06 August 2023: Article
Dynamics of Microbiome Changes in the Endometrium and Uterine Cervix during Embryo Implantation: A Comparative Analysis
Anna Bednarska-CzerwińskaDOI: 10.12659/MSM.941289
Med Sci Monit 2023; 29:e941289
Abstract
BACKGROUND: The microbiome is the collection of all micro-organisms and their genes, which naturally live in and on the body. The cervical and endometrial bacterial microbiome has previously been reported to affect fertility and influence the outcomes of assisted reproductive therapy (ART), including embryo transfer. This study aimed to evaluate the cervical and endometrial bacterial microbiome in 177 women treated for infertility before, during, and after embryo implantation, and the outcomes.
MATERIAL AND METHODS: Cervical and endometrial swabs were collected from 177 women diagnosed with infertility at 3 time points: (1) during the initial examination, (2) during implantation, (3) 10-14 days after implantation. Next-generation sequencing (NGS) was used to analyze the bacterial microbiome. Taxonomic identification was performed with the Usearch algorithm.
RESULTS: There was a significant change in the number of patients with Escherichia coli depending on the collection time. For the first swab collection, there were significant negative relationships between the percentage of Gardnerella vaginalis and Lactobacillus spp. For the second collection, there was a negative relationship between Lactobacillus helveticus and Lactobacillus jensenii. For the third collection, negative relationships were found between Escherichia coli and Lactobacillus spp. A similar distribution of the bacterial microbiome was observed in all 3 swab collections.
CONCLUSIONS: Lactobacillus spp. were the main bacteria identified in the cervix and endometrium, present before, during, and after successful embryo transfer. E. coli and G. vaginalis reduced the protective effect of Lactobacilli before, during, and after embryo transfer.
Keywords: Cervix Uteri, Endometrium, Fertilization in Vitro, High-Throughput Nucleotide Sequencing, Microbiota, Female, Humans, Escherichia coli, Uterine Cervical Neoplasms, Embryo Implantation, Bacteria, Infertility, Vagina
Background
Fertility and pregnancy disorders affect an increasing number of people around the world. They may be associated with anatomical anomalies of the reproductive organs, both congenital and acquired [1,2]. Hormonal balance is also important, as well as the presence of inflammatory processes that can lead, for example, to obstruction of the fallopian tubes [3]. Eating disorders, sleep disorders, and depression are also believed to have a negative impact on fertility. Problems with getting pregnant are a source of stress for many couples, which in turn can be the cause of idiopathic infertility [4,5].
Assisted fertilization techniques (ARTs), including in vitro fertilization (IVF), offer hope for parenthood. They are constantly being improved in order to maximize the chance of successful conception and live birth, reducing the number of possible complications [6,7]. Studies indicate that the IVF result is mainly influenced by the woman’s age, the number of oocytes, the length of infertility, and the level of basal (follicle-stimulating hormone) FSH [8]. However, additional factors that may affect the success of IVF are sought. One of them may be the bacterial microbiome of the female reproductive system [9].
The composition of the human microbiome is related to the state of health. Its change can lead to the expansion of certain bacteria or pathogens, which is referred to as dysbiosis [10]. Vaginal
Therefore, this study aimed to evaluate the cervical and endometrial bacterial microbiome in 177 women treated for infertility before, during, and after embryo implantation, and the outcomes.
Material and Methods
ETHICS:
This study was performed according to the guidelines of the Declaration of Helsinki and was approved by the Institution of the Bioethical Committee operating at the Regional Medical Chamber in Krakow (No. 161/KBL/OIL/2021). Informed consent for participation in the study and publication of this article was obtained from all patients.
PATIENTS:
Table 1 presents the inclusion and exclusion criteria, which are the same as in our previous publication [16].
Out of 250 women diagnosed with infertility who were qualified for the in vitro procedure at the Gyncentrum Clinic in Poland, 177 patients (71%) were enrolled for the study, from whom cervical and endometrial swabs were taken at 3 time points. Based on the assessment of beta-human chorionic gonadotropin (beta hCG) levels 14 days after embryo implantation, 67 women were pregnant. In 65 patients (97%) the pregnancy ended in childbirth, while the remaining 2 suffered a miscarriage. The characteristics of the patients included in the study are listed in Table 2. Measurable data were presented as mean and standard deviation (X±SD) and median (Me) and quartiles 1–3 (Q1–Q3).
MATERIAL COLLECTION:
Cervical and endometrial swabs were collected at 3 time points: (1) during the initial examination and Endometrial Receptivity Analysis (ERA) test, approximately 1 month before the planned embryo implantation, (2) during implantation, (3) 10–14 days after embryo implantation during routine diagnostic tests. At the third time point, the material for molecular testing was taken only from the cervix, as collection from the endometrium would be invasive. Swab Collection and DNA Preservation Tube Transport Medium (Norgen Biotek Corp., Thorold, ON, Canada) were used for cervical and endometrial swab collection.
DNA ISOLATION:
DNA isolation was performed using the NucleoSpin Tissue kit with glass beads (NucleoSpin Bead Tubes Type B, Macherey-Nagel, Oensingen, Switzerland), according to the manufacturer’s recommendation. The obtained DNA extracts were assessed qualitatively with 1% agarose gel electrophoresis. The concentration and purity were determined with spectrophotometric measurement (Nanodrop, Thermo Fisher Scientific, Waltham, MA, USA). The concentration of the extracts was evaluated at the wavelength of 260 nm, and their purity by determining the ratio of absorbance at the wavelength of 260 nm to 280 nm (standard 1.8–2.0). This allowed the qualification of DNA extracts for the analysis of cervical and endometrial bacterial microbiome using the next-generation sequencing (NGS) technique.
NEXT-GENERATION SEQUENCING:
The libraries were prepared according to the Ilumina 16S Metagenomic Sequencing Library Preparation (16S Sequencing) protocol, and their indexing was performed using the Nexter XT Kit (Illumina, San Diego, USA), as recommended by the manufacturer. The fragments were then purified using a MagSi-NGS apparatus (Magtivio, HK Nuth, The Netherlands) and analyzed using the Fragment Analyzer (Agilent, Santa Clara, CA, USA). The QuantiFluor ONE dsDNA Kit (Promega Corporation, Madison, WI, USA) was used to measure the concentration of libraries on a Quantus™ fluorometer (Promega Corporation, Madison, WI, USA) and normalized to 4 nM. Sequencing was performed on the Illumina Miseq 2×300 bp platform, and the analysis of the obtained sequences was based on the resources of the EzBioCloud platform (EzBiome Inc., Gaithersburg, MD, USA). The Usearch algorithm was used to taxonomically identify the bacteria down to the species level. Results were validated using the ZymoBIOMICS Microbial Community Standard Microbial Controls (Zymo Research, Irvine, CA, USA).
STATISTICAL ANALYSIS:
Statistical analysis was performed using Statistica 13.3 PL software (StatSoft, Cracow, Poland) and R 3.5.1 statistical software. The level of significance was set at α=0.05. In the case of measurable data, after the Shapiro-Wilk distribution normality test, further statistical analysis was performed using the Kruskal-Wallis and Dunn’s post hoc tests or Friedman’s and post hoc tests. For non-measurable data, chi-square (χ2) analysis was performed.
Results
BACTERIAL MICROBIOME COMPOSITION OF THE CERVIX AND ENDOMETRIUM BEFORE, DURING, AND AFTER EMBRYO IMPLANTATION:
A total of 105 strains of bacteria were distinguished during the observation (Table 3). The statistical analysis showed only 1 significant change in the number of patients in whom Escherichia coli was identified in the microbiome of the cervix and endometrium (P=0.03). This screening allowed for the selection of 10 strains of bacteria for further analysis, which were present in at least 2 samples in at least 6 patients: Bifidobacterium longum, Escherichia coli, Gardnerella vaginalis, Lactobacillus gasseri, Lactobacillus helveticus, Lactobacillus iners, Lactobacillus paracasei, Lactobacillus reuteri, Lactobacillus jensenii, and Staphylococcus aureus.
In addition, there were no significant changes between the selected bacterial strains and the place of residence (Table 4), BMI (Table 5), and the type of treatment (Table 6).
CORRELATION ANALYSIS BETWEEN THE CONTENT OF SELECTED BACTERIAL STRAINS BEFORE, DURING, AND AFTER EMBRYO IMPLANTATION:
Next, we assessed whether there was a statistically significant relationship between the selected strains of bacteria (Table 7).
In swabs obtained during the first collection, we showed significant negative relationships between the percentage of
We additionally assessed the correlations between the discussed bacterial strains and the patients’ anthropometric data and the period of infertility (Table 8).
In the first swab collection, significant relationships were observed between age and the percentage of
BACTERIAL MICROBIOME DIVISION BEFORE, DURING, AND AFTER EMBRYO IMPLANTATION:
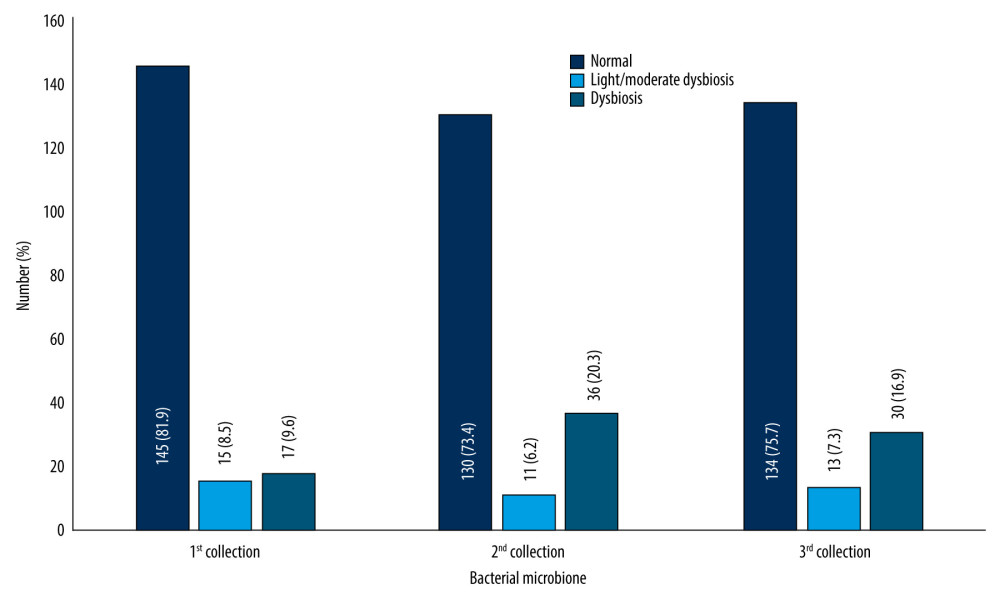
In the next step, the patients were divided according to the type of flora: normal, mild/moderate dysbiosis, and dysbiosis (Figure 1).
A similar distribution of the bacterial microbiome was observed in all 3 swab collections. The largest percentage were patients with normal microbiome, while the highest dysbiosis was noted for the second collection. The metric profile of the patients was also analyzed in relation to the nature of the microbiome (Table 9).
The analysis showed that the BMI significantly affected the type of the patient’s bacterial microbiome in the second swab collection. Then, we checked whether there were any relationships between the clinical profile of the patients and the type of microbiome (Table 10).
An association was observed between the use of metronidazole and the nature of the microbiome at the second swab collection. In addition, the type of microbiome at the third collection depended on fertilization methods other than insemination, cryotransfer, and embryo transfer.
Discussion
In our study, we determined the bacterial microbiome composition of the endometrium and uterine cervix at 3 time points: before, during, and after embryo implantation. The micro-organisms most common in patients were
Among the bacteria that contaminate the IVF medium,
The limitation of our study is the relatively small study group, which makes it difficult to analyze the results in more detail with so many species and strains of bacteria. It would be beneficial to expand the study group to be able to draw stronger conclusions. It would also be interesting to see if the cervical or endometrial bacterial microbiome is similar to the oral microbiome in order to simplify and popularize the use of microbiome changes in the context of infertility diagnosis. Another limitation is the single-center nature of our study.
Conclusions
Tables
Table 1. Inclusion and exclusion criteria.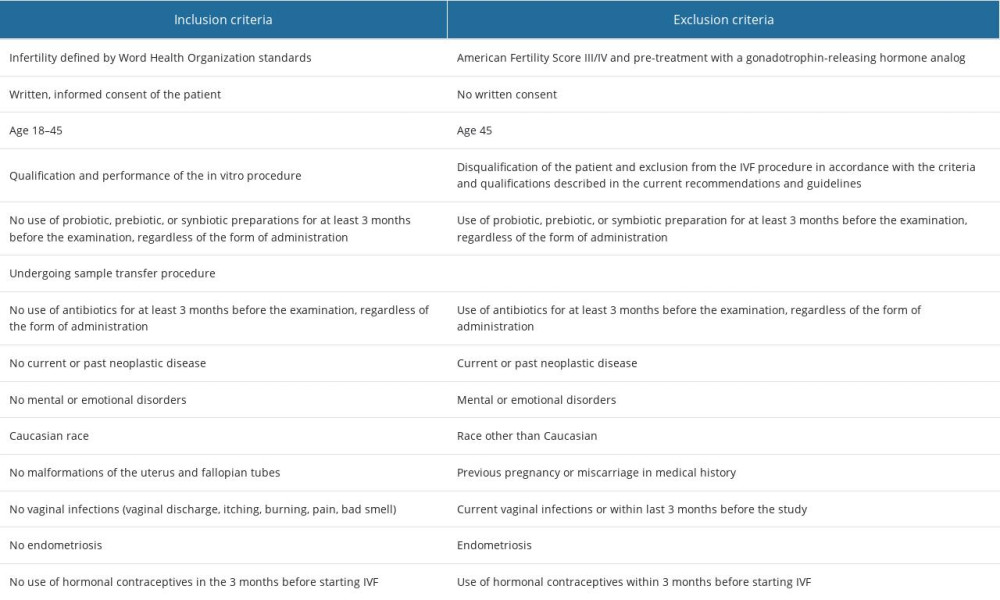 Table 2. Anthropometric data of patients.
Table 2. Anthropometric data of patients.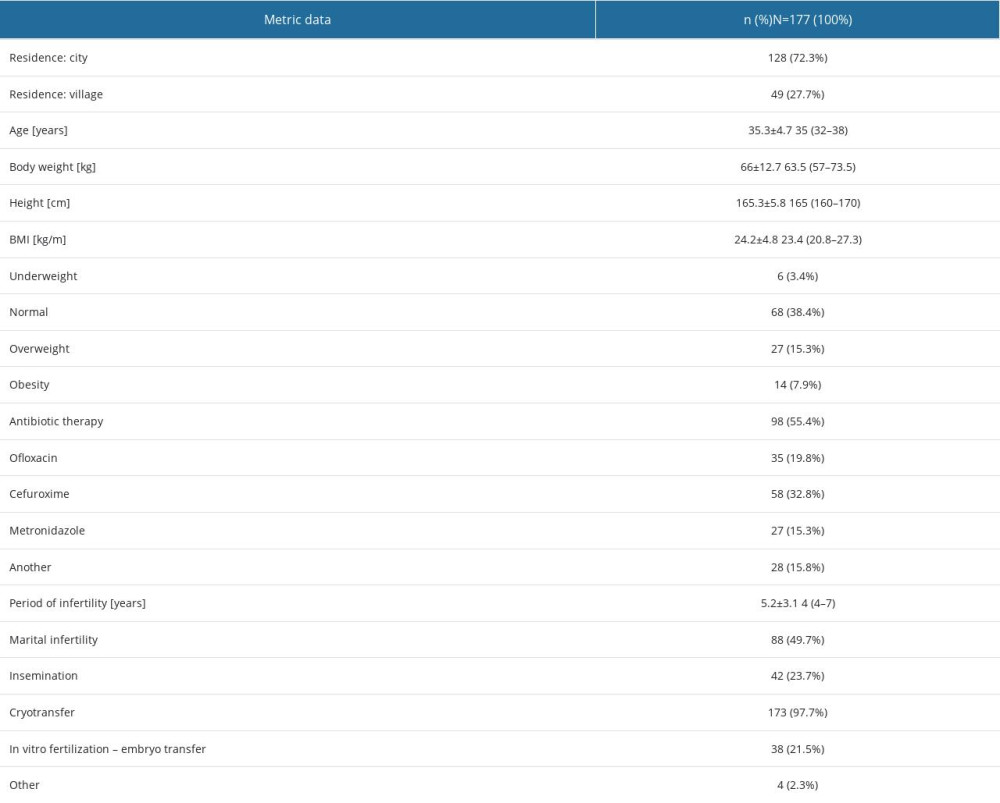 Table 3. Bacterial microbiome composition of the cervix and endometrium.
Table 3. Bacterial microbiome composition of the cervix and endometrium.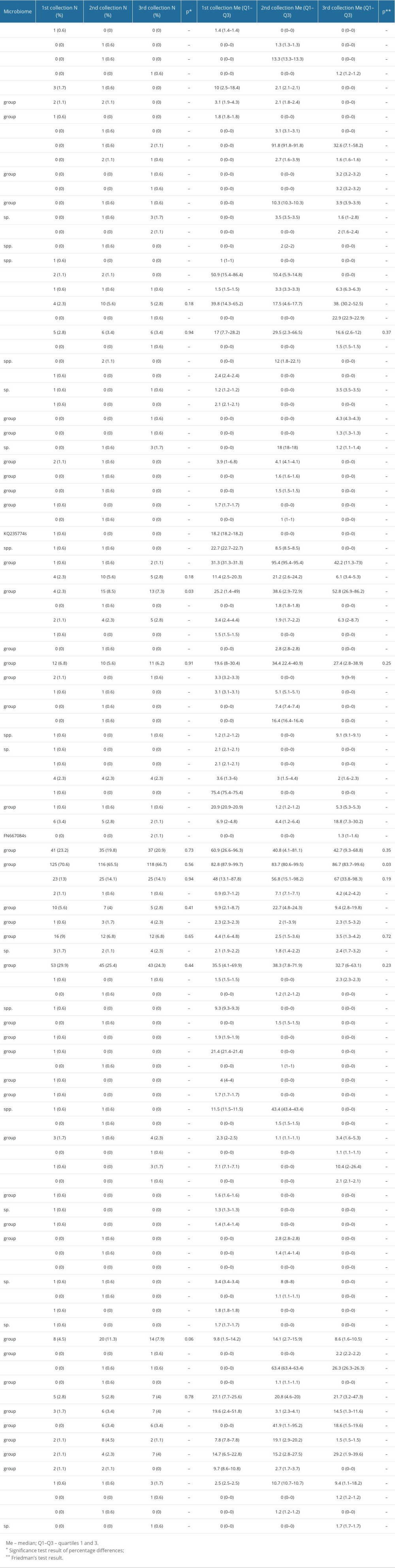 Table 4. Percentage of the selected bacterial strains by place of residence.
Table 4. Percentage of the selected bacterial strains by place of residence.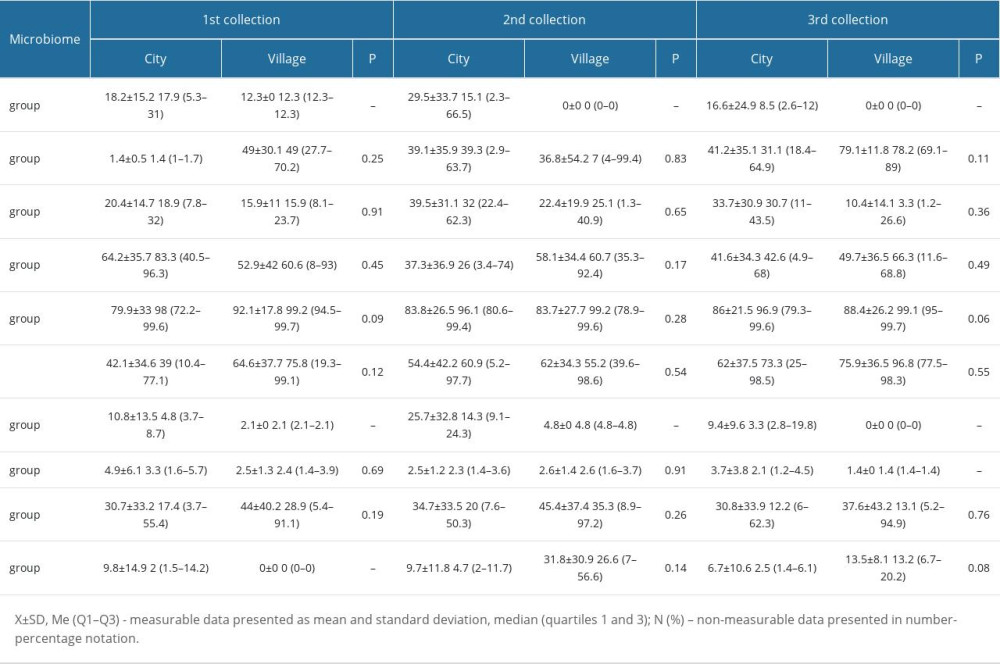 Table 5. Average percentage of selected bacterial strains by BMI.
Table 5. Average percentage of selected bacterial strains by BMI.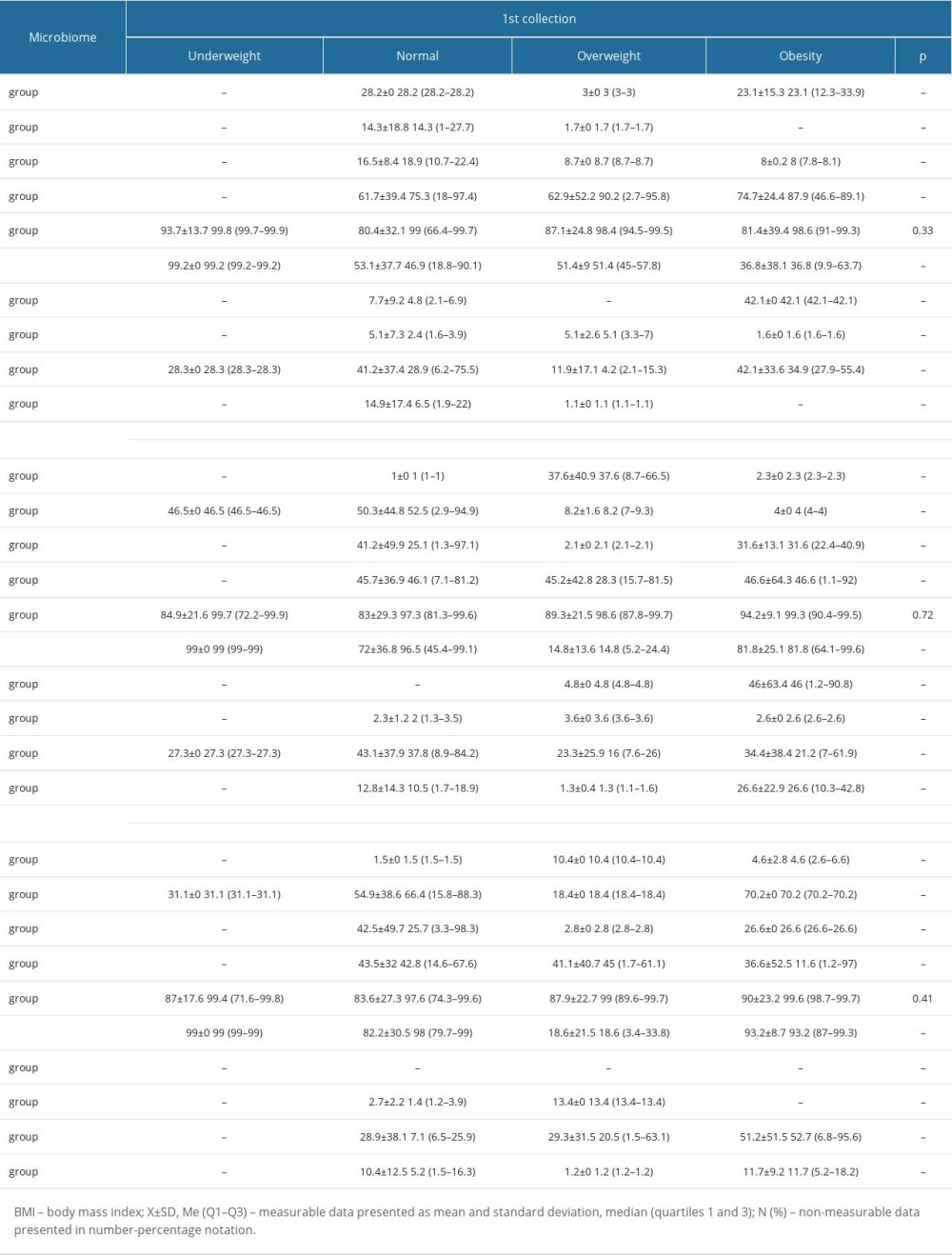 Table 6. Average percentage of selected bacterial strains by type of treatment.
Table 6. Average percentage of selected bacterial strains by type of treatment.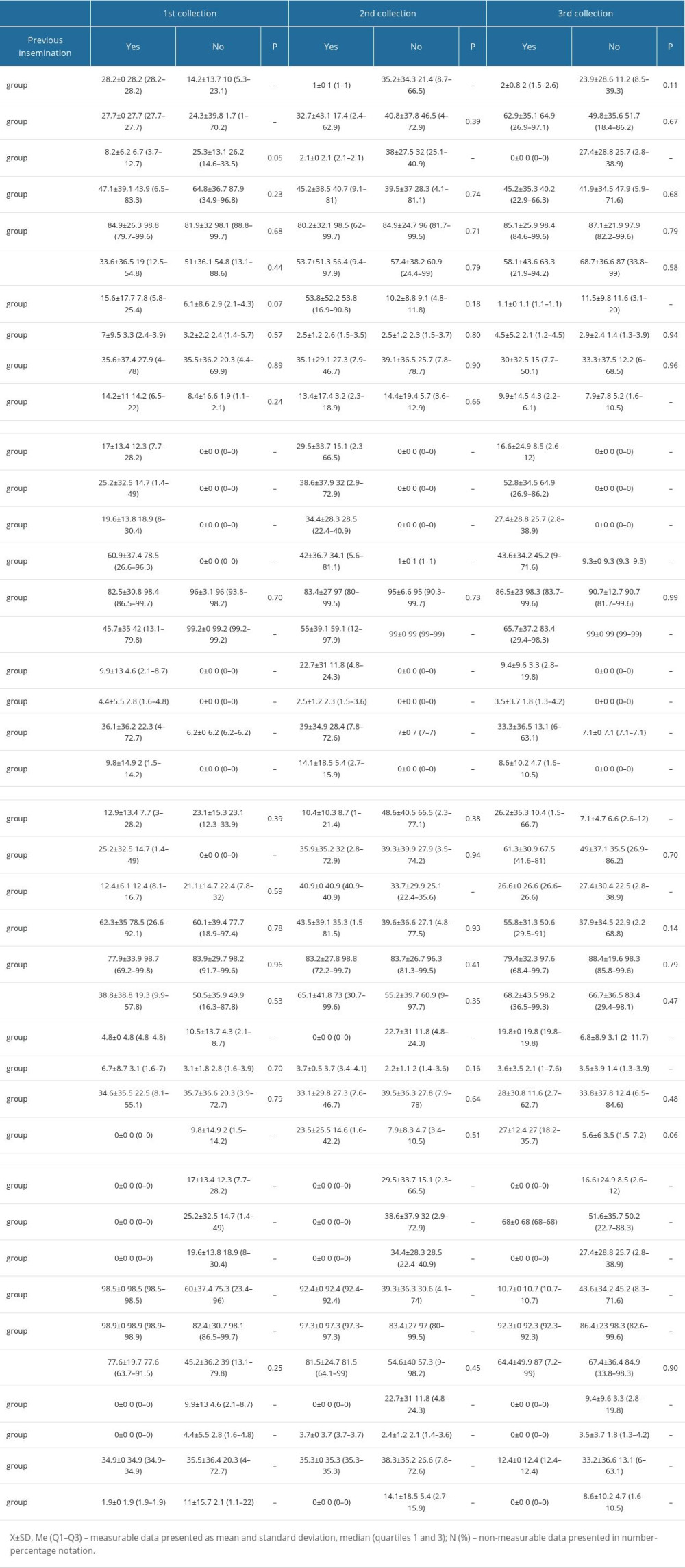 Table 7. Bacterial microbiome percentage correlation table.
Table 7. Bacterial microbiome percentage correlation table.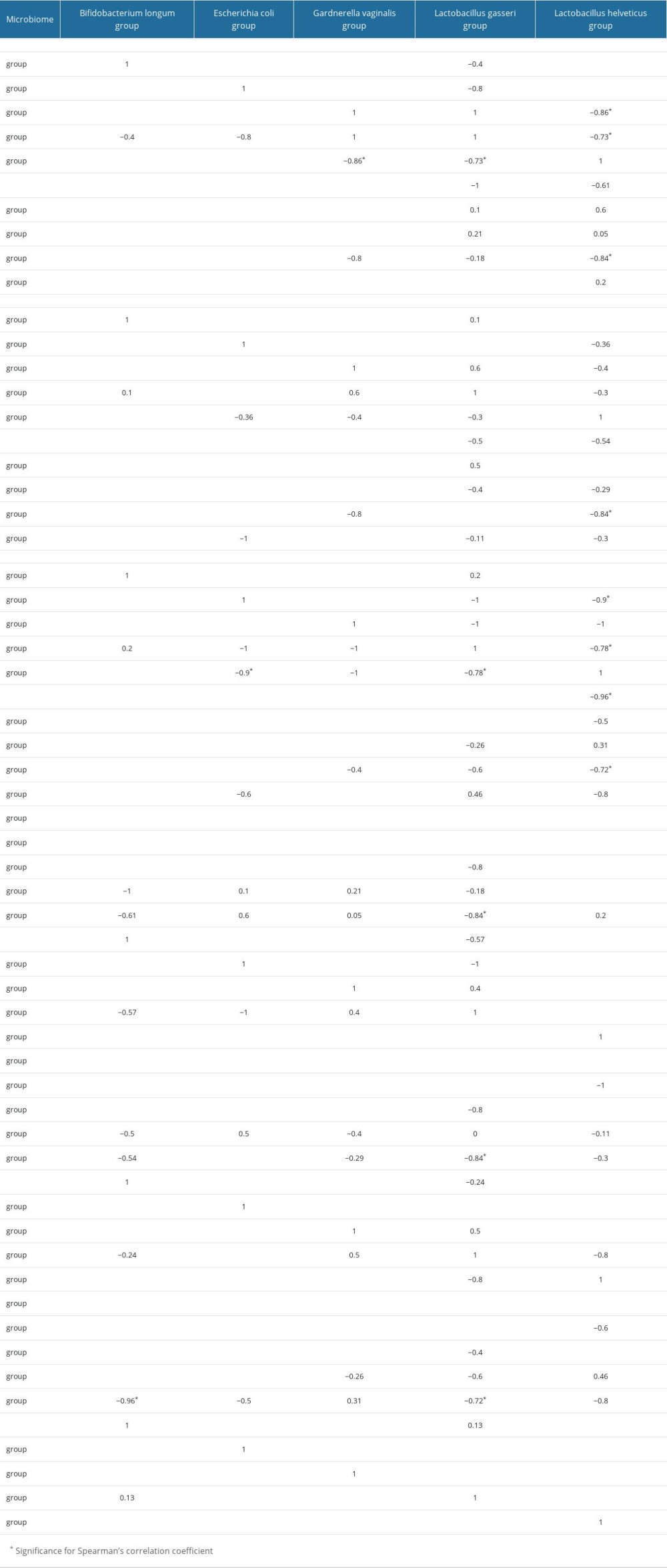 Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration.
Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration.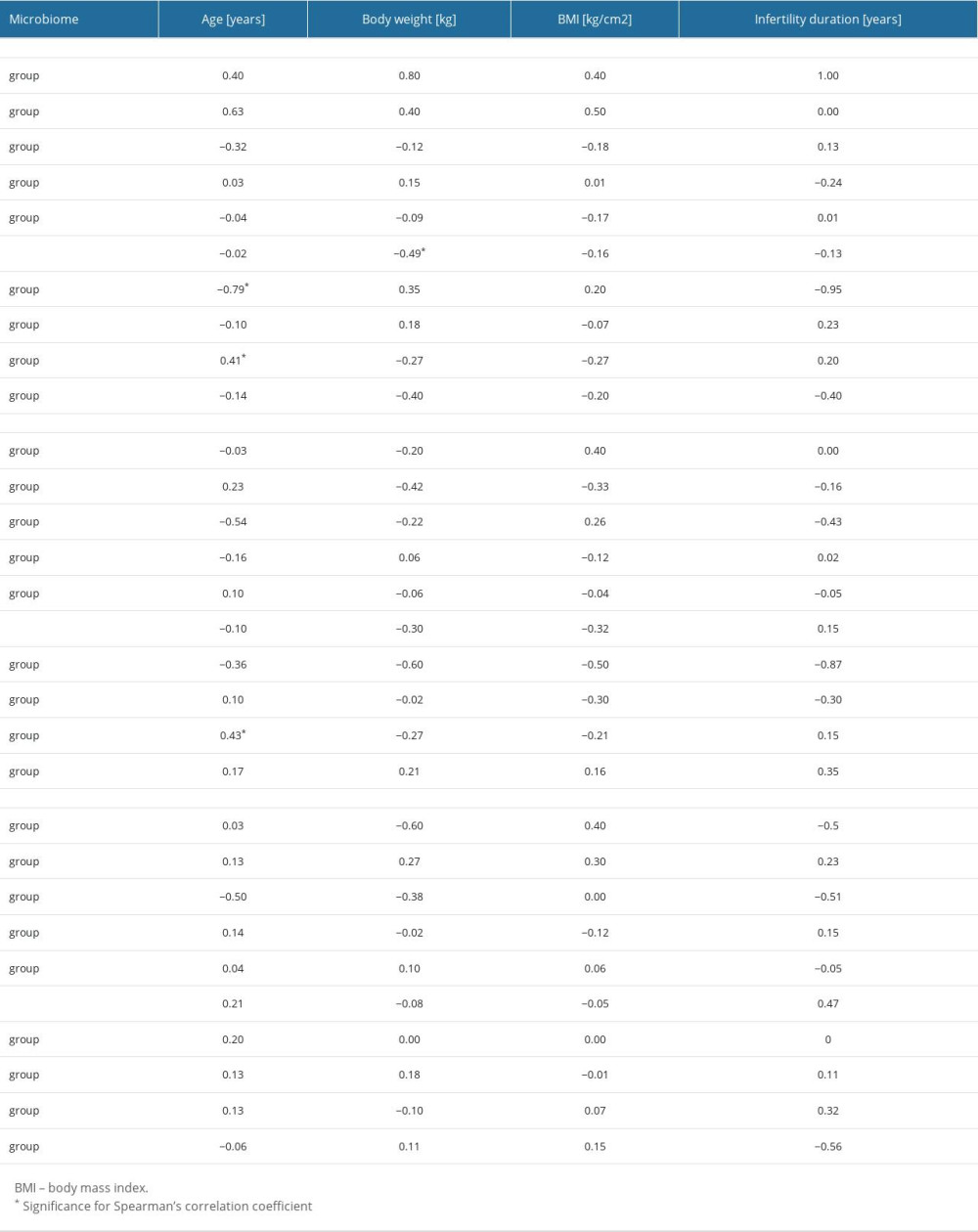 Table 9. Record profile of patients with regard to the type of microbiome.
Table 9. Record profile of patients with regard to the type of microbiome.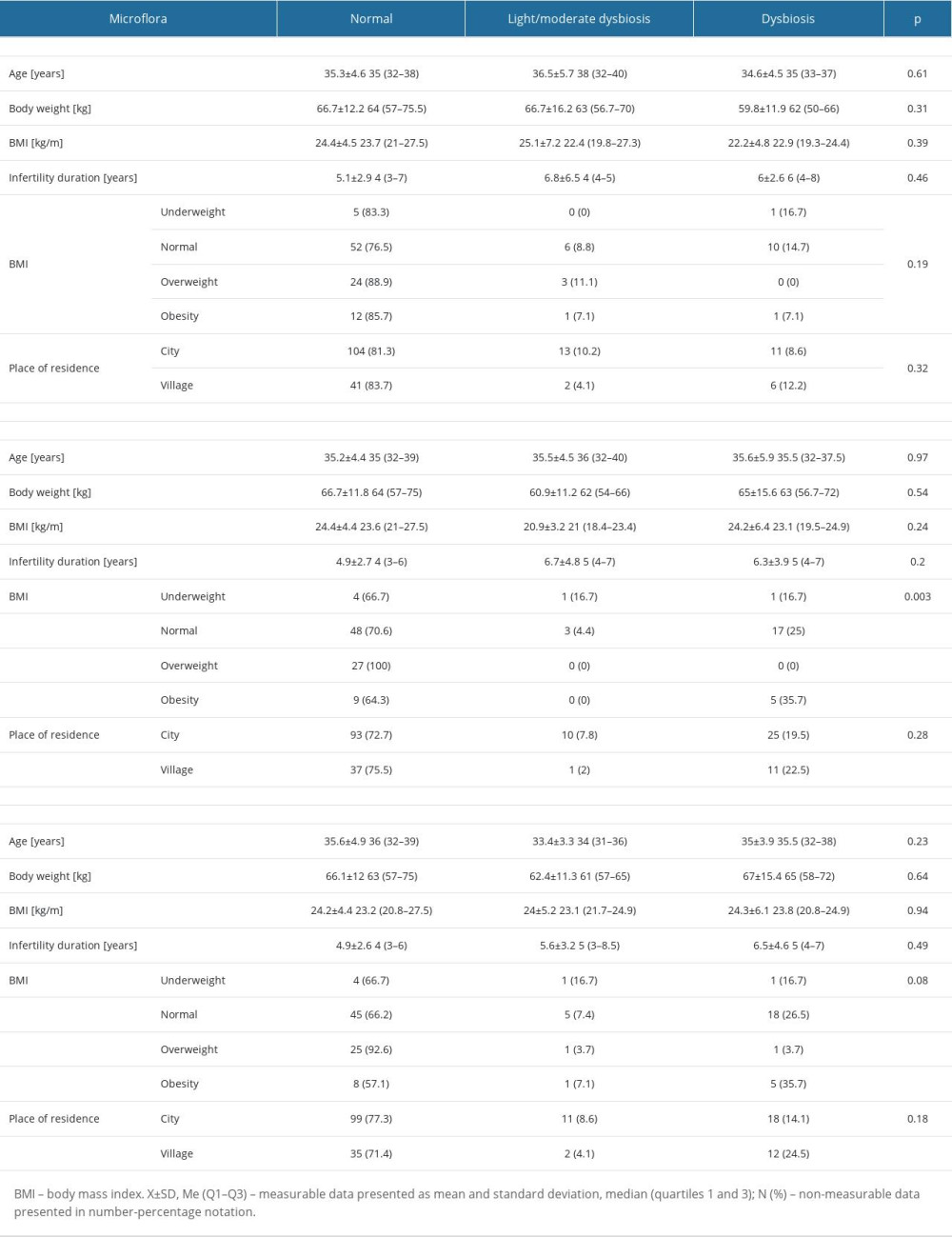 Table 10. Clinical profile of patients in relation to the type of microbiome.
Table 10. Clinical profile of patients in relation to the type of microbiome.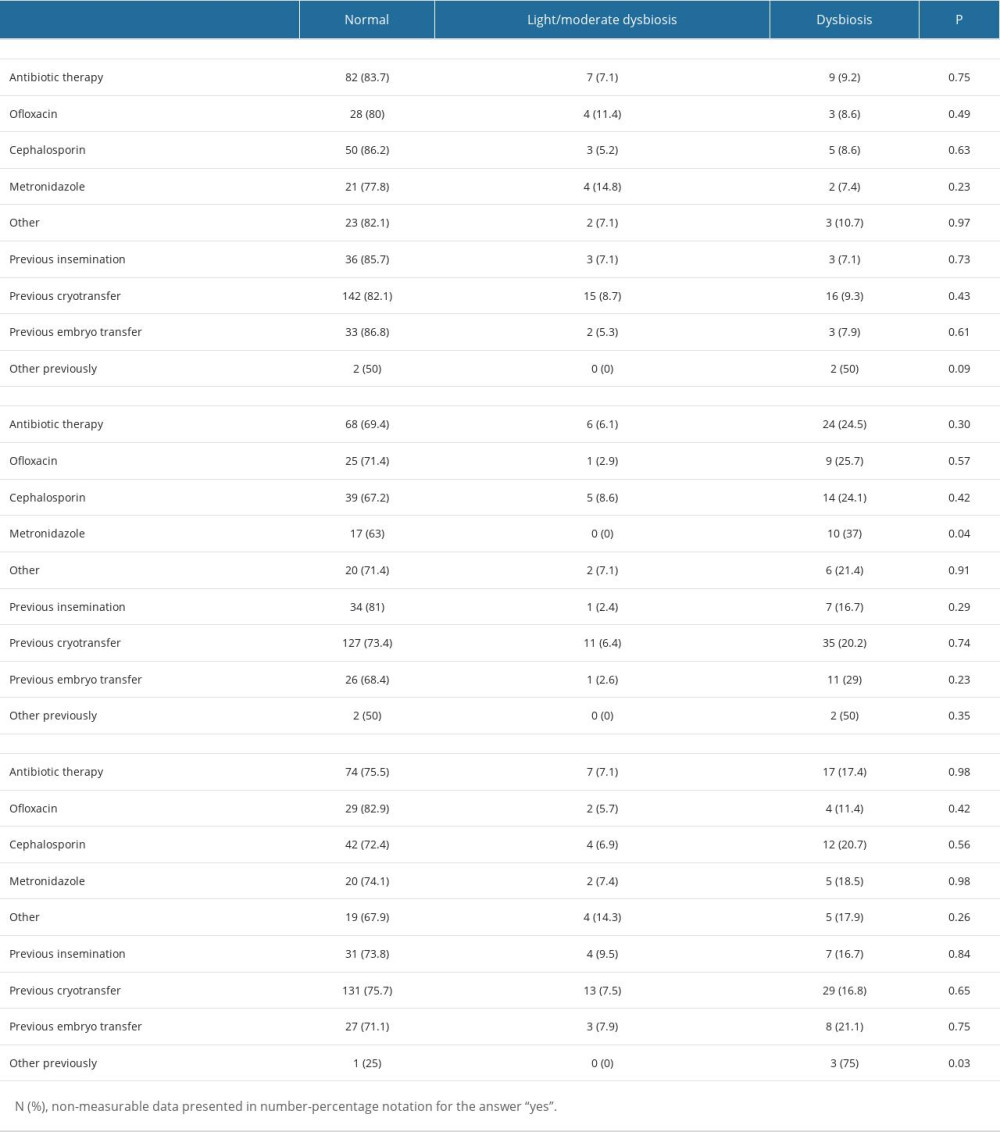
References
1. Yatsenko SA, Rajkovic A, Genetics of human female infertility: Biol Reprod, 2019; 101(3); 549-66
2. Abrao MS, Muzii L, Marana R, Anatomical causes of female infertility and their management: Int J Gynaecol Obstet, 2013; 123(Suppl 2); S18-24
3. Nardelli AA, Stafinski T, Motan T, Assisted reproductive technologies (ARTs): Evaluation of evidence to support public policy development: Reprod Health, 2014; 11(1); 76
4. Szkodziak F, Krzyżanowski J, Szkodziak P, Psychological aspects of infertility. A systematic review: J Int Med Res, 2020; 48(6); 300060520932403
5. Simionescu G, Doroftei B, Maftei R, The complex relationship between infertility and psychological distress (Review): Exp Ther Med, 2021; 21(4); 306
6. Troude P, Santin G, Guibert JDAIFI Group, Seven out of 10 couples treated by IVF achieve parenthood following either treatment, natural conception or adoption: Reprod Biomed Online, 2016; 33(5); 560-67
7. Eskew AM, Jungheim ES, A history of developments to improve in vitro fertilization: Mo Med, 2017; 114(3); 156-59
8. van Loendersloot LL, van Wely M, Limpens J, Predictive factors in in vitro fertilization (IVF): A systematic review and meta-analysis: Hum Reprod Update, 2010; 16(6); 577-89
9. Haahr T, Jensen JS, Thomsen L, Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients: Hum Reprod Oxf Engl, 2016; 31(4); 795-803
10. Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI, The human microbiome and its impacts on health: Int J Microbiol, 2020; 2020; 8045646
11. Valenti P, Rosa L, Capobianco D, Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense: Front Immunol, 2018; 9; 376
12. Moreno I, Franasiak JM, Endometrial microbiota-new player in town: Fertil Steril, 2017; 108(1); 32-39
13. Kyono K, Hashimoto T, Nagai Y, Sakuraba Y, Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study: Reprod Med Biol, 2018; 17(3); 297-306
14. Konstantinov SR, van der Woude CJ, Peppelenbosch MP, Do pregnancy-related changes in the microbiome stimulate innate immunity?: Trends Mol Med, 2013; 19(8); 454-59
15. Integrative HMP (iHMP) Research Network Consortium, The Integrative Human Microbiome Project: Nature, 2019; 569(7758); 641-48
16. Bednarska-Czerwińska A, Czerwiński M, Morawiec E, Marking the profile of the microflora of the endometrium and uterine cervix in women as a potential factor determining the effectiveness of in vitro fertilization: J Clin Med, 2022; 11(12); 3348
17. Vaduva CC, Cilica RM, Nastase A: Eur Rev Med Pharmacol Sci, 2022; 26(16); 5932-38
18. Li R, Du F, Ou S, A new method to rescue embryos contaminated by bacteria: FS Rep, 2022; 3(2); 168-71
19. Ricci S, De Giorgi S, Lazzeri E, Impact of asymptomatic genital tract infections on in vitro Fertilization (IVF) outcome: PLoS One, 2018; 13(11); e0207684
20. Fanchin R, Harmas A, Benaoudia F, Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome: Fertil Steril, 1998; 70(5); 866-70
21. Zeyad A, Hamad M, Amor H, Hammadeh ME, Relationships between bacteriospermia, DNA integrity, nuclear protamine alteration, sperm quality and ICSI outcome: Reprod Biol, 2018; 18(1); 115-21
22. Moretti E, Capitani S, Figura N, The presence of bacteria species in semen and sperm quality: J Assist Reprod Genet, 2009; 26(1); 47-56
23. Kroon SJ, Ravel J, Huston WM, Cervicovaginal microbiota, women’s health, and reproductive outcomes: Fertil Steril, 2018; 110(3); 327-36
24. Lynch SV, Pedersen O, The human intestinal microbiome in health and disease: N Engl J Med, 2016; 375(24); 2369-79
25. Taverniti V, Guglielmetti S: Front Microbiol, 2012; 3; 392
26. Johnson-Henry KC, Hagen KE, Gordonpour M: Cell Microbiol, 2007; 9(2); 356-67
27. Atassi F, Brassart D, Grob P: J Appl Microbiol, 2006; 101(3); 647-54
28. Wee BA, Thomas M, Sweeney EL, A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women: Aust NZJ Obstet Gynaecol, 2018; 58(3); 341-48
29. Moreno I, Codoñer FM, Vilella F, Evidence that the endometrial microbiota has an effect on implantation success or failure: Am J Obstet Gynecol, 2016; 215(6); 684-703
30. Moreno I, Garcia-Grau I, Perez-Villaroya D, Endometrial microbiota composition is associated with reproductive outcome in infertile patients: Microbiome, 2022; 10; 1
31. Koedooder R, Singer M, Schoenmakers S, The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study: Hum Reprod Oxf Engl, 2019; 34(6); 1042-54
32. Bernabeu A, Lledo B, Díaz MC, Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment: J Assist Reprod Genet, 2019; 36(10); 2111-19
33. Diaz-Martínez M, del C, Bernabeu A, Lledó B, Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes: J Clin Med, 2021; 10(18); 4063
34. Toson B, Simon C, Moreno I, The endometrial microbiome and its impact on human conception: Int J Mol Sci, 2022; 23(1); 485
35. Reschini M, Benaglia L, Ceriotti F, Endometrial microbiome: Sampling, assessment, and possible impact on embryo implantation: Sci Rep, 2022; 12; 8467
Tables
 Table 1. Inclusion and exclusion criteria.
Table 1. Inclusion and exclusion criteria. Table 2. Anthropometric data of patients.
Table 2. Anthropometric data of patients. Table 3. Bacterial microbiome composition of the cervix and endometrium.
Table 3. Bacterial microbiome composition of the cervix and endometrium. Table 4. Percentage of the selected bacterial strains by place of residence.
Table 4. Percentage of the selected bacterial strains by place of residence. Table 5. Average percentage of selected bacterial strains by BMI.
Table 5. Average percentage of selected bacterial strains by BMI. Table 6. Average percentage of selected bacterial strains by type of treatment.
Table 6. Average percentage of selected bacterial strains by type of treatment. Table 7. Bacterial microbiome percentage correlation table.
Table 7. Bacterial microbiome percentage correlation table. Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration.
Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration. Table 9. Record profile of patients with regard to the type of microbiome.
Table 9. Record profile of patients with regard to the type of microbiome. Table 10. Clinical profile of patients in relation to the type of microbiome.
Table 10. Clinical profile of patients in relation to the type of microbiome. Table 1. Inclusion and exclusion criteria.
Table 1. Inclusion and exclusion criteria. Table 2. Anthropometric data of patients.
Table 2. Anthropometric data of patients. Table 3. Bacterial microbiome composition of the cervix and endometrium.
Table 3. Bacterial microbiome composition of the cervix and endometrium. Table 4. Percentage of the selected bacterial strains by place of residence.
Table 4. Percentage of the selected bacterial strains by place of residence. Table 5. Average percentage of selected bacterial strains by BMI.
Table 5. Average percentage of selected bacterial strains by BMI. Table 6. Average percentage of selected bacterial strains by type of treatment.
Table 6. Average percentage of selected bacterial strains by type of treatment. Table 7. Bacterial microbiome percentage correlation table.
Table 7. Bacterial microbiome percentage correlation table. Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration.
Table 8. Correlation of bacterial microbiome percentage and metric data and infertility duration. Table 9. Record profile of patients with regard to the type of microbiome.
Table 9. Record profile of patients with regard to the type of microbiome. Table 10. Clinical profile of patients in relation to the type of microbiome.
Table 10. Clinical profile of patients in relation to the type of microbiome. In Press
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952