15 February 2024: Clinical Research
The Impact of Serum Uric Acid Levels on Hypertensive Disorders of Pregnancy in Advanced Maternal Age Women: A Retrospective Study from a Single Center in China
Li Luo12ADEFG, Sulan Huang2ABCEF, Zhijie Zhuang3BE, Yeping Mo4BE, Ning Guo2CE, Liangqing Ge12E*DOI: 10.12659/MSM.942629
Med Sci Monit 2024; 30:e942629
Abstract
BACKGROUND: In many countries, including China, women are delaying pregnancy until later in life; therefore, hypertensive disorders of pregnancy (HDP) are increasing. This retrospective study from a single center in China aimed to evaluate the association between serum uric acid (SUA) levels and HDP in 288 women of advanced maternal age >35 years.
MATERIAL AND METHODS: A total of 780 pregnant women of advanced maternal age were included in the study – 288 were had HDP (including gestational hypertension and preeclampsia) and 492 had normal blood pressure using 1: 2 (84: 168) propensity score matching. SUA (collected before 20 weeks’ gestation) and HDP incidence in advanced maternal age women were assessed using multivariate logistic modeling and 3 propensity score-based methods.
RESULTS: Median patient age was 37 years. The risk of developing HDP increases with higher SUA (30.19% vs 13.65%, P<0.001). In the PS-matched cohort, the risk ratio (OR) for HDP with high uric acid after adjusting for confounders was 2.88 (95% CI: 1.44-5.75, P=0.0027). It has been demonstrated that high uric acid is strongly associated with HDP incidence in both the crude population (OR=3.43, 95% CI: 2.01-4.66, P<0.0001) and the weighted cohorts (OR=3.62, 95% CI: 2.81-4.66, P<0.0001). As a successive variable, after adjusting for the clinical confounders, a 1-SD increase in SUA was related to a 135% increased risk of HDP (OR=2.35; 95% CI: 1.57-3.50; P<0.0001) based on the fully adjusted model. There were similar conclusions in the sensitivity analysis.
CONCLUSIONS: There was a significant association between SUA and HDP in women of advanced maternal age, supporting the importance of early detection of SUA in pregnant women.
Keywords: Uric Acid, Hypertension, Pregnancy-Induced, Risk Factors
Background
Hypertensive disorders of pregnancy (HDP) are among the top 3 causes of maternal, fetal, and neonatal morbidity and mortality [1]. Around 6–10% of pregnant women have HDP worldwide [2], while the incidence is 5.22–5.57% in China [3]. There are 4 types of HDP: gestational hypertension, preeclampsia-eclampsia, chronic hypertension, and chronic hypertension with superimposed preeclampsia [4,5]. The World Health Organization (WHO) warns that severe HDP during pregnancy can be dangerous for both the mother and fetus due to growth restrictions, placental abortion, neonatal death, poor placental transfer, and preterm birth [6,7]. Advanced maternal age is defined as childbearing in a woman ≥35 years and is a growing trend within high-income countries [8]. A high incidence of pregnancy-related hypertension occurs in overweight and older women, particularly in those with preexisting hypertension [8,9]. In China, the implementation of the 2-child policy in 2015 has led to increasing numbers of advanced maternal age pregnancies [10,11]. It is predicted that the number of women in their late 30s and early 40s wishing to have a second child will increase. Managing the expected increase in the number of older pregnant women will pose an additional challenge for obstetricians [12]. Advanced maternal age women are at risk of HDPs complications. Severe cases may have heart failure, renal failure, and other multiple-organ dysfunction symptoms, leading to increased risk of obstructed labor, postpartum bleeding, and fetal abnormalities [12]. The etiology and pathogenesis of HDPs remains unclear, and there is no effective prevention or treatment method at present [12]. These findings highlight that it is necessary to investigate the risk factors of HDPs in advanced-age pregnant women to provide a reference basis for its prevention and treatment.
It has been found that uric acid, one of the most common and earliest changes in HDP, is a much more reliable predictor of fetal and maternal risk than blood pressure [13]. The risk of new-onset hypertension in pregnancy to a mother and her baby has been conventionally differentiated by the presence or absence of proteinuria [13]. The presence of proteinuria in women with new-onset gestational hypertension is clinically diagnosed as preeclampsia and is associated with increased maternal/neonatal morbidity and mortality compared to isolated gestational hypertension [14]. Hyperuricemia has the same predictive value as proteinuria in predicting perinatal risk in high-risk women with gestational hypertension, according to Schmella et al [15]. Serum uric acid (SUA) is not necessarily indicative of disease severity, but rather plays a direct role in pathogenesis [16]. To minimize pregnancy complications and long-term consequences, early identification of pregnant women with high-complexity conditions is critical [17].
SUA’s efficacy as a biochemical marker is controversial [18]. A variety of factors can contribute to the production of uric acid, such as renal dysfunction, a breakdown of tissues, acidosis, and excessive activity of the enzyme xanthine oxidase. An early and consistent laboratory finding in pre-eclampsia is SUA, which is characteristic of patients affected by the condition [19]. In 2016, Elmas et al [20] conducted a study to determine the correlation between hypertension and plasma UA and its predictive utility in severe preeclampsia. Most studies conclude that uric acid is a useful predictor of hypertensive disorders of pregnancy [19,21] but others hold the view that SUA concentrations appear to be neither minimally predictive nor non-predictive in monitoring pregnancy hypertension [22]. Hawkins et al [23] found that elevated SUA levels in pregnant women with HDP had no obvious effect on maternal outcome. Some reports demonstrated no significant difference in serum uric acid levels in PE compared to normal pregnancies [24], perhaps because medications were generally administered at the start of gestation or as soon as symptoms began to appear [22]. The growing number of pregnant women of advanced maternal age is a worldwide trend [25]. Advanced-age women are prone to complications and numerous studies have analyzed the risk factors and outcomes for Chinese advanced-age women with HDP [26]. The impact of SUA on risk of HDPs among Chinese advanced-age women is unclear [12]. Few studies have examined the relationship between SUA and HDP in women of advanced maternal age. Therefore, this retrospective study from a single center in China, conducted from 2018 to 2022, of 780 pregnant women of advanced maternal age aimed to evaluate the association between serum uric acid levels in 288 women diagnosed with HDP.
Material and Methods
ETHICS STATEMENT:
This retrospective data-only study was approved by the Ethics Committee of the First People’s Hospital of Changde (No. 2020-155-02). As this was a retrospective study, informed consent was not required. All procedures were performed in compliance with the applicable guidelines and the Helsinki Declaration.
STUDY POPULATION:
This study enrolled pregnant women with advanced maternal age who were previously hospitalized or who delivered at the Obstetrics Department of Changde Hospital, China, from January 2018 to May 2022 (including preterm labor, delivery, and stillbirth). Pregnant women with gestational age above 28 weeks, attending the antenatal clinic for regular checkups in the Department of Obstetrics were enrolled in this study. An ongoing nonselective collection of data on Chinese women over the age of 35 years was conducted at Changde Hospital, with identifying information about patients removed. Data were collected using semistructured questionnaires. Training was provided to the data collectors on the study objectives, procedures, confidentiality, respondent rights, and informed consent. The data collection process was closely supervised. Written informed consent was obtained from women agreeing to participate in the study. Institutional Ethics Committee clearance was obtained. All patients were regularly followed up at the Outpatient Department at approximately 3 months, and physicians performed regular blood pressure monitoring and laboratory investigations. Patients were excluded if more than 10% of their data were missing. Missing data for records with 10% of missing variables were extrapolated using multiple imputations, by which missing observations can be replaced by plausible values drawn from the posterior predictive distribution based on the observations [27]. As a result, several complete datasets were generated with plausible values for the missing values.
The study recruited 990 pregnant women of advanced maternal age. The inclusion criteria were age ≥35 years, single pregnancy, available medical records, and complete laboratory test results. The exclusion criteria were missing SUA measurements after admission (n=58), renal failure (glomerular filtration rate <60 ml/min per 1.73 m2, n=10), and chronic hypertension (n=142). Finally, we included 780 women with advanced maternal age, including 288 with HDP (124 with gestational hypertension and 164 with preeclampsia) and 492 without hypertension (Figure 1).
DEFINITIONS OF ADVANCED MATERNAL AGE:
Advanced maternal age is defined as childbearing in a woman over 35 years and is a growing trend within high-income countries [8].
DEFINITIONS OF HDP:
The outcomes of the study were gestational hypertension and preeclampsia. Gestational hypertension was diagnosed when blood pressure140/90 mmHg without proteinuria occurred after 20 weeks’ gestation [13,28]. Preeclampsia is defined as 2 or more occasions of elevated systolic blood pressure or high diastolic blood pressure with 6-h intervals and proteinuria of >300 mg in 24-h urine specimens after 20 weeks of gestation [4,13,29].
SERUM URIC ACID QUANTIFICATION AND EVALUATION:
Study exposure was determined based on SUA levels before 20 weeks’ gestation. Three mL of venous blood was drawn from fasted pregnant women early in the morning after they were admitted in the hospital. SUA levels were analyzed using commercially available kits on an automatic biochemical analyzer (AU5800-A; Beckman Coulter, Japan). Reference values for women were 180–420 μmol/L. High uric acid (HUA) was defined as SUA of ≥420 μmol/L, whereas a normal concentration of uric acid (NUA) equals 420 mol/L in normal reference standards [30].
BLOOD PRESSURE MEASUREMENT METHOD:
In addition to monitoring blood pressure on admission, blood pressure was measured 3 times in the morning by a qualified nurse using an Omron HEM-705 automatic blood pressure monitor (Omron Healthcare, Ltd., Changsha, China) according to the standard measurement procedure recommended by the American Heart Association – in quiet room with comfortable temperature, no smoking, caffeine, or exercise for 30 min before measurements, empty bladder, remain seated and relaxed for 3–5 min, sitting with the arm resting on a table with mid-arm at heart level, back supported on chair, legs uncrossed, and feet flat on the floor) [31]. Analyses were based on the mean of 3 readings.
LABORATORY ANALYSIS AND EVALUATION:
Electronic medical records from the hospital were used to gather information on maternal characteristics such as age, blood pressure, body mass index (BMI), gestational week, parity, and gravidity. Blood samples were collected before 20 weeks’ gestation (at 14–19 weeks). A hospital laboratory information system was used to collect maternal SUA, blood lipid, creatinine, urea nitrogen (BUN), and blood glucose levels. An automatic analyzer (AU5800-A, Beckman Coulter, Japan) was used to measure SUA, blood lipid, creatinine, and BUN levels using oxidase, creatinine oxidase, catalase scavenging, and enzymatic procedures, respectively. A laboratory analyst performed all laboratory analyses prior to delivery without knowing the delivery outcome within the corresponding gestational period. Calibration and daily maintenance were performed according to the laboratory quality management system policy. Internal laboratory quality control was run to ensure the accuracy and reliability of laboratory testing before running patient samples. The control result was interpreted using a Levey-Jennings chart. The control result fell within the acceptable ranges (mean±2 SD). After samples were thoroughly mixed, they were processed by senior laboratory technologists.
STATISTICAL ANALYSES:
Variables with a continuous value are displayed as the average±standard deviation (SD) or the median (interquartile range). Based on the normality of the data distribution, the t test or Mann-Whitney U test was used. Comparisons between groups were made using the unpaired t test for normally distributed quantitative variables, whereas the non-parametric Mann-Whitney test was used for non-normally distributed quantitative variables. Categorical variables were analyzed using the chi-square test and are displayed as case quantities (%). Propensity score matching (PSM) was employed because it is difficult to achieve complete stochasticity during patient screening. This approach balances the selection bias and underlying confounding factors. We analyzed the PSM analysis based on previous literature, and data characteristics’ analysis was conducted using a logistic regression model developed using age, red blood cell count, creatinine level, BUN level, DBP, heart rate, gestational week, and gestational diabetes (GDM). The degree of PSM was assessed based on standardized mean differences (SMDs), with a threshold of 0.2 considered acceptable. For pairs of patients with normal SUA (<420) and high SUA (≥420), 1: 2 matching was performed with a caliper of 0.2. Univariate regression analysis and multivariate regression analysis were conducted to evaluate the diagnostic significance of SUA levels. After considering clinical significance, the final adjusted model retained the covariates that caused a significant change in the effect estimate of more than 10% (P<0.05) [32]. As part of the inverse probability of treatment-weighted (IPTW) calculation, the PS was estimated to perform sensitivity analyses. This example uses 1/PS to represent the HUA weight and 1/(1-PS) to represent the NUA weight. By using the IPTW model, a weighted cohort was created. In both the original and weighted cohorts, 2 relationship inference models were used for the sensitivity analysis [33,34]. In addition, we calculated E-values for statistically significant primary outcomes to assess the robustness of the observed associations to potential unmeasured confounding factors and then inspected the modification and interaction of the subgroups using the likelihood ratio test. Finally, we generated receiver operating characteristic (ROC) curves to measure the sensitivity and specificity of SUA and calculated the area under the curve to ascertain the quality of SUA as a predictor of HDP in women of advanced maternal age. Analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA), R (http://www.R-project.org; The R Foundation, Vienna, Austria), and Empower Stats (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA, USA).
Results
STUDY POPULATION AND CLINICAL CHARACTERISTICS RESULTS:
Of the 990 pregnant women with advanced maternal age, 780 met the inclusion and exclusion criteria. We analyzed the PSM analysis based on previous literature and data characteristics. The 2 groups of patients were matched using nearest-neighbor matching without replacement at a fixed ratio of 1: 2. We finally included 252 women in the final study through propensity score matching (1: 2) according to maternal age, red blood cell count, creatinine, BUN, diastolic blood pressure (DBP), heart rate (HR), gestational week, and GDM. With the use of propensity score matching, 84 participants with SUA levels ≥420 μmol/L were matched with 168 participants with SUA levels <420 μmol/L (Figure 1). Compared to preeclampsia without severe symptoms, SUA levels were higher in severe preeclampsia [510.0(428.7–563.0) vs 325.0(281.0–361.5), P<0.001]. In the study population, the average maternal age was 37.6±2.7 years.
Our preliminary analysis of the confounding variables identified significant differences between the HUA and NUA groups before PSM (OR=3.43, 95% CI: 2.01–5.84, P<0.0001). Patients with HUA appeared to have lower platelets, albumin (ALB) level, and gestational week, and a higher red blood cell distribution width (RDW), alanine transaminase (ALT), aspartate transaminase (AST), BUN, creatinine, potassium, total cholesterol (TC), triglyceride (TG), SUA, systolic pressure (SBP), and DBP levels, and were more likely to have GDM, urine protein, and HDP (all P<0.05). In the PSM-matched population, there were differences in platelets, ALB, AST, BUN, creatinine, potassium, TC, low-density lipoprotein (LDL), high-density lipoprotein (HDL), SUA, SBP, DBP, and prevalence of HDP (all P<0.05). Computation of standardized mean differences indicated a rate of <20% for all covariates (Table 1).
Figure 2 shows the HUA of different hypertension groups. HUA was more frequently observed in patients with preeclampsia (13.89% vs 11.1%, P=0.035).
UNIVARIATE AND MULTIVARIATE LOGISTIC ANALYSIS RESULTS:
The results of the univariate analysis are shown in Table 2 (the original population) and Table 3 (the matched population). Table 4 shows the estimated ORs and CIs for SUA levels in relation to HDP after PSM. As a categorical variable, HDP prevalence was significantly higher in the HUA group than in the NUA group (Crude Model: OR=3.25, 95% CI: 1.88–5.60, P<0.001; Model I: OR=3.31, 95% CI: 1.85–5.91, P<0.001; Model II: OR=2.88, 95% CI: 1.44–5.75, P=0.0027). As a successive variable, after adjusting for the clinical confounders, a 1-SD increase in SUA was related to a 135% increased risk of HDP (OR=2.35; 95% CI: 1.57–3.50; P<0.0001) based on the fully adjusted model. Moreover, we constructed 3 models to investigate the independent effects of the SUA ratio on HDP among advanced maternal age patients according to their SUA ratio tertiles (Crude Model: OR=1.96, 95% CI: 3.01–11.79, P<0.001; Model I: OR=5.96, 95% CI: 2.84–12.49, P<0.001; Model II: OR=4.90, 95% CI: 2.01–11.98, P=0.005). The SUA group counts were treated as continuous variables to ensure that our results would be robust (P for trend <0.001).
SENSITIVITY ANALYSIS RESULTS:
These findings led to a sensitivity analysis, which confirmed the association between SUA levels and HDP incidence in both cohorts (Table 5), and a weighted cohort was developed with the estimated PS through the development of an IPTW model. Moreover, we analyzed both cohorts with non-adjusted, partially adjusted, and fully adjusted models. In the original cohort, a high risk of HDP was noted in the cases with HUA (Crude Model: OR=4.49, 95% CI: 2.90–6.96, P<0.001; Model I: OR=4.89, 95% CI: 3.10–7.71, P<0.001; Model II: OR=3.43, 95% CI: 2.01–5.84, P<0.001) after adjusting different covariates. Using SUA as successive and tertile variables, the results (Crude Model: OR=2.02, 95% CI: 1.65–2.47, P<0.001; Model I: OR=2.80, 95% CI: 2.24–3.52, P<0.001; Model II: OR=3.62, 95% CI: 2.81–4.66, P<0.001) remained significant in the weighted cohort.
E-VALUE ANALYSIS RESULTS:
Using the Van der Weele and Ding’s E-value methodology, a sensitivity analysis was conducted to determine possible confounding effects. A contextually low E-value implies that there is weak evidence for an observed effect such that an unmeasured confounder could explain away the observed results. A relatively high E-value, on the other hand, renders the observed effect more probable since the strength and association of the unmeasured confounder with an exposure factor and outcome must be very high to explain away the observed effect [35]. For the current study, the adjusted ORs for the associations between SUA and HDP were 2.88 (95% CI: 1.44–5.75). An unmeasured confounder could fully account for the association between intensification and HDP if it was associated with exposure and outcome with an OR of 5.21(lower confidence limit, 3.46) (Figure 3). This E-value suggests that SUA’s current association between SUA and HDP has fewer confounding factors.
SUBGROUP ANALYSIS RESULTS:
Subgroup analyses are performed to identify subgroups of patients in whom a risk factor has the largest effect. To evaluate the robustness of our findings, a stratified analysis was conducted in a variety of subgroups after PSM. After adjusting for potential confounders, there were no interactions in GDM, maternal age, RDW, BMI, SBP, DBP, heart rate, gestational week, urine protein, and parity. Patients with anemia showed an increased risk of HUA (OR=1.13, 95% CI, 1.09–1.17, P<0.0001). Patients with high platelets also had an increased risk of HUA (OR=1.09, 95% CI, 1.06–1.13, P<0.0001) (Table 6, Figure 4).
PREDICTION OF HDP RESULTS:
Finally, we examined the performance of SUA as a biomarker for HDP diagnosis. ROC were constructed with SUA in the original (Figure 5) and the matched populations (Figure 6). The AUC was 0.684 [95% CI: 0.642–0.727) for the original population and 0.724 (95% CI: 0.655–0.782) for the matched population.
Discussion
STUDY STRENGTHS AND LIMITATIONS:
The strengths of our study include advanced maternal age and standardized measurement of BP and SUA levels. This study is the first to investigate the relationship between SUA levels and HDP in women of advanced maternal age in China. Although several confounding factors were controlled for, SUA and HDP still showed a relationship, indicating that our findings are stable. We performed non-linear regression analysis, which is a statistical modeling technique used to analyze and model relationships between variables when the relationship is not linear. In a non-linear regression model, the relationship between the dependent variable and the independent variables is represented by a non-linear equation. Unlike linear regression models, which assume a linear relationship between variables, non-linear regression models can capture more complex and flexible relationships and provided increased statistical power and reduced the loss of information that occurs when variables are categorized. Finally, the use of sensitivity analysis assured the robustness of our analyses and allowed us to examine the influence of subgroups.
Nevertheless, there are some limitations to our study. First, causal inferences were not possible because of the nature of the study. While propensity score matching is considered one of the most effective methods for mitigating inherent biases in nonrandomized studies, it is important to exercise caution when interpreting our findings as they may not completely eliminate residual biases. Second, the analysis may have introduced measurement errors due to the influence of specific factors on SUA and the exclusion of various SUA measurements. Finally, because only data from 1 SUA test were available upon admission, repeated-measures data were not analyzed. The SUA levels may have been influenced by variations in blood collection times, leading to bias in our findings. Therefore, we recommend further research, possibly through longitudinal or randomized controlled studies, to more conclusively determine the causal relationship between elevated SUA levels and HDP in this population.
Conclusions
Our study demonstrated a significant association between hyperuricemia and the development of HDP in women of advanced maternal age. This conclusion is substantiated by the robust statistical correlations observed between SUA levels and HDP incidences in our propensity score-matched analysis. These findings highlight the potential role of SUA as a key biomarker in predicting and managing HDP, particularly in older pregnant women. However, the nature of our study design precludes definitive conclusions about causality. Therefore, we recommend further research, possibly through longitudinal or randomized controlled studies, to more conclusively determine the causal relationship between elevated SUA levels and HDP in this population.
Figures
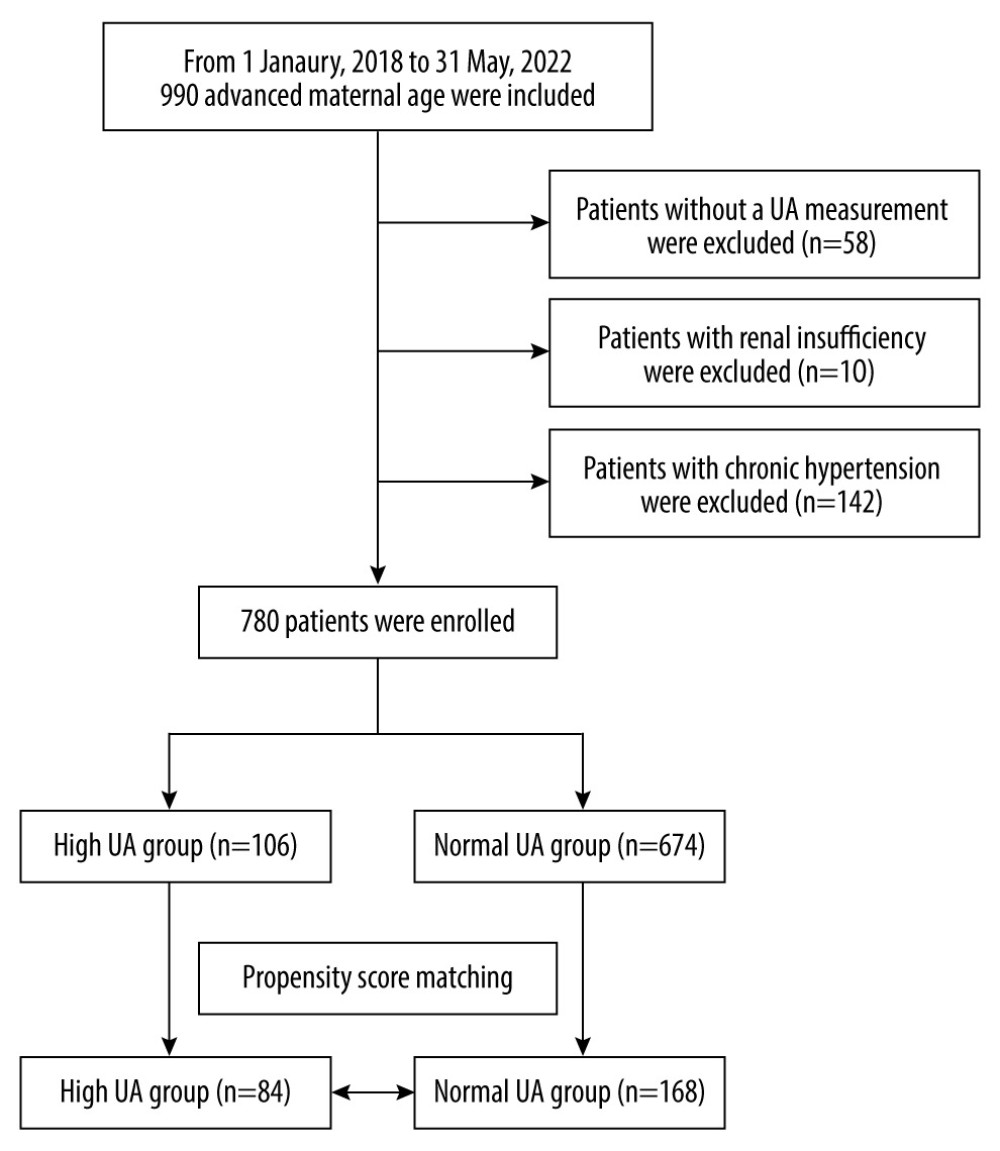 Figure 1. Flowchart of the participants. A total of 780 pregnant women of advanced maternal age were included in the analysis.
Figure 1. Flowchart of the participants. A total of 780 pregnant women of advanced maternal age were included in the analysis. 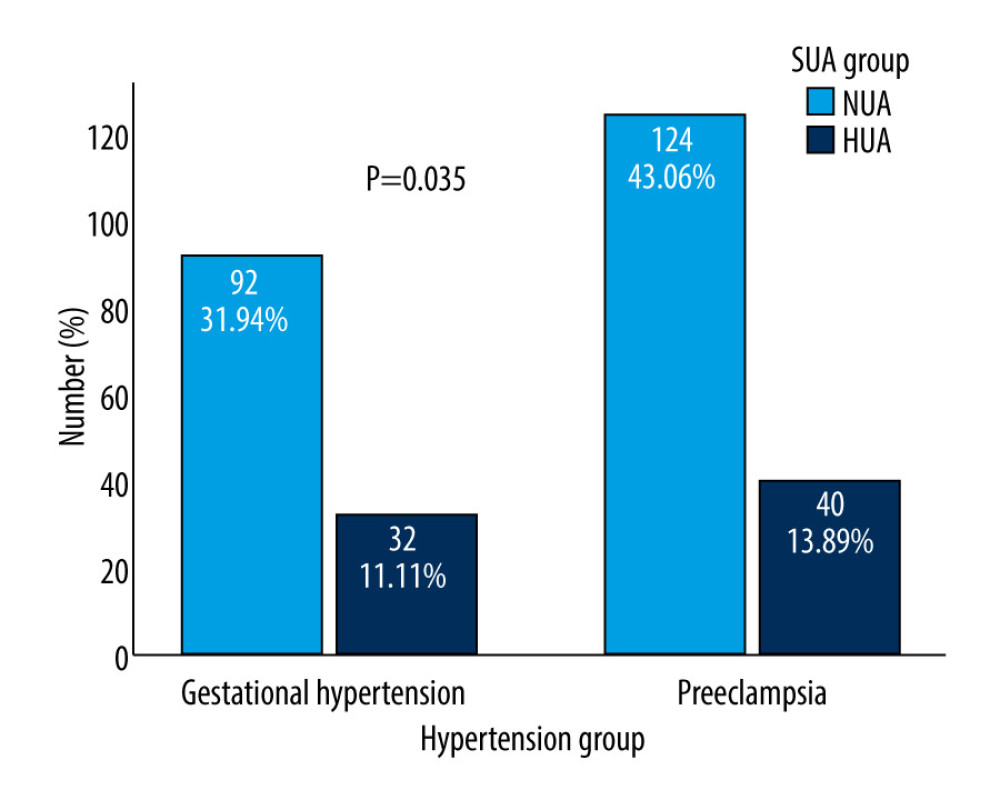 Figure 2. Differences in SUA between gestational hypertension and preeclampsia. HUA incidence was more frequently observed in patients with preeclampsia/eclampsia (13.89% vs 11.1%, P=0.035). SUA – serum uric acid; HUA – high uric acid.
Figure 2. Differences in SUA between gestational hypertension and preeclampsia. HUA incidence was more frequently observed in patients with preeclampsia/eclampsia (13.89% vs 11.1%, P=0.035). SUA – serum uric acid; HUA – high uric acid. 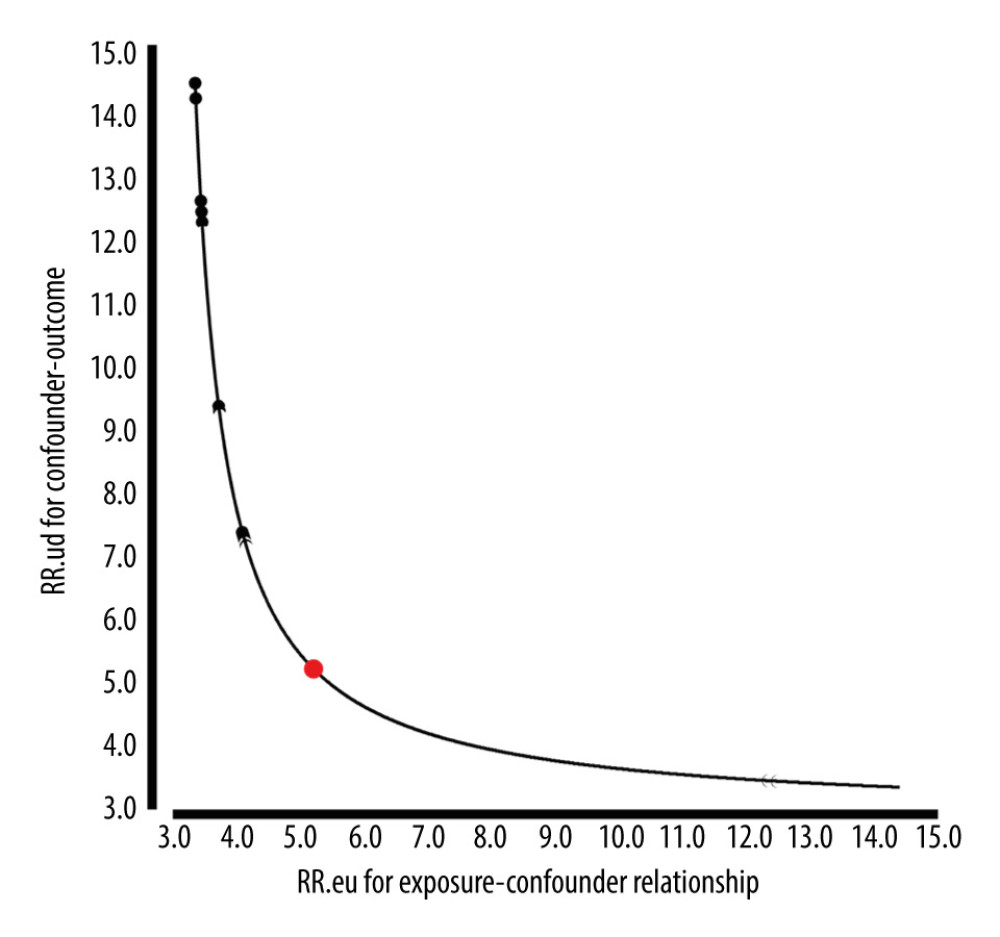 Figure 3. E-Value analyses. An unmeasured confounder could fully account for the association of intensification with HDP if it was associated with the exposure and outcome by an OR of 5.21(lower confidence limit, 3.46). HDP – hypertensive disorders of pregnancy; OR – ratio risk.
Figure 3. E-Value analyses. An unmeasured confounder could fully account for the association of intensification with HDP if it was associated with the exposure and outcome by an OR of 5.21(lower confidence limit, 3.46). HDP – hypertensive disorders of pregnancy; OR – ratio risk. 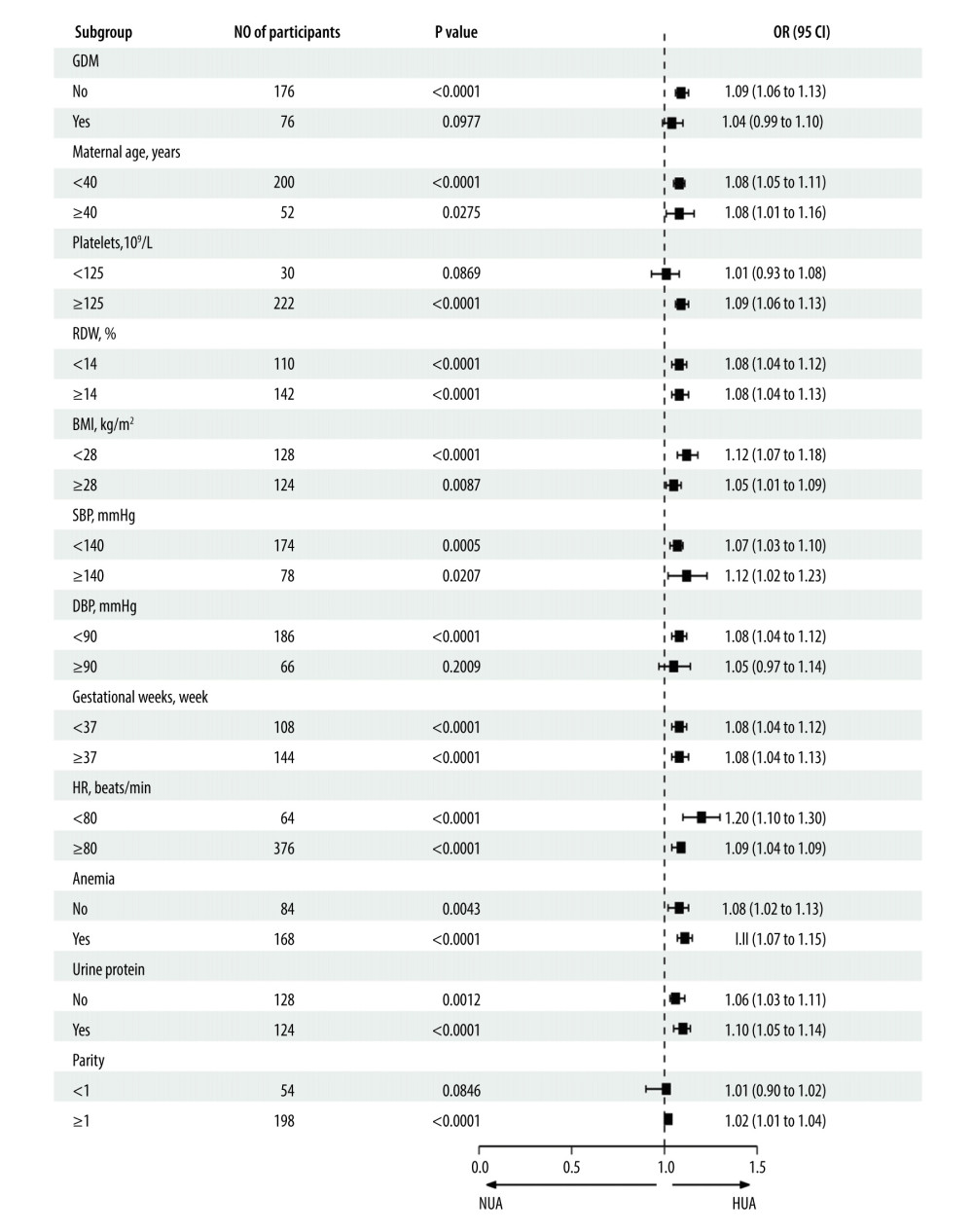 Figure 4. Forest plot of the association between SUA and HDP after adjusting for potential confounders, there were no interactions in GDM, maternal age, RDW, BMI, SBP, DBP, heart rate, gestational week, urine protein, and parity. Patients with anemia showed an increased risk of HUA (OR=1.13, 95% CI, 1.09–1.17, P<0.0001). Patients with high platelets also indicated an increased risk of HUA (OR=1.09, 95% CI, 1.06–1.13, P<0.0001). SUA – serum uric acid; HDP – hypertensive disorders of pregnancy; GDM – gestational diabetes; RDW – red blood cell distribution width; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; OR – ratio risk.
Figure 4. Forest plot of the association between SUA and HDP after adjusting for potential confounders, there were no interactions in GDM, maternal age, RDW, BMI, SBP, DBP, heart rate, gestational week, urine protein, and parity. Patients with anemia showed an increased risk of HUA (OR=1.13, 95% CI, 1.09–1.17, P<0.0001). Patients with high platelets also indicated an increased risk of HUA (OR=1.09, 95% CI, 1.06–1.13, P<0.0001). SUA – serum uric acid; HDP – hypertensive disorders of pregnancy; GDM – gestational diabetes; RDW – red blood cell distribution width; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; OR – ratio risk. 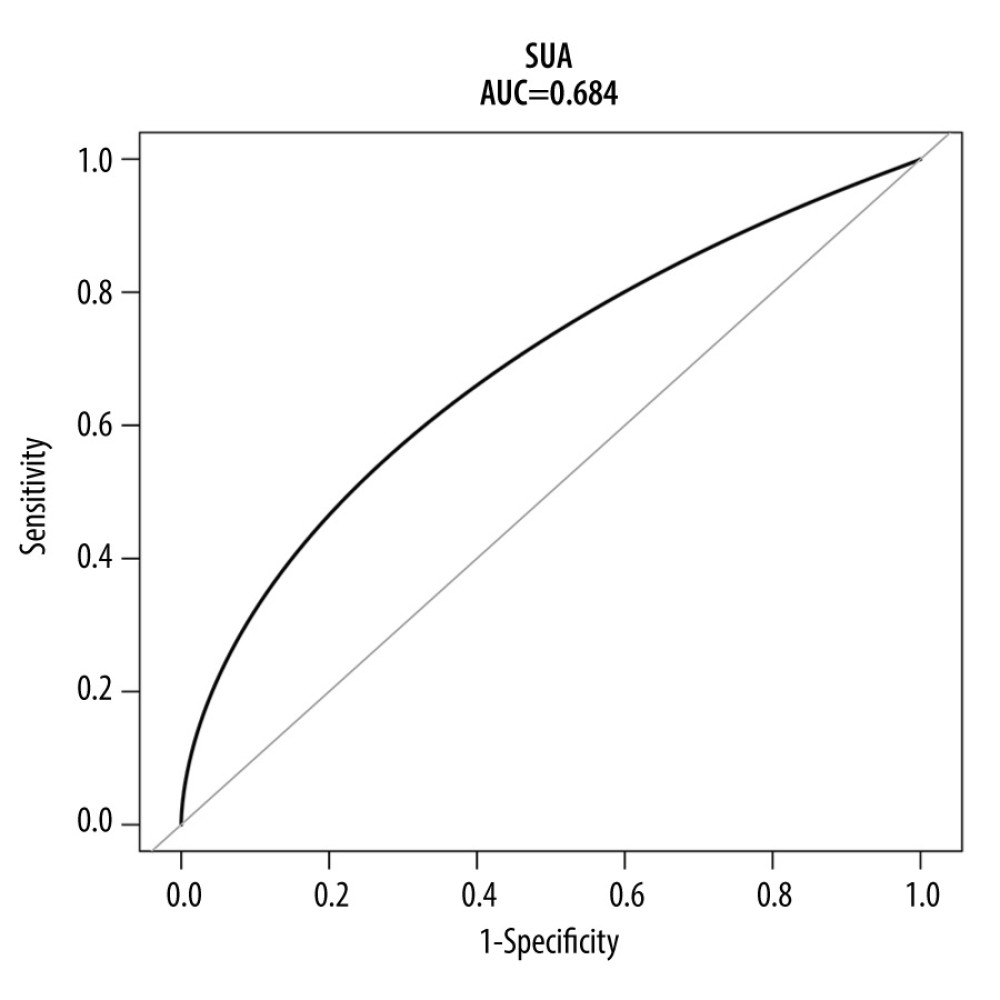 Figure 5. ROC curve for the prediction of HDP in the original population. The AUC was 0.684 [95% CI: 0.642–0.727) for the original population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve.
Figure 5. ROC curve for the prediction of HDP in the original population. The AUC was 0.684 [95% CI: 0.642–0.727) for the original population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve. 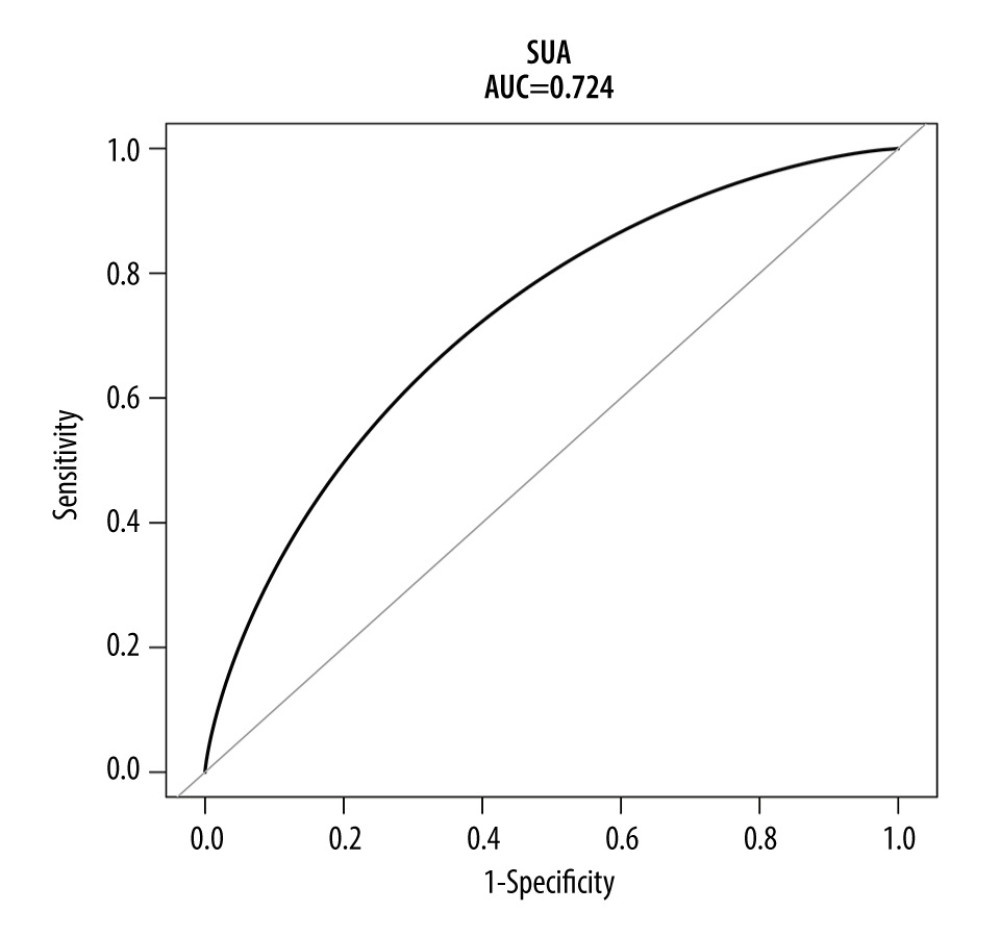 Figure 6. ROC curve for the prediction of HDP in the matched population. The AUC was 0.724 (95% CI: 0.655–0.782) for the matched population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve.
Figure 6. ROC curve for the prediction of HDP in the matched population. The AUC was 0.724 (95% CI: 0.655–0.782) for the matched population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve. Tables
Table 1. Baseline clinical characteristics before and after propensity score matching.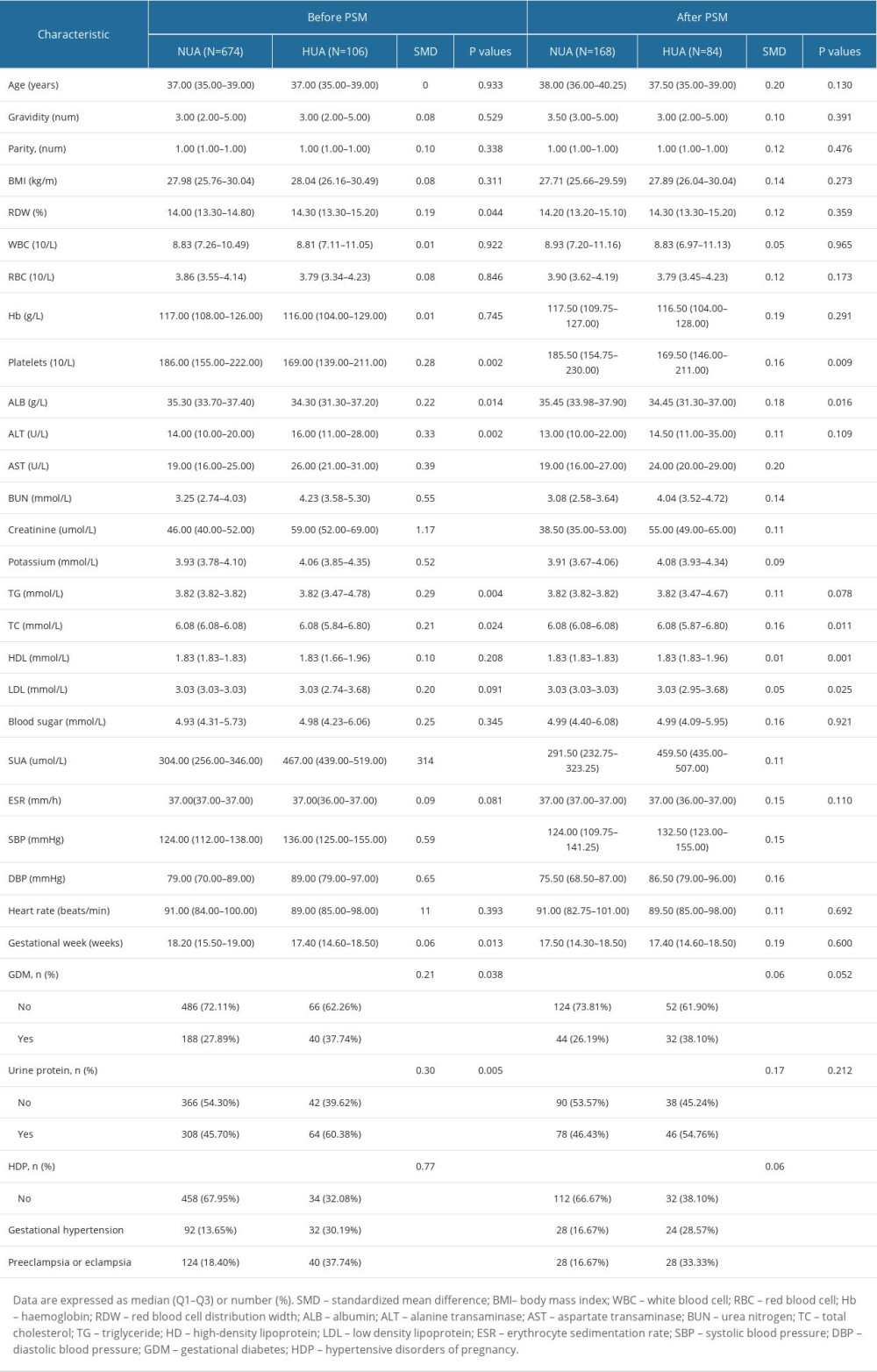 Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis.
Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis.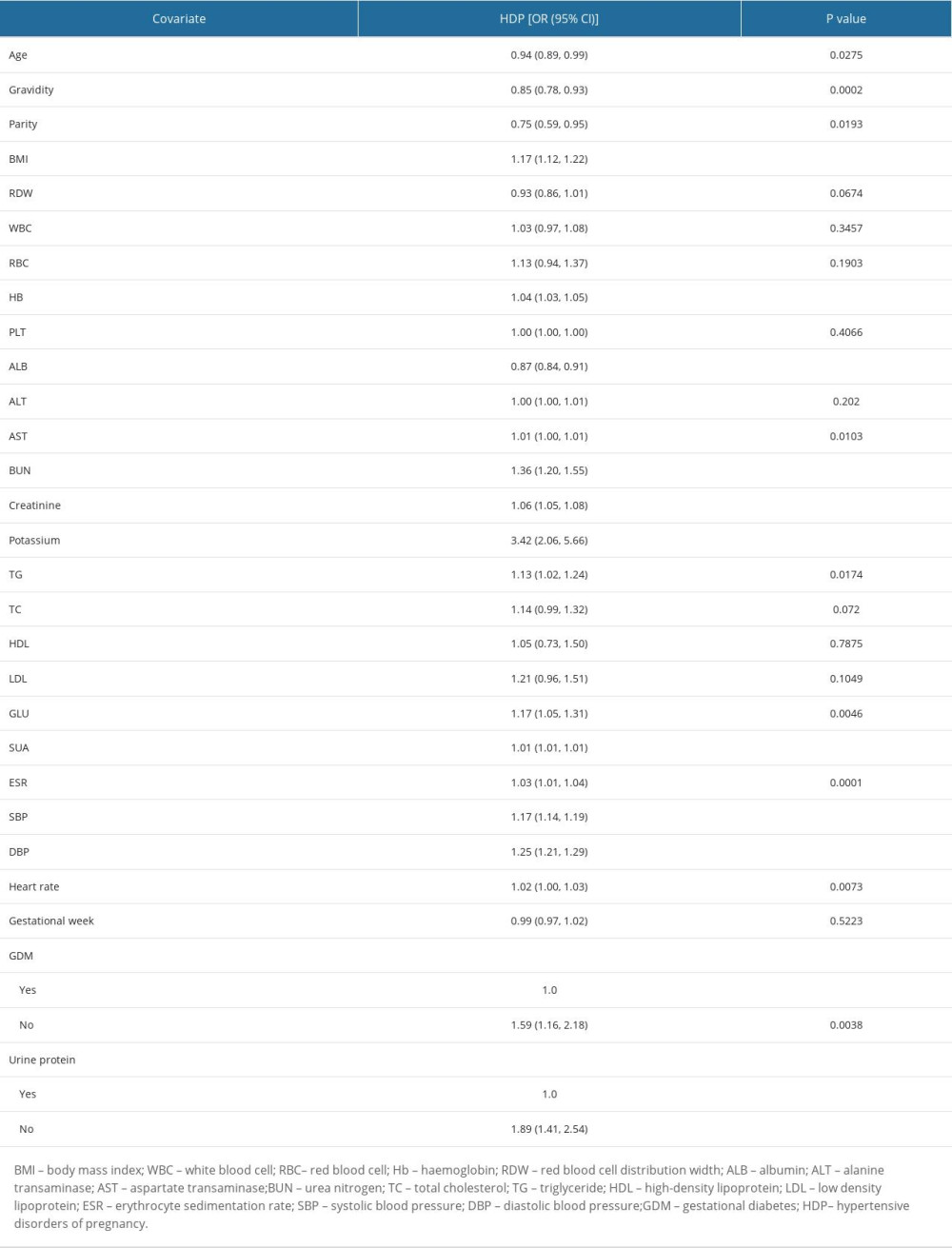 Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis.
Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis.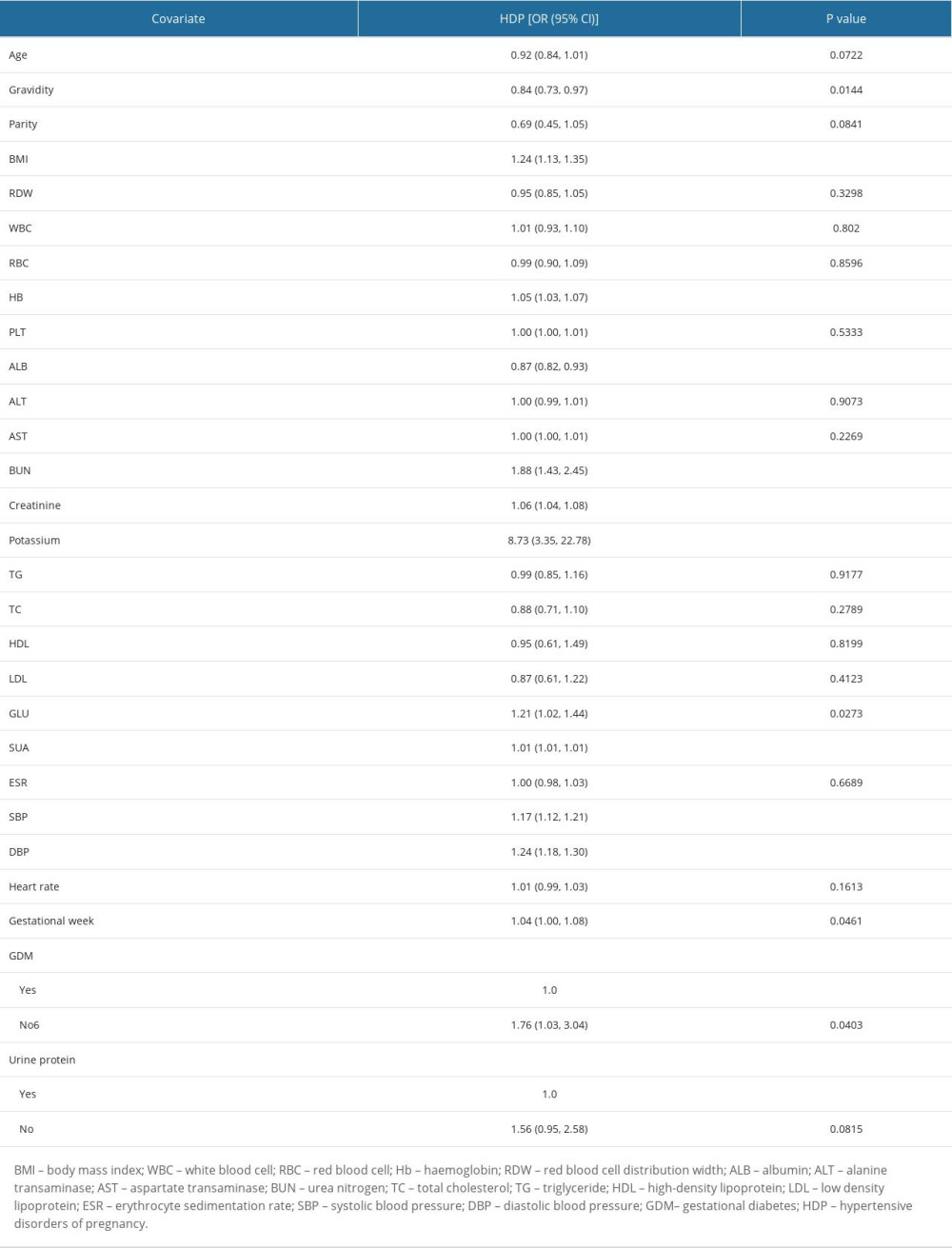 Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort.
Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort.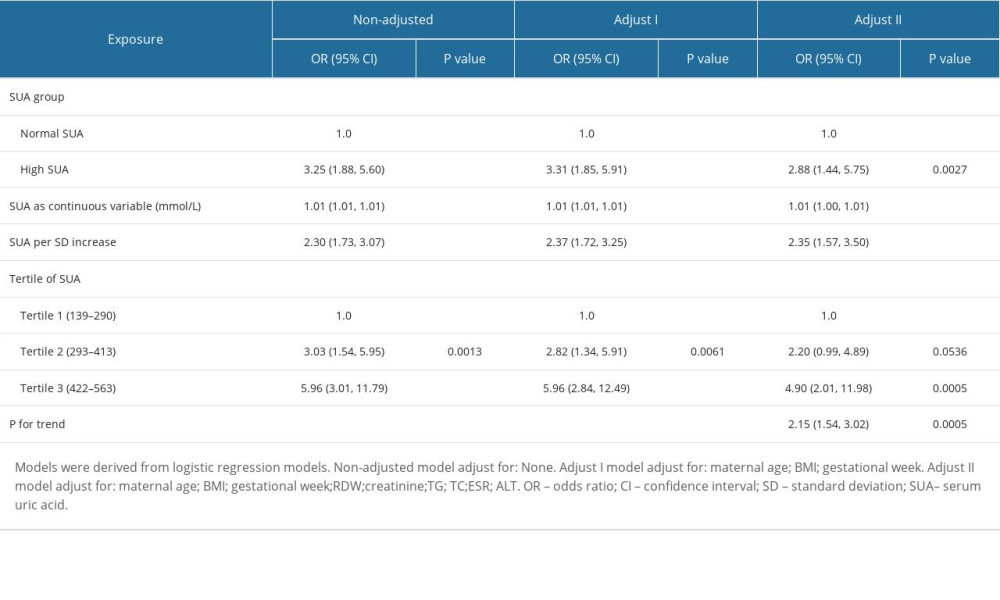 Table 5. Association of HUA and HDP in the original and the weighted cohorts.
Table 5. Association of HUA and HDP in the original and the weighted cohorts.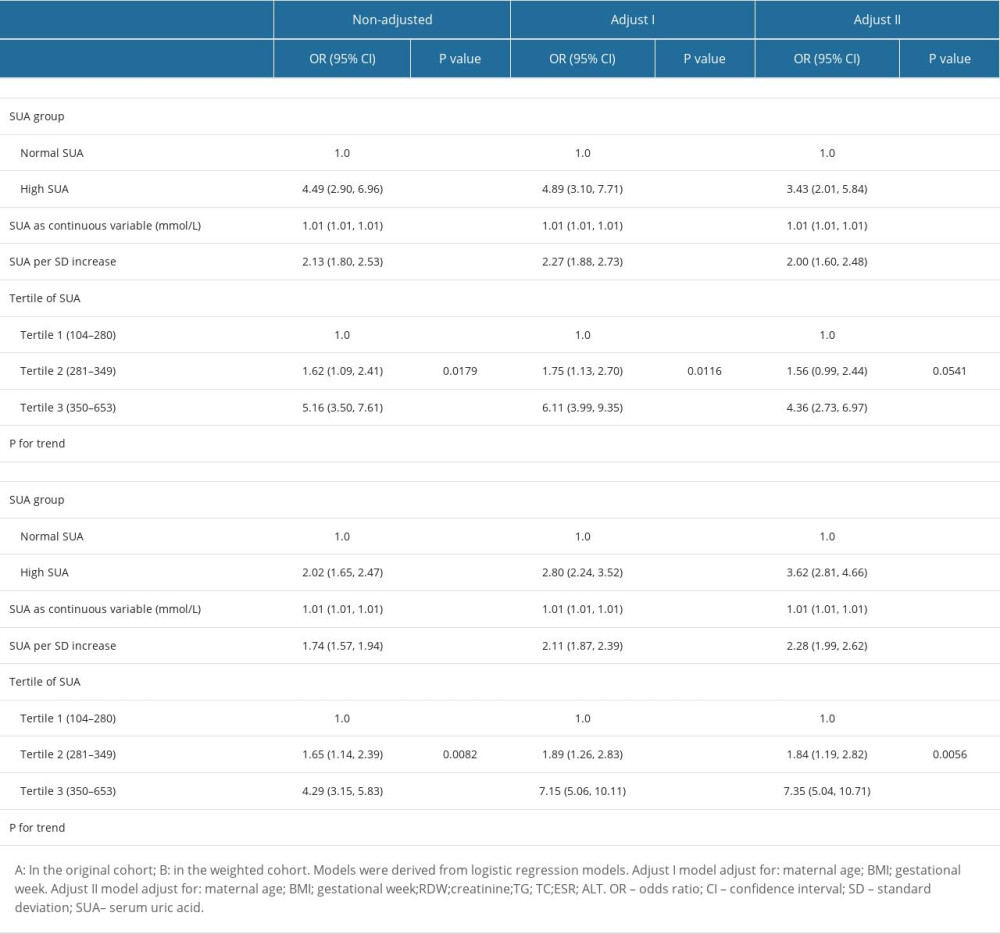 Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups.
Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups.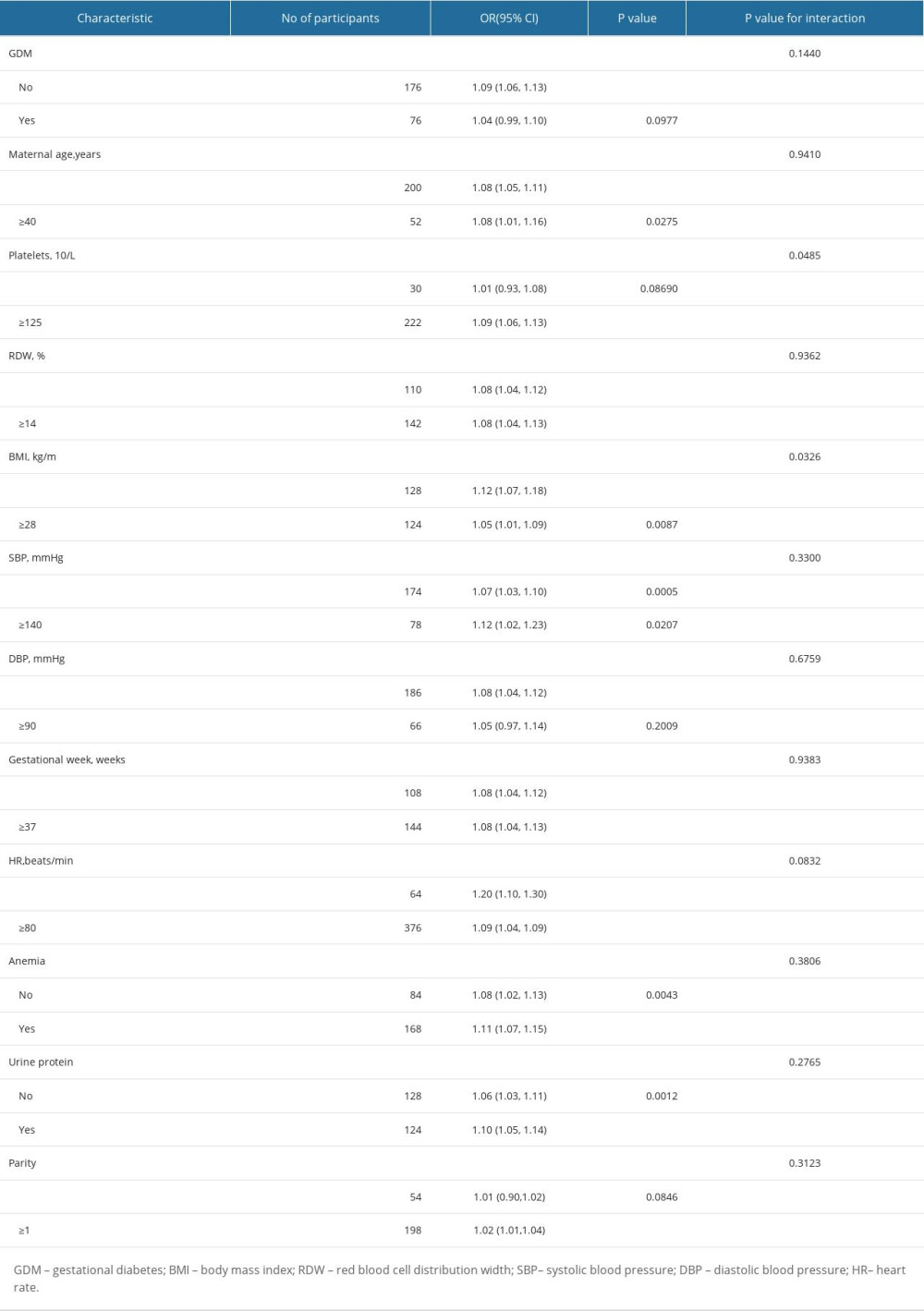
References
1. Coggins N, Lai S, Hypertensive disorders of pregnancy: Emerg Med Clin North Am, 2023; 41; 269-80
2. Umesawa M, Kobashi G, Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis: Hypertens Res, 2017; 40; 213-20
3. Ye C, Ruan Y, Zou L, The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: Prevalence, risk factors, complications, pregnancy and perinatal outcomes: PLoS One, 2014; 9; e100180
4. American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy: Obstet Gynecol, 2013; 122; 1122-31
5. Hassen FS, Malik T, Dejenie TA, Evaluation of serum uric acid and liver function tests among pregnant women with and without preeclampsia at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia: PLoS One, 2022; 17; e0272165
6. Phumsiripaiboon P, Suksai M, Suntharasaj T, Screening for pre-eclampsia: Performance of National Institute for Health and Care Excellence guidelines versus American College of Obstetricians and Gynecologists recommendations: J Obstet Gynaecol Res, 2020; 46; 2323-31
7. Areda BG, Gizaw ST, Berdida DH, Evaluation of serum lipid profiles, uric acid, and high sensitivity C-reactive protein levels between pregnancy-induced hypertension and normotensive pregnant women attending Ambo University Referral Hospital, Ambo, Ethiopia, 2020: A case-control study: Health Sci Rep, 2022; 5; e806
8. Lean SC, Derricott H, Jones RL, Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis: PLoS One, 2017; 12; e0186287
9. Jolly M, Sebire N, Harris J, The risks associated with pregnancy in women aged 35 years or older: Hum Reprod, 2000; 15; 2433-37
10. Cheng PJ, Duan T, China’s new two-child policy: Maternity care in the new multiparous era: BJOG, 2016; 123(Suppl 3); 7-9
11. Li F, Qin J, Zhang S, Prevalence of hypertensive disorders in pregnancy in China: A systematic review and meta-analysis: Pregnancy Hypertens, 2021; 24; 13-21
12. Zhang H, Wang W, Risk factors and adverse pregnancy outcomes in older pregnant women with hypertensive disorders of pregnancy: J Obstet Gynaecol Res, 2022; 48(7); 1710-20
13. Luger RK, Kight BP, Hypertension. Pregnancy: StatPearls [Internet[ Oct 3, 2022, Treasure Island (FL), StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK430839/
14. Rui M, Liping H, Yanmin W, Regional differences in clinical characteristics and fetal and maternal outcomes of hypertensive disorders in pregnancy in China: A retrospective study: Hypertens Pregnancy, 2023; 42(1); 2234490
15. Schmella MJ, Clifton RG, Althouse AD, Uric acid determination in gestational hypertension: Is it as effective a delineator of risk as proteinuria in high-risk women?: Reprod Sci, 2015; 22(10); 1212-19
16. Feig DI, Madero M, Jalal DI, Uric acid and the origins of hypertension: J Pediatr, 2013; 162; 896-902
17. Corominas AI, Medina Y, Balconi S, Assessing the role of uric acid as a predictor of preeclampsia: Front Physiol, 2021; 12; 785219
18. Pecoraro V, Trenti T, Predictive value of serum uric acid levels for adverse maternal and perinatal outcomes in pregnant women with high blood pressure. A systematic review and meta-analysis: Eur J Obstet Gynecol Reprod Biol, 2020; 252; 447-54
19. Colmenares-Mejia CC, Quintero-Lesmes DC, Bautista-Niño PK, Uric acid and risk of pre-eclampsia: Results from a large case-control study and meta-analysis of prospective studies: Sci Rep, 2023; 13; 3018
20. Elmas O, Elmas O, Aliciguzel Y, The relationship between hypertension and plasma allantoin, uric acid, xanthine oxidase activity and nitrite, and their predictive capacity in severe preeclampsia: J Obstet Gynaecol, 2016; 36(1); 34-38
21. Corominas AI, Balconi SM, Palermo MSerum uric acid levels and risk of developing preeclampsia: Medicina (B Aires), 2014; 74(6); 462-71 [in Spanish]
22. Moharana JJ, Mishra R, Nayak AK, A study on serum lactate dehydrogenase and uric acid in preeclampsia and eclampsia: Can they predict adverse fetomaternal outcome?: Int J Appl Basic Med Res, 2023; 13(2); 95-100
23. Hawkins TLA, Roberts JM, Mangos GJ, Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: A retrospective cohort study: BJOG, 2012; 119(4); 484-92
24. Punthumapol C, Kittichotpanich B, Serum calcium, magnesium and uric acid in preeclampsia and normal pregnancy: J Med Assoc Thai, 2008; 91(7); 968
25. Kim YN, Choi DW, Kim DS, Maternal age and risk of early neonatal mortality: A national cohort study: Sci Rep, 2021; 11(1); 814
26. Wang Z, Wang Y, Cai WW, High-risk factors of elderly pregnant woman with gestational hypertension and its influence on pregnancy outcome: Matern Child Health Care China, 2020; 35(22); 4204-6
27. Segura-Buisan J, Leyrat C, Gomes M, Addressing missing data in the estimation of time-varying treatments in comparative effectiveness research: Stat Med, 2023; 42(27); 5025-38
28. , Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy: Am J Obstet Gynecol, 2000; 183; S1-S22
29. Chen Y, Ou W, Lin D, Increased uric acid, gamma-glutamyl transpeptidase and alkaline phosphatase in early-pregnancy associated with the development of gestational hypertension and preeclampsia: Front Cardiovasc Med, 2021; 8; 756140
30. Borghi C, Agnoletti D, Cicero AFG, Uric acid and hypertension: A review of evidence and future perspectives for the management of cardiovascular risk: Hypertension, 2022; 79; 1927-36
31. Unger T, Borghi C, Charchar F, 2020 International Society of Hypertension global hypertension practice guidelines: J Hypertens, 2020; 38(6); 982-1004
32. Kernan WN, Viscoli CM, Brass LM, Phenylpropanolamine and the risk of hemorrhagic stroke: N Engl J Med, 2000; 343; 1826-32
33. Koch B, Vock DM, Wolfson J, Covariate selection with group lasso and doubly estimation of causal effects: Biometrics, 2018; 74(1); 8-17
34. Geleris J, Sun Y, Platt J, Observational study of hydroxychloroquine in hospitalized patients with Covid-19: N Engl J Med, 2020; 382; 2411-18
35. VanderWeele TJ, Ding P, Sensitivity analysis in observational research: Introducing the E-value: Ann Intern Med, 2017; 167; 268-74
36. Gupta S, Petras L, Tufail MU, Hypertension in pregnancy: What we now know: Curr Opin Nephrol Hypertens, 2023; 32; 153-64
37. Dröge LA, Perschel FH, Stütz N, Prediction of preeclampsia-related adverse outcomes with the sFlt-1(Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor)-ratio in the clinical routine: A real-world study: Hypertension, 2021; 77; 461-71
38. Correa-de-Araujo R, Yoon SSS, Clinical outcomes in high-risk pregnancies due to advanced maternal age: J Womens Health (Larchmt), 2021; 30(2); 160-67
39. Yue C, Ying C, Li X, Association of first trimester serum uric acid with preeclampsia: An observational cohort study with propensity score matching: Hypertens Res, 2023; 46; 377-85
40. Zhang H, Wang W, Risk factors and adverse pregnancy outcomes in older pregnant women with hypertensive disorders of pregnancy: J Obstet Gynaecol Res, 2022; 48(7); 1710-20
41. Sakr HI, Khowailed AA, Al-Fakharany RS, Serum uric acid level as a predictive biomarker of gestational hypertension severity; A prospective observational case-control study: Rev Recent Clin Trials, 2020; 15(3); 227-39
42. Kuwabara M, Kodama T, Ae R, Update in uric acid, hypertension, and cardiovascular diseases: Hypertens Res, 2023; 46; 1714-26
43. Mikolaitytė J, Badarienė J, Puronaitė R, Which clusters of metabolic syndrome are the most associated with serum uric acid?: Medicina (Kaunas), 2022; 58; 297
44. Lüscher BP, Schoeberlein A, Surbek DV, Hyperuricemia during pregnancy leads to a preeclampsia-like phenotype in mice: Cells, 2022; 11; 3703
45. Gao Y, Jia J, Liu X, Trimester-specific reference intervals of serum urea, creatinine, and uric acid among healthy pregnant women in Zhengzhou, China: Lab Med, 2021; 52; 267-72
46. Amini E, Sheikh M, Hantoushzadeh S, Maternal hyperuricemia in normotensive singleton pregnancy, a prenatal finding with continuous perinatal and postnatal effects, a prospective cohort study: BMC Pregnancy Childbirth, 2014; 14; 104
47. Schmella MJ, Clifton RG, Althouse AD, Uric acid determination in gestational hypertension: Is it as effective a delineator of risk as proteinuria in high-risk women?: Reprod Sci, 2015; 22; 1212-19
48. Niraula A, Lamsal M, Majhi S, Significance of serum uric acid in pregnancy induced hypertension: J Natl Med Assoc, 2017; 109; 198-202
49. Kumar N, Singh AK, Maternal serum uric acid and calcium as predictors of hypertensive disorder of pregnancy: A case control study: Taiwan J Obstet Gynecol, 2019; 58; 244-50
50. Yuan X, Han X, Jia C, Association of maternal serum uric acid and cystatin C levels in late pregnancy with adverse birth outcomes: An observational cohort study in China: Int J Womens Health, 2022; 14; 213-23
51. Wu Y, Xiong X, Fraser WD, Association of uric acid with progression to pre-eclampsia and development of adverse conditions in gestational hypertensive pregnancies: Am J Hypertens, 2012; 25; 711-17
52. Bellomo G, Venanzi S, Saronio P, Prognostic significance of serum uric acid in women with gestational hypertension: Hypertension, 2011; 58; 704-8
53. Kumar N, Singh AK, Maternal serum uric acid as a predictor of severity of hypertensive disorders of pregnancy: A prospective cohort study: Curr Hypertens Rev, 2019; 15(2); 154-60
54. Matias ML, Romão M, Weel IC, Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia: PLoS One, 2015; 10; e0129095
55. Borghese MM, Fisher M, Ashley-Martin J, Individual, independent, and joint associations of toxic metals and manganese on hypertensive disorders of pregnancy: Results from the MIREC Canadian pregnancy cohort: Environ Health Perspect, 2023; 131; 47014
56. Roberts JM, Bodnar LM, Lain KY, Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension: Hypertension, 2005; 46; 1263-69
Figures
 Figure 1. Flowchart of the participants. A total of 780 pregnant women of advanced maternal age were included in the analysis.
Figure 1. Flowchart of the participants. A total of 780 pregnant women of advanced maternal age were included in the analysis. Figure 2. Differences in SUA between gestational hypertension and preeclampsia. HUA incidence was more frequently observed in patients with preeclampsia/eclampsia (13.89% vs 11.1%, P=0.035). SUA – serum uric acid; HUA – high uric acid.
Figure 2. Differences in SUA between gestational hypertension and preeclampsia. HUA incidence was more frequently observed in patients with preeclampsia/eclampsia (13.89% vs 11.1%, P=0.035). SUA – serum uric acid; HUA – high uric acid. Figure 3. E-Value analyses. An unmeasured confounder could fully account for the association of intensification with HDP if it was associated with the exposure and outcome by an OR of 5.21(lower confidence limit, 3.46). HDP – hypertensive disorders of pregnancy; OR – ratio risk.
Figure 3. E-Value analyses. An unmeasured confounder could fully account for the association of intensification with HDP if it was associated with the exposure and outcome by an OR of 5.21(lower confidence limit, 3.46). HDP – hypertensive disorders of pregnancy; OR – ratio risk. Figure 4. Forest plot of the association between SUA and HDP after adjusting for potential confounders, there were no interactions in GDM, maternal age, RDW, BMI, SBP, DBP, heart rate, gestational week, urine protein, and parity. Patients with anemia showed an increased risk of HUA (OR=1.13, 95% CI, 1.09–1.17, P<0.0001). Patients with high platelets also indicated an increased risk of HUA (OR=1.09, 95% CI, 1.06–1.13, P<0.0001). SUA – serum uric acid; HDP – hypertensive disorders of pregnancy; GDM – gestational diabetes; RDW – red blood cell distribution width; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; OR – ratio risk.
Figure 4. Forest plot of the association between SUA and HDP after adjusting for potential confounders, there were no interactions in GDM, maternal age, RDW, BMI, SBP, DBP, heart rate, gestational week, urine protein, and parity. Patients with anemia showed an increased risk of HUA (OR=1.13, 95% CI, 1.09–1.17, P<0.0001). Patients with high platelets also indicated an increased risk of HUA (OR=1.09, 95% CI, 1.06–1.13, P<0.0001). SUA – serum uric acid; HDP – hypertensive disorders of pregnancy; GDM – gestational diabetes; RDW – red blood cell distribution width; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; OR – ratio risk. Figure 5. ROC curve for the prediction of HDP in the original population. The AUC was 0.684 [95% CI: 0.642–0.727) for the original population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve.
Figure 5. ROC curve for the prediction of HDP in the original population. The AUC was 0.684 [95% CI: 0.642–0.727) for the original population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve. Figure 6. ROC curve for the prediction of HDP in the matched population. The AUC was 0.724 (95% CI: 0.655–0.782) for the matched population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve.
Figure 6. ROC curve for the prediction of HDP in the matched population. The AUC was 0.724 (95% CI: 0.655–0.782) for the matched population. HDP-hypertensive disorders of pregnancy; ROC – receiver operating characteristic; AUC – area under the curve. Tables
 Table 1. Baseline clinical characteristics before and after propensity score matching.
Table 1. Baseline clinical characteristics before and after propensity score matching. Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis.
Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis. Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis.
Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis. Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort.
Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort. Table 5. Association of HUA and HDP in the original and the weighted cohorts.
Table 5. Association of HUA and HDP in the original and the weighted cohorts. Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups.
Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups. Table 1. Baseline clinical characteristics before and after propensity score matching.
Table 1. Baseline clinical characteristics before and after propensity score matching. Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis.
Table 2. Influencing factors for SUA in the original population by univariate logistic regression analysis. Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis.
Table 3. Influencing factors for SUA in the matched population by univariate logistics-regression analysis. Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort.
Table 4. Adjusted odds ratios for the prevalence of HDP according to the presence of HUA in the PS-matched cohort. Table 5. Association of HUA and HDP in the original and the weighted cohorts.
Table 5. Association of HUA and HDP in the original and the weighted cohorts. Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups.
Table 6. Effect size of SUA on HDP in prespecified and exploratory subgroups. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








