24 October 2023: Clinical Research
Comparison of Nanocrystalline Hydroxyapatite Bone Graft with Empty Defects in Long Bone Fractures: A Retrospective Case-Control Study
Jonas Pawelke1ABCDEF, Vithusha Vinayahalingam1ABE, Christian Heiss12D, Matthäus Budak2E, Thaqif El Khassawna1ABCDE*, Gero Knapp2ABCDEFDOI: 10.12659/MSM.941112
Med Sci Monit 2023; 29:e941112
Abstract
BACKGROUND: The regeneration of bone defects is indicated to restore lost tissue mass and functionality. Ostim®, an absorbable nanocrystalline hydroxyapatite (NCHA) paste, is indicated to enhance bone regeneration in bone defects due to trauma or surgery. This retrospective study of 110 patients with long-bone fracture defects presenting at a single trauma center between 2010 and 2012 aimed to compare outcomes with and without the use of Ostim® absorbable nanocrystalline hydroxyapatite paste.
MATERIAL AND METHODS: The study encompassed fractures in 110 patients – 55 patients received any defect augmentation (ED) and 55 patients were treated with NCHA augmentation. Fractures were located at the distal radius (66.4%, n=73), proximal humerus (5.5%, n=6), and proximal tibia (28.2%, n=31). Evaluating the clinical follow-up, the study encompassed post-surgery complications (eg, non-unions, infection). Bone healing was evaluated by conventional radiographs.
RESULTS: Postoperative complications occurred in 45.5% of patients regardless of the treatment (P=1.0). The non-union rate in both groups was 5.5% (n=8, P=1.0), and the risk for infection was lower in the NCHA group (3.6%, ED: n=3, NCHA: n=1, p=0.62). Patients suffered open fractures were treated in the NCHA group (100%, n=7, P=0.003). Radiological assessment demonstrated comparable healing of the fracture border, fracture gap, and articular surface (P>0.05).
CONCLUSIONS: The findings from this retrospective study support previous studies that have shown Ostim® absorbable nanocrystalline hydroxyapatite paste enhances outcomes and reduces the risk of complications when used to repair bone defects in long-bone fractures in trauma patients. NCHA paste augmentation is suitable for use in traumatic long-bone fractures.
Keywords: Radius Fractures, Tibial Fractures, humeral fractures, Bone Substitutes, Hydroxyapatite Cement, Orthopedic Procedures, Humans, Retrospective Studies, Case-Control Studies, Fractures, Bone, Durapatite, Fracture Healing, Treatment Outcome
Background
:
Since 2006, an absorbable paste, Ostim® (aap Biomaterials GmbH & Co. KG, Dieburg, Germany), has been used for bone defect augmentation. Ostim®, an injectable nanocrystalline paste, was already under evaluation for in vitro, in vivo, and clinical trials, mainly in maxillofacial surgery [1].
Autoplastic techniques (eg, Ostim®) have osteoinductive and osteoconductive effects and fewer graft-versus-host reactions [2,3]. Osteoconductive procedures using bone grafts are rarely performed [4]. Bone material demonstrates many structural similarities to the synthetic hydroxyapatite minerals used in autoplastic bone graft substitutes [5]; Ostim®, a nanocrystalline hydroxyapatite bone material paste, is an injectable bone substitute for filling bone defects that takes advantage of this fact [1,6]. Ostim® has undergone in vitro and in vivo evaluation for various conditions. Regarding revascularization, Ostim® demonstrated a total porosity (ratio between the volume of all pores and the total volume) of 52.66±10.14% and an open porosity (open pores) of 50.52±4.49% [7]. Comparing the total porosity of trabecular bone (50–90%), Ostim® shows a physiological porosity (Table 1) [7,8]. Furthermore, the recommend optimal pore sizes for bone ingrowth are pores with more than 50 μm and 100–300 μm [9–11]. It was reported that 95% of Ostim’s® pores were smaller than 85 μm, with a maximum of 100 μm [7]. Ostim® has a high porosity with a small pore size. Considering the primary stability, Ostim® has a density of 1.29±0.09 mg/mm3 and a compressive strength 0.24±0.05 MPa [7]. Bone density measured in femoral cortical bone is 1.85±0.06 g/cm3 [8]. Nanocrystalline hydroxyapatite paste was assessed for biocomparability and biodegeneration in controlling the amount of hydroxyapatite, chitosan, and gelatine due to the negative load of phosphate residues (PO3−4), carbonate groups (CO2−3), hydroxyl groups (OH−), and the imine group (C=N) [12].
:
Ostim® has been evaluated in a study using fluorescence labeling, which showed a resorption rate of 51.76% without optimal osseous integration [13]. Although there was no optimal osseous integration, void filling with Ostim® enhances the bone regeneration and leads to a stable bone [14]. In a rabbit model study, there were no differences between experimental and control groups in inflammation, bone formation, and residual graft material [15]. An in vivo trial of Ostim® paste in golden hamsters for bone defect filling showed better guided revascularization compared to other bone replacement materials [16]. Translating these results to clinical practice, improved bone regeneration and enhanced vascularization were noticed in periodontal bone defects [17,18].
The main problem of previous published studies are small investigation groups, just assessing dental disease or metaphysical trauma fractures, and lack of focus on intra-articular fractures [8,19–21]. Recently, reviews of alloplastic bone materials and nanocrystalline bone graft substitute in periodontal diseases were published and demonstrated good healing processes [17,22].
On the other hand, there was no histomorphometrically influence on de novo bone formation or inflammation in using hydroxyapatite in sinus cavity augmentation [23,24]. Furthermore, histopathological analysis found that patient age did not influence bone augmentation [25]. Recently, a clinical evaluation of bone void filling demonstrated enhanced healing in geriatric patients by using calcium phosphate and hydroxyapatite nanocrystalline bone material in traumatic fractures [26]. Most clinical studies on Ostim® have focused on atraumatic fractures and tumor-related bone defects, as well as in oral surgery, with good efficacy in void filling [21,27].
PURPOSE OF THIS STUDY:
This study of 110 patients with long-bone defects presenting at a single trauma center from 2010 to 2012 aimed to compare various outcome parameters with and without Ostim®. Proof of enhanced bone healing could promote the use of nanocrystalline bone material substitutes for bone material augmentation in large bone defects. A post-market clinical evaluation should assess the safety and performance of this medical material.
The presented study hypothesized that fracture void augmentation by Ostim® would not compromise the healing process, be safe, and would result in improved healing metrics.
Material and Methods
ETHICAL APPROVAL:
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the University Hospital Giessen (AZ225/20 of 11/29/21) for involving humans. The local Ethics Committee approved the analysis of demographic patient data, pre-existing conditions, surgery approach, and radiographs of patients with long-bone fractures of the proximal humerus, distal radius, and proximal tibia. Patient informed consent was ensured by providing general surgery information and explanations of the bone substitute material used prior to the surgery. Due to ethics approval of this retrospective trial, the requirement for separate patient consent was waived.
SURGICAL APPLICATION:
Acute long-bone fractures were treated by the highest standard of surgical treatment and by the intraoperative findings. The control group received the standard osteosynthesis and a bone graft with NCHA Ostim® during surgery. Ostim® was originally manufactured by Biomaterials GmbH & Co. KG, Dieburg, Germany in 2010, and now is made by Heraeus Kulzer GmbH, Hanau, Germany. Various package sizes of 0.2 mL up to 2 mL are available [28]. Ostim® was prepared by a surgical assistant as an injectable bone substitute material for use by the surgeon. Ready-to-use pre-filled syringes with NCHA Ostim® were bonded with cannulas for injecting into bone vaults. The bone defect zone was prepared by vacuum extraction and sterile dab dry for optimal contact of NCHA with the bone defect zone. Finally, NCHA Ostim® was injected to refill the fracture gap to the physiological bone corticalis. There were 55 patients in the NCHA group for data analysis.
GROUP FORMATTING:
Within a period of 2 years, 110 patients with traumatic bone fractures were surgically treated. All patients were divided into a group without defect filling (Empty Defects [ED]), and a group with bone substitute augmentation using nanocrystalline hydroxyapatite paste Ostim® (NCHA).
Between January 2010 and February 2012, 58 patients were additionally treated with the injectable hydroxyapatite bone substitute Ostim® for defect filling during fracture treatment. Every patient received up to 5 post-surgery examinations, with at least 3 documented examinations. We excluded patients who were under age 18 years, those who had reoperations caused by complications and pathological fractures, and those with less than 3 examinations or unavailable data.
Based on the control group, a matched reference group of 55 patients was formed from a cohort of patients with trauma-related fractures who did not receive defect filling (Figure 2). For matching the patients, gender, and age, with a range of 15 years, were set as primary matching criteria. Clinical conditions measured using the ASA classification were checked for generating comparable conditions (P>0.05). Due to the long observation period chosen, it was possible to generate a homogeneously matched comparison group. Since there are no recommendations for defect augmentation in fracture treatment, it was possible to select patients from a much larger patient population. Similarity in both groups was ensured thanks to the matching criteria whenever possible. For comparability between the empty defect and the NCHA Ostim® groups, similarity for gender, age, and severity of fracture were controlled while matching subjects in both groups.
DEMOGRAPHIC CHARACTERISTICS:
Demographic data and pre-existing conditions were collected for each patient. Based on the pre-existing conditions, a clinical scoring system, the ASA Physical Status System, was used to categorize the preoperative conditions into groups. The ASA score was assessed as standard in each patient in preparation for surgery. Furthermore, pre-existing conditions were illustrated due to patient’s sex and the body mass index (BMI) according to the WHO definition of obesity. Patient age was classified by review of previous trials into a geriatric patient group (aged over 65 years) and a non-geriatric group (aged 65 years and younger).
Surgical treatment of the fractures was performed with the respective osteosynthesis as the criterion standard, depending on the region. In the NHA group, Ostim® was applied directly into the respective defect with a syringe.
LOCATION OF FRACTURE:
Based on the inclusion criteria for traumatic fractures, we assessed fractures at 3 distinct locations: distal radius (66.4%, n=73), proximal humerus (5.5%, n=6), and proximal tibia (28.2%, n=31). Utilizing the globally recognized Association for Osteosynthesis (AO) fracture classification system, we categorized the fracture regions into 3 groups: long bone, humerus (type 1), and radius (type 2), and tibial fractures were classified as type 4 AO fractures. Fracture morphology was subdivided using a numeric code.
RADIOLOGICAL ANALYSIS:
Data analysis was focused on 2 parameters: the primary outcome parameter for clinical practice was the number and type of complications, and the secondary outcome parameter assessed was the bone healing process.
In clinical practice, physical examinations are used along with radiographs to assess the bone healing process. Based on previous publications by Bohndorf et al and Islam et al, we have developed a new classification system for bone healing in standard radiographs for improving the clinical relevance of the data. For each patient, 2 perpendicular planes (a.p. and lateral plane) were used for evaluating the bone healing process and secondary dislocations. To improve the evaluation, computer tomographics were used. The evaluations were conducted by 2 independent and blinded researchers. Later in the study, the researchers were partially controlled by a senior trauma surgery doctor and by a professional for evaluating X-rays. Four areas were distinguished to evaluate the bone healing process: fracture propensity, fracture gap, articular surface, and osteosynthesis material [29–31]. For example, the fracture gap demonstrated bone healing in case of increased density in the fracture gap. If there was a restitutio ad integrum healing, the fracture gap was evaluated with the same trabecular bone surrounding bone. Fracture healing was evaluated (modified from Bohndorf et al 2006) by measuring fracture borders and gaps with increasing density between the fracture borders (Figure 3). Healing evaluation were ordinally scaled (Table 2) based on the German school grading system, (1–5: best to worst). Additionally, the bone substance was evaluated in the same manner to determine the local bone strength.
POST-SURGERY COMPLICATIONS:
Data on typical-occurrence pathologies of fracture healing were collected, including an expansion fracture tendency, a new fracture gap, and long-term problems such as subchondral sclerosis, osteophytes, reduction of the joint gap, and pseudarthrosis (Table 3).
Complications were also evaluated. Based on previous trials, severe complications in trauma and orthopedics surgery, pseudarthrosis, and infection were evaluated. According to the FDA definition of pseudarthrosis, we defined pseudarthrosis as any non-union for more than 6 months without any progress in healing for more than 3 months [32]. Delayed bone healing was defined as delayed bone healing of more than 6 weeks without any improvement in the healing process. Postoperative complications included persistent severe pain, paresthesia, dysesthesias, and hyperesthesia for more than 6 weeks. Long-term complications and cartilage damage, such as post-traumatic osteoarthritis, were also considered.
This observational study was performed in a single center and during a fixed time period; therefore, no power analysis was carried out. Data from all patients that matched the inclusion criteria were analyzed.
LIMITATION OF STUDY METHODS:
A limitation of this study is that the surgeries were performed between 2010 and 2012, and the bone replacement material was used at a level one hospital in Giessen, Germany. Data collection and curation were done in the years 2020 to 2021 with the current scientific knowledge, but there have been some recent advances in bone vault treatment in the last 10 years (eg, bone material paste with an antibiotic coating).
STATISTICAL ANALYSIS:
Continuous variables are presented as mean and standard deviation (SD) and the range with the minimum and the maximum in each variability. Clarifying the proportion according to all patients in each cohort, the weighted mean or percentage was calculated. For statistical analysis, the demographic data were nominally scaled, while the variables for evaluating post-surgery radiographs were ordinally scaled. The subsequent analysis was performed using IBM® SPSS® Statistics. Descriptive data were evaluated as minimum, maximum, mean, and standard deviation. Post-surgery outcome was assessed using the Mann-Whitney U test in unequal distribution. Comparing equal distributed data, the
Results
SURGICAL APPLICATION:
The surgical approach was open surgery in all types of fractures (n=110, 100%). Initial osteosynthesis was performed by external fixation (n=16, 14.5%) with a subsequent plate osteosynthesis for permanent stability in all cases (n=110, 100%). Using an external fixator was particularly determined in the nanocrystalline hydroxyapatite paste Ostim® paste cohort (23.6%, n=13), while initial external fixature therapy in the empty defect treatment group was less common (5.5%, n=3,
GROUP FORMATTING:
A total of 110 patients underwent fracture management surgery in a level one trauma center between the years 2010 and 2012. Patients were divided into 2 matched groups (empty defects and nanocrystalline hydroxyapatite paste) and each included half of the patients (ED: 50%, n=55; NCHA: 50%, n=55). Based on the matching criteria, 55 female (50%, n=55) and 55 male patients (50%, n=55) were under evaluation, with no differences between treatment groups (
Patients age at the time injury was [17: 91; 57.7±18.18] years. Patients age in the ED group was [17: 91; 56.36±19.36] years, while the age in the NCHA paste group was [22: 89; 57.67±17.07] years. However, there was no statistically significant difference between the groups (P=0.97), (Table 4).
DEMOGRAPHIC CHARACTERISTICS:
The body mass index (BMI) values were [18.03: 44.44; 26.93±5.17] kg/m2, in all patients. The values for each group were [19.03: 44.08; 27.28±5.32] kg/m2 for the ED group, and [18.03: 44.44; 26.51±5.02] kg/m2 for the NCHA paste group, but the difference was not significant (
Almost half of the investigated fractures were located on the left side (51.8%, n=57), while the rest (46.4%, n=51) were located on the right side (
High impact trauma fractures comprised 41.81% (n=46) of total fractures, including sport accidents (16.4%, n=18), traffic accidents (16.4%, n=18), and fall from a height (9.1%, n=10). Low-impact trauma comprised 50.0%, n=55), including SSF (stumbles, slips, falls) accidents (29.1%, n=32), domestic injuries (13.5%, n=15) and falling down stairs (7.3%, n=8). Due to incomplete patient files, accurate cause of injury could not be evaluated in 9 patients (8.19%). There was no difference in energetic impact between the ED and the NCHA paste cohort (
LOCATION OF FRACTURE:
Most fractures were located at the upper extremities (71.82%, n=79), with 7.59% (n=6) at the proximal humerus and 92.41% (n=73) at the distal radius. The remaining (28.18%, n=31) fractures were located at the lower extremity, all at the proximal tibia (100%) (
PATIENT’S PREVIOUS CONDITION:
Patient condition before surgery is a determinant of healing; therefore, ASA classification was considered. The smallest group showed no comorbidity and were graded with ASA I in 14 (12.7%) patients. Most patients (60.9%, n=67) were graded ASA II as per their moderate diseases and previous surgeries. ASA III, the worse calculation of comorbidities, was present in 21 patients (19.1%). Eight (7.3%) patients had missing data.
Eight (14.5%) patients in the ED group had ASA I, 37 (67.3%) had ASA II, and 10 (18.2%) had ASA III. In the NCHA paste group, 6 (10.9%), patients had ASA I, 20 (54.5%) had ASA II, and 11 (20.0%, n=11) had ASA III. Because of missing anesthesiologic patient files, 8 (14.5%) cases in the NCHA paste group could not be classified. Severe ASA types (IV to VI) were not seen in either group. Pre-existing conditions were equivalent in both groups (
All of the above descriptive parameters were compared and demonstrated good comparability among both study groups (
POST-SURGERY COMPLICATIONS:
Being a fundamental parameter regarding medical device’s safety and the primary outcome parameter in this trial, clinical complications were evaluated (Table 5). Both groups had the same probability for complications in quantity and quality (P>0.05). One of the most challenging complications, mal-union and non-union, occurred in 4 (7.3%) cases in each group (P=1.0). Assuming that infection could occur by implanting foreign material, there was just 1 infection in the NCHA paste group (1.8%, n=1) and 3 in the ED group (5.5%, n=3), and the difference between groups was not significant (P=0.62). Various secondary neurological disease, such as a lower rate of reduction of pain and paresthesia for more than 6 weeks, were detected in 3 patients in the NCHA paste cohort (5.5%, n=3) and in 4 patients in the ED group (7.3%, n=4, P=1.0). Secondary dislocation was detected in both groups, and the difference between groups was not significant (ED: 5.5%, n=3; NCHA: 5.5%, n=3, P=1.0).
RADIOLOGICAL OUTCOME PARAMETERS:
In daily clinical practice, the secondary outcome parameter, bone healing, was evaluated. Radiographs for reassessment were performed at 2 days [0: 10;2.0±1.6], 28.1 days [6: 85;28.1±15.3], 59.5 days [16: 161;59.5±25.7], and 178.5 days [20: 811;178.5±140.8], and a long-term follow-up after 453.4 [84: 1986;451.4±312.6] days after surgery. The time points for reassessment were adapted in each patient based on the clinical outcome and by recommendation of the doctor. Fewer significant results were determined by comparing the cluster treated with NCHA paste with the ED group in each follow-up examination (FU), (Table 6). We have noticed evidence of statistical similarity for all biological pattern, fracture border, fracture gap, and articular surface in radiographs (P>0.05), (Figures 4, 5). The composition of osteosynthesis partially demonstrated significant differences (Figure 6). Next to the second reassessment (P=0.03), the last one mainly showed lower counts in the grading system (ED [1: 6;1.9±1.1], NCHA paste [1: 2;1.2±0.4], P<0.001). Metal removal was defined as the smallest number of osteosynthesis. Considering this, the group treated with additional NCHA paste augmentation demonstrated a larger number of metal removals (38.2%, n=21) than the ED groups (23.6%, n=13, P<0.001).
SUBGROUP ANALYSIS – GERIATRIC FRACTURES:
Based on the fact of belated bone healing in osteoporotic and geriatric patients, the dataset was subdivided into 2 subsets, 1 group being under age 65 years and mainly without postmenopausal patients, and 1 group being older than 65 years, and some significant difference was seen. It appears that delayed bone healing in geriatric patients is possible. The main difference was in the bone healing process according to the fracture’s border. Earlier radiographs detected reduced grades for patients treated with NCHA paste than patients treated in the ED group (FU1,
SUBGROUP ANALYSIS – REGION OF FRACTURE:
Subdividing the data set once again based on the 3 regions of fracture, less difference was seen regarding complications. The reason for subdividing the data set based on fracture localization was seen in the influence of force and pressure in fracture healing. Comparing distal radius fractures, the NCHA paste group showed decreased rates of post-traumatic arthrosis compared to the ED group (
Discussion
SUMMARY OF FINDINGS:
Comparing descriptive data, both groups demonstrated a good comparability in gender, age, affected body side, and pre-existing diseases between the cohort of empty defect treatment (ED) and nanocrystalline hydroxyapatite bone paste Ostim® (NCHA) (P>0.05). The BMI (26.93 kg/m2) in this patient cohort was similar to the typical European average [33].
Essential for clinical treatment, a general risk of complications of 45.5% was found in both groups (45.5%,
COMPARISON TO PREVIOUS TRIALS:
In daily clinical practice, risk of complications must be considered when choosing the optimal treatment option. The current study confirms the results of previous studies showing that one must be careful in making assumptions about any influence on the bone healing and the rate of complications [34]. We did not find any significant differences in complications between the NCHA paste and the ED group. A previous metanalysis reported a non-union rate of 0.6% and a mal-union rate of 1.2%, which are lower than the rates we found in each treatment cohort [35]. Because managing long-bone fractures with an external fixation is associated with a larger number of non-unions [36], the larger number of non-unions in this study (n=4, 7.3%) compared to previous trials may be due to soft tissue injuries and the treatment option.
We also investigated the hypothesis that bone defects become contaminated by implanting bone substitute materials. The NCHA paste treatment group had fewer infections than the ED treatment group (P=0.62). Previous histomorphology trials found no inflammation caused by using alloplastic bone substitution, supporting use of NCHA paste [8,15]. Collectively, the current data refute the assumption of infection by implanting any foreign material (eg, nanocrystalline hydroxyapatite paste) in a sterile setting during surgery [37]. Using NCHA paste to treat most patients with open fractures reduced the infection rate caused by bacterial contamination, suggesting that NCHA achieves better overall infection results in patients with open fractures.
We mainly found non-significant differences between the 2 groups in the bone healing process. Use of a mixture of calcium and hydroxyapatite pastes has previously been demonstrated to improve bone healing [38]. Despite previous findings that nanocrystalline hydroxyapatite paste Ostim® does not affect bone healing in periodontal defects [39], it did not significantly enhance bone healing in this trial compared with the empty defect cohort, consistent with previous histomorphogenetic trials [15]. Based on improved vascular genesis, the osteoconductive effect of NCHA paste is not clinically significantly different from that of control groups [6,7,16–18]. Contrary to the results of the present study, previous studies have shown poorer osteoconductive performance [4]. This disagreement may be due to the differences between our study and previous in vivo and in vitro trials that mainly assessed bone substitute materials focused in patient populations undergoing vertebral body fusion or oral and maxillofacial surgical procedures.
Previous studies demonstrated an improved bone healing process caused by negative-loaded chemical groups [12]. Trauma patients have lower pH values and higher lactate levels, which impedes the bone healing process [40]. Although trauma patients are thought to have slight lactate acidosis, the patients treated with nanocrystalline bone graft material augmentation demonstrated good bone healing.
Recently, bone substitute material in geriatric fracture treatment demonstrated improved bone healing [26]. Supporting previous findings of fewer complications and decreased rates of pseudarthrosis in treating geriatric patients with various bone material pastes, our study showed early stability in using nanocrystalline hydroxyapatite bone paste based on conventional radiographs. In contrast, the bone healing process in geriatric patients treated with NCHA bone paste demonstrated an insignificant difference. It seems appropriate that calcium phosphate bone substitute material improved the bone healing process more significantly than NCHA [26].
We found insignificantly fewer complications and insignificantly enhanced bone healing process, supporting the hypothesis that NCHA promotes osteoinduction and osteoconduction, despite the insignificant difference in bone healing.
Discussing the limitations of this investigation, it should be noted that data were evaluated retrospectively between the years 2010 to 2012. In the level one trauma center, NCHA Ostim® was the most commonly used bone substitute between 2010 and 2012. Data collection and evaluation were examined in 2020 to 2021 with the current status of science. Due to the retrospective analysis, no impact on patient population was possible. We highly recommend other clinical trials, especially RCT, for review of these retrospective clinical findings regarding use of NCHA paste. Future studies should perform comparative analysis with a larger number of patients in each group to extend the ability to generalize findings beyond long tubular bones. We acknowledge that due to the relatively low population distribution per group in our observational study, calculating meaningful effect sizes and confidence intervals may be challenging. Additionally, the nature of our study design as an observational study limited our ability to control for potential confounders. However, we recognize the importance of addressing these limitations in future studies. We are actively working on expanding our study to involve multiple centers in a prospective study, which will allow for larger sample sizes, more robust analyses, and better control of confounding variables.
Conclusions
LOCAL ETHICS COMMITTEE CONSENT:
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of the University Hospital Giessen (AZ225/20 of 11/29/21) for involving humans.
Figures
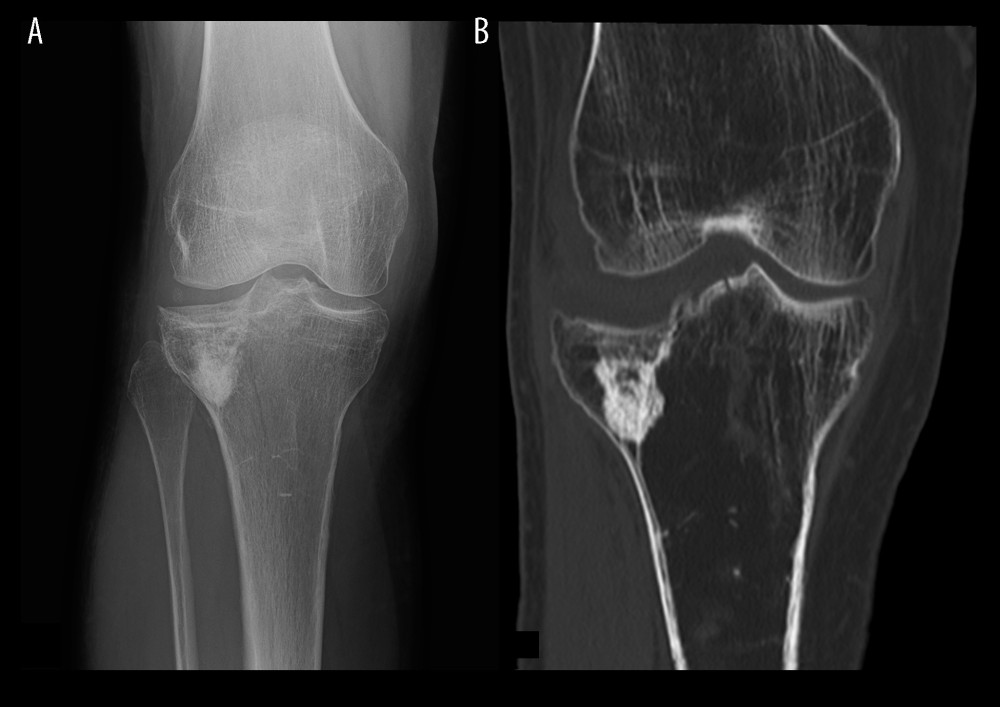 Figure 1. Fracture of the proximal tibia treated by bone substitute material augmentation. (A) Radiograph and (B) CT image following material removal.
Figure 1. Fracture of the proximal tibia treated by bone substitute material augmentation. (A) Radiograph and (B) CT image following material removal. 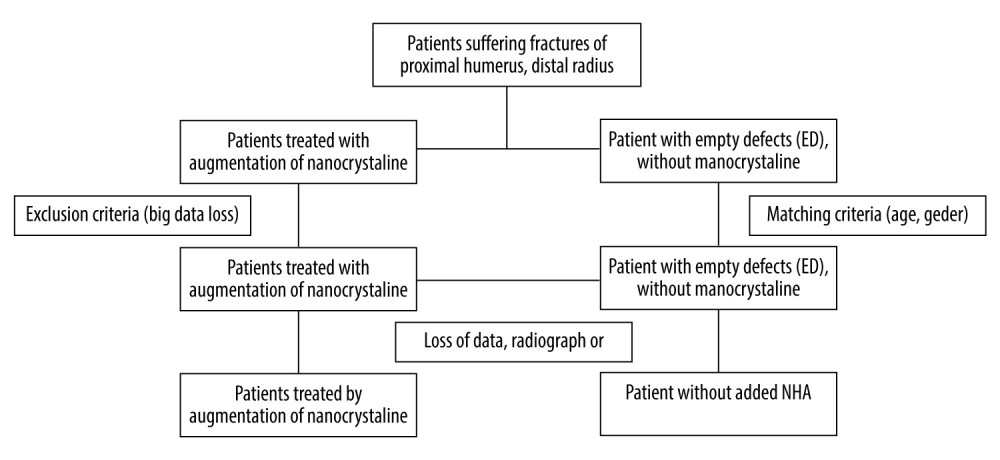 Figure 2. Flow diagram of participants in this trial. Exclusion of 13 patients due to loss of data, missing radiographs, or patient death.
Figure 2. Flow diagram of participants in this trial. Exclusion of 13 patients due to loss of data, missing radiographs, or patient death. 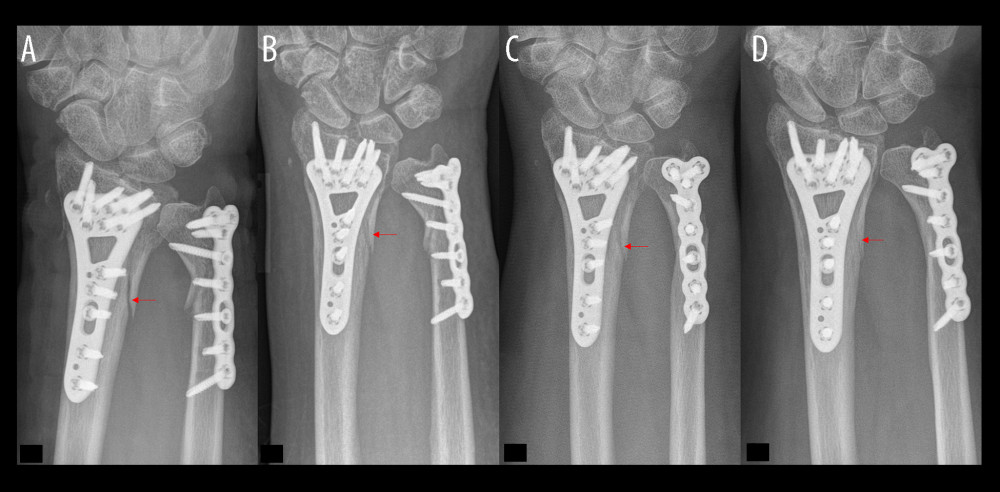 Figure 3. Distal forearm fracture treatment without bone defect augmentation demonstrates the bone healing process. (A) Plate osteosynthesis stabilized a fracture gap without bony filling. (B) Increased bone density in the first follow-up examination of the distal radius fracture. (C) In evaluating the bone healing process, fewer sharp bone edges were noticed. (D) Optimal interosseus integration demonstrate a slightly unclear edge and a fracture gap with bone density.
Figure 3. Distal forearm fracture treatment without bone defect augmentation demonstrates the bone healing process. (A) Plate osteosynthesis stabilized a fracture gap without bony filling. (B) Increased bone density in the first follow-up examination of the distal radius fracture. (C) In evaluating the bone healing process, fewer sharp bone edges were noticed. (D) Optimal interosseus integration demonstrate a slightly unclear edge and a fracture gap with bone density. 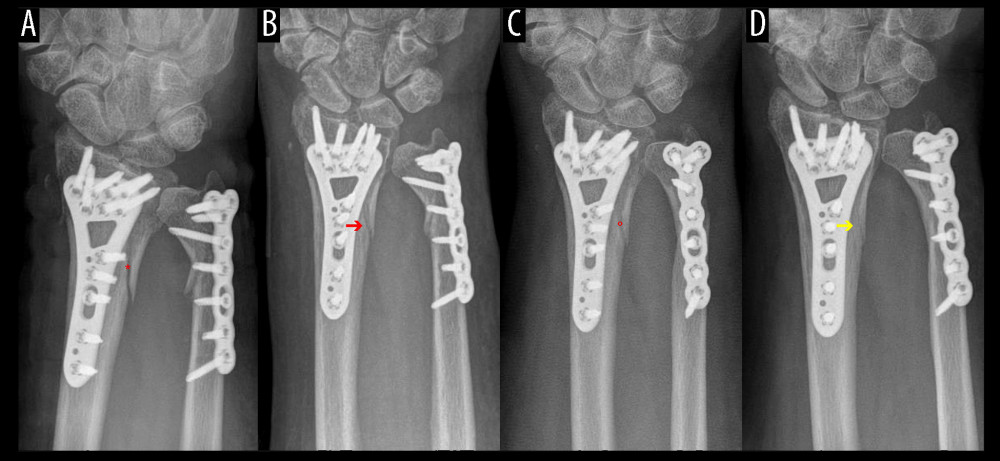 Figure 4. Follow-up (FU) examination demonstrating pattern of bone healing. (A) Border of fracture is sharply edged, with a (*) loss of density in the fracture gap. (B) Border of fracture with (red arrow) dimly visible edges and slightly increasing density of fracture gap. (C) Commencing consolidation with (°) increasing density in fracture gap. (D) Concentration of density in the ulnar part of fracture (yellow arrow) and maximal osseous integration in the radial part of fracture compared to previous examinations. A similar consolidation pattern was seen on the ulna.
Figure 4. Follow-up (FU) examination demonstrating pattern of bone healing. (A) Border of fracture is sharply edged, with a (*) loss of density in the fracture gap. (B) Border of fracture with (red arrow) dimly visible edges and slightly increasing density of fracture gap. (C) Commencing consolidation with (°) increasing density in fracture gap. (D) Concentration of density in the ulnar part of fracture (yellow arrow) and maximal osseous integration in the radial part of fracture compared to previous examinations. A similar consolidation pattern was seen on the ulna. 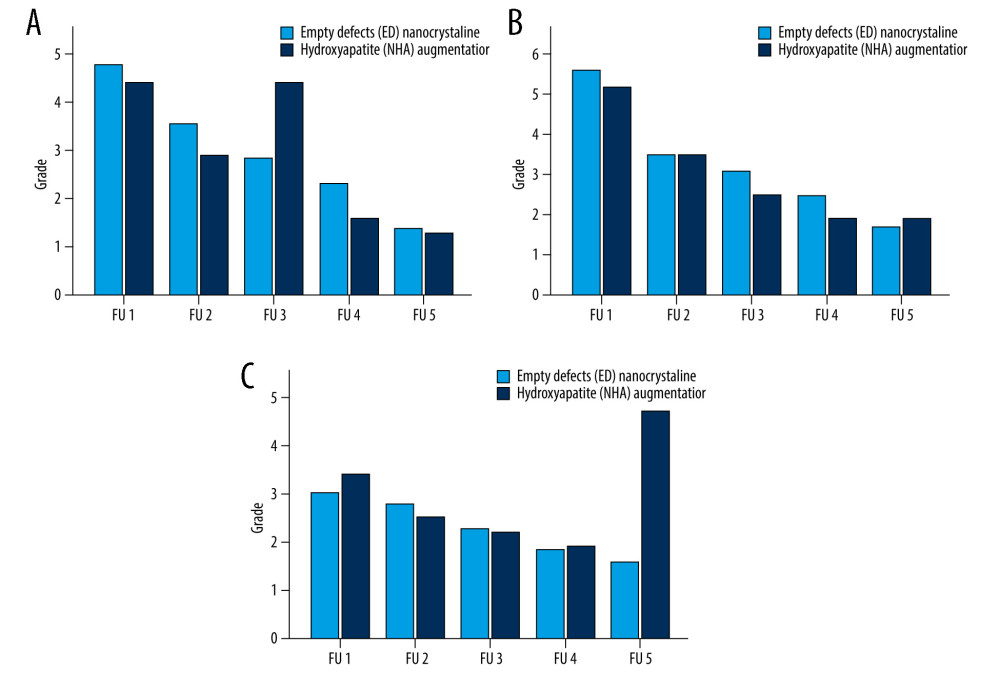 Figure 5. Radiological assessment of bone healing in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). Healing process determined by (A) fracture border, (B) fracture gap (bridging) and (C) articular surface, with no significant differences.
Figure 5. Radiological assessment of bone healing in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). Healing process determined by (A) fracture border, (B) fracture gap (bridging) and (C) articular surface, with no significant differences. 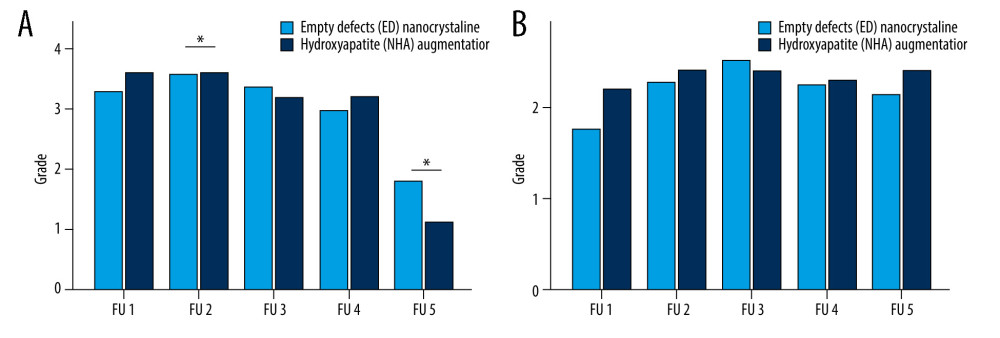 Figure 6. Structural assessment in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). (A) Osteosynthesis demonstrates significant differences in second and fifth FU. Significance in last FU is explained by osteosynthesis removal. (B) Bone substance is comparable between groups.
Figure 6. Structural assessment in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). (A) Osteosynthesis demonstrates significant differences in second and fifth FU. Significance in last FU is explained by osteosynthesis removal. (B) Bone substance is comparable between groups. Tables
Table 1. Micro- and macrostructural parameters of the nanocrystalline hydroxyapatite paste (NCHA), Ostim®, compared with normal bone structure.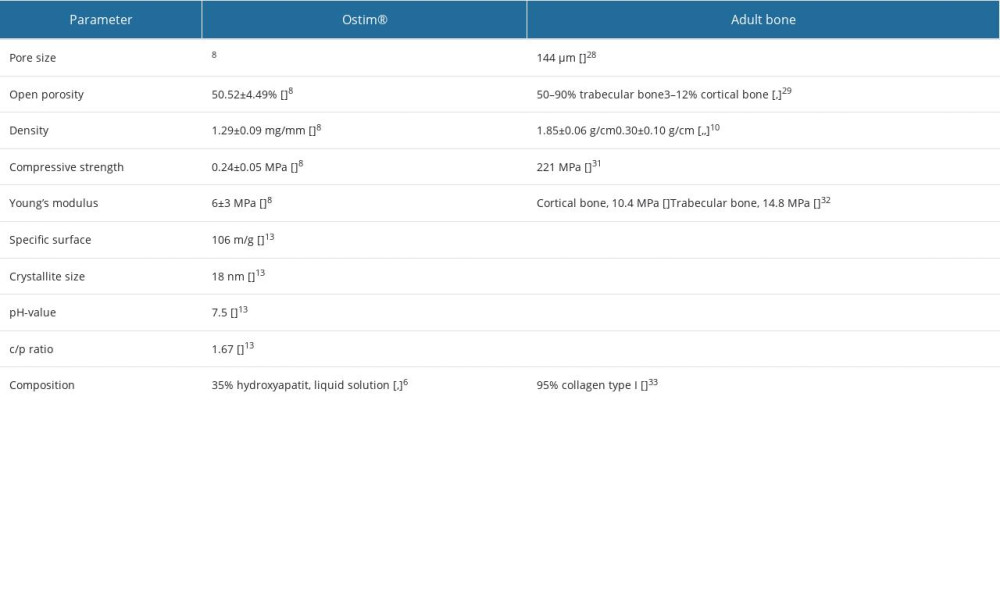 Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs.
Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs.![General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs.](https://jours.isi-science.com/imageXml.php?i=t2-medscimonit-29-e941112.jpg&idArt=941112&w=1000) Table 3. General pathologies of fracture healing evaluated in this trial.
Table 3. General pathologies of fracture healing evaluated in this trial.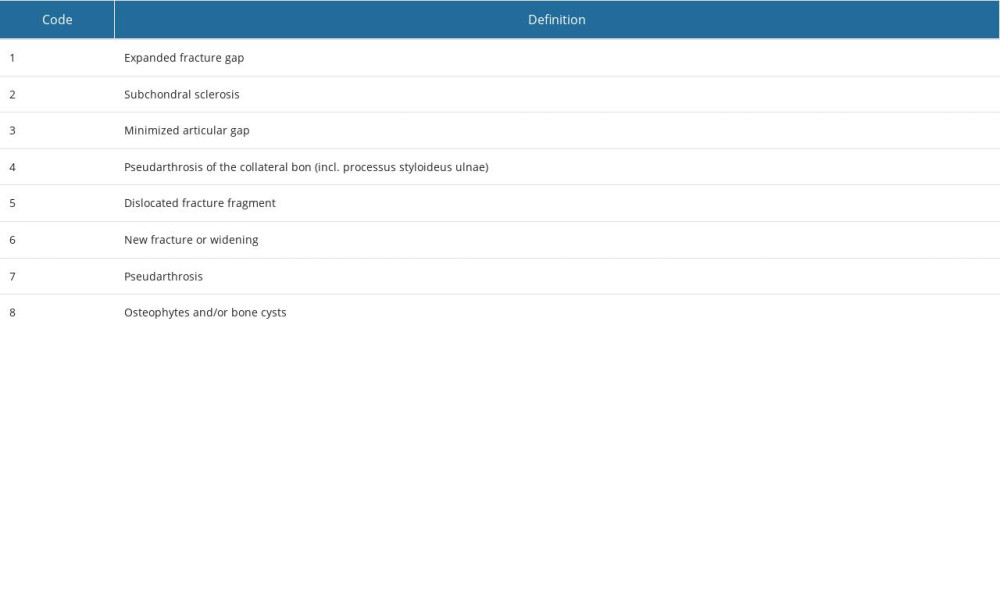 Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity.
Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity. Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group.
Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group.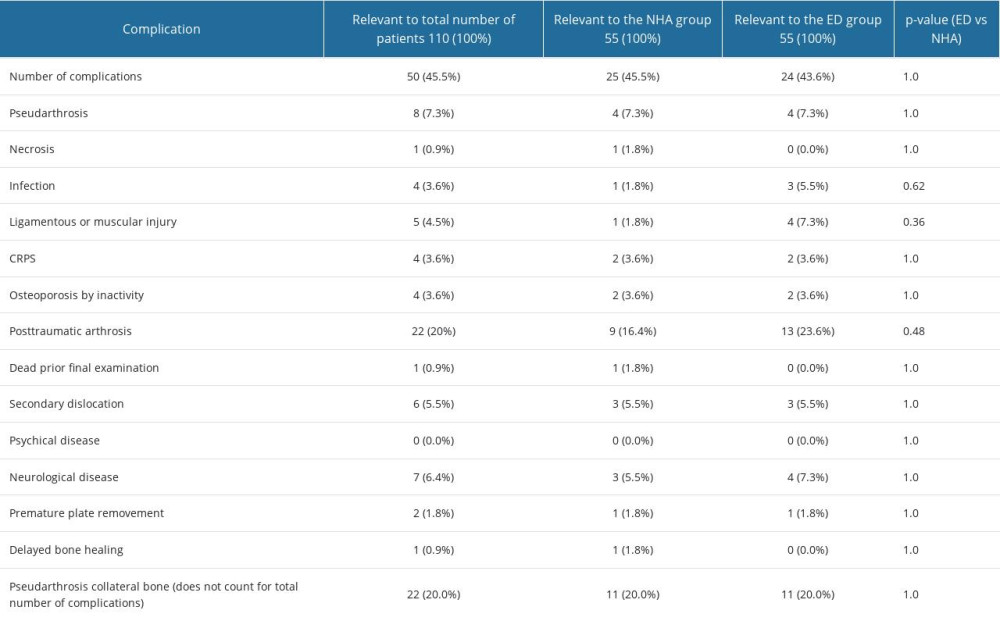 Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options.
Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options.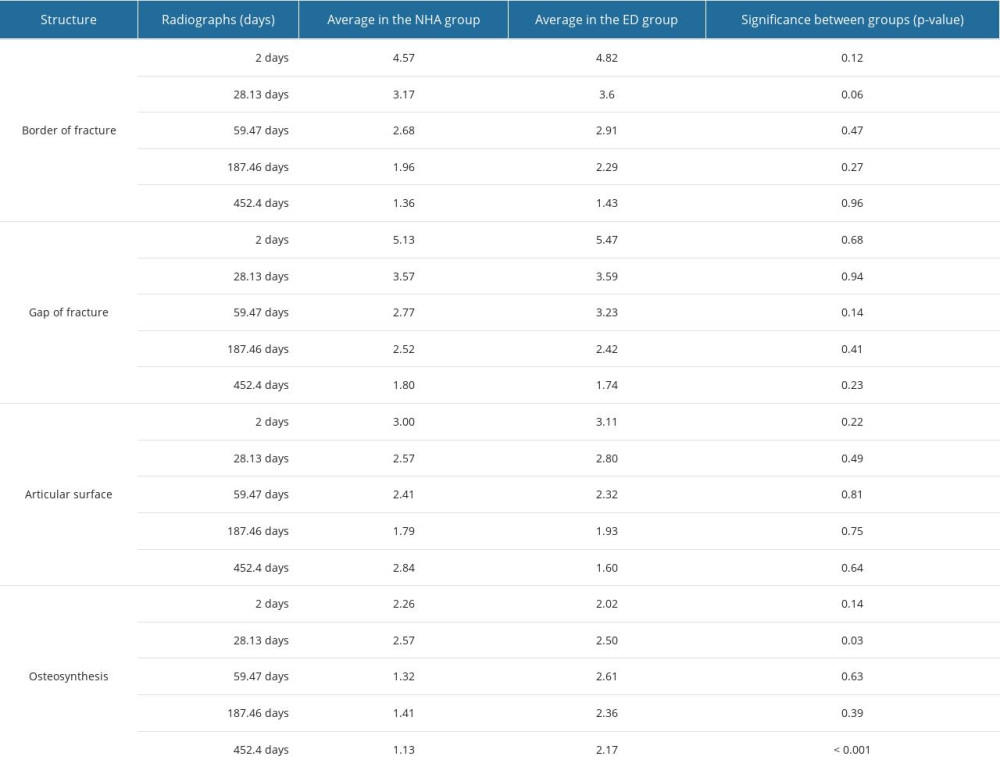
References
1. : Biomet, 2009 in German
2. Heiss C, Rupp M, Knapp G, Bone replacement and bone formation: Z Orthop Unfall, 2019; 157(6); 715-28
3. Marakusha IGOsteo-cartilaginous autoplasty of the joints: Vestn Khir Im I I Grek, 1985; 135(8); 56-60 in Russian
4. Albrektsson T, Johansson C, Osteoinduction, osteoconduction and osseointegration: Eur Spine J, 2001; 10(Suppl 2); S96-101
5. Von Euw S, Wang Y, Laurent G, Bone mineral: New insights into its chemical composition: Sci Rep, 2019; 9(1); 8456
6. Huber FX, McArthur N, Hillmeier J, Void filling of tibia compression fracture zones using a novel resorbable nanocrystalline hydroxyapatite paste in combination with a hydroxyapatite ceramic core: First clinical results: Arch Orthop Trauma Surg, 2006; 126(8); 533-40
7. Van Lieshout EM, Van Kralingen GH, El-Massoudi Y, Microstructure and biomechanical characteristics of bone substitutes for trauma and orthopaedic surgery: BMC Musculoskelet Disord, 2011; 12; 34
8. Huber FX, Belyaev O, Hillmeier J, First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste OSTIM in human cancellous bone: BMC Musculoskelet Disord, 2006; 7; 50
9. Karageorgiou V, Kaplan D, Porosity of 3D biomaterial scaffolds and osteogenesis: Biomaterials, 2005; 26(27); 5474-91
10. Holmes R, Mooney V, Bucholz R, Tencer A, A coralline hydroxyapatite bone graft substitute. Preliminary report: Clin Orthop Relat Res, 1984; 188; 252-62
11. Hulbert SF, Morrison SJ, Klawitter JJ, Tissue reaction to three ceramics of porous and non-porous structures: J Biomed Mater Res, 1972; 6(5); 347-74
12. Rahman S, Maria KH, Ishtiaque MS, Evaluation of a novel nanocrystalline hydroxyapatite powder and a solid hydroxyapatite/Chitosan-Gelatin bioceramic for scaffold preparation used as a bone substitute material: Turk J Chem, 2020; 44(4); 884-900
13. Spies CK, Schnurer S, Gotterbarm T, Breusch S, The efficacy of Biobon and Ostim within metaphyseal defects using the Gottinger Minipig: Arch Orthop Trauma Surg, 2009; 129(7); 979-88
14. Zaffe D, Traversa G, Mozzati M, Behavior of aqueous nanocrystalline hydroxyapatite in oral bone regeneration: J Appl Biomater Biomech, 2011; 9(1); 19-25
15. Sadeghi R, Najafi M, Semyari H, Mashhadiabbas F, Histologic and histomorphometric evaluation of bone regeneration using nanocrystalline hydroxyapatite and human freeze-dried bone graft: An experimental study in rabbit: J Orofac Orthop, 2017; 78(2); 144-52
16. Laschke MW, Witt K, Pohlemann T, Menger MD, Injectable nanocrystalline hydroxyapatite paste for bone substitution: In vivo analysis of biocompatibility and vascularization: J Biomed Mater Res B Appl Biomater, 2007; 82(2); 494-505
17. Shaheen MY, Nanocrystalline hydroxyapatite in periodontal bone regeneration: A systematic review: Saudi Dent J, 2022; 34(8); 647-60
18. Ghanaati S, Barbeck M, Lorenz J, Synthetic bone substitute material comparable with xenogeneic material for bone tissue regeneration in oral cancer patients: First and preliminary histological, histomorphometrical and clinical results: Ann Maxillofac Surg, 2013; 3(2); 126-38
19. Huber FX, Hillmeier J, Kock HJFilling of metaphyseal defects with nanocrystalline hydroxyapatite (Ostim) for fractures of the radius: Zentralbl Chir, 2008; 133(6); 577-81 in German
20. Shirmohammadi A, Roshangar L, Chitsazi MT, Comparative study on the efficacy of anorganic bovine bone (Bio-Oss) and nanocrystalline hydroxyapatite (Ostim) in maxillary sinus floor augmentation: Int Sch Res Notices, 2014; 2014; 967091
21. Kamboj M, Arora R, Gupta H, Comparative evaluation of the efficacy of synthetic nanocrystalline hydroxyapatite bone graft (Ostim((R))) and synthetic microcrystalline hydroxyapatite bone graft (Osteogen((R))) in the treatment of human periodontal intrabony defects: A clinical and dental scan study: J Indian Soc Periodontol, 2016; 20(4); 423-28
22. Trimmel B, Gede N, Hegyi P, Relative performance of various biomaterials used for maxillary sinus augmentation: A Bayesian network meta-analysis: Clin Oral Implants Res, 2021; 32(2); 135-53
23. Ghanaati S, Barbeck M, Willershausen I, Nanocrystalline hydroxyapatite bone substitute leads to sufficient bone tissue formation already after 3 months: Histological and histomorphometrical analysis 3 and 6 months following human sinus cavity augmentation: Clin Implant Dent Relat Res, 2013; 15(6); 883-92
24. Belouka SM, Strietzel FP, Sinus floor elevation and augmentation using synthetic nanocrystalline and nanoporous hydroxyapatite bone substitute materials: Preliminary histologic results: Int J Oral Maxillofac Implants, 2016; 31(6); 1281-91
25. Wolf M, Wurm A, Heinemann F, The effect of patient age on bone formation using a fully synthetic nanocrystalline bone augmentation material in maxillary sinus grafting: Int J Oral Maxillofac Implants, 2014; 29(4); 976-83
26. Pawelke J, Vinayahalingam V, El Khassawna T, Complication and infection risk using bone substitute materials to treat long bone defects in geriatric patients: An observational study: Medicina (Kaunas), 2023; 59(2); 365
27. Liu JM, Chen XY, Zhou Y, Is nonstructural bone graft useful in surgical treatment of lumbar spinal tuberculosis?: A retrospective case-control study: Medicine (Baltimore), 2016; 95(35); e4677
28. GmbH HK: Produktinformation, 2013 in German
29. Klaus Bohnhof HI: Wolfgang Fischer Frakturheilung Radiologische Diagnostik der Knochen und Gelenke: Georg Thieme Verlag KG, 2006 in German
30. Freyschmidt J: Klinisch-radiologische Diagnose und Differentialdiagnose, 2016, Skeletterkankungen, Springer Verlag in German
31. Islam O, Soboleski D, Symons S, Davidson LK, Development and duration of radiographic signs of bone healing in children: Am J Roentgenol, 2000; 175(1); 75-78
32. Thomas JD, Kehoe JL: Bone Non-union, 2022, StatPearls, Treasure Island (FL)
33. Boeing HaUB, Deskriptive Epidemiologie von Übergewicht und Adipositas: Handbuch Essstörungen und Adipositas, 2015; 371-78, Berlin, Heidelberg, Springer Berlin Heidelberg in German
34. Reichl M, Piatek S, Adolf DUnrepaired fracture of the styloid process of the ulna: Not a bad treatment result at distal radius fracture: Unfallchirurg, 2011; 114(12); 1099-104 in German
35. Beeres FJP, van de Wall BJM, Hug U, Temporary spanning plate wrist fixation of complex distal radius fractures: A systematic review of 353 patients: Eur J Trauma Emerg Surg, 2022; 48(3); 1649-62
36. Liu J, Xie L, Liu L, Comparing external fixators and intramedullary nailing for treating open tibia fractures: A meta-analysis of randomized controlled trials: J Orthop Surg Res, 2023; 18(1); 13
37. Rochford ET, Richards RG, Moriarty TF, Influence of material on the development of device-associated infections: Clin Microbiol Infect, 2012; 18(12); 1162-67
38. Medvecky L, Giretova M, Stulajterova R, Tetracalcium phosphate/monetite/calcium sulfate hemihdrate biocement powder mixtures prepared by the one-step synthesis for preparation of nanocrystalline hydroxyapatite biocement-properties and in vitro evaluation: Materials (Basel), 2021; 14(9); 2137
39. Al Machot E, Hoffmann T, Lorenz K, Clinical outcomes after treatment of periodontal intrabony defects with nanocrystalline hydroxyapatite (Ostim) or enamel matrix derivatives (Emdogain): A randomized controlled clinical trial: Biomed Res Int, 2014; 2014; 786353
40. Refaeli E, Zarour S, Lior Y, Prevalence of preoperative metabolic disturbances in elderly patients with hip fracture and their association with mortality – a retrospective cohort study: J Clin Anesth, 2023; 88; 111137
Figures
 Figure 1. Fracture of the proximal tibia treated by bone substitute material augmentation. (A) Radiograph and (B) CT image following material removal.
Figure 1. Fracture of the proximal tibia treated by bone substitute material augmentation. (A) Radiograph and (B) CT image following material removal. Figure 2. Flow diagram of participants in this trial. Exclusion of 13 patients due to loss of data, missing radiographs, or patient death.
Figure 2. Flow diagram of participants in this trial. Exclusion of 13 patients due to loss of data, missing radiographs, or patient death. Figure 3. Distal forearm fracture treatment without bone defect augmentation demonstrates the bone healing process. (A) Plate osteosynthesis stabilized a fracture gap without bony filling. (B) Increased bone density in the first follow-up examination of the distal radius fracture. (C) In evaluating the bone healing process, fewer sharp bone edges were noticed. (D) Optimal interosseus integration demonstrate a slightly unclear edge and a fracture gap with bone density.
Figure 3. Distal forearm fracture treatment without bone defect augmentation demonstrates the bone healing process. (A) Plate osteosynthesis stabilized a fracture gap without bony filling. (B) Increased bone density in the first follow-up examination of the distal radius fracture. (C) In evaluating the bone healing process, fewer sharp bone edges were noticed. (D) Optimal interosseus integration demonstrate a slightly unclear edge and a fracture gap with bone density. Figure 4. Follow-up (FU) examination demonstrating pattern of bone healing. (A) Border of fracture is sharply edged, with a (*) loss of density in the fracture gap. (B) Border of fracture with (red arrow) dimly visible edges and slightly increasing density of fracture gap. (C) Commencing consolidation with (°) increasing density in fracture gap. (D) Concentration of density in the ulnar part of fracture (yellow arrow) and maximal osseous integration in the radial part of fracture compared to previous examinations. A similar consolidation pattern was seen on the ulna.
Figure 4. Follow-up (FU) examination demonstrating pattern of bone healing. (A) Border of fracture is sharply edged, with a (*) loss of density in the fracture gap. (B) Border of fracture with (red arrow) dimly visible edges and slightly increasing density of fracture gap. (C) Commencing consolidation with (°) increasing density in fracture gap. (D) Concentration of density in the ulnar part of fracture (yellow arrow) and maximal osseous integration in the radial part of fracture compared to previous examinations. A similar consolidation pattern was seen on the ulna. Figure 5. Radiological assessment of bone healing in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). Healing process determined by (A) fracture border, (B) fracture gap (bridging) and (C) articular surface, with no significant differences.
Figure 5. Radiological assessment of bone healing in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). Healing process determined by (A) fracture border, (B) fracture gap (bridging) and (C) articular surface, with no significant differences. Figure 6. Structural assessment in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). (A) Osteosynthesis demonstrates significant differences in second and fifth FU. Significance in last FU is explained by osteosynthesis removal. (B) Bone substance is comparable between groups.
Figure 6. Structural assessment in 5 follow-up (FU) radiographs of empty defect (ED) treatment and nanocrystalline hydroxyapatite paste augmentation (NCHA). (A) Osteosynthesis demonstrates significant differences in second and fifth FU. Significance in last FU is explained by osteosynthesis removal. (B) Bone substance is comparable between groups. Tables
 Table 1. Micro- and macrostructural parameters of the nanocrystalline hydroxyapatite paste (NCHA), Ostim®, compared with normal bone structure.
Table 1. Micro- and macrostructural parameters of the nanocrystalline hydroxyapatite paste (NCHA), Ostim®, compared with normal bone structure.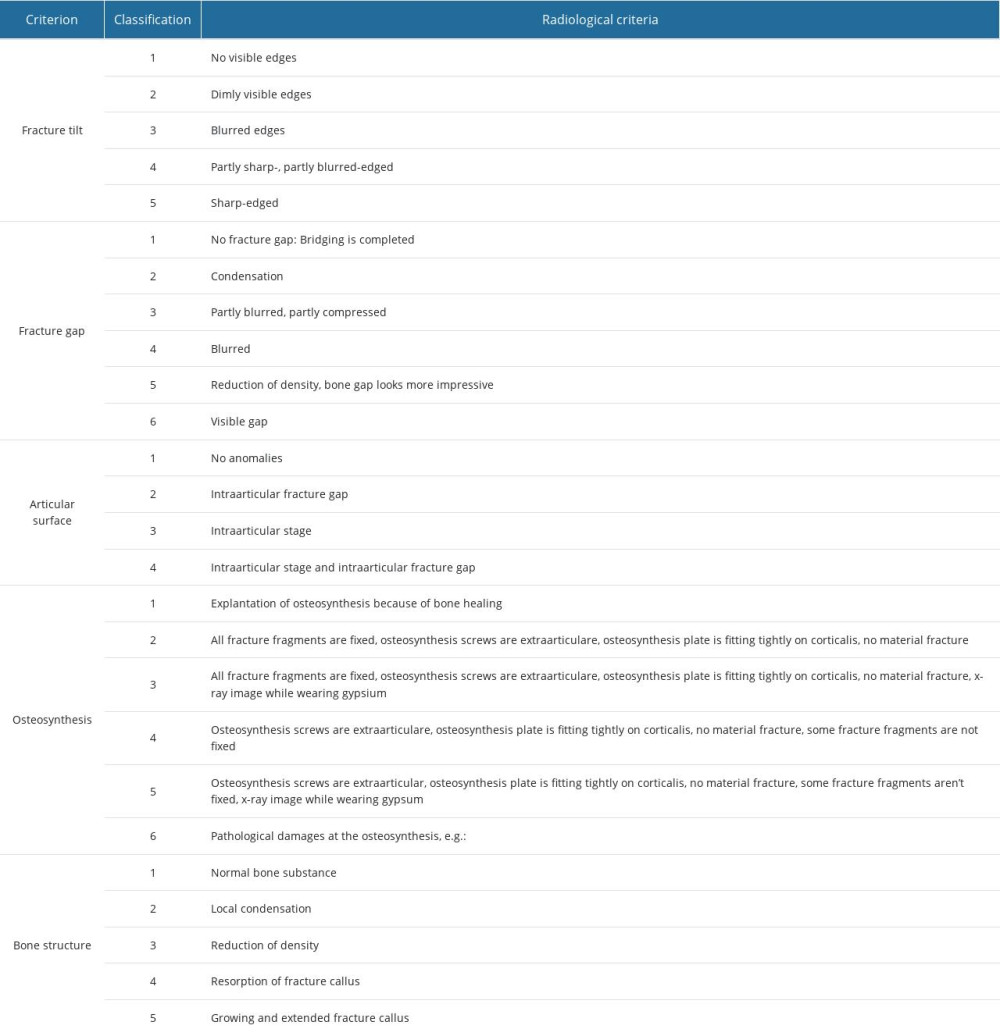 Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs.
Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs. Table 3. General pathologies of fracture healing evaluated in this trial.
Table 3. General pathologies of fracture healing evaluated in this trial. Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity.
Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity. Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group.
Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group. Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options.
Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options. Table 1. Micro- and macrostructural parameters of the nanocrystalline hydroxyapatite paste (NCHA), Ostim®, compared with normal bone structure.
Table 1. Micro- and macrostructural parameters of the nanocrystalline hydroxyapatite paste (NCHA), Ostim®, compared with normal bone structure. Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs.
Table 2. General radiological criteria of bone healing, modified after Bohnhof et al [29], Freyschmidt et al [30], and Islam et al [31] for evaluating conventional radiographs. Table 3. General pathologies of fracture healing evaluated in this trial.
Table 3. General pathologies of fracture healing evaluated in this trial. Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity.
Table 4. Frequency analysis of main descriptive parameters showed homogeneity of the investigated population in this examination. In all parameters, statistic equality was demonstrated between the empty defect (ED) and nanocrystalline hydroxyapatite paste (NCHA) treatment group (P>0.05). Comparing the frequency of gender in the groups demonstrated equality in (A) all patients, in the (B) ED, and in the (C) NCHA group. Comparing age, divided into 2 groups, showed equality in (D) all patients, in the (E) ED, and in the (F) NCHA group. Statistical analysis of the frequency of affected side in (G) all patients, in the (H) ED, and in the (I) NCHA group demonstrated homogeneity. Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group.
Table 5. Overview of the number of complications in both treatment groups and significance between the empty defect (ED) group and the nanocrystalline hydroxyapatite paste treatment (NCHA) group. Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options.
Table 6. Overview of pattern of the bone healing in post-surgery radiographs in the empty defects (ED) group and nanocrystalline hydroxyapatite paste (NCHA) group, showing statistically significant differences between the 2 treatment options. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








