hospital-related costs of sepsis around the world: A systematic review exploring the economic burden of sepsis: J Crit Care, 2022; 71; 154096
3. Kempker JA, Martin GS, A global accounting of sepsis: Lancet, 2020; 395(10219); 168-70
4. Xie J, Wang H, Kang Y, The epidemiology of sepsis in Chinese ICUs: A national cross-sectional survey: Crit Care Med, 2020; 48(3); e209-e18
5. Schuetz P, Birkhahn R, Sherwin R, Serial procalcitonin predicts mortality in severe sepsis patients: Results from the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) study: Crit Care Med, 2017; 45(5); 781-89
6. Yang Y, Xie J, Guo F, Combination of C-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients: Ann Intensive Care, 2016; 6(1); 51
7. Dellinger RP, Levy MM, Rhodes A, Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012: Intensive Care Med, 2013; 39(2); 165-228
8. Rhodes B, Merriman ME, Harrison A, A genetic association study of serum acute-phase C-reactive protein levels in rheumatoid arthritis: Implications for clinical interpretation: PLoS Med, 2010; 7(9); e1000341
9. Becker KL, Snider R, Nylen ES, Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations: Crit Care Med, 2008; 36(3); 941-52
10. Schuetz P, Christ-Crain M, Muller B, Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections – hope for hype?: Swiss Med Wkly, 2009; 139(23–24); 318-26
11. Wu C, Ma J, Yang H, Interleukin-37 as a biomarker of mortality risk in patients with sepsis: J Infect, 2021; 82(3); 346-54
12. Tu H, Lai X, Li J, Interleukin-26 is overexpressed in human sepsis and contributes to inflammation, organ injury, and mortality in murine sepsis: Crit Care, 2019; 23(1); 290
13. Wu YL, Yo CH, Hsu WT, Accuracy of heparin-binding protein in diagnosing sepsis: A systematic review and meta-analysis: Crit Care Med, 2021; 49(1); e80-e90
14. Nguyen HB, Kuan WS, Batech M, Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: A multi-national evaluation: Crit Care, 2011; 15(5); R229
15. Yin M, Si L, Qin W, Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: A prospective cohort study: J Intensive Care Med, 2018; 33(12); 687-94
16. Gao X, Liu Y, Xu F, Assessment of apoptosis inhibitor of macrophage/CD5L as a biomarker to predict mortality in the critically ill with sepsis: Chest, 2019; 156(4); 696-705
17. Li F, Zhang Y, Yu B, Evaluation of the diagnostic and prognostic values of serum HSP90alpha in sepsis patients: A retrospective study: Peer J, 2022; 10; e12997
18. Povoa P, Coelho L, Dal-Pizzol F, How to use biomarkers of infection or sepsis at the bedside: guide to clinicians: Intensive Care Med, 2023; 49(2); 142-53
19. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG, The immunopathology of sepsis and potential therapeutic targets: Nat Rev Immunol, 2017; 17(7); 407-20
20. Pierrakos C, Velissaris D, Bisdorff M, Biomarkers of sepsis: Time for a reappraisal: Crit Care, 2020; 24(1); 287
21. Gharipour A, Razavi R, Gharipour M, Mukasa D, Lactate/albumin ratio: An early prognostic marker in critically ill patients: Am J Emerg Med, 2020; 38(10); 2088-95
22. Li Y, Li D, Yuan XPredictive value of early lactate/albumin ratio in the prognosis of sepsis: Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2023; 35(1); 61-65 [in Chinese]
23. Bou Chebl R, Geha M, Assaf M, The prognostic value of the lactate/albumin ratio for predicting mortality in septic patients presenting to the emergency department: a prospective study: Ann Med, 2021; 53(1); 2268-77
24. Li T, Li X, Liu X, Association of procalcitonin to albumin ratio with the presence and severity of sepsis in neonates: J Inflamm Res, 2022; 15; 2313-21
25. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315(8); 801-10
26. Calcagno V, De CM, glmulti: An R package for easy automated model selection with (generalized) linear models: Journal of Statistical Software, 2010; 34(12); 1-29
27. Lemeshow S, Hosmer DW, A review of goodness of fit statistics for use in the development of logistic regression models: Am J Epidemiol, 1982; 115(1); 92-106
28. Coopersmith CM, De Backer D, Deutschman CS, Surviving Sepsis Campaign: Research priorities for sepsis and septic shock: Crit Care Med, 2018; 46(8); 1334-56
29. Casserly B, Phillips GS, Schorr C, Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database: Crit Care Med, 2015; 43(3); 567-73
30. Wang S, Ding S, Luo H, Chai X, International Normalized Ratio to Albumin Ratio (PTAR): An objective risk stratification tool in patients with sepsis: Int J Gen Med, 2021; 14; 1829-41
31. Yu YT, Liu J, Hu B, Expert consensus on the use of human serum albumin in critically ill patients: Chin Med J (Engl), 2021; 134(14); 1639-54
32. Wang X, Jing M, Li L, Xu Q, The prognostic value of procalcitonin clearance and procalcitonin to albumin ratio in sepsis patients: Clin Lab, 2023; 69(3); 220613
33. Nelson J, Hansen C, Scupp T, Brainard J, Implications of procalcitonin testing in critically ill patients with sepsis: Am J Respir Crit Care Med, 2019; 199(2); 232-34
34. Arnau-Barres I, Guerri-Fernandez R, Luque S, Serum albumin is a strong predictor of sepsis outcome in elderly patients: Eur J Clin Microbiol Infect Dis, 2019; 38(4); 743-46
35. Karakike E, Kyriazopoulou E, Tsangaris I, The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: Analysis through a derivation and a validation cohort: Crit Care, 2019; 23(1); 387
36. Hu H, Li L, Zhang Y, A prediction model for assessing prognosis in critically ill patients with sepsis-associated acute kidney injury: Shock, 2021; 56(4); 564-72
37. Liu H, Zhang L, Xu F, Establishment of a prognostic model for patients with sepsis based on SOFA: A retrospective cohort study: J Int Med Res, 2021; 49(9); 3000605211044892
38. Zhao L, Yang J, Zhou C, A novel prognostic model for predicting the mortality risk of patients with sepsis-related acute respiratory failure: a cohort study using the MIMIC-IV database: Curr Med Res Opin, 2022; 38(4); 629-36
39. Vrieze SI, Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC): Psychol Methods, 2012; 17(2); 228-43
Figures
 Figure 1. The levels of LA/ALB, PCT/ALB, and the SOFA score in survival and non-survival of septic patients. (A) The levels of LA/ALB across survival and non-survival groups. (B) The levels of PCT/ALB across survival and non-survival groups. (C) The levels of SOFA score across survival and non-survival groups. n=238 (survival group) and 102 (non-survival group), respectively. **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 1. The levels of LA/ALB, PCT/ALB, and the SOFA score in survival and non-survival of septic patients. (A) The levels of LA/ALB across survival and non-survival groups. (B) The levels of PCT/ALB across survival and non-survival groups. (C) The levels of SOFA score across survival and non-survival groups. n=238 (survival group) and 102 (non-survival group), respectively. **** P<0.0001. R version 4.2.3, The R Foundation, Vienna, Austria. Figure 2. Correlation network of SOFA score with laboratory indexes (Spearman analysis). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 2. Correlation network of SOFA score with laboratory indexes (Spearman analysis). R version 4.2.3, The R Foundation, Vienna, Austria. Figure 3. Kaplan-Meier survival curves of 340 adult septic patients based on the LAC/ALB (A) cutoff value (0.079) and PCT/ALB (B) cutoff value (0.256) on the day of ICU admission. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 3. Kaplan-Meier survival curves of 340 adult septic patients based on the LAC/ALB (A) cutoff value (0.079) and PCT/ALB (B) cutoff value (0.256) on the day of ICU admission. R version 4.2.3, The R Foundation, Vienna, Austria. Figure 4. Nomogram for predicting the prognosis of septic patients. Nomogram, to draw an upward vertical line to the “Points” bar to calculate points. Based on the sum, draw a downward vertical line from the “Total Points” line to calculate the probability of 28-d mortality in sepsis for each patient. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 4. Nomogram for predicting the prognosis of septic patients. Nomogram, to draw an upward vertical line to the “Points” bar to calculate points. Based on the sum, draw a downward vertical line from the “Total Points” line to calculate the probability of 28-d mortality in sepsis for each patient. R version 4.2.3, The R Foundation, Vienna, Austria. Figure 5. The validation of a predictive model for the prognosis of septic patients. (A) ROC curve of the prediction model for the prognosis of septic patients. (B) Calibration curve of the model for the prognosis of septic patients. R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 5. The validation of a predictive model for the prognosis of septic patients. (A) ROC curve of the prediction model for the prognosis of septic patients. (B) Calibration curve of the model for the prognosis of septic patients. R version 4.2.3, The R Foundation, Vienna, Austria. Figure 6. Clinical decision curve for the prognosis of septic patients. (X: probability with value, Y: net benefit, black line: hypothesis of no death in all septic patients, gray line: hypothesis of death in all septic patients). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 6. Clinical decision curve for the prognosis of septic patients. (X: probability with value, Y: net benefit, black line: hypothesis of no death in all septic patients, gray line: hypothesis of death in all septic patients). R version 4.2.3, The R Foundation, Vienna, Austria. Figure 7. The validation of a predictive model for the prognosis of septic patients (validation cohort). (A) ROC curve of the prediction model for the prognosis of septic patients (validation cohort). (B) Calibration curve of the model for the prognosis of septic patients (validation cohort). (C) Clinical decision curve for the prognosis of septic patients (validation cohort). R version 4.2.3, The R Foundation, Vienna, Austria.
Figure 7. The validation of a predictive model for the prognosis of septic patients (validation cohort). (A) ROC curve of the prediction model for the prognosis of septic patients (validation cohort). (B) Calibration curve of the model for the prognosis of septic patients (validation cohort). (C) Clinical decision curve for the prognosis of septic patients (validation cohort). R version 4.2.3, The R Foundation, Vienna, Austria. Tables
 Table 1. The comparisons of clinical features between the survival and non-survival groups of septic individuals.
Table 1. The comparisons of clinical features between the survival and non-survival groups of septic individuals. Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis.
Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis. Table 3. Logistic regression with best subsets variable reduction.
Table 3. Logistic regression with best subsets variable reduction. Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients.
Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients. Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort).
Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort). Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort).
Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort). Table 1. The comparisons of clinical features between the survival and non-survival groups of septic individuals.
Table 1. The comparisons of clinical features between the survival and non-survival groups of septic individuals. Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis.
Table 2. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis. Table 3. Logistic regression with best subsets variable reduction.
Table 3. Logistic regression with best subsets variable reduction. Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients.
Table 4. The comparisons of clinical features between the derivation cohort and validation cohort of septic patients. Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort).
Table 5. The comparisons of clinical characteristics between the survival and non-survival groups of septic individuals (validation cohort). Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort).
Table 6. ROC analysis of laboratory indexes for predicting the prognosis of individuals with sepsis (validation cohort). In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








